HEMATOPOIESIS is governed by a number of cytokines that promote the survival, proliferation, and differentiation of hematopoietic stem cells and progenitor cells.1 Stem cell factor (SCF; also known as kit ligand, mast cell growth factor, or steel factor) is a hematopoietic cytokine that triggers its biologic effects by binding to its receptor, c-kit.2-5 A host of naturally occurring mutations at the Sl locus or at the W locus, which encode SCF and the c-kit receptor, respectively, has been identified6,7 and molecularly characterized (reviewed in Besmer et al8 ). These mutations provide insights into the role of SCF and the c-kit receptor in vivo. Absence of SCF protein (the Sl mutation) or absence of cell surface c-kit receptor display (the W mutation) result in death in utero or in the perinatal period with severe macrocytic anemia. Absence of c-kit receptor kinase activity (the W42 mutation)9 also causes perinatal death with severe macrocytic anemia. These observations indicate that SCF plays an essential role during development in utero.
Point mutations in the c-kit receptor that diminish its tyrosine kinase activity or mutations that alter SCF production are associated with a spectrum of phenotypic abnormalities, including variable degrees of macrocytic anemia, decreased numbers of tissue mast cells, decreased fertility, and decreased pigmentation.10 These observations demonstrate a role for SCF and its receptor c-kit in hematopoiesis and in development of germ cells and melanocytes. Development of the interstitial cells of Cajal, which are responsible for intestinal pacemaker activity, is defective in mice with mutations at the Sl or W loci.11,12 In general, the severity of the defect in c-kit receptor kinase activity parallels the severity of the phenotypic abnormalities that result.13
SCF is normally found in both soluble and transmembrane forms14 (Fig 1, described in detail below). The Sld mutation (deletion of the transmembrane and cytoplasmic domains of SCF ) results in production of soluble SCF and absence of transmembrane SCF.14,15Sl/Sld mice are viable, but have severe macrocytic anemia, markedly reduced tissue mast cells, and are sterile and white.6 The phenotype of the Sl/Sld mouse indicates that the presence of soluble SCF is not sufficient to completely compensate for the lack of transmembrane SCF and suggests that the transmembrane form of SCF may play a unique role in vivo.
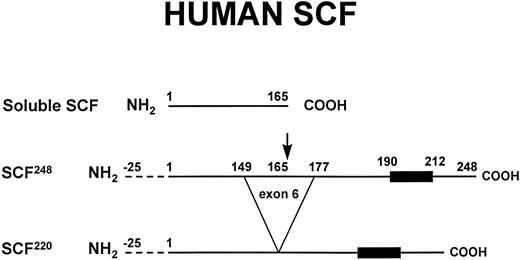
Soluble and transmembrane forms of human SCF. The primary proteolytic cleavage site of SCF248 in exon 6 is indicated by the arrow. Cleavage at this site generates the soluble form of SCF. The transmembrane form of SCF, SCF220, lacks the primary proteolytic cleavage site in exon 6. The 25 amino acid signal sequence (dotted lines) and the hydrophobic transmembrane domain (dark box) are also shown. As described in the text, murine SCF248 and murine SCF220 can also be cleaved at an alternative site in exon 7.
Soluble and transmembrane forms of human SCF. The primary proteolytic cleavage site of SCF248 in exon 6 is indicated by the arrow. Cleavage at this site generates the soluble form of SCF. The transmembrane form of SCF, SCF220, lacks the primary proteolytic cleavage site in exon 6. The 25 amino acid signal sequence (dotted lines) and the hydrophobic transmembrane domain (dark box) are also shown. As described in the text, murine SCF248 and murine SCF220 can also be cleaved at an alternative site in exon 7.
During embryonic life, SCF and c-kit receptor RNA are expressed along the migratory pathways and in destinations of primordial germ cells and melanocytes, in sites of hematopoiesis (including the yolk sac, fetal liver, and bone marrow), in the gut, and in the central nervous system.16-18 This pattern of expression suggests that SCF may influence the migration of germ cells, melanocytes, and hematopoietic cells to their ultimate destinations during development. Hematopoietic cells expressing the c-kit receptor protein were detected at gestational day 8 in the embryonic yolk sac and by day 10 in fetal liver, where they progressively increased until day 15 and then decreased,19 paralleling the transition from yolk sac to fetal liver to bone marrow hematopoiesis. Fetal thymus also contains c-kit receptor-positive primitive T-lymphocyte and B-lymphocyte progenitors.19 Although the brain and spinal cord develop normally in mice with mutations at the Sl or W loci, these mice demonstrate subtle learning and memory defects.20
In addition to its essential role during development, SCF is also important during adult life. Treatment of adult mice with a neutralizing anti–c-kit receptor monoclonal antibody (ACK2) causes pancytopenia and markedly decreases bone marrow cellularity,21 suggesting that constitutive production of SCF by marrow endothelial cells and fibroblasts22,23 may be required for maintenance of normal basal hematopoiesis. SCF is also required for acute erythroid expansion during recovery from hemolytic anemia in adult mice.24 Spermatogenesis,25 melanocyte development,26 gut motility,12 and response to intestinal helminth infection27 are impaired by ACK2 antibody treatment.
This review will briefly discuss the production and structure of SCF and will focus on the physiologic role of SCF in hematopoiesis. The cellular distribution of the c-kit receptor will also be described. Potential clinical uses for SCF and therapeutic approaches based on the c-kit receptor will be discussed.
SCF PRODUCTION
SCF is encoded by the Sl locus on mouse chromosome 104,5,28 and has been mapped to human chromosome 12q22-12q24.29,30 The structural organization of the SCF gene has been recently reviewed.31
The soluble and transmembrane forms of SCF are generated by alternative splicing that includes or excludes a proteolytic cleavage site (Fig 1).29,32 Both the soluble and the transmembrane form of SCF are biologically active.33,34 SCF248 includes exon 6, which encodes a proteolytic cleavage site, resulting in the production of soluble SCF. The cleavage occurs after Ala165. The lack of exon 6 in human SCF220 results in production of the transmembrane form of human SCF. In SCF220, amino acids 149-177 are replaced by a Gly residue. The ratio of SCF248 mRNA to SCF220 mRNA varies considerably in different tissues, ranging from 10:1 in the brain and 4:1 in the bone marrow to 0.4:1 in the testis.32,35 Studies of normal human bone marrow fibroblasts confirm that these marrow stromal cells contain predominantly SCF248 mRNA.23 The mechanisms that control the tissue-specific32 and developmentally regulated36 production of SCF248 vs SCF220 are not well understood.
Soluble murine SCF can be generated by cleavage of murine SCF248 at the site in exon 6 or by cleavage of murine SCF248 or murine SCF220 at an alternative site in exon 7. A fibroblast cell line (Sl/Sl4 ), derived from the liver of a fetal Sl/Sl mouse that contains no SCF mRNA,34 was engineered to express murine SCF248 or murine SCF220.37 Both the Sl/Sl4murine SCF248 and the Sl/Sl4murine SCF220 cell lines were found to produce soluble SCF,37 suggesting the existence of a secondary proteolytic cleavage site for murine SCF.32,37 Deletion of 12 nucleotides (encoding Lys178-Lys181 ) in exon 7 of murine SCF prevented release of soluble SCF, thus identifying a second proteolytic cleavage site that is unique to murine SCF.
The cleavage of SCF from the cell surface can be induced by activation of protein kinase C or by agents that increase cytosolic calcium levels.32 Two serine protease inhibitors prevented the cleavage of murine SCF248 but not that of murine SCF220, suggesting that cleavage at the sites in exon 6 and exon 7 may be differentially regulated.38 However, certain protease inhibitors affect protein transport to the cell surface rather than protein cleavage at the cell surface, and a recent report suggests that a family of metalloproteases may release the soluble form of many cell surface proteins.39
SCF is constitutively produced by endothelial cells and by fibroblasts.22,23,40 These cells display the transmembrane form of SCF on the cell surface and also release soluble SCF. Keratinocytes in normal skin41 and epithelial cells in the gut42,43 produce SCF, and SCF protein can be detected in the thymus44 as well as in other sites.45-47 Enriched populations of human hematopoietic stem cells and progenitor cells (CD34+c-kit receptor+) are reported to contain SCF mRNA detectable by reverse transcription polymerase chain reaction.48 Inflammatory stimuli such as interleukin-1 (IL-1) or tumor necrosis factor (TNF ) may modestly enhance SCF protein production by marrow stromal cells,22,23 in contrast to their profound ability to increase granulocyte-macrophage colony-stimulating factor (GM-CSF ) and granulocyte colony-stimulating factor (G-CSF ) production.49 Exposure of endothelial cells to transforming growth factor β1 (TGFβ1 ) can decrease SCF mRNA content and SCF production in vitro.50
The concentration of SCF in normal human serum is, on average, 3.3 ng/mL.51 Studies of patients with aplastic anemia, myelodysplasia, and a number of other types of chronic anemia have shown no increase in serum SCF levels.52-54 Thus, the level of SCF in the circulation, unlike the level of erythropoietin (Epo), is not inversely related to the hematocrit. Serum SCF levels do not increase during the period of profound pancytopenia in patients undergoing marrow ablative chemoradiotherapy and stem cell transplantation.55 Myelosuppressive chemotherapy or radiation therapy can increase SCF mRNA levels in murine bone marrow,56,57 but whether this results in increased display of the transmembrane form of SCF within the marrow microenvironment is not known. Alterations in the local distribution of SCF within the skin have been described in patients with cutaneous mastocytosis41 and may play a role in the pathogenesis of this disorder.
SCF STRUCTURE
The soluble form of SCF circulates as a noncovalently bonded dimer, is glycosylated, and has considerable secondary structure, including regions of α helices and β sheets.5,58-60 The molecular weight of the soluble form of SCF calculated from its amino acid sequence is approximately 18,500 daltons. Expression of SCF in Chinese hamster ovary cells results in proteins of 28,000 to 40,000 daltons, indicating the presence of extensive and heterogenous glycosylation.58,61 Human SCF expressed in Chinese hamster ovary cells is approximately 30% carbohydrate by weight58 and contains both N-linked and O-linked carbohydrate, both with attached sialic acid. The glycosylation sites of SCF have been characterized in detail.62
The 4 Cys residues of SCF are involved in intramolecular disulfide bonds62 (Fig 2). The disulfide pairs are Cys4-Cys89 and Cys43-Cys138. Truncation mutagenesis of the carboxy-terminal region of soluble SCF reveals that biologic activity is diminished when the region containing Cys138 is deleted, suggesting that the Cys43-Cys138 bond may be important for full biologic activity63; subsequent work suggests that both intramolecular disulfide bonds are critical to maintain SCF in a fully biologically active conformation.64 Although an active dimeric form of SCF with 4 intermolecular disulfide bonds has been identified during oxidation and refolding of recombinant SCF expressed in Escherichia coli,65 neither Chinese hamster ovary-expressed SCF nor native SCF dimers have been reported to contain intermolecular disulfide bonds, so it seems unlikely that this form of SCF plays a major role in vivo.
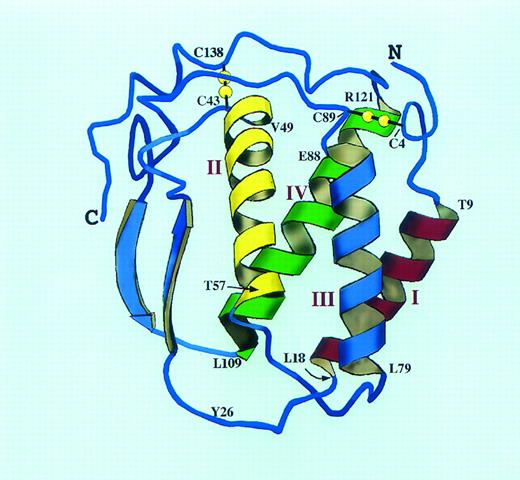
A molecular model of human SCF structure. The model is based extensively on the published structure of human M-CSF (Modified and reprinted with permission from Pandit et al.69 Copyright 1992 American Association for the Advancement of Science.) and the M-CSF/SCF alignment of Bazan.66 The SCF four helix bundle with two long overhand loops is shown as a ribbon diagram. The location of the two intramolecular disulfide bonds is shown in yellow, and the helix boundaries are indicated in the single amino acid code. The interhelical loop lengths have been altered from the M-CSF structure to account for the slightly longer predicted loops of SCF. Tyr26 may be part of the dimer interface for SCF; this residue corresponds to Cys31 of M-CSF, the site of the single M-CSF interchain disulfide bond (Cys31-Cys31 ).
A molecular model of human SCF structure. The model is based extensively on the published structure of human M-CSF (Modified and reprinted with permission from Pandit et al.69 Copyright 1992 American Association for the Advancement of Science.) and the M-CSF/SCF alignment of Bazan.66 The SCF four helix bundle with two long overhand loops is shown as a ribbon diagram. The location of the two intramolecular disulfide bonds is shown in yellow, and the helix boundaries are indicated in the single amino acid code. The interhelical loop lengths have been altered from the M-CSF structure to account for the slightly longer predicted loops of SCF. Tyr26 may be part of the dimer interface for SCF; this residue corresponds to Cys31 of M-CSF, the site of the single M-CSF interchain disulfide bond (Cys31-Cys31 ).
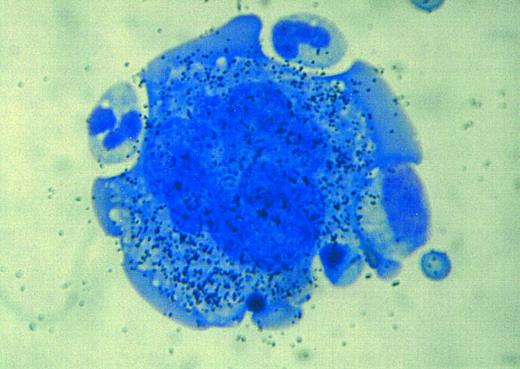
Autoradiographic analysis of 125I-SCF binding to a normal human megakaryocyte. A multitude of grains is associated with the megakaryocyte, indicating that these cells display the c-kit receptor.
Autoradiographic analysis of 125I-SCF binding to a normal human megakaryocyte. A multitude of grains is associated with the megakaryocyte, indicating that these cells display the c-kit receptor.
SCF shares a number of features with macrophage colony-stimulating factor (M-CSF ),66 including dimeric structure, the existence of soluble and transmembrane forms generated by alternative splicing of a proteolytic cleavage site in exon 6, homologous intramolecular disulfide bonds, and homology between their receptors, c-kit and c-fms.67,68 M-CSF contains 3 additional Cys residues (beyond the 4 Cys residues found in SCF ), one of which is involved in an intermolecular disulfide bond between the two M-CSF monomers.69 It has been speculated that the relatively large area of contact at the surface of the two SCF monomers, even in the absence of an intermolecular disulfide bond, may suffice for stable dimer formation.69 Whether the transmembrane form of SCF is a dimer like the soluble form of SCF, is not known.
Rat and human SCF have roughly equivalent bioactivity on human hematopoietic cells, but rat SCF is 800-fold more active on a murine cell line than is human SCF.70 Rat SCF has a 100-fold higher binding affinity for the murine c-kit receptor than does human SCF.71 These observations have permitted the use of interspecies chimera to investigate the sequences of SCF required for bioactivity.72 Regions of the first, third, and fourth helices of SCF have been implicated as being essential for biologic activity.72 A splicing defect in the cytoplasmic tail of SCF in Sl/Sl17H mice results in male sterility,73 suggesting that an intact cytoplasmic domain of SCF may be important for normal function, although this mutation may also alter the stability of transmembrane SCF.
Amino-terminal and carboxy-terminal truncation of soluble human SCF has identified amino acids 1-141 as being essential for full biologic activity in vitro, assessed by ability to support proliferation of a factor-dependent cell line and ability to competitively displace binding of 125I-SCF1-165 to the c-kit receptor.74 Absence of amino acids 1-3 partially impaired both cell proliferation and receptor binding activity, whereas deletion of amino acid 4 or beyond completely ablated both activities. It is of interest that SCF truncation mutant 1-127 retained full receptor binding activity but had reduced ability to support cell proliferation, suggesting that receptor binding and receptor activation can be dissociated.74
SCF AND HEMATOPOIESIS
Elizabeth Russell first suggested that the W and Sl loci might encode a receptor-ligand pair that was critical for hematopoiesis in vivo.6 The seminal observations that marrow from W mutant mice could not reconstitute hematopoiesis when transplanted into irradiated hosts,75 that Sl marrow microenvironmental cells could not fully support hematopoiesis in vitro,76 and that transplantation of normal splenic tissue into Sl/Sld mice improved hematopoiesis77,78 indicated that the W defect was intrinsic to hematopoietic cells, whereas the Sl defect was intrinsic to marrow and splenic microenvironmental cells. The marrows of W/Wv and Sl/Sld mice contain fewer colony-forming units-spleen (CFU-S; W/Wv), burst-forming unit-erythroid (BFU-E), colony-forming unit–granulocyte-macrophage (CFU-GM), and colony-forming unit-erythroid (CFU-E) than do littermate controls.79-83 Subsequent studies in vitro have shown that SCF acts on hematopoietic stem cells and progenitor cells and, in some lineages, precursor cells and mature cells as well.
SCF can act directly on an enriched population containing hematopoietic stem cells to accelerate their entry into cell cycle.84 SCF alone transiently maintained the long-term repopulating ability of Rhodaminelow, Lin−, Sca-1+ populations of murine hematopoietic cells, suggesting that SCF can promote the survival of hematopoietic stem cells in vitro.85 Self-renewal of stem cells in vitro in the presence of SCF alone or in the presence of SCF, IL-6, and Epo was not found.85,86 Stem cells can survive on a stromal cell line even in the presence of the ACK2 anti–c-kit receptor antibody, suggesting that other cytokines produced by the stromal cells can support stem cell survival in the absence of SCF activity.87 Brief exposure of murine marrow cells to a cytokine cocktail (SCF, IL-3, IL-6, and IL-11) expanded the number of progenitor cells but impaired long-term repopulating ability, sounding a cautionary note for ex vivo expansion protocols.88 Inclusion of IL-3 in a combination of growth factors used for ex vivo expansion reduced the B- and T-lymphocyte potential and the long-term reconstituting ability of murine hematopoietic cells,89-91 suggesting that IL-3 can promote differentiation at the expense of maintenance of true stem cell activity. However, murine marrow cells cultured in SCF plus IL-11 retained long-term repopulating activity and could be serially transplanted up to quartenary recipients.92 Sustained stem cell self-renewal in vivo may also be dependent on SCF.93
Cells that give rise to spleen colonies in vivo (CFU-S) survive in vitro in the presence of SCF, and SCF in conjunction with IL-3 can increase the production of CFU-S over a 2-week period in vitro.94 Blocking the c-kit receptor with the ACK2 antibody markedly reduced the survival of CFU-S in vitro,95 although cells capable of initiating long-term bone marrow cultures (LTBMC-IC; defined by the ability to generate CFU-GM over a 5-week period in vitro) survived. Sl/Sl and normal fibroblasts supported LTBMC-IC equivalently, arguing that SCF is not required for LTBMC-IC survival.96 Human LTBMC-IC underwent self renewal and 50-fold expansion in the presence of a cytokine cocktail that, for optimal LTBMC-IC expansion, included SCF.97
SCF, in concert with IL-3 or other cytokines, increased the number of BFU-E, CFU-GM, and colony-forming unit granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) produced approximately 20-fold in liquid culture over a 1- to 4-week period in vitro,98,99 indicating that SCF can act on a more primitive cell (pre–CFU-C) capable of generating the direct colony-forming cells. These observations98-101 have formed the basis for ex vivo expansion protocols for human hematopoietic progenitor cells. Combinations of 5 cytokines (SCF, Epo, IL-1, IL-3, and IL-6) resulted in up to a 200-fold expansion of BFU-E, CFU-GM, and CFU-GEMM over a 2-week period in vitro from mobilized human peripheral blood CD34+ cells102; LTBMC-IC cells did not expand under these conditions.103 Ex vivo expanded populations of cells have been reinfused into patients following myelosuppressive chemotherapy.104 Because these were autologous transplants in patients who did not receive myeloablative chemotherapy, the contribution of the infused stem cells to long-term hematopoiesis could not be stringently assessed,104 and it is not clear that the infusion of these cells accelerated hematopoietic reconstitution. Other SCF-containing combinations of cytokines are also effective in amplifying the number of BFU-E, CFU-GM, and CFU-GEMM generated in liquid culture.105-108
SCF synergizes with other cytokines (including Epo, IL-3, GM-CSF, and G-CSF ) to support the direct colony growth of BFU-E, CFU-GM, and CFU-GEMM in semisolid media.98,109-114 Although SCF alone has modest effect on colony growth, in the presence of other cytokines SCF increases both the size and the number of colonies. The combinations of SCF plus IL-9 or SCF plus IL-7 have also been reported to synergistically promote BFU-E or CFU-GM colony growth, respectively.115,116 SCF can also promote progenitor cell survival.117 SCF is required for BFU-E growth under serum-free conditions,118 and CD34+ hematopoietic cells cultured in the presence of SCF plus IL-3 can generate CFU-E,119 suggesting that the deficiency of CFU-E numbers in the marrow or fetal liver of Sl or W mutant mice120 may be due to lack of SCF synergistic activity.119,121,122 Interaction between the c-kit and Epo receptors123 may be essential to enable CFU-E to proliferate and differentiate in response to Epo.124 The combination of SCF, IL-6, and soluble IL-6 receptor can support the proliferation, differentiation, and terminal maturation of BFU-E in vitro, even in the absence of Epo.125 Reports that SCF can reactivate fetal hemoglobin synthesis in BFU-E colonies126 and can retard differentiation of CFU-E in vitro127 may reflect the potent synergistic effect of SCF on the proliferation of less mature erythroid progenitor cells. BFU-E, CFU-GM, and CFU-GEMM can migrate along an SCF gradient, suggesting that SCF can also function as a chemotactic and chemokinetic factor for progenitor cells.128
Mice with mutations at the Sl and W loci have normal platelet counts,6 indicating that full SCF bioactivity is not an absolute requirement for platelet production in vivo. SCF alone is not a potent stimulant of colony-forming unit-megakaryocyte (CFU-Meg) proliferation or of megakaryocyte nuclear endoreduplication or cytoplasmic maturation in vitro.129-132 However, SCF can synergistically enhance the ability of other cytokines such as Tpo, IL-3, and GM-CSF to promote CFU-Meg colony growth and to increase the size of CFU-Meg colonies.129,130 The addition of SCF to IL-3 doubled the number of CFU-Meg generated in human long-term bone marrow cultures over a 3-month period, indicating the SCF can act on a more primitive population of cells capable of producing CFU-Meg.130 SCF also promotes the growth but not the differentiation of human megakaryocytic cell lines in vitro.131 Thus, SCF predominantly exhibits synergistic proliferative effects on megakaryocytic progenitor cells, especially in combination with Tpo or IL-3.129,130,132 SCF can also potentiate the ability of epinephrine and ADP to induce the secondary wave of platelet aggregation and serotonin secretion.133
SCF AND MAST CELLS
The profound mast cell deficiency in W/Wv and Sl/Sld mice suggested that SCF may be required for mast cell production. Although the tissues of W/Wv or Sl/Sld mice contain less than 1% of the normal levels of mast cells,134,135 mast cell progenitors are found in the marrow of these mice,136,137 and treatment of Sl/Sld mice with SCF increases the number of mast cells in the skin.4 SCF promotes the survival, proliferation, and maturation of mast cells in vitro138,139 (Table 1). The earliest committed mast cell progenitor, the pro-mastocyte, proliferates and differentiates in vitro in the presence of SCF plus IL-3.140 Mast cells can be cultured from human bone marrow CD34+ cells,141 peripheral blood mononuclear cells,142 cord blood cells,143 or fetal liver cells,144 or from rodent marrow cells145 in the presence of SCF. Optimal murine mast cell proliferation and differentiation in response to SCF may require cofactors such as IL-3, IL-4, or IL-10.146 In cultures maintained in SCF for more than 3 months, mast cell production may predominate over granulocyte-macrophage production.143
Effects of SCF on Mast Cells
| In vitro | Survival |
| Proliferation | |
| Maturation | |
| Mediator release | |
| Chemotaxis | |
| Adhesion to fibroblasts or extracellular matrix | |
| In vivo | Survival |
| Proliferation | |
| Locally at injection site | |
| Systemically | |
| Mediator release | |
| Histamine | |
| α Tryptase | |
| Migration of mast cell precursors |
| In vitro | Survival |
| Proliferation | |
| Maturation | |
| Mediator release | |
| Chemotaxis | |
| Adhesion to fibroblasts or extracellular matrix | |
| In vivo | Survival |
| Proliferation | |
| Locally at injection site | |
| Systemically | |
| Mediator release | |
| Histamine | |
| α Tryptase | |
| Migration of mast cell precursors |
SCF also promotes mast cell secretory function,147,148 chemotaxis,149 and adhesion150 and can enhance secretion of mediators in response to IgE-dependent activation148,151 (Table 1). At concentrations similar to that found in normal human serum, SCF stimulates histamine and prostaglandin D2 release from cultured human cutaneous mast cells.148 However, brief preincubation of mast cells with substantially lower concentrations of SCF can potentiate IgE-dependent cutaneous mast cell mediator release.147,148 At concentrations 10- to 100-fold lower than required to stimulate mast cell proliferation, SCF enhances histamine and leukotriene C4 release from human lung mast cells in response to IgE receptor cross-linking.151 SCF can also potentiate IgE-dependent histamine released by human basophils, although basophils appear to be much less sensitive to SCF than are mast cells.148 In murine mast cells, SCF can induce secretion of cytokines (including IL-6 and, to a lesser extent, TNF-α) at concentrations that induce little or no release of preformed mediators such as histamine.152 Mast cell chemotaxis in the direction of a gradient of SCF has also been reported.149 The effects of SCF on mast cell adhesion to fibronectin or to fibroblasts are described in a subsequent section.
In preclinical studies, SCF treatment increased tissue mast cell numbers by greater than 100-fold153,154 and induced mast cell activation in vivo.147 The studies documenting the effects of SCF on mast cell proliferation, maturation, chemotaxis, and degranulation provide important information that may explain the principal toxicity that has been encountered with the use of SCF in vivo.155
SCF AND LYMPHOPOIESIS
B lymphopoiesis is relatively normal in mice with mutations at the Sl or the W loci.156,157 B lymphopoiesis was not impaired in mice injected with the ACK2 anti–c-kit receptor antibody,158 although erythroid and myeloid cells were virtually eliminated from the marrow.21 In fact, the number of pro-B cells, pre-B cells, and mature B cells increased twofold to fourfold in the mice treated with the ACK2 antibody.158 Transplantation of W/W fetal liver cells into lymphocyte-deficient RAG-2−/− mice resulted in development of all stages of B cells, demonstrating that signal transduction through the c-kit receptor is not essential for B lymphopoiesis in vivo.159
The initial stages of B-cell development in vitro (production of pro-B cells and pre-B cells) requires factors produced by stromal cells, whereas the maturation of B lymphocytes does not depend on stromal cells.160 Whether SCF is one of the stromal cell-derived factors that is essential for B lymphopoiesis has been intensely investigated. When individual primitive hematopoietic cells (Sca-1+ Lin− cells that retain the potential to differentiate along the lymphoid or the myeloid lineages) were cultured in combinations of cytokines, SCF was effective in maintaining the B-lymphoid potential of these cells.161 However, the combination of flk-2/flt3 ligand plus IL-7 may be more potent that the combination of SCF and IL-7 in this respect.162 The most primitive cells that are committed to the B lineage (pro-B cells) do not proliferate in response to SCF, IL-7, or the combination of these two cytokines.163 Pro-B–cell numbers increase as readily on Sl/Sl stromal cells (that do not produce SCF ) as on S17 stromal cells (that do produce SCF ), demonstrating that the growth of the pro-B cells depends on stromal cell factors distinct from SCF.163 The differentiation of pro-B cells to pre-B cells also depends on stromal cell factors other than SCF.164 SCF and IL-7 synergistically stimulate the proliferation of pre-B cells, but SCF is not required for the differentiation of pre-B cells to surface Ig+ B lymphocytes.164,165 Likewise, the proliferative response of B lymphocytes to mitogens does not require SCF/c-kit receptor interaction.165 These reports suggest that SCF exerts its predominant effect on B lymphopoiesis in vitro by enhancing the proliferative response of pre-B cells to IL-7.19
SCF plays a role in the early stages of T lymphocyte development in vivo.166 The size of the most immature thymocyte compartment in neonatal W/W mice is 1/40 of that found in littermate control mice,166 and W/W fetal liver cells do not contribute to thymopoiesis after transplantation into RAG-2−/− mice.159 Populations of normal CD4− CD8− CD3− thymocytes can proliferate in vitro in response to the combination of SCF plus IL-7.44,167 The ability of CD4− CD8− CD3− thymocytes to reconstitute T-cell differentiation in explants of thymic tissue was ablated by the presence of the ACK2 anti–c-kit receptor antibody,167 suggesting that SCF is critical for early T-lymphocyte development in this in vitro model. Likewise, Sl/Sl fetal thymic tissue, when implanted into a normal host, did not support production of CD4−CD8−CD3− thymocytes as effectively as did normal fetal thymic tissue implants,166 implying that SCF presentation by the thymic microenvironment is important. The development of intestinal intraepithelial lymphocyte populations is dysregulated in W/Wv and Sl/Sld mice,168 suggesting that SCF/c-kit receptor interaction is important for normal function of the intestinal immune system. SCF may also affect mature T-cell function by potentiating the allogeneic mixed lymphocyte reaction.169
Natural killer (NK) cells are a subset of large granular lymphocytes that are important for the immune response to viral infection and tumor cells. CD34+ human hematopoietic cells can differentiate into NK cells in vitro in the presence of SCF plus IL-2.170 SCF also enhances the ability of CD56bright NK cells to proliferate in response to IL-2.171 However, exposure to SCF did not increase the cytotoxicity of the CD56bright NK cells against K562 cells (NK activity) or augment IL-2–induced LAK activity.171
Dendritic cells are efficient antigen-presenting cells that play a role in T-cell immunity. When CD34+ human marrow cells were cultured in GM-CSF plus TNF-α, the addition of SCF increased the number of dendritic cell progenitors generated up to 100-fold, demonstrating that SCF can amplify dendritic cell progenitor numbers in vitro.172 SCF also synergized with GM-CSF and TNF-α to directly increase the number of dendritic cells produced from CD34+ human marrow cells; the dendritic cells thus generated were capable of stimulating resting T cells in the allogeneic mixed leukocyte reaction.172 173
SCF AND ADHESION
Hematopoiesis occurs in close proximity to bone marrow stromal cells and to extracellular matrix molecules such as fibronectin.174 A number of adhesive interactions maintain the intimate association of hematopoietic stem cells and progenitor cells and marrow stromal elements. Normal hematopoietic progenitor cells express the integrins VLA-4 and VLA-5175 and can adhere in vitro to stromal cells that display VCAM-1176 or to specific sites on fibronectin.177,178 Adhesion to fibronectin declines with terminal erythroid differentiation179 and may modulate progenitor cell proliferation.180 Other adhesive interactions may also be important.174 181
Evidence that SCF can modulate the adhesive behavior of hematopoietic cells is derived from studies of mast cells,14,150,182-185 of hematopoietic cell lines,186,187 and of normal hematopoietic cells.187,188 Exposure to SCF increases adherence of CD34+ marrow cells to fibronectin,187 and hematopoietic progenitor cells from W/Wv mice exhibit diminished basal adhesion to stromal cells.189 Stimulation of mast cell adhesion to fibronectin requires 10- to 100-fold less SCF than does stimulation of mast cell proliferation in vitro.182,185 SCF treatment initially increases (over 30 to 60 minutes) and then decreases (over 24 hours) adhesion of hematopoietic cell lines to VCAM-1 or to fibronectin.186 These effects were mediated not by a change in the number of VLA-4 or VLA-5 molecules displayed on the hematopoietic cell surface, but by an alteration in their avidity for VCAM-1 or fibronectin. Other cytokines can also modulate VLA-4 and VLA-5 adhesive function.187 Several of these reports document that SCF-induced cellular adhesion requires c-kit receptor kinase activity. These results suggest that the mechanism of SCF-induced progenitor cell adhesion to VCAM-1 or fibronectin may involve alteration of integrin avidity. An alternative possibility is that the transmembrane form of SCF displayed on fibroblasts binds directly to the c-kit receptor on the surface of hematopoietic cells and thus helps to anchor the hematopoietic cells in the microenvironment.14,183,190 The report that mast cells derived from W/W42 mice (that display the c-kit receptor protein on the cell surface but lack c-kit receptor kinase activity) can adhere normally to fibroblasts184 provides support for the latter model. However, the preponderance of data argues that SCF binding to the c-kit receptor and activation of c-kit receptor kinase activity results in an inside-out signal that modulates integrin avidity on the surface of hematopoietic cells, thereby altering integrin adhesive function.186-188
Because of the strong correlation between the ability of cytokines to induce hematopoietic progenitor cells to proliferate and to adhere to fibronectin, it has been proposed that the cytokine-induced inside-out signal that alters integrin avidity on the hematopoietic cell surface may be followed by an outside-in signal transmitted via the integrins that may promote cell proliferation in cooperation with the hematopoietic growth factors.188 Likewise, SCF-induced enhancement of integrin avidity for fibronectin may potentiate phosphorylation of the focal adhesion kinase pp125FAK,191 which plays a key role in integrin-mediated signalling.
Normal human endothelial cells display high-affinity c-kit receptors,40 and it is possible that SCF binding to endothelial cells or to other marrow stromal cells could alter the adhesive phenotype of these cells. Stimulation of endothelial cells with SCF in vitro did not induce display of the adhesion molecules VCAM-1, ELAM-1, or ICAM-1.40 A preliminary report indicates that SCF treatment of a marrow stromal cell line induced the expression of hemonectin, which could potentially mediate adhesion of progenitor cells.192,193 Expression of human c-kit cDNA in porcine aortic endothelial cells conferred the ability to proliferate, to undergo cytoskeletal reorganization, and to migrate in response to human SCF,194 demonstrating that these cells have the capacity to respond to SCF. The potential effects of SCF on the adhesive function of marrow stromal cells has not been fully explored.
SCF AND HEMATOPOIETIC STEM CELL/PROGENITOR CELL MOBILIZATION
The mechanisms that govern the trafficking of hematopoietic stem cells and progenitor cells between the bone marrow and blood in vivo are not understood in detail. Injection of mice with SCF results in a profound redistribution of primitive hematopoietic cells from the bone marrow into the blood and spleen.195,196 Primates treated with SCF demonstrate a 10- to 100-fold increase in the number of circulating progenitor cells and mobilization of cells that engraft lethally irradiated recipients.197,198 Treatment of primates or mice with antibodies against the integrin VLA-4 or against its counter receptor VCAM-1 also results in egress of progenitor cells from the marrow into the blood,199,200 suggesting that interaction of VLA-4 expressed on hematopoietic cells and VCAM-1 constitutively displayed by microenvironmental cells may play a role in the trafficking of primitive hematopoietic cells in vivo. It is possible that SCF-induced mobilization of stem cells and progenitor cells from the bone marrow into the blood may be mediated in part by alterations in the interactions of hematopoietic cell integrins with VCAM-1 or fibronectin, but multiple mechanisms are likely to be involved.201
Phase I clinical studies showed that treatment with SCF increases the numbers of progenitor cells of many types (including BFU-E, CFU-GM, CFU-Meg, and CFU-GEMM) in the marrow.202 A randomized clinical trial in patients with ovarian cancer compared the ability of SCF plus G-CSF with that of G-CSF alone to mobilize LTBMC-IC203; all patients also received cyclophosphamide. Treatment with SCF resulted in a fivefold to sixfold increase in the number of LTBMC-IC mobilized, as well as an increase in the total number of CD34+ cells mobilized.203
Preclinical studies in mice showed that SCF treatment can expand the total number of stem cells capable of lymphohematopoietic reconstitution, as well as the numbers of CFU-S and direct colony-forming cells.204-207 Hematopoietic cells obtained from mice previously treated with SCF or with SCF plus G-CSF were shown to be excellent targets for retrovirally mediated gene transfer206,208; these studies have recently been extended to primates.209 The combination of SCF plus G-CSF synergistically increased the number of CFU-S in the blood and increased the short-term and long-term repopulating ability of the circulating murine hematopoietic cells.196,205,210 The murine hematopoietic cells mobilized by SCF plus G-CSF maintained marrow repopulating ability on serial transplantation, whereas the hematopoietic cells mobilized by G-CSF had poor repopulating function on secondary transplantation.210 Thus, the population of cell mobilized by SCF plus G-CSF is functionally different than that mobilized by G-CSF alone. Autologous peripheral blood stem cell transplant studies in baboons showed that the use of cells mobilized with SCF plus G-CSF significantly accelerated neutrophil and platelet recovery in comparison to the use of cells mobilized with G-CSF alone.211 Whether the results of these preclinical studies will translate into clinical benefit in human peripheral blood stem cell transplantation remains to be seen.
SCF AND RADIATION
Treatment with SCF alters sensitivity to radiation therapy. The increased radiosensitivity of mice with Sl and W mutations has long been known212 and may be due to the ability of SCF to suppress apoptosis and promote cell cycle progression.213 Mice receiving SCF at specific times before radiation therapy were protected from radiation-induced death, possibly because SCF promotes hematopoietic cell entry into the relatively radioresistant S phase of the cell cycle.214 Pretreatment with SCF also increases the survival of murine duodenal crypt stem cells, which may decrease radiation-induced gut toxicity.215 Mice receiving SCF after radiation therapy demonstrated accelerated recovery of white blood cell and platelet counts.216 Treatment of dogs with SCF after radiation therapy permitted survival despite otherwise lethal doses of radiation.217 Conversely, neutralization of SCF activity increased sensitivity to radiation-induced death.218 SCF as a single agent has generally not accelerated hematopoietic recovery when administered after myelosuppressive chemotherapy or stem cell/progenitor cell transplantation,219-221 although the combination of SCF plus IL-11 may enhance neutrophil and platelet recovery.220
DIFFERENTIAL EFFECTS OF SOLUBLE AND TRANSMEMBRANE SCF
The soluble and transmembrane forms of SCF display somewhat different effects in vitro. Transmembrane SCF expressed by a fibroblast cell line was able to support hematopoiesis (production of CFU-GM) for a several week longer period of time than was soluble SCF produced by the fibroblast cell line.34 Transmembrane SCF is a more potent stimulant of primordial germ cell survival in vitro than is soluble SCF222,223 and may also mediate binding of germ cells to Sertoli cells in the testis.36 Soluble SCF appears to activate the c-kit receptor more transiently and to induce more rapid downregulation of cell surface c-kit receptor display than does transmembrane SCF.224 The duration of activation of the MAP kinase pathway can influence the cellular response (proliferation v differentiation) to the signal in a neuronal cell line225; it is possible that this concept could apply to signaling via the c-kit receptor as well. However, the basis for the differing effects of soluble and transmembrane SCF is not fully understood at present.
The c-kit receptor. The ATP binding site is found in the kinase domain proximal to the cell membrane. The phosphotransferase region is in the kinase domain distal to the cell membrane. As described in the text, a soluble form of the c-kit receptor generated by cleavage at a site near the membrane-spanning region has also been identified.
The c-kit receptor. The ATP binding site is found in the kinase domain proximal to the cell membrane. The phosphotransferase region is in the kinase domain distal to the cell membrane. As described in the text, a soluble form of the c-kit receptor generated by cleavage at a site near the membrane-spanning region has also been identified.
The phenotypes of Sl/Sld mice6,14,15 and of male Sl/Sl17H mice73 suggest that the presence of soluble SCF cannot fully overcome the lack of normally expressed transmembrane SCF. The Sld mutation may also diminish the quantity of soluble SCF produced; SCF activity was not detected in the supernatant of fibroblasts from Sl/Sld mice,137 and administration of exogenous SCF to Sl/Sld mice increased mast cell numbers and hematocrit.4 Despite the low binding affinity of soluble human SCF for the murine c-kit receptor,71 transgenic expression of the transmembrane form of human SCF in mice resulted in abnormalities of coat color reminiscent of certain W alleles,226 suggesting that the human transmembrane SCF blocked the ability of native murine SCF to activate the c-kit receptor in melanocytes.
Transgenic expression of an obligate transmembrane form of murine SCF in Sl/Sld mice dramatically expanded erythropoiesis, but had little effect on myelopoiesis. In contrast, enforced expression of soluble murine SCF in the Sl/Sld mice increased myelopoiesis but not erythropoiesis.227 These results suggest that the presentation of SCF, whether in a transmembrane form by marrow microenvironmental cells or in a soluble form, may differentially influence the proliferation of erythroid and myeloid progenitor cells. As previously discussed, the transmembrane form of SCF may guide the migration of hematopoietic cells, germ cells, and melanocytes to their final destinations during embryogenesis17,18 or may be required for melanocyte survival in the skin.228
THE c-kit RECEPTOR
The c-kit receptor229 is a member of the type III receptor tyrosine kinase family67,68 (reviewed in Ullrich and Schlessinger230 ). This family of cytokine receptors also encompasses the c-fms receptor, the platelet-derived growth factor (PDGF ) receptors, and flk-2/flt3 receptor. The structure of these receptors includes an extracellular domain with five Ig-like motifs, a single short membrane-spanning domain, and a cytoplasmic domain with tyrosine kinase activity (Fig 3). The kinase domain is interrupted by a kinase insert sequence that divides the kinase domain into an ATP binding region and phosphotransferase region. The c-kit receptor is a 145,000-dalton glycoprotein67 and has been given the designation CD117. Binding of SCF (which circulates as a noncovalently associated dimer)58 triggers c-kit receptor homodimerization and intermolecular tyrosine phosphorylation of the receptor, creating docking sites for a number of SH2-containing signal transduction molecules.231 The signal transduction pathways employed by the c-kit receptor will not be covered in this review. An elegant genetic analysis suggests that a protein tyrosine phosphatase, SHP1, may terminate c-kit receptor signal transduction by modulating phosphorylation of downstream substrates of the c-kit receptor.232 233
Deletion analysis and construction of chimeric human-mouse c-kit receptors have suggested that the three amino-terminal Ig-like domains of the c-kit receptor contain the SCF binding site and that the fourth Ig-like domain may contain a site required for c-kit receptor dimerization.234-236 A recombinant soluble form of the c-kit receptor, truncated at the juncture of the extracellular and transmembrane domains, can bind SCF and undergo ligand-induced dimerization,237-239 demonstrating that all of the structural information required for these processes is present in the extracellular domain of the receptor.
Alternative splicing results in two naturally occurring isoforms of the c-kit receptor that contain (designated kit A) or lack (designated kit) four amino acids (Gly Asn Asn Lys) at codon 510 just outside the transmembrane domain.240 These two c-kit receptor isoforms coexist in normal tissues. The ratio of kit A to kit mRNA in normal human bone marrow is approximately 1:5.241 In marrow from patients with acute myelogenous leukemia, the ratio varies considerably, but there is no association with French-American-British subtype or clinical outcome.241 The biologic significance of these two isoforms of the c-kit receptor remains unclear.
Point mutations in the c-kit receptor cytoplasmic domain have been identified in murine and human mast cell lines and in hematopoietic cells from patients with mast cell disorders. Mutation of Asp814 in the kinase domain in a murine mast cell line and mutation of the corresponding amino acid (Asp816 ) in a human mast cell line confer factor-independent growth, constitutive tyrosine phosphorylation of the c-kit receptor, mast cell differentiation, and tumorigenicity in vivo.242-246 The Asp816 mutation has also been found in peripheral blood mononuclear cells from patients with mastocytosis and an associated myelodysplastic disorder247 and in mast cells from patients with urticaria pigmentosa.248 Mutation of Asp814 alters the substrate specificity of the c-kit receptor and results in ubiquitinization and accelerated degradation of SHP1,249 the tyrosine phosphatase that normally attenuates c-kit receptor signal transduction. A mutation of Val559 in the juxtamembrane region of the c-kit receptor, which has been identified in a human mast cell line, results in ligand-independent dimerization and constitutive activation of the c-kit receptor.250 Deletion of seven amino acids (Thr573-His579 ) in the juxtamembrane domain likewise resulted in constitutive activation of the c-kit receptor in a murine mast cell line.251 Thus, structural and functional characterization of the c-kit receptor in mast cell lines or in cells from mastocytosis patients has identified single amino acid mutations and a short deletion in the cytoplasmic domain of the c-kit receptor that can be associated with neoplastic transformation of mast cells.
Mutations in the c-kit receptor have also been found in patients with the autosomal dominant disorder piebaldism.252-255 Human piebaldism is characterized by a white hair forelock and hypopigmented patches on the trunk and extremities, reminiscent of the murine W (dominant white spotting) mutation. The hypopigmented areas of skin in individuals with piebaldism are devoid of melanocytes, possible due to failure of melanocyte migration during embryogenesis. Unlike the murine W mutation, anemia and infertility are not features of human piebaldism.
CELLULAR DISTRIBUTION OF THE c-kit RECEPTOR
The c-kit receptor is broadly distributed within the hierarchy of hematopoietic cells and is also found in other tissues.25,40,47,120 Cell populations enriched for murine hematopoietic stem cells, defined by the ability to reconstitute donor-derived lymphohematopoiesis in lethally irradiated hosts for more than 6 months, display the c-kit receptor on the cell surface.256 A study in which primitive murine hematopoietic cells were separated into c-kit receptor<low and c-kit receptorlow populations and then transplanted into lethally irradiated recipients showed that both populations of cells could reconstitute lymphohematopoiesis in the primary recipients for at least 6 months.257 A report using a different transplant model (human hematopoietic cells transplanted into fetal sheep) showed that the CD34+c-kitlow population of cells contained the long-term reconstituting activity.258 However, most reports,256,258-260 although not all reports,261 agree that c-kit receptor− purified populations of hematopoietic cells lack long-term reconstituting activity. Injection of a single murine hematopoietic cell with the phenotype CD34low/−, c-kit receptor+, Sca-1+, Lin− was capable of reconstituting lymphohematopoiesis for more than 6 months in a portion of recipient mice,262 and donor-derived cells from recipient mice could repopulate lethally irradiated secondary recipients, convincingly demonstrating that the true lymphohematopoietic stem cell with extensive self-renewal capacity displays the c-kit receptor.262 263
The cells that confer radioprotection when transplanted into irradiated recipients, also known as short-term repopulating cells, are also c-kit receptor+,260 as are CFU-S. In populations of human CD34+ hematopoietic cells, approximately 60% to 75% of the cells coexpress the c-kit receptor.264 265 Thus, there is substantial but not complete overlap between the cellular distribution of the CD34 antigen and the c-kit receptor on hematopoietic cells.
Hematopoietic progenitor cells exhibit the c-kit receptor. When c-kit receptor+ hematopoietic cells are selected using a monoclonal antibody and cultured in direct colony-forming assays, the c-kit receptor+ population of cells is greatly enriched in BFU-E, CFU-GM, CFU-Meg, CFU-GEMM, LTBMC-IC, and CFU-E, whereas the c-kit receptor− population of cells is depleted of colony-forming cells.21,256,264-267 Cell sorting experiments based on detection of biotinylated SCF binding to marrow cells suggested that the density of c-kit receptor display on BFU-E is higher than on CFU-GM or on CFU-E.268 However, experiments using an anti–c-kit receptor monoclonal antibody showed similar density of c-kit receptor display on BFU-E, CFU-GM, and CFU-GEMM.269 The latter result suggests that the profound effect of SCF on erythropoiesis in vivo and in vitro, in comparison to its effects on myelopoiesis, is not due to differential receptor density at the progenitor cell level. The c-kit receptor can physically associate with the cytoplasmic domain of the Epo receptor in cells responsive to both cytokines; this observation may provide a molecular explanation for the potent synergistic effects of SCF and Epo on erythropoiesis.123
Recognizable hematopoietic precursor cells also exhibit c-kit receptor on the cell surface. Autoradiographic analysis of 125I-SCF binding to human or murine marrow cells demonstrates a plenitude of grains associated with recognizable erythroblasts, myeloblasts, and megakaryocytes (Fig 4) and a decrease in grain density in parallel to maturation.114,265 Mast cells also exhibit c-kit receptors.270 Platelets display the c-kit receptor after activation with ADP; resting platelets are devoid of cell surface c-kit receptors.133
The c-kit receptor is found on normal B- and T-lymphocyte progenitor cells as well.19 Approximately 85% of pro-B cells display the c-kit receptor.163 A portion of pre-B cells also exhibit the c-kit receptor at low density; c-kit expression is lost as these cells mature to surface Ig+ B lymphocytes.158,165 Of CD4− CD8− CD3− thymocytes, approximately 30% exhibit the c-kit receptor.44 As is true for B-lymphocyte progenitor cells, c-kit receptor density is highest on the least mature subpopulation of the CD4− CD8− CD3− thymocytes.167 These reports provide insights into c-kit receptor display during differentiation and maturation of normal hematopoietic cells and suggest a model in which the c-kit receptor is found at low density on primitive hematopoietic cells capable of long-term lymphohematopoietic reconstitution, increases in density to reach a peak at the progenitor cell level, and then decreases with terminal maturation of hematopoietic precursor cells.271
Neoplastic human hematopoietic cells can also display the c-kit receptor. Virtually all patients presenting with acute myelogenous leukemia have c-kit receptor+ blasts, and SCF can stimulate the proliferation of these cells.272-274 In contrast, leukemic blasts from patients with acute lymphocytic leukemia rarely display the c-kit receptor.272,275 Most non-Hodgkin's lymphomas lack c-kit receptor expression,40,276,277 with the exception of anaplastic large-cell lymphomas that can display the c-kit receptor.276 Lymph nodes from patients with Hodgkin's disease also express the c-kit receptor.276 The c-kit receptor is ubiquitously found on human hematopoietic cell lines of the erythroid and megakaryocytic phenotypes and on some myeloid and lymphoid cell lines as well.131,265,278 Receptor density is highest in erythroleukemia cell lines, which may express up to 50,000 to 100,000 c-kit receptors per cell.238 The majority of cell lines studied display a single class of high-affinity (kd of 50 to 200 pmol/L) binding sites; this is similar to the binding affinity found on normal human hematopoietic cells.279 A number of human solid tumor cell lines (including small cell lung cancer, breast cancer, and neuroblastoma) as well as a variety of fresh human tumor tissues (particularly small cell lung cancer, testicular seminoma, glioblastoma, and some breast cancer samples) have been shown to display the c-kit receptor protein.280-284
MODULATION OF c-kit RECEPTOR EXPRESSION
Display of the c-kit receptor on hematopoietic cells is modulated in a number of ways. Binding of SCF induces rapid internalization of the SCF–c-kit–receptor complex (see cover figure),270,285,286 likely via clathrin-coated pits.287 In parallel, the c-kit receptor is ubiquitinated and targeted for degradation by the proteasome proteolytic pathway.285,286 Internalization and ubiquitination of the c-kit receptor requires the tyrosine kinase activity of the c-kit receptor, but not association and activation of PI 3′ kinase.286 Treatment of cells with chloroquine partially blocks c-kit receptor degradation, suggesting that lysosomal proteases may also be involved.285 Whether a portion of the internalized c-kit receptors is parsimoniously recycled to the cell surface for reuse is unclear at present. Exposure of hematopoietic cells to TGF-β, TNF-α, or IL-4 can downregulate cell surface c-kit receptor display by transcriptional or posttranscriptional mechanisms.288-291 As described below, the c-kit receptor can also be proteolytically cleaved in the juxtamembrane region and shed from the cell surface40,238,270,292; this process can be accelerated by activators of protein kinase C.
SOLUBLE c-kit RECEPTOR
A number of cell surface cytokine receptors have soluble counterparts that are generated by alternative mRNA splicing or by proteolytic cleavage of the cell surface form of the receptor.293,294 Many soluble receptors function as natural antagonists by competing with the cell surface receptor for cytokine binding. Soluble receptors may also function as carriers for the ligand to prolong ligand half-life in vivo,295 may mediate cytokine binding to transmembrane signal transduction subunits of the receptor,296 or may potentiate signal transduction via transmembrane receptor subunits even in the absence of the ligand by as yet unidentified mechanisms.297 Decreased cytokine receptor density on the cell surface is one mechanism that can attenuate cellular response to the cytokine.298 Whether soluble cytokine receptors normally serve to modulate ligand bioactivity in vivo or whether they are a byproduct of cellular desensitization to the ligand remains unclear at present.
Soluble c-kit receptor is released by human hematopoietic cells, mast cells, and endothelial cells40,238,270,292 and circulates in normal human plasma at a concentration of approximately 325 ng/mL.299 The quantity of soluble c-kit receptor in the plasma exceeds that of SCF by a 30-fold molar ratio. Native soluble human c-kit receptor binds SCF with an affinity comparable to that of the cell surface form of the receptor.238 Recombinant soluble c-kit receptor can bind SCF and can antagonize SCF-induced tyrosine phosphorylation of the cell surface c-kit receptor in vitro.237 A form of recombinant soluble murine c-kit receptor, fused to the Fc domain of human IgG and thus an obligate dimer, binds murine SCF with high affinity (kd of 300 pmol/L) and blocks SCF-induced cell proliferation in vitro.300 The stoichiometry of interaction of SCF and recombinant soluble c-kit receptor is 2:2.239 These reports suggest that the quantity and binding affinity of the soluble c-kit receptor present in vivo are sufficient to modulate SCF bioactivity, although this has not been directly demonstrated.
POTENTIAL CLINICAL USES FOR SCF
The potential cornucopia of clinical uses for SCF has been limited by the adverse events encountered in initial clinical trials.155,203 Dermal mast cell degranulation resulting in a pruritic wheal with surrounding edema at the SCF injection site occurs in most patients and resolves within 24 hours.155,203 Melanocyte proliferation and increased melanization of the epidermis, resulting in 3- to 5-cm diameter areas of increased skin pigmentation at SCF injection sites, has been reported in some patients receiving SCF at 5 to 50 μg/kg/d for 14 days.155,301 The hyperpigmentation generally resolves after 2 to 12 months. Approximately 10% to 20% of the patients in two studies developed an allergic-like reaction characterized by urticaria, with or without respiratory symptoms suggestive of laryngeal edema, that required discontinuation of SCF treatment.155,203 The effects of SCF on mast cells appear to be dose-dependent,155 and it is possible that the potent synergistic effects of SCF noted in vitro could be exploited in vivo. For example, low doses of SCF could be used in conjunction with G-CSF or other cytokines as a stem cell and progenitor cell mobilizing agent; clinical trials of SCF plus G-CSF are currently underway.203,302,303 The latter studies have shown an improved safety profile for SCF when used at lower doses. The incidence of allergic-like reactions was approximately 2% in 400 SCF-treated patients.303 Analogs of SCF with enhanced ability to stimulate the proliferation and modulate the adhesive function of hematopoietic cells yet no increased effect on mast cell activation have been designed304,305 and are currently in preclinical trials (K. Nocka, personal communication, November 1996). Hematopoietic cells exposed to SCF either in vivo206,208,209 or in vitro306 are more efficiently transduced by retroviral vectors; this may prove useful for gene therapy. Ex vivo expansion of hematopoietic stem cells and progenitor cells is another potential use for SCF; many of the cytokine cocktails used for this purpose contain SCF.104 Although the number of progenitor cells can be expanded many fold, whether the number of stem cells can actually be increased ex vivo with presently available techniques remains an area of debate.307 308
Therapeutic approaches based on the c-kit receptor protein may eventually have clinical utility. Because the c-kit receptor is displayed on the surface of hematopoietic stem cells and progenitor cells, selection of these cells by immunoaffinity techniques (similar to those currently in clinical use to select hematopoietic cells expressing the CD34 antigen) may provide a population of cells useful for gene targeting and for transplantation.309 The recent success of retrovirally mediated gene transfer by targeting the erythropoietin receptor310 and the use of an adenoviral vector linked to SCF to target cells expressing the c-kit receptor311 underscore the potential of this approach.
NOTE ADDED IN PROOF
ACKNOWLEDGMENT
The author thanks Drs Andrew Bohm and Kenneth Kaushansky for their assistance with Fig 2 and Drs Robert Andrews, George Demetri, Stephen Galli, Keith Langley, Karl Nocka, Thalia Papayannopoulou, and David Williams for helpful discussions and/or providing access to unpublished information.
Supported by National Institutes of Health Grant No. DK44194 and by an American Cancer Society Faculty Research Award.
Address reprint requests to Virginia C. Broudy, MD, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195-7710.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal