Abstract
Hematopoietic progenitor cells (HPC) from mice nullizygous at the Fanconi anemia (FA) group C locus (FAC −/−) are hypersensitive to the mitotic inhibitory effects of interferon (IFN-γ). We tested the hypothesis that HPC from the bone marrow of Fanconi group C children are similarly hypersensitive and that the fas pathway is involved in affecting programmed cell death in response to low doses of IFN-γ. In normal human and murine HPC, IFN-γ primed the fas pathway and induced both fas and interferon response factor-1 (IRF-1) gene expression. These IFN-γ-induced apoptotic responses in HPC from the marrow of a child with FA of the C group (FA-C) and in FAC −/− mice occurred at significantly lower IFN doses (by an order of magnitude) than did the apoptotic responses of normal HPC. Treatment of FA-C CD34+ cells with low doses of recombinant IFN-γ, inhibited growth of colony forming unit granulocyte-macrophage and burst-forming unit erythroid, while treatment with blocking antibodies to fas augmented clonal growth and abrogated the clonal inhibitory effect of IFN-γ. Transfer of the normal FAC gene into FA-C B-cell lines prevented mitomycin C–induced apoptosis, but did not suppress fas expression or inhibit the primed fas pathway. However, the kinetics of Stat1-phosphate decay in IFN-γ–treated cells was prolonged in mutant cells and was normalized by transduction of the normal FAC gene. Therefore, the normal FAC protein serves, in part, to modulate IFN-γ signals. HPC bearing inactivating mutations of FAC fail to normally modulate IFN-γ signals and, as a result, undergo apoptosis executed through the fas pathway.
FANCONI ANEMIA (FA) is an autosomal recessive disorder characterized by cellular hypersensitivity to chemical cross-linking agents, bone marrow (BM) failure, diverse congenital anomalies, and a marked increase in the incidence of acute myelogenous leukemia.1-4 Phenotypically, the sine qua non of this disorder is hypersensitivity of FA cells to DNA cross-linking agents such as diepoxybutane and mitomycin C.5,6 The disorder is genetically heterogeneous, with at least five different complementation groups having been identified by somatic cell hybrid analysis.7,8 The group A, C, and D genes have been localized to chromosomes 16q24.3, 9q22.3, and 3p, respectively, but the cDNAs for only the group C (FAC) and the group A genes9,10 have been cloned and sequenced.7,11-15 The FAC gene is constitutively expressed in most cells,16 and encodes a 63-kD protein17,18 that has no strong amino acid sequence homology with any known gene family and is of unknown function. Although it is clear that the protein plays some role in either facilitating repair of cross-linked DNA or resisting the effects of cross-linking agents on nuclear DNA, the protein is largely cytoplasmic17,18 and at least some critical function of the gene product requires cytoplasmic localization.19
The product of the FAC gene clearly plays a supportive role in growth and/or differentiation of hematopoietic progenitor cells.20,21 We recently described a murine FA model in which FAC −/− mice with a targeted deletion of exon 9 of the murine FAC gene were found to have a progressive decline in colony-forming capacity of committed progenitor cells. We also found that low doses of interferon-γ (IFN-γ) (doses that had minimal effects on cells from FAC −/+ mice) potently inhibited clonal growth of burst-forming unit erythroid (BFU-E) and colony-forming unit granulocyte-macrophage (CFU-GM) in FAC −/− mice.22
IFN-γ suppresses growth of committed progenitor cells and long-term culture-initiating cells (LTCIC)23-25 and has been proposed as an important mediator of aplastic anemia.26-31 IFN-γ induces progenitor cells to undergo programmed cell death,31,32 at least in part through its capacity to induce fas expression in these cells.31,33,34Fas, a 43- to 48-kD a membrane glycoprotein member of the tumor necrosis factor/nerve growth factor (TNF/NGF ) receptor superfamily,35,36 induces apoptosis when engaged by either its ligand (fas-L, a type II membrane protein homologous to members of the TNF family)37,38 or by specific anti-fas antibodies that mimic the effects of fas-L.39-41Fas expression alone is insufficient to account for an apoptotic cellular response. Other peptides are required, including FADD42,43 and FLICE,44,45 and at least in lymphocytes, downstream signals that include interleukin-1B (IL-1B) converting enzyme (ICE) and CPP32.46 47 The ordered activation of these death mediators is less well defined in hematopoietic progenitor cells exposed to IFN-γ. In addition, the inductive effect of IFN-γ on fas expression may not be sufficient to account for the overall effect of IFN-γ on the activation of the fas pathway. Accordingly, it is appropriate to describe the overall effect of IFN-γ as fas “priming.”
There is indirect evidence to suggest that the fas pathway is involved in the pathophysiology of acquired BM failure. Specifically, fractional expression of fas by CD34+ cells from aplastic anemia patients is higher than in CD34+ cells from normal volunteers.48 Three of the aplastic patients reported in that study were children with FA,48 suggesting that: (1) production of fas inducing cytokines is increased in such children, (2) FA group C progenitors and stem cells constitutively express fas, or (3) FA group C progenitor cells and stem cells express high levels of fas because they are hypersensitive to inductive cytokines. Because we have recently discovered that hematopoietic progenitor cells of FAC −/− mice are hypersensitive to IFN-γ,22 we performed studies designed to test the third model.
We hypothesize that BM failure in children with FA is a result of cumulative losses of progenitors and stem cells, in part, due to their IFN-γ hypersensitive phenotype. If this model is correct, it would follow that: (1) IFN-γ hypersensitivity should be present in human FA-C progenitors, (2) IFN-γ–induced priming of the fas pathway would occur at lower-than-expected doses of IFN in vitro and in vivo, (3) a higher than normal fraction of FAC progenitor cells would express IFN-γ–inducible apoptosis effector genes fas and interferon response factor-1 (IRF-1) constitutively as a result of exposure to low endogenous levels of IFN-γ in vivo, and (4) the product of the FAC gene should directly modulate IFN-γ signals. The studies on both murine and human FA-C cells described below have confirmed each one of these predictions.
MATERIALS AND METHODS
FAC Nullizygous Mice
FAC-deficient mice, homozygous for the targeted deletion of exon 9 of the murine FA complementation group C gene on a mixed genetic background of C57BL and 129Sv, were generated as described.22
Murine BM, Liver, and Spleen Cell Cultures
Samples were obtained from an equal number of FAC −/− and FAC −/+ mice for every experiment. Femoral marrow samples were obtained from mice after cervical dislocation, washed twice, and resuspended in RPMI 1640 (Life Technologies, Grand Island, NY) for viable cell counts (trypan blue dye exclusion). Liver and spleen cells were prepared by forcing the organs through sterile screens followed by sequential passage through a series of 21-, 23-, and 25-gauge needles to yield single cell suspensions. Cells were washed twice and resuspended in RPMI 1640 for viable cell counting using trypan blue.
Unfractionated murine marrow cells (1 × 105) or murine spleen cells (1.25 × 105) were cultured in 1 mL of MethoCult H4230 (Stem Cell Technologies, Vancouver, BC, Canada), penicillin-streptomycin (Life Technologies), and three recombinant growth factors: human erythropoietin (2 U/mL; Amgen, Thousand Oaks, CA), murine Steel factor (10 ng/mL; R & D Systems, Minneapolis, MN), and murine IL-3 (10 ng/mL; R & D Systems). CFU-GM and BFU-E were cultured in 35-mm tissue culture dishes at 37°C in 5% CO2 in air and counted after 7 and 14 days, using a dissecting microscope. Colony growth results were expressed as mean (of triplicate plates) ±SD colonies and bursts per plate. Between-group comparisons were made using one-way analysis of variance.
Mitotic inhibitory factors were added, in each experiment, to methylcellulose cultures containing growth factors. Multiple doses of recombinant murine IFN-γ (0.05 to 10 ng/mL; Genzyme, Cambridge, MA) were tested. In some cases, an agonistic hamster antimurine fas antibody (clone Jo2; PharMingen, San Diego, CA) was added at a concentration of 500 ng/mL for 3 hours with IFN-γ before plating the cells in methylcellulose.
Human CD34+ Marrow Cells
BM cells were obtained under a protocol of informed parental consent from a patient with FA-C on three separate occasions over a period of 14 months and from adult normal volunteers. Low-density human BM cells were prepared using Ficoll-Paque (Pharmacia Piscataway, NJ).20 CD34+ cells were isolated using magnetic microspheres covalently linked to antihuman CD34+ Ig (Miltenyi Biotec, Inc, Auburn, CA) according to the manufacturer's instructions. Human colony growth assays using CD34+ cells (5 × 103/mL) were carried out in methylcellulose medium, as above, using the following recombinant human growth factors: Steel factor (50 ng/mL; R & D Systems), IL-3 (10 ng/mL; R & D Systems), and erythropoietin (2 U/mL; Amgen).
Epstein-Barr Virus (EBV)-Immortalized Cell Lines
The EBV-transformed lymphoblast cell line HSC536N (gift of Manuel Buchwald, The Hospital for Sick Children, Toronto, Ontario, Canada) was derived from peripheral blood cells of a child with FA-C. In the cells of this patient, one FAC allele is deleted and the other carries a leucine-to-proline substitution at amino acid position 554. The EBV-transformed cell line JY was derived from EBV-infected normal peripheral blood mononuclear leukocytes. HSC536N/FAC cells were derived by transducing the HSC536N cells with a retrovirus (pLFACSN) encoding both FAC and neomycin phosphotransferase (neo) (see below). The HSC536N/neo cells were derived from the same parent cells by transduction with the pLXSN retroviral vector which encodes neomycin phosphotransferase alone.49 Similar isogenic B-cell lines were generated from the peripheral blood cells of two unrelated children with FA-C. All lymphoblast cell lines were grown in RPMI 1640 (Life Technologies) supplemented with 15% fetal calf serum (FCS) (heat inactivated, low endotoxin; HyClone, Logan, UT), 1% glutamine (Life Technologies), and 50 μg/mL gentamicin (Life Technologies) at 37°C and 5% CO2 in a humidified atmosphere.
Retroviral-Mediated Gene Transfer of FAC cDNA
Plasmids.pLXSN was a generous gift of Dr A.D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) and has been previously described.49 pLFACSN was constructed as follows: a 314-bp polymerase chain reaction (PCR) product (FAC ATG start to EcoRI site [bases 256-569 of GenBank sequence X66893]) was cloned into the pCR Script SK(+) vector (Stratagene, LaJolla, CA) to form pCR Script FAC ATG-RI. The sense primer was designed to add an Xho I site immediately 5′ of the FAC start codon. A 1,452-bp HindIII to Xba I (stop codon) fragment of FAC cDNA derived from pFAC3 was cloned into the HindIII and Xba I sites of pUC18 to create pUC FAC-D1. The pFAC3 plasmid contains the entire human FAC cDNA sequence and was the generous gift of Dr Manuel Buchwald. pLFACSN was constructed in a three-way ligation of an Xho I/EcoRI fragment from pCR-Script FAC ATG-RI and EcoRI/BamHI fragment from pUC FAC-DI and pLXSN linearized with Xho I/BamHI pLFACSN contains the full-length coding sequence for human FAC.
Packaging, transduction, selection, and complementation analysis.Purified pLFACSN or pLXSN plasmid DNA (10 μg) was transfected as a calcium phosphate precipitate using protocol A, as previously described, into culture dishes that contained a 1:1 mixture of ψ2 and PA12 cells.50 Supernatants from the ping-pong culture were procured, filtered, and used for transduction of FAC lymphoblasts. Polybrene (Sigma Chemical Co, St Louis, MO) was added during the transduction procedure to achieve a final concentration of 8 μg/mL. Cell lines were exposed to supernatants from four to six times in as many days before selection in G418 (Life Technologies). Sets of isogeneic lines were selected in G418, then exposed to multiple doses of mitomycin C in cytotoxicity (trypan blue viability) and apoptosis assays (TUNEL and annexin-V binding assays) to ensure that the mutant cells transduced with the normal cDNA were fully complemented.
Detection of fas, fas-Ligand (fas-L), and IRF-1 mRNA
Total RNA was isolated from cells using Tri Reagent (Molecular Research Center, Inc, Cincinnati, OH) in accordance with the manufacturer's instructions. First-strand cDNA was reverse transcribed from the indicated RNA using random hexanucleotide primers (life Technologies) and MMLV RNase H− reverse transcriptase (Life Technologies) as previously described.51 The cDNA was then amplified by PCR for 36 cycles (denatured at 94°C for 10 seconds, primer annealed at 54°C for 30 seconds, primer extended at 72°C for 10 seconds). Control reactions in which no cDNA (ie, water only) was added to the PCR mixture were analyzed with each experiment. For fas amplification, the primers used were 5′-ACAGACAAAGCCCATTTTTC-3′ and 5′-TTGCCACTGTTTCAGGATT-3′ and produced an amplimer with a predicted length of 328 nucleotides. The primers used for fas-L amplification were 5′-GCCTGTGTCTCCTTGTG-3′ and 5′-TCATCTTCCCCTCCATC-3′ and produced an amplimer with a predicted length of 450 nucleotides. The amplification products were separated by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, and visualized by UV transillumination. IRF-1 mRNA was analyzed semi-quantitatively by reverse transcriptase (RT)-PCR using the primers 5′-GAGCTGGGCCATTCACACAG-3′ and 5′-CATGGCGACAGTGCTGGAGT-3′ to amplify bases 365 to 754 of IRF-1 cDNA (GenBank Accession No. X14454). Messenger RNA for studies on IRF-1 was obtained from CD34+ and CD34− BM cells from normal volunteers, and from CD34− BM cells from a child with FA-C. Cells were exposed to multiple doses (0, 0.5, and 50 ng/mL) of IFN-γ for 2 to 24 hours before isolation of mRNA as above. When it became clear that FA-C cells expressed at least some IRF-1 mRNA constitutively, serial dilutions of cDNAs from informative time points were carried out to determine relative amounts of IRF-1 present in FA-C and normal cells.
Immunoblotting
Immunoblot analyses were performed using lysates from CD34+ cells. CD34+ cells (1 × 108/mL) were preincubated overnight in RPMI 1640 with 10% FCS in the presence of recombinant human Steel factor (50 ng/mL), IL-3 (10 ng/mL), and erythropoietin (2 U/mL). Recombinant human (rh) IFN-γ (R & D Systems) was added at 100 ng/mL for the interval indicated. The cells were procured, washed twice with phosphate-buffered saline (PBS), and the cell pellets were solubilized in RIPA (10 mmol/L Tric-Cl cell [pH 7.6], 150 mmol/L NaCl cell, 1% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X-100, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], and freshly added 1% [vol/vol] aprotinin, 2 mmol/L Na3VO4 , leupeptin [1 μg/mL], and 1 mmol/L phenylmethylsulfonylfluoride [PMSF ]. Lysates were centrifuged at 16,250g for 20 minutes at 4°C, and protein concentrations were determined using a protein microassay of the Bradford method (Bio-Rad, Hercules, CA). Cell lysates were heated at 94°C for 5 minutes in the presence of SDS and dithiothreitol, and the proteins separated by polyacrylamide gel electrophoresis (SDS-PAGE) in 10% polyacrylamide gels. Proteins were electroblotted onto Bio-Blot nitrocellulose (Costar, Cambridge, MA) as previously described.52 Nonspecific binding was blocked by incubating the blots for 1 hour in 5% (wt/vol) nonfat dry milk in Tris-buffered saline plus 0.05% Tween 20 (TBS-T). Fas was detected by incubating blots for 2 hours with a polyclonal antihuman fas antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) diluted 1:2,000 in 5% nonfat dry milk/TBS-T. Fas-L was detected by incubating blots for 2 hours with a monoclonal anti-fas ligand antibody (Transduction Laboratories, Lexington, KY) diluted 1:2,000 in 5% nonfat dry milk/TBs-T. Primary antibody incubations were followed by six (5-minute) washes of TBS-T. The blots were incubated with secondary antibodies (donkey-antirabbit IgG-horseradish peroxidase (HRP) conjugate and sheep-antimouse Ig-HRP conjugate for anti-fas and anti-fas-L antibodies, respectively [Amersham, Arlington Heights, IL]) for 30 minutes at 1:2,000 dilution in 5% nonfat dry milk/TBS-T, then washed with TBS-T. Antibody-reactive proteins were detected using ECL (Enhanced Chemiluminescence) reagents (Amersham).
Flow Cytometry: fas and Annexin-V Binding
Inducible expression of fas was assessed, in part, using flow cytometry. CD34+ BM cells were cultured at 1 × 106/mL in RPMI 1640 supplemented with 10% FCS in the presence of the following human growth factors: Steel factor (50 ng/mL). IL-3 (10 ng/mL), and erythropoietin (2 U/mL). Cells were cultured with 0, 100, and 1,000 ng/mL rhIFN-γ (R & D Systems) for 18 hours and then collected and washed in 3 mL cold (4°C) staining buffer (PBS with 0.1% bovine serum albumin, fraction V [Sigma]). Cells were resuspended to 1 × 106/50 μL staining buffer, and the following antibodies were added: 125 ng murine IgG1 -FITC isotypic control (Coulter, Miami, FL) and 125 ng murine IgG1 -PE isotypic control (Coulter), or 250 ng antihuman fas-FITC (murine IgG1 ) (Coulter) and 1 μg antihuman CD117(c-kit )-PE (murine IgG1 ) (Coulter). The suspensions were gently mixed, then incubated in the dark for 30 minutes, at 4°C. Cells were washed twice in 3 mL staining buffer, and then analyzed by flow cytometry (BD FacScan; Becton Dickinson, San Jose, CA). Fifty microliters of propidium iodide solution (50 μg/mL propidium iodide and 0.1% [wt/vol] sodium citrate in PBS) was added to each sample just before data acquisition to exclude necrotic cells (FL3-bright).
We took steps to confirm the apoptotic phenotype revealed using the TUNEL assay by using annexin-V binding analysis in the B-cell lines we studied. Phosphatidylserine, normally located exclusively on the cytoplasmic side of the plasma membrane,53 is externalized during apoptosis in a variety of cells, including transformed B lymphocytes,53 and can be detected using annexin V as a probe.53 54 Cells were cultured in RPMI 1640 (Life Technologies) supplemented with 15% fetal bovine serum (Life Technologies), 2 mmol L-glutamine (Life Technologies), 100 U/mL penicillin G (Life Technologies), and 100 μg/mL streptomycin sulfate (Life Technologies). Cells were washed once in binding buffer (10 mmol L HEPES/NaOH pH 7.4, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2 , 1.8 mmol/L CaCl2 ) and stained in binding buffer with annexin-V-FITC (Caltag Laboratories, Burlingame, CA) at a final concentration of 2 μg/106 cells/0.8 mL. Staining reactions were incubated in the dark for 15 minutes and washed once with 4 vol of binding buffer. Just before fluorescence-activated cell sorter (FACS) analysis, freshly prepared propidium iodide (50 μg/mL, Sigma; 0.1% [wt/vol] sodium citrate in PBS) was added to each sample at a final concentration of 5 μg/mL. All washes and incubations were performed at room temperature.
Activation of the fas Mediated Apoptotic Pathway
An agonistic antihuman fas antibody (Upstate Biotechnology, Inc [UBI], Lake Placid, NY) was used at 100 ng/mL and an agonistic antimurine fas antibody (UBI) was used at 500 ng/mL. Initially the doses of the antihuman antibodies were optimized for apoptotic activity by carrying out apoptosis-response curves (5 to 200 ng/mL) using the EBV-transformed cell line JY. Cells were exposed to the antibody for 24 and 48 hours, and apoptosis was then quantified using the deoxyribonucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay (see below).55 The assay detects free 3-hydroxyl groups in DNA, an indicator of cells undergoing apoptosis. In additional experiments, with both human and murine cells, 0.5 to 100 ng/mL recombinant human or murine IFN-γ (R & D Systems) was used to treat cells in suspension culture for 1 hour before the addition of anti-fas antibodies. In some experiments, a blocking antihuman fas antibody was used (100 ng/mL; UBI) in parallel with the agonistic antibody.
Quantification of Apoptotic Cells
A portion of the cells cultured under various conditions were quantified for apoptosis using fluorescence microscopy and the TUNEL assay (ApopTag in situ Apoptosis Detection Kit; Oncor, Gaithersburg, MD). Cells with nuclear blebbing and/or green fluorescence were scored as apoptotic. Annexin V binding was also used as a marker of phosphatidylserine externalization, a process induced, at least in lymphocytes, by activation of the fas pathway.56
IFN-γ–Induced Stat1 Phosphorylation in Normal and FA-C B Cells
Immunoblot analyses were performed using lysates from JY, HSC536N, HSC536N/FAC, and HSC536N/neo cells treated with 1 ng/mL IFN-γ for 15, 30, and 60 minutes. Samples containing 50 μg of total cell lysate were immunoblotted for Stat1-phosphate. The primary antibody, which specifically recognizes only the Tyr701 phosphorylated form of Stat-1 (New England Biolabs, Beverly, MA), was diluted 1:1,000 and incubated with blots overnight at 4°C. The secondary antibody, donkey antirabbit IgG-HRP, was used at a 1:3,000 dilution for 30 minutes at room temperature. The remainder of the immunoblotting procedure is as described above.
RESULTS
IFN-γ Mediates Hematopoietic Suppression Through the fas Pathway
We obtained BM cells from a child with FA-C on three separate occasions over a period of 14 months. Parallel studies were performed using BM cells obtained from normal volunteers. CD34+ cells from the FA-C patient were hypersensitive to the clonal inhibitory effects of IFN-γ (Fig 1) in all three experiments. Studies on the IFN-γ/fas pathway were performed using CD34+ cells from the second and third specimens at which time there was no cytogenetic evidence for clonal hematopoiesis. Having documented IFN-γ hypersensitivity in progenitor cells from this child, we next confirmed the findings of other investigators34 57 that IFN-γ induces fas expression in normal human CD34+ cells. Although fas is constitutively expressed in normal CD34+ cells (Fig 2), the IFN-γ–mediated inductive effect was clear-cut and involved the accumulation of fas mRNA (Fig 2A), and an increase in both total cellular protein (Fig 2B) and per-cell protein (Fig 2C). The histogram shown in Fig 2C represents cells exposed to a dose of 100 ng/mL IFN-γ. Exposure of cells to 1,000 ng/mL did not further increase fas expression, as assessed by flow cytometry (not shown).
Human FA-C progenitor cells are hypersensitive to the mitotic inhibitory influence of IFN-γ. A parallel study of CD34+ cells from the BM of a normal volunteer (□) (67.5 ± 19 BFU-E/5,000 cells, 85.5 ± 6.3 CFU-GM/5,000 cells, and from a child with FA-C () (4 ± 1.4 BFU-E/5,000 cells, and 7 ± 4 CFU-GM/5,000 cells) are shown here. Both BFU-E (A) and CFU-GM (B) growth (mean ± SEM of replicate methylcellulose plates) were inhibited by doses of IFN-γ that have never suppressed clonal growth of normal human progenitor cells in more than 14 studies.
Human FA-C progenitor cells are hypersensitive to the mitotic inhibitory influence of IFN-γ. A parallel study of CD34+ cells from the BM of a normal volunteer (□) (67.5 ± 19 BFU-E/5,000 cells, 85.5 ± 6.3 CFU-GM/5,000 cells, and from a child with FA-C () (4 ± 1.4 BFU-E/5,000 cells, and 7 ± 4 CFU-GM/5,000 cells) are shown here. Both BFU-E (A) and CFU-GM (B) growth (mean ± SEM of replicate methylcellulose plates) were inhibited by doses of IFN-γ that have never suppressed clonal growth of normal human progenitor cells in more than 14 studies.
IFN-γ induces fas expression in normal human CD34+ cells. (A) RT-PCR analysis of RNA obtained from cells exposed for 6 hours to various doses of IFN-γ are shown. Fas is expressed constitutively and mRNA increases slightly with all doses of IFN-γ tested except the highest which suppressed fas RNA. (B) Fas immunoblot: CD34+ cells were incubated in the absence (lane 1) or presence (lane 2) of IFN-γ for 6 hours. Each lane was loaded with 50 μg of protein from whole cell lysates. (C) Flow cytometry: Flow cytometric analysis of surface fas expression on CD34+ cells purified from normal BM. The horizontal axis illustrates linear fluorescence intensity, and the vertical axis indicates absolute cell number. The dotted line represents CD34+ cells cultured without IFN-γ exposed to the isotypic control antibody, the dot-dashed line shows fas expression in CD34+ cells cultured in the absence of IFN-γ. The shaded histogram indicates fas expression by CD34+ cells exposed to 100 ng/mL IFN-γ for 18 hours. Treatment of cells with 1,000 ng/mL IFN-γ yielded an identical histogram. (D) IFN-γ primes the fas pathway in human CD34+ cells. Results of one representative experiment (of three separate experiments) is shown. Human CD34+ cells exposed to various doses of IFN-γ for 60 minutes followed by exposure to antihuman fas monoclonal antibodies (“anti-fas,” ▪), or isotype control antibodies (“control,” □), for 3 hours were suspended in methylcellulose medium containing the same dose of IFN-γ. CFU-GM (not shown) and BFU-E (mean ± SD of triplicate plates) were counted on day 14 of culture. In the absence of IFN (control) BFU-E were 154 ± 14/5,000 CD34+ cells. Although IFN-γ suppresses clonal growth of normal BFU-E, the addition of anti-fas augments clonal inhibition by IFN-γ, particularly at IFN levels above 0.5 ng/mL.
IFN-γ induces fas expression in normal human CD34+ cells. (A) RT-PCR analysis of RNA obtained from cells exposed for 6 hours to various doses of IFN-γ are shown. Fas is expressed constitutively and mRNA increases slightly with all doses of IFN-γ tested except the highest which suppressed fas RNA. (B) Fas immunoblot: CD34+ cells were incubated in the absence (lane 1) or presence (lane 2) of IFN-γ for 6 hours. Each lane was loaded with 50 μg of protein from whole cell lysates. (C) Flow cytometry: Flow cytometric analysis of surface fas expression on CD34+ cells purified from normal BM. The horizontal axis illustrates linear fluorescence intensity, and the vertical axis indicates absolute cell number. The dotted line represents CD34+ cells cultured without IFN-γ exposed to the isotypic control antibody, the dot-dashed line shows fas expression in CD34+ cells cultured in the absence of IFN-γ. The shaded histogram indicates fas expression by CD34+ cells exposed to 100 ng/mL IFN-γ for 18 hours. Treatment of cells with 1,000 ng/mL IFN-γ yielded an identical histogram. (D) IFN-γ primes the fas pathway in human CD34+ cells. Results of one representative experiment (of three separate experiments) is shown. Human CD34+ cells exposed to various doses of IFN-γ for 60 minutes followed by exposure to antihuman fas monoclonal antibodies (“anti-fas,” ▪), or isotype control antibodies (“control,” □), for 3 hours were suspended in methylcellulose medium containing the same dose of IFN-γ. CFU-GM (not shown) and BFU-E (mean ± SD of triplicate plates) were counted on day 14 of culture. In the absence of IFN (control) BFU-E were 154 ± 14/5,000 CD34+ cells. Although IFN-γ suppresses clonal growth of normal BFU-E, the addition of anti-fas augments clonal inhibition by IFN-γ, particularly at IFN levels above 0.5 ng/mL.
We also noted that induction of fas in both human and murine BM cells by IFN-γ occurs in parallel with priming of the fas-mediated apoptotic program in committed progenitor cells (Fig 2D). Specifically, in four separate experiments, exposure of normal CD34+ cells to the anti-fas antibody did not suppress clonal growth of BFU-E or CFU-GM in vitro, but when cells were exposed for 60 minutes to IFN-γ, followed by exposure to anti-fas, clonal growth of BFU-E (Fig 2D) and CFU-GM (not shown) was inhibited.
The fas pathway Mediates IFN-γ Hypersensitivity in FA Cells
We tested the hypothesis that the priming effects of IFN-γ occur at a lower dose of IFN-γ in FAC −/− mice when compared with FAC −/+ mice. The results of four separate studies confirmed this notion. Responses of murine FA-C cells were distinctly abnormal in three ways (Fig 3A): (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of an anti-fas antibody to FAC cells without prior exposure to IFN-γ resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC cells (the degree to which the addition of an anti-fas antibody augmented BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ than in FAC −/+ progenitor cells.
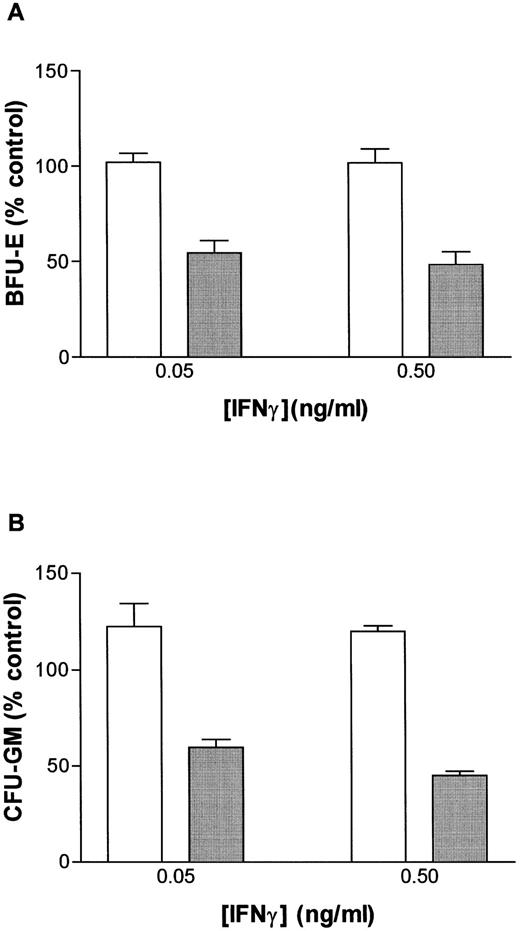
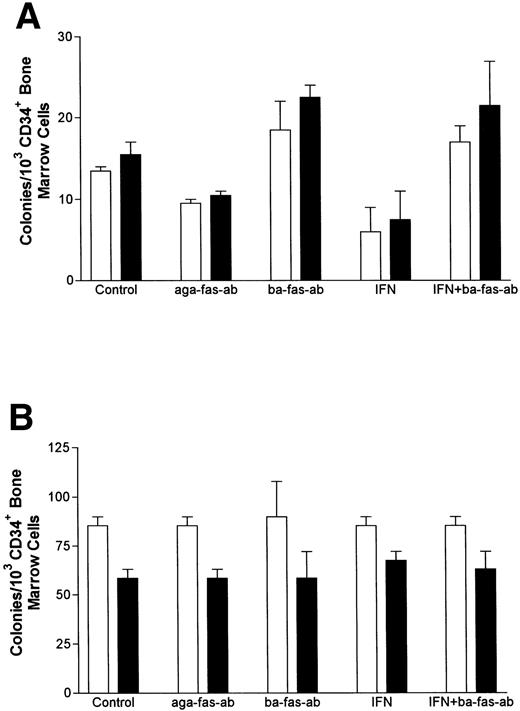
IFN-γ primes the fas pathway in vitro and in vivo. (A) Results of one representative experiment (of four separate experiments) of BM cells from an FAC −/− mouse (□) and from an FAC −/+ (○) littermate. Cells were exposed to various doses of recombinant murine IFN-γ for 60 minutes, followed by 3-hour exposure to antimurine fas antibodies (“FA-C/fas” [▪] and “hetero/fas” [•]) or medium alone (“FA-C” [□] and “Hetero” [○]). Open and closed circles represent clonal growth of BFU-E from FAC −/+ mice. Open and closed squares represent BFU-E growth of marrow cells from FAC mice. Responses of FAC cells were distinctly abnormal in three ways: (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of anti-fas to FAC cells, even without prior exposure to IFN-γ, resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC-cells (the degree to which the addition of anti-fas augmented fractional BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ as compared with FAC −/+ progenitor cells. Fas priming occurred in FAC −/+ mice at every dose of IFN, but was most notable at 5.0 ng/mL. At that dose, in two consecutive studies (in cells from each mouse, each variable was quantified using mean colony counts of triplicate plates) exposure of FAC −/− marrow cells to agonistic fas antibody reduced BFU-E to 10.7% control growth while exposure of heterozygote marrow to the antibody reduced BFU-E growth to 52% of control values (P < .005, by Student's t-test). (B) IFN-γ administration in vivo induces fas expression and reduces progenitor cell number in spleen and BM of FAC mice. Two sets of paired mice were treated with 105 U IFN-γ/d intraperitoneally for 5 days. Results were comparable in both experiments (bars reflect means ± SD). BM BFU-E were significantly suppressed in IFN-γ–treated heterozygote mice, but the fractional reduction was significantly greater in BM of FAC −/− mice. Splenic BFU-E increased in treated heterozygotes but were markedly suppressed in FAC −/− mice. (C) Treatment of unfractionated marrow cells from FAC −/+ mice with anti-fas antibody did not suppress BFU-E growth but did suppress BFU-E growth in the FA-C cells by 50%.
IFN-γ primes the fas pathway in vitro and in vivo. (A) Results of one representative experiment (of four separate experiments) of BM cells from an FAC −/− mouse (□) and from an FAC −/+ (○) littermate. Cells were exposed to various doses of recombinant murine IFN-γ for 60 minutes, followed by 3-hour exposure to antimurine fas antibodies (“FA-C/fas” [▪] and “hetero/fas” [•]) or medium alone (“FA-C” [□] and “Hetero” [○]). Open and closed circles represent clonal growth of BFU-E from FAC −/+ mice. Open and closed squares represent BFU-E growth of marrow cells from FAC mice. Responses of FAC cells were distinctly abnormal in three ways: (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of anti-fas to FAC cells, even without prior exposure to IFN-γ, resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC-cells (the degree to which the addition of anti-fas augmented fractional BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ as compared with FAC −/+ progenitor cells. Fas priming occurred in FAC −/+ mice at every dose of IFN, but was most notable at 5.0 ng/mL. At that dose, in two consecutive studies (in cells from each mouse, each variable was quantified using mean colony counts of triplicate plates) exposure of FAC −/− marrow cells to agonistic fas antibody reduced BFU-E to 10.7% control growth while exposure of heterozygote marrow to the antibody reduced BFU-E growth to 52% of control values (P < .005, by Student's t-test). (B) IFN-γ administration in vivo induces fas expression and reduces progenitor cell number in spleen and BM of FAC mice. Two sets of paired mice were treated with 105 U IFN-γ/d intraperitoneally for 5 days. Results were comparable in both experiments (bars reflect means ± SD). BM BFU-E were significantly suppressed in IFN-γ–treated heterozygote mice, but the fractional reduction was significantly greater in BM of FAC −/− mice. Splenic BFU-E increased in treated heterozygotes but were markedly suppressed in FAC −/− mice. (C) Treatment of unfractionated marrow cells from FAC −/+ mice with anti-fas antibody did not suppress BFU-E growth but did suppress BFU-E growth in the FA-C cells by 50%.
If IFN-γ–dependent fas priming of progenitor cells accounts for BM failure, FAC mutant mice should be hypersensitive in vivo. We treated 2-month-old mice (an age at which myeloid and erythroid clonal growth of BM- and spleen-derived progenitors is consistently identical) for 5 consecutive days with 105 U recombinant murine IFN-γ, after which we performed clonal assays for hematopoietic progenitor cells using both BM and spleen cells from the same animals. Both BM and spleen-derived progenitor cells in the IFN-γ-treated FAC −/− mice were significantly reduced (Fig 3B). Because both femoral BM (25 × 106 [FAC −/−] 35 × 106 [FAC −/+]) and spleen (340 × 106 [FAC −/−], 350 × 106 [FAC −/+]) total cell counts were reduced in FAC −/− mice, values were consistently different whether they were expressed as colonies per cells plated (Fig 3B), or colonies per organ (not shown). The priming effect of IFN-γ on fas antibody-mediated apoptosis was noted only in FAC −/− mice (Fig 3C), demonstrating: (1) differential sensitivity of progenitor cells to IFN-γ and (2) the involvement of the fas pathway in IFN-γ–mediated clonal suppression.
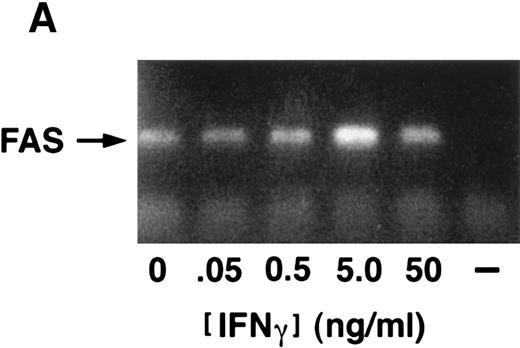
(A) IFN-γ hypersensitivity and fas priming in progenitors from an FA-C child. All values represent mean clonal growth ± SD. Parallel studies with the same reagents were performed using BM CD34+ cells from a normal volunteer (B). Neither the agonistic nor the blocking anti-fas antibodies influence clonal growth of CD34+ cells from normal volunteers. IFN-γ (0.05 ng/mL) had no effect (nor has it had an effect in more than 15 separate studies in normal volunteers). As was the case in the FA mice, the fas pathway was primed in progenitors from the child. Specifically, the agonistic antibody suppressed clonal growth and the blocking antibody enhanced clonal growth of both CFU-GM (▪) and BFU-E (□). Moreover, both BFU-E and CFU-GM were suppressed by IFN-γ exposure and the suppressive effect of IFN-γ was blocked completely by the exposure of CD34+ cells to the blocking antihuman fas antibody. (C) IRF-1 mRNA is expressed constitutively by BM cells from an FA-C child. CD34+ low-density BM cells were exposed to IFN-γ at concentrations of 0.5 ng/mL (lanes 3, 6, and 9) and 50 mg/mL (lanes 4, 7, and 10) for 2 hours (lanes 2, 3, and 4), 6 hours (lanes 5, 6, and 7), and 24 hours (lanes 8, 9, and 10). Lane 1 is a zero time point control (RNA isolated at time zero with no IFN-γ exposure), and lanes 2, 5, and 8 are zero IFN-γ controls at 2, 6, and 24 hours, respectively. The upper set of lanes show IRF-1 amplification from CD34− BM cells from a normal volunteer, and the lower set of lanes show IRF-1 amplification from CD34− BM cells from an FA-C child.
(A) IFN-γ hypersensitivity and fas priming in progenitors from an FA-C child. All values represent mean clonal growth ± SD. Parallel studies with the same reagents were performed using BM CD34+ cells from a normal volunteer (B). Neither the agonistic nor the blocking anti-fas antibodies influence clonal growth of CD34+ cells from normal volunteers. IFN-γ (0.05 ng/mL) had no effect (nor has it had an effect in more than 15 separate studies in normal volunteers). As was the case in the FA mice, the fas pathway was primed in progenitors from the child. Specifically, the agonistic antibody suppressed clonal growth and the blocking antibody enhanced clonal growth of both CFU-GM (▪) and BFU-E (□). Moreover, both BFU-E and CFU-GM were suppressed by IFN-γ exposure and the suppressive effect of IFN-γ was blocked completely by the exposure of CD34+ cells to the blocking antihuman fas antibody. (C) IRF-1 mRNA is expressed constitutively by BM cells from an FA-C child. CD34+ low-density BM cells were exposed to IFN-γ at concentrations of 0.5 ng/mL (lanes 3, 6, and 9) and 50 mg/mL (lanes 4, 7, and 10) for 2 hours (lanes 2, 3, and 4), 6 hours (lanes 5, 6, and 7), and 24 hours (lanes 8, 9, and 10). Lane 1 is a zero time point control (RNA isolated at time zero with no IFN-γ exposure), and lanes 2, 5, and 8 are zero IFN-γ controls at 2, 6, and 24 hours, respectively. The upper set of lanes show IRF-1 amplification from CD34− BM cells from a normal volunteer, and the lower set of lanes show IRF-1 amplification from CD34− BM cells from an FA-C child.
We next sought to confirm our prediction that IFN-γ hypersensitivity and fas priming occurs in progenitors of FA-C children. Low doses of IFN-γ did not suppress CFU-GM growth of CD34+ cells from BM of normal volunteers (n = 14) but did consistently suppress colony growth significantly in CD34+ cells from the BM of a child with FA of the C complementation group (Fig 4A). As was the case in the FAC mice, the fas pathway was primed in progenitors from the child. Specifically, clonal growth was suppressed by exposure of CD34+ cells to the agonistic anti-fas antibody and augmented by exposure to a blocking antibody. The blocking antibody also abrogated the inhibitory effect of low-dose IFN-γ in FA-C CD34+ cells (Fig 4A). None of the treatments inhibited clonal growth of normal CD34+ cells (Fig 4B). Activation of IFN-γ signalling in vivo was also suggested by our finding that IRF-1 mRNA was readily detectable in human FA-C BM cells, but not in normal marrow cells (Fig 4C).
Fas-L Is Expressed and Functional in CD34+ Cells
Because (1) fewer than 0.5% of our CD34+ BM cells express CD3 (not shown), (2) IFN-γ can suppress clonal growth even in the absence of agonistic antibody, and (3) blocking anti-fas antibodies relieved IFN-γ-induced suppression (Fig 4A), we sought to determine if CD34+ cells expressed fas-L. Fas-L was detected in unstimulated CD34+ cells and was uninfluenced by exposure of the cells to IFN-γ for up to 48 hours (Fig 5A and B).
Fas-ligand expression in CD34+ cells. (A) RT-PCR of cells exposed to various doses of IFN-γ showed constitutive expression, no inductive effect of IFN-γ, and repression of fas-L at the 50 ng/mL dose. (B) Immunoblot of CD34+ cell lysates probed with anti-fas-L antibody. CD34+ cells were incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of IFN-γ for 24 hours (lanes 1 and 2) or 48 hours (lanes 3 and 4). (Each lane was loaded with 50 μg total protein.)
Fas-ligand expression in CD34+ cells. (A) RT-PCR of cells exposed to various doses of IFN-γ showed constitutive expression, no inductive effect of IFN-γ, and repression of fas-L at the 50 ng/mL dose. (B) Immunoblot of CD34+ cell lysates probed with anti-fas-L antibody. CD34+ cells were incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of IFN-γ for 24 hours (lanes 1 and 2) or 48 hours (lanes 3 and 4). (Each lane was loaded with 50 μg total protein.)
Expression of the Normal FAC Gene Product in FA Cells Does Not Directly Repress Expression of fas But Does Influence IFN-γ Signaling
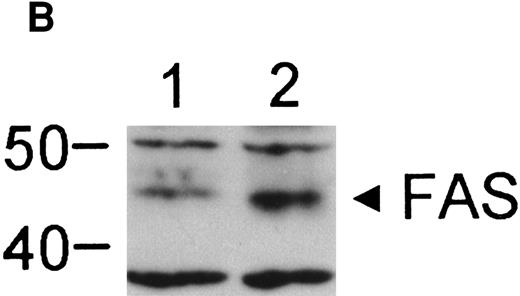
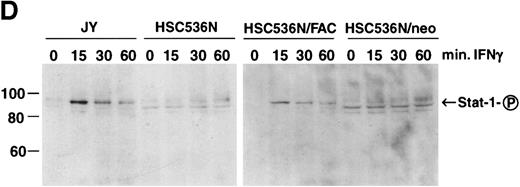
The finding that IFN-γ primes the fas pathway in normal and FA-C cells, but the FA-C cells are hypersensitive to IFN-γ (as measured by both IRF-1 induction and fas priming at very low doses of IFN) is compatible with our view that constitutive fas priming in vivo is caused by IFN-γ hypersensitivity in vivo. However, it remained theoretically possible that the FA-C protein functions to directly repress fas pathway activation or to repress both the IFN-γ and fas pathways. To examine these possibilities, we performed studies in which B-cell lines from normal volunteers and isogenic cell lines from children with FAC were exposed to mitomycin C, or to agonistic anti-fas antibodies, and subsequently analyzed for apoptosis using the TUNEL assay. As shown in Fig 6, transduction of normal FAC cDNA corrects mitomycin C-induced apoptosis in FAC B cells as measured by the TUNEL assay (Fig 6A) and by annexin V-binding (not shown), but neither represses the apoptotic response of those cells to agonistic anti-fas antibody (Fig 6B) nor represses expression of fas in these cells (Fig 6C). Reasoning that the FAC gene product functions in the IFN-signaling pathway more proximal than fas expression, we sought to determine whether the kinetics of activation and deactivation of an essential rapidly acting signaling molecule, Stat1, might be somehow perturbed in FA-C cells. Immunoblot analysis was used to describe kinetics of Stat1 phosphorylation in response to IFN-γ in isogenic FA-C cell lines. The immunoblot shown in Fig 6D reveals clear differences in the decay and induction kinetics of Stat1 phosphate that depends on expression of a normal FAC gene product. EBV-transformed normal B cells (JY) and the complemented FA-C cells (HSC536N fac ) show a normal inductive response involving largely Stat-1α that declines after 30 minutes. HSC536N neo and HSC536N cells demonstrate constitutive phosphorylation of Stat1 (largely the β form), a minor inductive effect of IFN-γ, and slow increase of the phosphoprotein at 60 minutes and 120 minutes (not shown) after exposure to IFN-γ (Fig 6D).
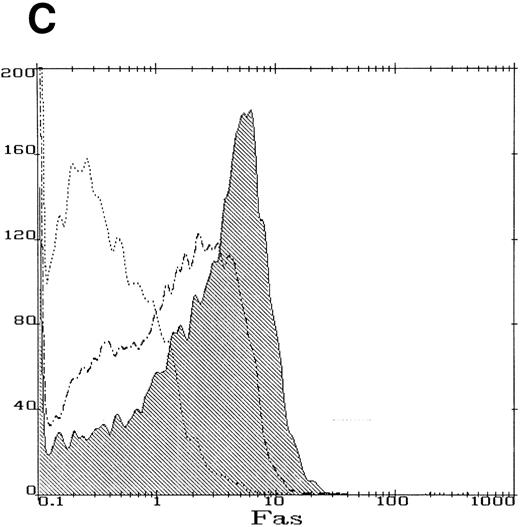
The product of the FAC gene does not directly inactivate the fas pathway. B-cell lines from normal volunteers and isogenic cell lines from children with FA-C were exposed to mitomycin C, or to agonistic anti-fas antibodies, and were analyzed for apoptosis using the TUNEL assay. Results are expressed as percent apoptotic cells (mean ± SD). (A) Mitomycin C hypersensitivity was noted in FAC mutant cells (HSC536N). HSC536N cells transduced with a retroviral vector expressing only the neomycin phosphotransferase gene (HSC536N/neo) were also hypersensitive. Transduction of normal FAC cDNA (HSC536N/FAC/neo) corrects mitomycin C–induced apoptosis in FAC cells. JY cells are EBV-transformed cells from a normal volunteer. (B) Exposure of the isogenic cells to agonistic anti-fas antibody induced substantial apoptosis (quantified 48 hours after exposure to the antibody) in all three of the isogenic sets (only one is shown here). (C) Flow cytometric analysis of the isogenic cells shown in (B) indicated that fas expression in HSC536N and HSC536N/neo was similar to that of normal EBV-transformed cells and the corrected FA-C cells (HSC536N FAC/neo). The group of low-intensity peaks in the histogram (on the left) represent binding of an isotypic control antibody with each of the four cell types; the high-intensity group on the right shows binding of an anti-fas antibody with the same cell lines. (D) Kinetics of Stat1 phosphorylation differed between isogenic lines. Shown is an immunoblot analysis of phosphorylated Stat1 0, 15, 30, and 60 minutes after exposure of the cell lines to IFN-γ. JY and the complemented FA-C cells (HSC536N fac ) demonstrate a normal inductive response that declines after 30 minutes. HSC536N neo and HSC536N cells show constitutive phosphorylation of Stat1, largely the β splice form, a minor inductive effect of IFN-γ on Stat1α, and persistence of the phosphoprotein for more than 60 minutes after exposure to IFN-γ.
The product of the FAC gene does not directly inactivate the fas pathway. B-cell lines from normal volunteers and isogenic cell lines from children with FA-C were exposed to mitomycin C, or to agonistic anti-fas antibodies, and were analyzed for apoptosis using the TUNEL assay. Results are expressed as percent apoptotic cells (mean ± SD). (A) Mitomycin C hypersensitivity was noted in FAC mutant cells (HSC536N). HSC536N cells transduced with a retroviral vector expressing only the neomycin phosphotransferase gene (HSC536N/neo) were also hypersensitive. Transduction of normal FAC cDNA (HSC536N/FAC/neo) corrects mitomycin C–induced apoptosis in FAC cells. JY cells are EBV-transformed cells from a normal volunteer. (B) Exposure of the isogenic cells to agonistic anti-fas antibody induced substantial apoptosis (quantified 48 hours after exposure to the antibody) in all three of the isogenic sets (only one is shown here). (C) Flow cytometric analysis of the isogenic cells shown in (B) indicated that fas expression in HSC536N and HSC536N/neo was similar to that of normal EBV-transformed cells and the corrected FA-C cells (HSC536N FAC/neo). The group of low-intensity peaks in the histogram (on the left) represent binding of an isotypic control antibody with each of the four cell types; the high-intensity group on the right shows binding of an anti-fas antibody with the same cell lines. (D) Kinetics of Stat1 phosphorylation differed between isogenic lines. Shown is an immunoblot analysis of phosphorylated Stat1 0, 15, 30, and 60 minutes after exposure of the cell lines to IFN-γ. JY and the complemented FA-C cells (HSC536N fac ) demonstrate a normal inductive response that declines after 30 minutes. HSC536N neo and HSC536N cells show constitutive phosphorylation of Stat1, largely the β splice form, a minor inductive effect of IFN-γ on Stat1α, and persistence of the phosphoprotein for more than 60 minutes after exposure to IFN-γ.
DISCUSSION
Because IFN-γ-exposed progenitor cells undergo apoptosis,58,59 we hypothesize that BM failure in children with FA results from accumulated losses of progenitors and stem cells caused by repeated episodes of low-level IFN-γ release in response to recurrent minor inflammatory stimuli. In three separate studies on BM cells obtained over a period of 14 months, progenitor cells from a child with FA of the C type proved to be consistently hypersensitive to the clonal inhibitory activity of IFN-γ (Fig 1). As is the case with fetal liver CD34+ cells,57 we found that that CD34+ cells from adult BM express fas constitutively and confirmed findings of Nagafuji et al33 that IFN-γ augments fas expression (Fig 2) and primes cells for apoptosis after fas ligation (Fig 2D). Based on our prior findings of IFN-γ hypersensitivity in FAC cells,22 we reasoned that the fas pathway in FAC cells exposed to IFN-γ would be primed at doses that would have no influence on normal progenitor cells. We confirmed this notion both in vitro and in vivo (Fig 3). We also found that murine (Fig 3A) and human (Fig 4A) FAC −/− progenitor cells express fas constitutively but that FAC −/+ mice (Fig 3A) and normal human progenitors do not. Evidence of in vivo activation of other elements of the IFN-γ signaling pathway was also found in marrow cells derived from the FA-C patient in which IRF-1 mRNA was readily detectable even before IFN-γ exposure (Fig 4C) and increased at doses of IFN-γ that had no inductive effect on normal cells. At no time in six identical experiments on normal marrow cells have we ever detected IRF-1 mRNA unless the cells were first exposed to IFN-γ in vitro. Therefore, we attribute this aberrant pattern of IRF-1 gene expression to be the result of IFN-γ hypersensitivity.
Although the fas pathway was essential in mediating the IFNγ hypersensitivity of FA cells, the ordered relationship of the FAC gene product to IFN-γ hypersensitivity and fas activation was less clear. To determine whether the FAC protein functioned to directly modulate the state of activation of the fas pathway or the IFN-γ signaling pathway or both, we studied sets of B-cell lines derived from three children with FA of the C complementation group.
Retroviral-mediated gene transfer clearly reversed the FA phenotype by reducing mitomycin C–induced apoptosis (Fig 6A), but expression of the normal FAC gene had no impact on either the activation state of the fas pathway (Fig 6B) or on the expression of fas (Fig 6C). Having been persuaded that the FAC gene product is a likely component of the IFN signaling pathway, we tested the notion that the protein might influence the pattern of activation and decay of a molecule known to be indispensable for IFN-γ activity, Stat1,60.61 the phosphorylation and dimerization of which is IFN-γ dependent. Indeed, we discovered that exposure of these cell lines to IFN-γ showed that FAC protein had a major influence on the decay kinetics of phosphorylated Stat1 molecules. Specifically, mutant cells transduced with a control retroviral vector largely contained the Stat1β splice form and Stat1α and β disappearance was delayed (most noticeable at the 60-minute time point, Fig 6D). The pattern of Stat1 induction in mutant cells was normalized by transduction of the normal FAC gene (Stat1α was the dominant form in both normal cells and corrected FA-C cells) as were the kinetics of Stat1-phosphate decay (Fig 6D). This suggests that the FA protein may function to modulate IFN-γ signals through a pathway that catabolizes or dephosphorylates Stat1-phosphate, phenomena known to involve either the ubiquitin-proteasome system, as yet uncharacterized phosphatases, or both,62 63 or through an alternative pathway that modulates IFN-γ signals by accelerated splicing of Stat1 mRNA.
Despite these mechanistic uncertainties, our data are most consistent with the view that high level fas expression in progenitor cells of FA-C mice and humans results from their primary IFN-γ hypersensitivity, not because the FAC gene product functions to directly inhibit the activation state of the fas pathway. Future experiments ordering the IFN-γ–fas pathway in hematopoietic progenitor cells will be required because the FAC protein might be disconnected from fas modulation in B-cell lines (either by a lineage specific phenomenon or by an effect of EBV proteins) but not disconnected in diploid hematopoietic progenitor cells.
The apoptotic model is applicable to BM failure so prevalent in children with FA. The capacity of IFN-γ to induce TNF-α gene expression64 may well explain recent observations by Shahidi's group that levels of TNF-α are increased in the serum of children with Fanconi anemia.65 Finally, considering the mutability of somatic cells of children with FA, we speculate that the apoptotic phenotype in hematopoietic progenitor cells and stem cells creates a perfect environment for the selection of fully IFN-γ–resistant clones. Because IFN-γ functions as a normal hematopoietic “braking” factor, inactivating mutations of any one of many molecules required to transduce IFN-γ signals in hematopoietic precursor cells may represent the earliest events in the evolution of myelodysplasia and myeloid leukemia, clinical disorders for which children with FA are at great risk.66 67 Consequently, therapies designed to correct the abnormal pattern of IFN-γ sensitivity in FA cells may at once relieve BM failure and prevent or delay the onset of acute leukemia.
ACKNOWLEDGMENT
We thank Dr Lyle Sensenbrenner for most helpful suggestions and his insights into the nature of graft-versus-host disease in children with FA.
Supported by grants from the National Institutes of Health HL48546 (G.C.B. and M.G.) and a Department of Veterans Affairs Merit Review Grant (G.C.B).
Address reprint requests to Grover C. Bagby, MD, Division of Hematology and Medical Oncology, Oregon Health Sciences University, L580, 3181 SW Sam Jackson Park Road, Portland, OR 97201-3098.


![Fig. 3. IFN-γ primes the fas pathway in vitro and in vivo. (A) Results of one representative experiment (of four separate experiments) of BM cells from an FAC −/− mouse (□) and from an FAC −/+ (○) littermate. Cells were exposed to various doses of recombinant murine IFN-γ for 60 minutes, followed by 3-hour exposure to antimurine fas antibodies (“FA-C/fas” [▪] and “hetero/fas” [•]) or medium alone (“FA-C” [□] and “Hetero” [○]). Open and closed circles represent clonal growth of BFU-E from FAC −/+ mice. Open and closed squares represent BFU-E growth of marrow cells from FAC mice. Responses of FAC cells were distinctly abnormal in three ways: (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of anti-fas to FAC cells, even without prior exposure to IFN-γ, resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC-cells (the degree to which the addition of anti-fas augmented fractional BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ as compared with FAC −/+ progenitor cells. Fas priming occurred in FAC −/+ mice at every dose of IFN, but was most notable at 5.0 ng/mL. At that dose, in two consecutive studies (in cells from each mouse, each variable was quantified using mean colony counts of triplicate plates) exposure of FAC −/− marrow cells to agonistic fas antibody reduced BFU-E to 10.7% control growth while exposure of heterozygote marrow to the antibody reduced BFU-E growth to 52% of control values (P < .005, by Student's t-test). (B) IFN-γ administration in vivo induces fas expression and reduces progenitor cell number in spleen and BM of FAC mice. Two sets of paired mice were treated with 105 U IFN-γ/d intraperitoneally for 5 days. Results were comparable in both experiments (bars reflect means ± SD). BM BFU-E were significantly suppressed in IFN-γ–treated heterozygote mice, but the fractional reduction was significantly greater in BM of FAC −/− mice. Splenic BFU-E increased in treated heterozygotes but were markedly suppressed in FAC −/− mice. (C) Treatment of unfractionated marrow cells from FAC −/+ mice with anti-fas antibody did not suppress BFU-E growth but did suppress BFU-E growth in the FA-C cells by 50%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.974/4/m_bl_0072f3a.jpeg?Expires=1767781481&Signature=Gv3TmYHmyVupe02ZPUxHwbyH1nWLKOyvjiZ3fia76JXw4dJqFzcZCh2F8eq4QUiNU1v2~kc3z-S0kc4l~re9sF0X0E7h0HmUtLg~vsaOA6qmcXE-5uqGW7vJfMkWG5Uk6oG3DxmgMWbWVHJ8stpyf4YPo2m4qA61Dj9a2ggbq4PmXswf7IbIAdSDJ-a0wkLDZQl4Uc-3jbTlJZZcjeZov6DITo3jLQMQcF9DrKHw14jCTOqMAMvcxux-osXXmhOLdrbsmXUVHNPqhABuTzFDCo34jVn5WyP-tb-1Ese5pxrHQ2Ys~lSK9SC2R9sgCX4oqgcKYnyFuf9aEOwE0HHJ-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. IFN-γ primes the fas pathway in vitro and in vivo. (A) Results of one representative experiment (of four separate experiments) of BM cells from an FAC −/− mouse (□) and from an FAC −/+ (○) littermate. Cells were exposed to various doses of recombinant murine IFN-γ for 60 minutes, followed by 3-hour exposure to antimurine fas antibodies (“FA-C/fas” [▪] and “hetero/fas” [•]) or medium alone (“FA-C” [□] and “Hetero” [○]). Open and closed circles represent clonal growth of BFU-E from FAC −/+ mice. Open and closed squares represent BFU-E growth of marrow cells from FAC mice. Responses of FAC cells were distinctly abnormal in three ways: (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of anti-fas to FAC cells, even without prior exposure to IFN-γ, resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC-cells (the degree to which the addition of anti-fas augmented fractional BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ as compared with FAC −/+ progenitor cells. Fas priming occurred in FAC −/+ mice at every dose of IFN, but was most notable at 5.0 ng/mL. At that dose, in two consecutive studies (in cells from each mouse, each variable was quantified using mean colony counts of triplicate plates) exposure of FAC −/− marrow cells to agonistic fas antibody reduced BFU-E to 10.7% control growth while exposure of heterozygote marrow to the antibody reduced BFU-E growth to 52% of control values (P < .005, by Student's t-test). (B) IFN-γ administration in vivo induces fas expression and reduces progenitor cell number in spleen and BM of FAC mice. Two sets of paired mice were treated with 105 U IFN-γ/d intraperitoneally for 5 days. Results were comparable in both experiments (bars reflect means ± SD). BM BFU-E were significantly suppressed in IFN-γ–treated heterozygote mice, but the fractional reduction was significantly greater in BM of FAC −/− mice. Splenic BFU-E increased in treated heterozygotes but were markedly suppressed in FAC −/− mice. (C) Treatment of unfractionated marrow cells from FAC −/+ mice with anti-fas antibody did not suppress BFU-E growth but did suppress BFU-E growth in the FA-C cells by 50%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.974/4/m_bl_0072f3b.jpeg?Expires=1767781481&Signature=PeaiQg~nE2RKS19dCMry2JI2thGg0UK3vFUBRdDverDZ8BmmViLskl5mPbcfUzKQI57CAAi30Szs5fn2MyMnBvKY7L0N91NPKolEOturflq4NJ9ayvqnyFABT1mwjDfhQEQNarP7IKyzOcwq94NcAyR2ho9MS7RClrEugbyVi11060nhNCCwCsVCbPPmV4ToA7qLisKApV5havDeylw6z9KIrOnres2~jY2BiwTF4vs9vLk7esbDNUE1VF5qQxq10xqQUy~QjGIADYXYXVBA7FGS~i9OFlwH8LbHMZbvBa8Z7R67wnrkfA9~0AEcuGsPgqPChazPDvLcW-79Kzs-sA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. IFN-γ primes the fas pathway in vitro and in vivo. (A) Results of one representative experiment (of four separate experiments) of BM cells from an FAC −/− mouse (□) and from an FAC −/+ (○) littermate. Cells were exposed to various doses of recombinant murine IFN-γ for 60 minutes, followed by 3-hour exposure to antimurine fas antibodies (“FA-C/fas” [▪] and “hetero/fas” [•]) or medium alone (“FA-C” [□] and “Hetero” [○]). Open and closed circles represent clonal growth of BFU-E from FAC −/+ mice. Open and closed squares represent BFU-E growth of marrow cells from FAC mice. Responses of FAC cells were distinctly abnormal in three ways: (1) BFU-E were suppressed by IFN-γ alone at doses (0.05 and 0.5 ng/mL) that had no suppressive effect on FAC −/+ cells, (2) the addition of anti-fas to FAC cells, even without prior exposure to IFN-γ, resulted in clonal suppression, and (3) the “priming” effect of IFN-γ in FAC-cells (the degree to which the addition of anti-fas augmented fractional BFU-E suppression at every IFN-γ dose) was greater at these low doses of IFN-γ as compared with FAC −/+ progenitor cells. Fas priming occurred in FAC −/+ mice at every dose of IFN, but was most notable at 5.0 ng/mL. At that dose, in two consecutive studies (in cells from each mouse, each variable was quantified using mean colony counts of triplicate plates) exposure of FAC −/− marrow cells to agonistic fas antibody reduced BFU-E to 10.7% control growth while exposure of heterozygote marrow to the antibody reduced BFU-E growth to 52% of control values (P < .005, by Student's t-test). (B) IFN-γ administration in vivo induces fas expression and reduces progenitor cell number in spleen and BM of FAC mice. Two sets of paired mice were treated with 105 U IFN-γ/d intraperitoneally for 5 days. Results were comparable in both experiments (bars reflect means ± SD). BM BFU-E were significantly suppressed in IFN-γ–treated heterozygote mice, but the fractional reduction was significantly greater in BM of FAC −/− mice. Splenic BFU-E increased in treated heterozygotes but were markedly suppressed in FAC −/− mice. (C) Treatment of unfractionated marrow cells from FAC −/+ mice with anti-fas antibody did not suppress BFU-E growth but did suppress BFU-E growth in the FA-C cells by 50%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/3/10.1182_blood.v90.3.974/4/m_bl_0072f3c.jpeg?Expires=1767781481&Signature=cE1Z2KzkTbbUjoQlpXu3yM8pG7OAG0zHbomsEVu7Nmgjeqh-RJs-Y-1TvvEAtnOv9l9xC9y-AG3RMX~yuhDoTAZTZbq6kpGn0ScBUr1~0wkg4rY5D3J-z5Lk7pBE2pnm0M8GO9usGmvcAT04a5YTAEJsEswJlUUzvOceD0w3TEXbnmQuekJPkAXLBuVx6VcUZsFCO4uIIMTsC~eQyMEez6Gb4gmKuISjsGKwlRqZYVQy9J6kwq-j~cIF8CTyXL3TpfHmNNuEGLWtKDNn6cw1n~txiQISdw0iAHW0QJrsoRT1uDtqPBv93HIyvuR-EH9JwrvSB82DqXSCx-yQ6wgtCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal