Abstract
Activation of p38 MAP kinase (p38) as well as JNK/SAPK has been described as being induced by a variety of environmental stresses such as osmotic shock, ultraviolet radiation, and heat shock, or the proinflammatory cytokines tumor necrosis factor-α and interleukin-1 (IL-3). We found that the hematopoietic cytokines erythropoietin (Epo) and IL-3, which regulate growth and differentiation of erythroids and hematopoietic progenitors, respectively, also activate a p38 cascade. Immunoblot analyses and in vitro kinase assay clearly showed that Epo and IL-3 rapidly and transiently phosphorylated and activated p38 in Epo– or IL-3–dependent mouse hematopoietic progenitor cells. p38 can generally be activated by the upstream kinase MKK3 or MKK6. However, in vitro kinase assays in the immunoprecipitates with anti-MKK6 antibody and anti-phosphorylated MKK3/MKK6 antibody showed that activation of neither MKK3 nor MKK6 was detected after Epo or IL-3 stimulation, while osmotic shock clearly induced activation of both MKK3/MKK6 and p38. Together with previous observations, these results suggest that both p38 and JNK cascades play an important role not only in stress and proinflammatory cytokine responses but also in hematopoietic cytokine actions.
MITOGEN-ACTIVATED protein kinases (MAPKs) form a large family of serine-threonine protein kinases activated by separate cascades conserved through evolution.1 In mammalian cells, four distinct MAPK cascades have been identified: the extracellular signal-regulated kinases (ERKs),2,3 c-Jun amino-terminal kinases (JNKs) or stress-activated protein kinases (SAPKs),4,5 p38 MAP kinase (p38) or cytokine suppressive anti-inflammatory drug binding protein (CSBP),6,7 and Erk5/BMK1.8 9 These cascades have become the prototype for the study of structurally related but functionally distinct pathways.
Detailed studies of the JNK and ERK subgroups of MAPK have led to significant insight into the physiological function of these signaling pathways.10-15 In contrast, the role of the p38 signal transduction pathway is poorly understood.7,16-19 The signal transduction pathway leading to p38 activation is related, in part, to a pathway in yeast leading to activation of a MAPK known as Hog1p. To date the activation of this yeast pathway has been shown to occur principally in response to increased extracellular osmolarity,20 and recently two distinct pathways leading to Hog1p activation have been defined in Saccharomyces cerevisiae.21 22
In mammalian cells p38, the Hog1p homologue, is activated by multiple stimuli acting through different receptors. For example, it was shown that p38 is involved in bacterial endotoxin (lipopolysaccharide)-induced cytokine production through the use of pharmacologic inhibitors that are specific for p38.18 p38 is also activated by other bacterial components, proinflammatory cytokines, and physical-chemical changes in the extracellular environments.19 The contribution of the p38 pathway to the cellular response to these stimuli has not been established. However, recent studies have implicated p38 in the phosphorylation of the small heat shock protein Hsp27,7,16 in increased cytokine expression,18 and in programmed cell death.23 In vitro protein kinase assays showed that p38 phosphorylates MAPKAP kinase-27,16 and the transcription factor ATF-2,19 24 and thus these two proteins have been identified as a substrate of p38.
p38 is activated by at least two dual-specific kinases, MKK324,25 and MKK6,25,26 which phosphorylate on Thr and Tyr residues within Thr-Gly-Tyr motif located in subdomain VIII.19 MKK3 and MKK6 phosphorylate and activate p38 but do not phosphorylate the related JNKs or ERKs24 and, therefore, are specific activators of p38. Recently, it was reported that mixed lineage kinase-3 (MLK-3) can activate the p38 and JNK pathways via MKK3/MKK6 and SEK1.27 Furthermore, the Rho family GTPases Rac1 and Cdc4228-32 and the STE20-related protein kinases PAK-1,32 PAK-3,28 and GC kinase33 have been implicated in the p38 and JNK signaling pathways. However, the other components of the p38 pathway have not been identified.
The p38 and JNK cascades are primarily activated by various environmental stresses: osmotic shock, ultraviolet radiation, heat shock, x-ray radiation, hydrogen peroxide and protein synthesis inhibitors, and by the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1).7,16-19,34-38 It can also be weakly activated by such mitogenic factors as epidermal growth factor and phorbol esters, and by T-cell activation signaling.39 40 The exact mechanism of how the p38 and JNK cascades integrate with other signaling pathways to achieve specific response to different stimuli remains to be elucidated.
The hematopoietic cytokine receptor-mediated signaling pathways have been extensively studied, and activation of ERK by various hematopoietic cytokines has been evidenced.41-46 We also recently showed that JNK cascade can be activated by hematopoietic cytokines,47 although possible involvement of p38 cascade in hematopoietic cytokine signal transduction has not been determined. Therefore, we examined the possible activation of MKK3/MKK6 and p38 by erythropoietin (Epo) and IL-3, which are hematopoietic cytokines regulating the growth and differentiation of erythroids and hematopoietic progenitors, respectively. Using Epo-dependent FD-EPO cells, which are derived from IL-3–dependent mouse hematopoietic progenitor FDC-P2 cells, we measured the activities of p38 and MKK3/MKK6 after Epo and IL-3 stimulation. We found that Epo and IL-3 induced activation of p38, but could not detect the activation of either MKK3 or MKK6. Taken together with previous observations, these results suggested that p38 as well as JNK cascades represent an important signaling pathway that mediates the actions of hematopoietic cytokines as well, and that hematopoietic cytokines might activate p38 and JNK cascades through a kinase other than MKK3, MKK6, and SEK1/MKK4.
MATERIALS AND METHODS
Cytokines and antibodies.Antibody against p38 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibodies against Ser189/207-phosphorylated MKK3/MKK6 and against Thr223-phosphorylated SEK1/MKK4 were purchased from New England Biolabs (Beverley, MA). Human Epo (2.6 × 105 U/mg) was a gift of Kirin Brewery (Tokyo, Japan). Mouse IL-3 (1 × 106 U/mg) was obtained from Genzyme (Cambridge, MA). Polyclonal anti-MKK6-specific antibody was prepared as described.48
Cell culture.Epo-dependent FD-EPO cells, which were derived from IL-3–dependent FDC-P2 cells as previously described,47 were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 0.5 U/mL of human Epo. FDC-P2 cells were maintained with 500 U/mL of mouse IL-3.
Immunoprecipitation and immunoblotting.Cells were starved in RPMI 1640 medium containing 0.4% FCS, 0.125 μg/mL of transferrin, and 0.01% bovine serum albumin without Epo or IL-3 for 12 hours, and restimulated with or without 0.5 U/mL of Epo or 500 U/mL of IL-3 for up to 60 minutes. The stimulated and unstimulated cells were immediately lysed in a lysis buffer: 50 mmol/L Tris-HCl, pH 7.5, 0.5% Nonidet P-40 (Calbiochem, La Jolla, CA), 150 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 2 mmol/L Pefabloc (Boehringer Mannheim, Mannheim, Germany), 10 ng/mL leupeptin, and 10 ng/mL aprotinin. Insoluble material was then removed by centrifugation and the precleared cell lysate was incubated with a specific antibody at 4°C for 2 hours. The immunocomplexes were then bound to protein A-Sepharose (Pharmacia, Uppsala, Sweden) at 4°C for 1 hour. The beads were washed five times with lysis buffer containing 0.1% Nonidet P-40 before being boiled in Laemmli sample buffer. Samples were fractionated in 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred to ECL membrane (Amersham, Buckinghamshire, UK). The membrane was blocked in 5% bovine serum albumin (BSA) in 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.5% Tween 20 (TBS-T), and incubated with anti-phosphotyrosine antibody or anti-p38 antibody for 2 hours. After washing three times with TBS-T, the membrane was incubated with antimouse or antirabbit IgG conjugated horseradish peroxidase antibody, and the antibody complexes were visualized by an ECL system (Amersham).
Preparation of substrate proteins.Glutathione-S-transferase (GST)-human ATF-2 fusion protein (amino terminal domain corresponding to amino acids 1 to 96) was obtained from Santa Cruz Biotechnology. His-tagged human p38 in pET28a vector was constructed as described,50 and the plasmid was transfected into BL21(DE3)pLysS. The bacteria grew at OD600 = 0.7 and was incubated with 0.5 mmol/L isopropyl β-D-thiogalactopyranoside for 4 additional hours. Cells were suspended in IMAC-5 (20 mmol/L Tris-HCl, pH 7.9, 500 mmol/L NaCl, 10% glycerol, 5 mmol/L imidazole), and sonicated. To purify the His-p38 protein in the lysates, 1 mL of 50% slurry of His-Bind Resin (Novagen, Madison, WI) was added to 20 mL cell extract and mixed at room temperature for 60 minutes. The His-Bind Resin was washed with 10 vol of IMAC-5, and washed sequentially with 4 vol of each 10% IMAC-200 (20 mmol/L Tris-HCl, pH 7.9, 500 mmol/L NaCl, 10% glycerol, 200 mmol/L imidazole)/90% IMAC-5, 20% IMAC-200/80% IMAC-5, and finally 30% IMAC-200/70% IMAC-5. The bound proteins were eluted with 4 vol of 50% IMAC-200/50% IMAC-5. The amounts of purified fusion proteins were estimated by the method of Bradford.51
In vitro protein kinase assay.Immunoprecipitates with anti-p38 antibody, anti-MKK6 antibody, or anti-phosphorylated MKK3/MKK6 antibody were mixed with 1 μg of purified substrates, either GST-ATF-2 or His-p38, in 20 μmol/L adenosine triphosphate (ATP) and 5 μCi of [γ-32P]ATP in 30 μL of kinase buffer (25 mmol/L HEPES, pH 7.4, 25 mmol/L β-glycerophosphate, 25 mmol/L MgCl2 , 0.1 mmol/L sodium orthovanadate, 2 mmol/L dithiothreitol [DTT]), and incubated at 30°C for 30 minutes. The reactions were terminated by mixing with Laemmli sample buffer and boiling. The samples were resolved by 10% SDS-polyacrylamide gel electrophoresis, and autoradiographed.
RESULTS
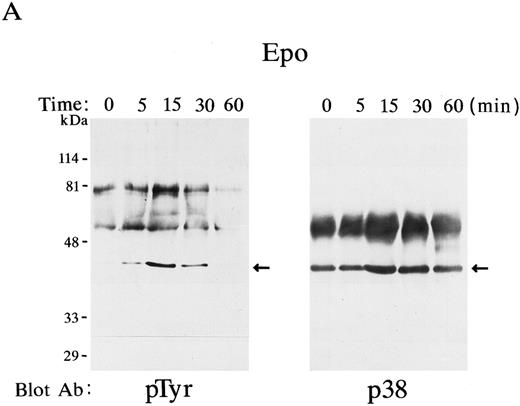
p38 was phosphorylated by Epo and IL-3 stimulation.Possible p38 phosphorylation was examined in Epo-stimulated FD-EPO cells. This cell line expresses endogenous Epo receptors and responds with Epo in a dose-dependent manner. Figure 1A shows the time course of p38 phosphorylation after Epo stimulation. p38 immunoprecipitated with anti-p38–specific antibody was immunoblotted with antiphosphotyrosine antibody 4G10. It was found that p38 was rapidly and transiently tyrosine-phosphorylated by Epo stimulation (Fig 1A, left panel). Little tyrosine-phosphorylated p38 was detected before stimulation, the level of tyrosine-phosphorylation reached the maximum at 15 minutes after Epo stimulation and decreased thereafter (Fig 1A, left panel). The blot was reprobed by the anti-p38 antibody to ensure that equal amounts of p38 were immunoprecipitated during the separation of p38 from the cell lysates (Fig 1A, right panel); it was confirmed that this was the case.
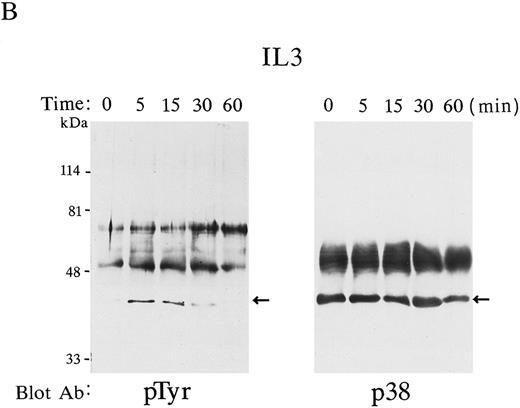
p38 was phosphorylated by Epo and IL-3 stimulation. p38 was immunoprecipitated at various time points (0 to 60 minutes) in Epo-stimulated FD-EPO cell lysates (A) or IL-3–stimulated FDC-P2 cell lysates (B). The immunoprecipitates were immunoblotted with antiphosphotyrosine antibody 4G10 (left panels) or with p38 antibody (right panels). Arrows indicate the phosphorylated p38 (left panels) and total p38 (right panels).
p38 was phosphorylated by Epo and IL-3 stimulation. p38 was immunoprecipitated at various time points (0 to 60 minutes) in Epo-stimulated FD-EPO cell lysates (A) or IL-3–stimulated FDC-P2 cell lysates (B). The immunoprecipitates were immunoblotted with antiphosphotyrosine antibody 4G10 (left panels) or with p38 antibody (right panels). Arrows indicate the phosphorylated p38 (left panels) and total p38 (right panels).
Possible p38 phosphorylation was similarly examined in IL-3–stimulated FDC-P2 cells. Figure 1B shows the time course of p38 phosphorylation after IL-3 stimulation; clearly p38 was rapidly and transiently tyrosine-phosphorylated by this stimulation (Fig 1B, left panel). The maximal level of tyrosine-phosphorylation was detected after 15 minutes (Fig 1B, left panel), and it was confirmed that equal amounts of p38 were immunoprecipitated (Fig 1B, right panel).
In vitro kinase assay showed that Epo and IL-3 activate p38.Next, we examined in vitro p38 activity in the cell lysates after Epo or IL-3 stimulation. The p38 was immunoprecipitated by anti-p38–specific antibody at various time points after Epo or IL-3 stimulation, and the protein kinase activity in the immunoprecipitates was measured in the presence of [γ-32P]ATP and the purified GST-ATF-2 protein (molecular weight, 40 kD) as a substrate.
As shown in Fig 2, both Epo and IL-3 rapidly and transiently activated p38. p38 activity was rarely seen in unstimulated cells, but a rapid and marked increase in the activity was observed within 5 minutes of treatment with Epo (Fig 2A) or IL-3 (Fig 2B). The activity then reached the maximal level at 15 minutes and decreased thereafter in both cases (Fig 2A and B). Thus, both Epo and IL-3 rapidly and transiently induce phosphorylation and activation of p38.
In vitro p38 activity is induced by Epo and IL-3 stimulation. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with anti-p38–specific antibody were incubated with [γ-32P]ATP and GST-ATF-2 as a substrate. Arrows indicate the phosphorylated GST-ATF-2 (molecular weight, 40 kD).
In vitro p38 activity is induced by Epo and IL-3 stimulation. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with anti-p38–specific antibody were incubated with [γ-32P]ATP and GST-ATF-2 as a substrate. Arrows indicate the phosphorylated GST-ATF-2 (molecular weight, 40 kD).
Activation of MKK6 was not detected after Epo or IL-3 stimulation.It has been reported that MKK3 and MKK6 phosphorylate and activate p38,24 and thus we sought to learn whether or not either of these kinases is indeed activated upon Epo or IL-3 stimulation. The protein kinase activity in the immunoprecipitates with anti-MKK6-specific antibody was measured in the presence of [γ-32P]ATP and the purified His-p38 as a substrate (Fig 3). His-p38 could phosphorylate itself without the immunoprecipitates (Fig 3A through C, lane C). At various time points after Epo or IL-3 stimulation, the levels of phosphorylated His-p38 did not change but were the same level as lane C (Fig 3A and B); in contrast, MKK6 activity was clearly enhanced by osmotic shock (Fig 3C). Therefore, in these assays we detected no MKK6 activation after Epo or IL-3 stimulation.
In vitro MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. MKK6 activity was measured in the immunoprecipitates with antispecific MKK6 antibody in the presence of [γ-32P]ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.
In vitro MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. MKK6 activity was measured in the immunoprecipitates with antispecific MKK6 antibody in the presence of [γ-32P]ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.
Similarly, the protein kinase activity in the immunoprecipitates with antiphosphorylated-MKK3/MKK6 antibody was measured with His-p38 as a substrate (Fig 4). This antibody can immunoprecipitate both Ser189-phosphorylated MKK3 and Ser207-phosphorylated MKK6. Once again, the MKK3 and/or MKK6 activities at various time points after Epo or IL-3 stimulation were the same as those without the immunoprecipitates (Fig 4A and B), whereas MKK3 and/or MKK6 activity was clearly induced by osmotic shock (Fig 4C). Because MKK3-specific antibody, which can be used for in vitro protein kinase assay, is not available at present, we used these two antibodies. Although we may not be able to completely eliminate the possibility that Epo and IL-3 weakly activate MKK3 and/or MKK6, it is possible that p38 is activated by a kinase other than these two.
In vitro MKK3/MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with antiphosphorylated MKK3/MKK6 antibody were incubated in the presence of [γ-32P] ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.
In vitro MKK3/MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with antiphosphorylated MKK3/MKK6 antibody were incubated in the presence of [γ-32P] ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.
We concluded that hematopoietic cytokines, at least Epo and IL-3, clearly induce activation of p38, though the primary activation may not be by MKK3 or MKK6, and that the p38 signaling pathway plays an important role not only in the response to environmental stresses and proinflammatory cytokines, but also to hematopoietic cytokines.
DISCUSSION
We showed in this report that hematopoietic cytokines, at least Epo and IL-3, whose receptors belong to the type I cytokine superfamily, clearly activate the p38 signaling pathway, which has heretofore been believed to be activated only by the environmental stresses of osmotic shock, UV radiation and heat shock, or by proinflammatory cytokines like TNF-α and IL-1.7,16-19 We also observed that thrombopoietin phosphorylates and activates p38 (data not shown). Hematopoietic cytokines reportedly activate the ERK cascade,41-46 and we recently showed that the JNK cascade is also activated by these cytokines.47 Thus, it appears that hematopoietic cytokines simultaneously activate the entire known MAPK family, ERK cascade, JNK cascade, and p38 cascade.
We observed that p38 was clearly activated by hematopoietic cytokines, but activation of neither MKK3 nor MKK6 was detected after IL-3 and Epo stimulation. It has also been reported that epidermal growth factor and nerve growth factor induced activation of neither MKK324 nor MKK6,52 while p38 was clearly activated.19 Although we may not be able to completely eliminate the possibility that MKK3 and/or MKK6 partially activates p38 in these hematopoietic cytokine-stimulated cells, it is possible that a kinase other than one of these is mainly involved in the activation due to factors such as epidermal growth factor, nerve growth factor, and hematopoietic cytokines.
It was reported that a novel hematopoietic-specific protein kinase, hematopoietic progenitor kinase 1 (HPK1), activates JNK cascade.53 Although ubiquitously expressed MKK3, MKK6, and SEK1/MKK4 may also act as upstream kinases of p38 and JNK cascades in hematopoietic cells, other unidentified hematopoietic-specific kinases may exist that activate p38 and/or JNK cascades in a hematopoietic cytokine-specific manner. The MKK that specifically activates p38 and/or JNK cascades in hematopoietic cells remains to be identified.
Cellular stresses and inflammatory cytokines that activate the p38 and JNK pathways reportedly induce cell death characteristic of apoptosis.54,55 In PC12 cells, dominant-interfering or constitutively activated forms of various components of the p38, JNK, and ERK signaling pathways showed that activation of p38 and JNK and concurrent inhibition of ERK are critical for induction of apoptosis.23 However, TNF-α–induced apoptosis was not affected by a specific p38 inhibitor SB203580 in L929 cells.56 Dominant negative JNK or SEK1 also did not affect apoptosis in 3T3 cells.57 The targets of hematopoietic cytokine-induced p38 and the JNK signaling pathway, and the role of the p38 and JNK signaling cascade in hematopoietic cytokine actions, ie, cell differentiation, proliferation, tissue-specific functions, inhibition or stimulation of apoptosis and/or cell survival, require clarification.
ACKNOWLEDGMENT
The authors thank Dr M Hibi (Osaka University, Osaka, Japan) for valuable discussions, Kirin Brewery for Epo, and C. Hisano and I. Mogi for their technical assistance.
Supported in part by a Special Grant for Promotion of Research from The Institute of Physical and Chemical Research (RIKEN).
Address reprint requests to Kazuo Todokoro, PhD, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305, Japan.

![Fig. 2. In vitro p38 activity is induced by Epo and IL-3 stimulation. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with anti-p38–specific antibody were incubated with [γ-32P]ATP and GST-ATF-2 as a substrate. Arrows indicate the phosphorylated GST-ATF-2 (molecular weight, 40 kD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.929/4/m_bl_0066f2.jpeg?Expires=1769127268&Signature=HiACeNTUzUVyCTqVge~mRvU0UF3607bhGQ7-IHWw27ijddsIpOKdEOpEZqp43SQQAbMw0ZdHvi0Ofno1S~jIOWNyPnFhQaRgd64al8fOtyhjoSJhMqP16hFdpZ4SeCDc4Qfmsenz1imYtU1nxtDQx1oUanONcEP1QP60AW5tG6mqQV5d8DaBo~SYSOr95cYEUfQpAX8hqgphMX8tEqH3CrXi3iwpuR22ALfzjnt~r3YZ2GC7nLzPoHGTYShkGh7rZKBKs8nXlmPq~ghLfWto3IEEWPRySEMcbP9zsDeZSOjOUMHrNWgcCHm06qqWjpsPLBHjbUmM4A9rQ-lamT9q5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. In vitro MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. MKK6 activity was measured in the immunoprecipitates with antispecific MKK6 antibody in the presence of [γ-32P]ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.929/4/m_bl_0066f3.jpeg?Expires=1769127268&Signature=jhVPflfzRB0KXvq8rHAzl~Zza1M56pHagYLCqgMJyKjaG7YrTKtRNGfsXlLoop~U9mVQykWot-aiHpdRwXMDP2a7LNFRpIcGcfh0XCtONilUeNLwJ3TrNwpFoP-U9OZrNlkxBQpasGFVpn9uTSXLl~57y2kgzaL8FioAZlBAw-o0PJ7eZUQIqFgS1uMEw3oMQ6M4Jdu8VCBw8rP14cIths8~GZXqb1NGXNhNueLc~oZepDfcD6gqOrHwlXPKnjnF-lFSMvcVQMxK4tOFej426OKYGuc2jAXEM1m76Cw7vKJKCePx7eu2gHke0mBAIFLuQRM3Pu6sZEtkJrvAGXMxBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. In vitro MKK3/MKK6 assay. FD-EPO cells (A) and FDC-P2 cells (B and C) were stimulated with Epo (A) and IL-3 (B), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) NaCl (C) for 30 minutes. The immunoprecipitates with antiphosphorylated MKK3/MKK6 antibody were incubated in the presence of [γ-32P] ATP and His-p38 as a substrate. Lane C, the kinase assay was performed without immunoprecipitates (only His-p38). Arrows indicate the phosphorylated His-p38.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.929/4/m_bl_0066f4.jpeg?Expires=1769127268&Signature=UaoKJ9gxO8028Og1csIcEvuqnq1oe5eysz7Lh3qvkRx7T7OZCm9JDwDhojXtEC6nlAU2XPTjmHwpmaydOiO9HT7F5MqX7NGVCedDOlGW7NsnxE8J5CP~qJOomFSFOMFPReO~g-YJzw1nkTUXcdbpyu5KCmn9739D7F5GrTSaO29OkXuEjUEawkI7KEZeM~k2nyTpPvxInVM4-JwUwt4fk-MZuQOV4o1-9PqobMsz1CUgklpOmmIwRvQBI-ruE5srNF5laKYfhJQxCjI6cmqCXeYZncJ6DrvwOcEB4jiTz9b74~pGdYrw3P04hVJNLRSX5vNo43ZRGWCzDgpAtU0t3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal