Abstract
To further characterize the molecular mechanisms of platelet function, we have sought to identify some of the proteins that mediate the secretory events of the platelet release reaction. We report that platelets contain the general elements of the membrane transport apparatus: N-ethylmaleimide sensitive fusion protein (NSF ), p115/transcytosis-associated protein (p115/TAP), and the soluble NSF attachment proteins (α- and, γ-SNAP). The cDNAs encoding two of these proteins, α- and γ-SNAP, have been cloned from a human platelet-derived cDNA library. Platelet membrane extracts possess SNAPreceptor (SNARE) activity, suggesting that the class of proteins (SNAREs) proposed to provide the specificity for vesicle docking and membrane fusion are present in platelets. To identify these proteins, we have used specific antibodies against known SNAREs to probe platelet extracts. Syntaxin 2 and 4 can be readily detected in platelet membrane preparations and are shown to participate in 20 S complex formation. Syntaxin 1, 3, and 5 could not be detected. Other known SNARE and SNARE-associated proteins such as vesicle-associated membrane protein (VAMP)/synaptobrevin 2, SNAP-25, synaptophysin, or synaptotagmin I could not be immunochemically detected in platelet membrane preparations. The presence of both the general transport proteins (NSF and SNAPs) and specific transport proteins (syntaxin 2 and 4) indicates that platelet exocytosis uses a molecular mechanism similar to other secretory cells such as neurons. However, the subcellular concentrations of these proteins suggest that, unlike neuronal secretion, granule-to plasma membrane docking may be the limiting step in platelet exocytosis.
THE PLATELET'S ROLE is to sense vascular lesions or damage and to respond by secreting components that promote hemostasis and tissue repair. Initially, receptors on the platelet surface bind to elements, which are made accessible as the result of tissue damage, such as collagen, thrombin, and adenosine diphosphate (ADP).1 In one model of platelet activation,2-4 these stimuli trigger morphological changes in the platelet resulting in the apparent movement of the secretory granules to the center of the cell and their subsequent fusion with the surface connected canalicular system (SCCS).1 These granule-plasma membrane (PM) fusion steps result in the release of platelet components critical to the formation of the hemostatic thrombus. Three types of regulated targeting and fusion events occur in the activated platelet: dense core granules to PM, resulting in the release of small molecules such as ADP, serotonin, and calcium; α-granule to PM, resulting in the release of proteins such as von Willebrand's factor, thromboglobulin, and platelet derived growth factor; and lysosome to PM, resulting in the release of degradative lysosomal enzymes.4 5 Each of these secretion events must be tightly controlled to prevent inappropriate release of hemostatic agents. The goal of this study is to begin to identify the molecular components that mediate the membrane fusion events in activated platelets.
Studies of secretion in mammalian systems and in yeast have led to the identification of a number of proteins that appear to play a role in the docking and fusion of vesicles to their target membranes.6-8 A model explaining this process was first proposed by Söllner et al9 and was termed the SNARE hypothesis. This model holds that there are membrane proteins from the vesicle (v-SNAREs) and the target membrane (t-SNAREs) which, through their mutual recognition, govern the specificity of vesicle targeting and docking. Once the two classes of SNAREs bind to each other, they form a complex (7 S docking complex) that recruits the general elements of the fusion apparatus (NSF and SNAPs) to the site of membrane fusion, thereby forming the so-called 20 S fusion complex.9,10 Although the exact mechanism for the final step of membrane fusion is yet to be determined, it is clear that adenosine triphosphate (ATP) hydrolysis by N-ethylmaleimide sensitive fusion (NSF ) is a critical step for disassembly of the 20 S complex and subsequent bilayer fusion.11
Several SNARE molecules have been identified from various sources suggesting that there is sufficient diversity in these molecules to provide the specificity required for vesicular transport.12 v-SNAREs, such as synaptobrevin 1 and 2 and cellubrevin, are small molecular weight (≈20 kD), type II integral membrane proteins, which contain a highly conserved central domain that is important for function.13 Specific v-SNAREs only interact with a limited set of t-SNAREs, demonstrating that these v/t-SNARE molecules are able to recognize each other and thereby specifically target vesicles to their appropriate destinations.14 t-SNAREs can be divided into two families. The syntaxin family are type II integral membrane proteins with a very small lumenal domain15 and have been shown to be important for vesicular transport in both mammals and yeast.12 The second family of t-SNAREs are generally hydrophilic proteins, such as SNAP-25, that appear to be either peripherally associated with a membrane or anchored by fatty acylation of several cysteine residues.16-18
Control of the interactions that mediate the formation of the docking and fusion complexes is critical for regulated secretory events. Several proteins have been shown to affect either v- or t-SNARE activities by modulating how one SNARE binds to its cognate SNARE.19 Specifically, syntaxin 1 has been shown to bind to a cytosolic protein, n-Sec1, which in turn inhibits the t-SNARE's ability to bind to synaptobrevin 2.14 Furthermore, genetic and biochemical data have suggested that the p115/TAP protein and the rab family of small guanosine triphosphate (GTP)-binding proteins may play a role in controlling this n-Sec1/syntaxin interaction.20 Synaptobrevin 2 has been shown to be associated with synaptophysin, which inhibits the v-SNARE's ability to interact with its cognate t-SNARE.21 Some of these regulatory proteins (n-Sec1, synaptophysin, and rabs) possess potential protein kinase sites or are known to be phosphorylated in neurons and platelets.22-24 Rab 3B, rab 6, and rab 8 are phosphorylated in response to thrombin stimulation of platelet secretion.24 n-Sec1 when phosphorylated in vitro by protein kinase C fails to bind to syntaxin 1, suggesting that phosphorylation could affect nSec1p/syntaxin interactions.22 These potential regulatory proteins may be the targets of platelet signalling pathways and thus convert extracellular signals into control of platelet exocytosis.
In this report, we show the presence in platelets of several elements of the general fusion apparatus such as NSF, α- and γ-SNAPs, and p115/TAP. cDNAs encoding two of these proteins, α- and γ- SNAP, were cloned from a platelet-derived cDNA library. We show that SNARE activity is present in platelet membrane extracts and by western blotting, we show the presence of two of the known t-SNAREs, syntaxin 2 and 4. The cellular concentrations of the elements of the secretory machinery suggest a potential mechanism for controlling the membrane fusion events of platelet exocytosis.
MATERIALS AND METHODS
Materials.Outdated platelets were procured as units from the Central Kentucky Blood Center (Lexington, KY). Platelet preparations were routinely checked to insure that they were able to aggregate and secrete serotonin in response to thrombin (2 U/mL) stimulation. HepG2 cells were kindly provided by Dr Daniel Noonan (Lexington, KY).
Antibodies were generously provided as follows: anti-α–SNAP, anti-γ–SNAP,25 anti-NSF26 and anti-syntaxin 1a and b (HPC-1)27 from Dr James E. Rothman (Sloan-Kettering Institute, New York, NY), anti-synaptobrevin 2 (VAMP/synaptobrevin 2) (C1 69.1),21 and anti-synaptotagmin I (C1 41.1)28 from Dr Reinhard Jahn (Yale University, New Haven, CT), antisyntaxin 2, 3, 4, and 515 from Dr Mark K. Bennett (University of California, Berkeley, CA), antihuman SNAP-25 (SP-12)29 from Dr William G. Honer (University of British Columbia, Vancouver, British Columbia, Canada), anti-p115/TAP (7D1)30 from Dr M.G. Waters (Princeton University, Princeton, NJ). Anti-synaptophysin (SY 38) was purchased from Boehringer Mannheim Biochemica (Indianapolis, IN).
A platelet derived human cDNA library in the λZAP vector was constructed with the mRNA from freshly acquired platelets of a thrombocytotic patient and was generously provided by Dr Gerald J. Roth (VA Medical Center, Seattle, WA) and Dr Si Lok (Zymogenetics Inc, Seattle, WA). An additional cDNA library constructed from mRNA of a megakaryocyte cell line (CMK),31 which had been treated with phorbol ester, was a generous gift of Dr Takeyuki Sato and obtained through Mochida Pharmaceuticals (Tokyo, Japan).
Platelet membrane extracts were prepared by a modification of the procedure used to produce the bovine membrane extracts used in Söllner et al.9 The platelet suspensions were cleared of contaminating cells by centrifugation at 600g for 25 minutes at room temperature. Platelets were then harvested from the supernatant by centrifugation at 3,000g for 25 minutes. At this stage, the platelets were >99% pure as determined by microscopy. For the generation of human platelet membrane extracts (HPE) and human platelet homogenates (HPH), the cells were washed three times in buffer A (20 mmol/L HEPES/NaOH, pH 6.5, 137 mmol/L NaCl, 2.7 mmol/L KCl, 50 mmol/L glucose, 5 mmol/L EDTA). The cells were disrupted in 20 mmol/L HEPES/NaOH, pH 7.4, 150 mmol/L KCl, 1 mmol/L dithiothreitol (DTT), 5 mmol/L MgCl2 , 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 10 μg/mL leupeptin, 1 μmol/L pepstatin by five cycles of freeze/thaw. At this stage, the samples were either taken as HPH or further processed to generate HPE. For HPE, membranes from the disrupted cells were harvest by centrifugation at 100,000g for 1 hour at 4°C. The membranes were then washed once to remove peripheral membrane proteins with 20 mmol/L HEPES/NaOH, pH 7.4, 1 mol/L KCl, 1 mmol/L DTT, 5 mmol/L MgCl2 , 1 mmol/L PMSF, 10 μg/mL leupeptin, 1 μmol/L pepstatin. The pellet was then resuspended in 1/100 of the original platelet suspension volume in 20 mmol/L HEPES/NaOH, pH 7.4, 150 mmol/L KCl, 1 mmol/L DTT, 5 mmol/L MgCl2 , 1 mmol/L PMSF, 10 μg/mL leupeptin, 1 μmol/L pepstatin, and solubilized by the addition of Triton X-100 to a final concentration of 7% for 45 minutes on ice. Insoluble material was removed by centrifugation at 100,000g for 1 hour, and the supernatant was dialyzed overnight at 4°C in 25 mmol/L Tris/HCl, pH 7.8, 50 mmol/L KCl, 1 mmol/L DTT, 1% Triton X-100. After dialysis, the protein concentration of the extract was measured by the bicinchoninic acid assay (Pierce, Rockford, IL), and the extract was frozen in aliquots at −80°C. It should be noted that HPE preparations with protein concentrations less than 5.0 mg/mL appeared to have significantly lower SNARE activity, and this has been noted previously for bovine brain SNARE activity.9 Extracts from Hep G2 cells were prepared in a similar manner.
Western blotting and quantification.Western blotting onto nitrocellulose was performed according to standard techniques11 using 5% powdered milk in Tris-buffered saline and horseradish peroxidase conjugated to anti-Ig secondary antibodies (Sigma, St Louis, MO). SuperSignal Substrate (Pierce) was used to visualize the antigens using X-OMAT X-ray film (Eastman Kodak, Rochester, NY). For these experiments, bovine brain cytosol was used as a positive control for blotting conditions. The molecular weights of the bovine proteins serve as useful standards for the human proteins but the relative amounts cannot be used for comparative analysis.
Platelets were washed in buffer A to remove serum proteins before quantification experiments. The cells were counted with hemocytometer and aliquotted based on cell number. To quantify the proteins present in the cells, samples of the washed platelets (0.5 to 1 × 108 for α-SNAP and NSF and 1 to 4.5 × 108 for γ-SNAP) were solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer at 95°C for 10 minutes, separated by SDS-PAGE,32 blotted onto nitrocellulose, and probed with specific polyclonal antiserum. The blots were developed using the SuperSignal system so as not to exceed the linear exposure range of the film. The films were scanned with an Apple One Scanner (Apple Computer Inc, Brea, CA) and the digitized images were integrated using the Scan Analysis software from BioSoft (Cambridge, UK). The amount of the specific proteins in a defined number of human platelets was determined by comparison to standard curves made with known amounts of recombinant proteins that were analyzed on the same SDS-PAGE gel. Because the human proteins show such a high degree of amino acid identity to the bovine and Chinese hamster proteins (97% for NSF Chinese hamster ovary [CHO], 96% for α-SNAP [bovine], and 95% for γ-SNAP [bovine]), it is assumed that the polyclonal antibodies used for this quantification will equally recognize the platelet proteins analyzed and the recombinant proteins used as standards.
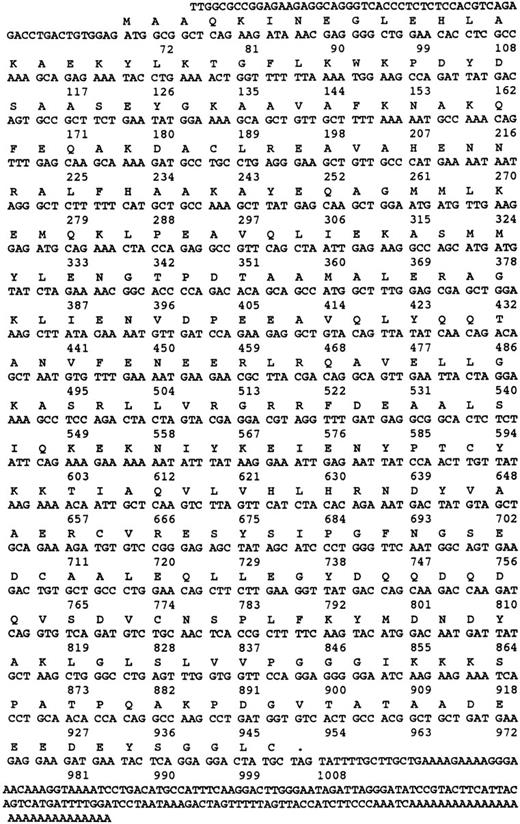
Cloning of cDNA encoding α and γ SNAP.Cloning of the cDNA encoding α-SNAP was performed by initially amplifying a region of the coding sequence using degenerate polymerase chain reaction (PCR) primers 5′-GAYTAYTAYAARGGNGARG and 5′-GCDGCYTTRAARAARTARTC based on conserved regions of the known α-SNAP proteins from bovine, rat, squid, and Drosophila. The cDNA library from CMK cells was used as template. Once positive PCR products were generated and sequenced, they were used as templates for the generation of [32P]-labeled RNA riboprobes.25 The nucleotide sequence of this human-megakaryocyte–derived PCR product was 91% identical to the bovine α-SNAP and 77% identical to the bovine β-SNAP. Bovine γ-SNAP sequences were compared with a database of human expressed sequence tags (EST).33 One clone from a melanocyte cDNA library, accession no. N39674 (I.M.A.G.E. Consortium cDNA Clones) showed a high degree of sequence identity (86%) to the bovine γ-SNAP nucleotide sequence. This clone was purchased from Genome Systems Inc (St Louis, MO) and subjected to dideoxy sequencing (see Fig 3). On completion of the sequencing of this EST, it was apparent that this clone contained the complete melanocyte, γ-SNAP coding sequence. This clone was used to generate [32P]-labeled RNA riboprobes.25 Standard protocols were used to screen the λZAP cDNA library and the resulting positive phagemids were rescued using the Exassist/SOLR system (Stratagene, La Jolla, CA). The phagemid plasmids were then sequenced on both strands by dideoxy sequencing (USB/Amersham, Cleveland, OH). The sequence for human platelet α-SNAP was deposited in Genbank accession no. U39412. The sequence for human platelet and human melanocyte γ-SNAP were deposited in Genbank accession no. U78107.
Nucleotide and amino acid sequence of γ-SNAP. The amino acid and nucleotide sequence of human γ-SNAP cDNA isolated from a human platelet-derived cDNA library and from the melanocyte EST (N39674) are presented. Both nucleotide sequences were identical. Numbers correspond to the number of nucleotides.
Nucleotide and amino acid sequence of γ-SNAP. The amino acid and nucleotide sequence of human γ-SNAP cDNA isolated from a human platelet-derived cDNA library and from the melanocyte EST (N39674) are presented. Both nucleotide sequences were identical. Numbers correspond to the number of nucleotides.
SNARE activity assay.SNARE activity assays were performed according to the method in Nagiec et al.11 [35S]-labeled α-SNAP was produced by in vitro transcription and translation using [35S]-methionine34 (Du Pont/NEN, Boston, MA). Reactions were performed by incubating HPE (40 μg) together with radiolabeled α-SNAP (70,000 cpm) and NSFmyc (12 μg) and the samples were incubated for 15 minutes on ice. An excess of anti-myc antibody (9E10) coupled to Protein G Superose beads (Pharmacia, Piscataway, NJ) was then added to the reactions, and they were incubated for an additional 2 hours. The beads were then collected on glass fiber filters, and the bound [35S]-α–SNAP was measured by liquid scintillation counting. Particle formation was assessed by measuring the amount of [35S]-α–SNAP associated with immunoprecipitated NSFmyc. Production and purification of mass amounts of 20 S complex made from recombinant α-SNAP and NSFmyc, using HPE as a source of platelet SNAREs was performed as described by Söllner et al.9 Briefly, 3.8 mg of HPE were incubated with 300 μg of α-SNAP, 30 μg γ-SNAP, and 112 μg of NSFmyc in the presence of 0.5 mmol/L ATPγS and 1 mmol/L EDTA. The NSFmyc was then precipitated with an excess of 9E10 antibody coupled to Protein G-Superose beads. Following immunopreciptation of NSFmyc, the beads were first washed in binding buffer (20 mmol/L HEPES/NaOH, pH 7.0, 100 mmol/L KCl, 1 mmol/L DTT, 1% [wt/vol] Triton X-100, 1% [wt/vol] PEG-3000, 1% [wt/vol] glycerol), then washed with buffer containing 0.5 mmol/L ATPγS/4 mmol/L MgCl2 and eluted with buffer containing 5 mmol/L ATP/4 mmol/L MgCl2 . Material not eluted was harvested by treatment of the beads with 200 mmol/L glycine/HCl pH 2.7, 0.5%(wt/vol) Triton X-100. All eluted proteins were precipitated with 15% (wt/vol) trichloroacetic acid and analyzed by SDS-PAGE and western blotting.
RESULTS
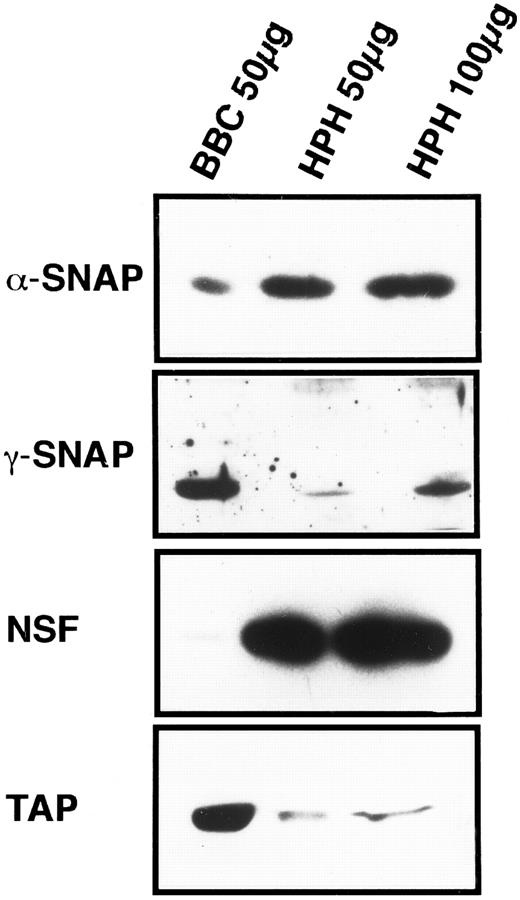
General vesicular transport machinery is present in platelets.If the SNARE hypothesis applies to platelet exocytosis, then the general proteins of the fusion pathway should be present in platelets. As shown in Fig 1, the two SNAPs, α and γ, are readily detectable in 50 μg of total platelet homogenate (HPH) and both proteins have apparent molecular weights that are similar to their bovine counterparts. Resolution of the brain-specific β-SNAP (36 kD) from the general, α-SNAP (35 kD) was not possible with this type of electrophoresis. However, when that analysis was performed, only α-SNAP could be detected in the platelet samples (data not shown). The general fusion protein NSF is also readily detectable in 50 μg of platelet homogenate, and its apparent molecular weight is equivalent to its bovine counterpart (Fig 1). These three proteins, α-, γ- SNAP, and NSF, have been shown to be expressed in other tissues25 35 confirming the generality of the SNAPs and NSF. This also suggests a mechanistic similarity between platelet exocytosis and other forms of regulated and constitutive secretion.
Platelets contain general proteins of the vesicular transport machinery. HPH was probed for the presence of general vesicular transport proteins. A total of 50 and 100 μg of HPH was analyzed by SDS-PAGE followed by western blotting. Western blots were probed with antisera specific for α-SNAP, γ-SNAP, NSF, and p115/TAP. As a positive control for the specific antisera, 50 μg of bovine brain cytosol (BBC) was examined along side the HPH samples. Exposure times for each of the four antigens were not consistent and therefore should not be regarded as indicative of relative amounts.
Platelets contain general proteins of the vesicular transport machinery. HPH was probed for the presence of general vesicular transport proteins. A total of 50 and 100 μg of HPH was analyzed by SDS-PAGE followed by western blotting. Western blots were probed with antisera specific for α-SNAP, γ-SNAP, NSF, and p115/TAP. As a positive control for the specific antisera, 50 μg of bovine brain cytosol (BBC) was examined along side the HPH samples. Exposure times for each of the four antigens were not consistent and therefore should not be regarded as indicative of relative amounts.
p115/TAP was isolated based on its ability to mediate intra-Golgi transport36 and to support transcytosis in polarized epithelial cells.37 Its yeast homologue, USO1p, has been shown to be required for transport between the endoplasmic reticulum and the Golgi apparatus.38 p115/TAP is readily detectable in 50 μg of total platelet homogenates (Fig 1).
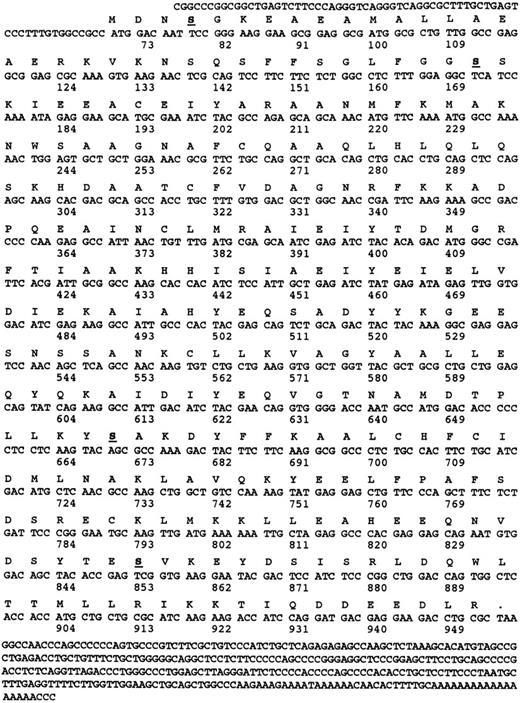
Cloning of cDNA encoding human platelet α- and γ-SNAP.To further characterize the general proteins of the platelet fusion apparatus, we cloned a cDNA encoding α-SNAP from a platelet-derived cDNA library. The nucleotide and predicted amino acid sequence of one of the full-length clones is shown in Fig 2. Of the 600,000 individual plaques screened, 21 were positive and 18 of those contained full-length clones as shown by PCR analysis. Primers with 100% identity to the 5′ and 3′ end of the human α-SNAP coding region together with primers to the T7 and T3 promoters of the pSKII+ phagemid were used to analyze the clones that were not characterized by sequencing. Two of the clones appeared to be partial products that lacked the 5′ end of the coding region. One clone, which was shorter than α-SNAP but contained the 5′ and 3′ sequences of α-SNAP, was sequenced and appeared to be the result of an in-frame deletion of 198 nucleotides (170 to 367) from the coding region (Fig 2). While this internally deleted clone could encode a protein product similar in sequence to α-SNAP, no truncated species were detected by Western blotting (data not shown). Using PCR and sequence analysis of the 21 positive clones, none appeared to correspond to a larger β-SNAP encoding cDNA.
Nucleotide and amino acid sequence of α-SNAP. The amino acid and nucleotide sequences of human α-SNAP cDNA isolated from a human platelet-derived cDNA library are presented. The numbers correspond to the number of nucleotides and the underlined and bolded amino acids denote potential protein kinase C phosphorylation sites. Only two of the potential sites Ser 35 and Ser 201 are conserved in all of the known α-SNAP sequences.
Nucleotide and amino acid sequence of α-SNAP. The amino acid and nucleotide sequences of human α-SNAP cDNA isolated from a human platelet-derived cDNA library are presented. The numbers correspond to the number of nucleotides and the underlined and bolded amino acids denote potential protein kinase C phosphorylation sites. Only two of the potential sites Ser 35 and Ser 201 are conserved in all of the known α-SNAP sequences.
Comparison of the amino acid sequence of the human α-SNAP with other forms from yeast,39 (37% identical), Drosophila,40 (60% identical), and squid41 (67% identical) suggests that α-SNAP is a highly conserved protein. This sequence conservation is consistent with the fact that α-SNAP homologues from one species can participate in the vesicular transport processes of another divergent species.41 This level of conservation also appears to be true for NSF,40 but interestingly does not apply to γ-SNAP (see below).
The message for γ-SNAP was much rarer in the platelet cDNA library. From the original 600,000 phage plaques screened with a melanocyte cDNA-based riboprobe, one positive clone was recovered. This clone appears to be the result of an incomplete splicing event at the 5′ end of the coding region. When compared with the sequence of the melanocyte EST clone (Fig 3), the platelet clone's nucleotide sequence was 100% identical up to the 5′-most 9 bases of the coding region of the EST clone, but diverged greatly further 5′ from that point. Because the 5′ untranslated regions of the platelet and melanocyte clones are divergent and a splicing acceptor site is present at this site in the platelet clone, it is possible that the platelet clone represents an incompletely spliced mRNA. PCR primers based on nucleotide sequence of the melanocyte cDNA were used to determine if the platelet cDNA library contained a completely processed γ-SNAP cDNA. An appropriately sized PCR product was obtained when the platelet library was used as template. This product was subjected to dideoxy sequencing and was shown to be identical to the sequence derived from the melanocyte EST clone. From these data, it is concluded that the platelet cDNA library does contain a completely processed γ-SNAP encoding cDNA, which is identical to the melanocyte EST clone.
When the deduced amino acid sequences for the human γ-SNAPs are compared with the bovine sequence, there is a sequence difference in the putative amino termini. Two potential initiator ATGs exist in the open reading frame of the bovine cDNA. The 5′-most ATG was originally proposed to be the initiator methionine, as neither ATG was in the context of an optimal Kozak sequence.25,42 The two human sequences reported in this study contain only the second methionine in their open reading frame, but again the Kozak sequence is not optimal.42 While the three (bovine and two human) amino acid sequences 3′ (C-terminal) from this second methionine are almost identical (95%), there is no similarity when comparing the sequence 5′ (N-terminal) from this second methionine. This pattern of sequence conservation suggests that the correct initiation methionine corresponds to the second methionine of the bovine open reading frame, not the first, as was originally thought.25 Supporting this is the fact that the proteins detected in human platelets and in bovine brain are of similar apparent molecular weight by SDS-PAGE (Fig 1). This would probably not be true if the two proteins used different initiator methionines because the bovine brain protein would contain 16 more amino acids than the human platelet protein.25 The recombinant bovine γ-SNAP, made using the first (5′-most/N-terminal–most) methionine as initiator, migrates more slowly by SDS-PAGE (5 kD slower) indicating that the recombinant protein is larger than the authentic γ-SNAP.25 Unless the bovine brain form of γ-SNAP is proteolytically processed to remove this N-terminal extension, it seems likely that the second methionine in the bovine open reading frame is the true start site.
When the sequence of γ-SNAP was compared with the S. cerevisiae genome database, no homologues were detected, suggesting that yeast do not contain a γ-SNAP homologue. The closest match was to Sec17p, which has already been shown to be the yeast α-SNAP homologue.39 Likewise, when the sequence of γ-SNAP was compared with the C. elegans database, the closest match was a protein that has previously been identified as a potential homologue of α-SNAP.43 The peptide sequence for γ-SNAP is incredibly well conserved between human and bovine (95% identical), yet to date, we have not been able to identify other γ-SNAP homologues in the available databases.
SNARE activity in platelet extracts.Wilson et al10 devised a functional assay to measure SNARE activity in detergent-solubilized membrane extracts. In this assay, the SNARE-dependent association of α-SNAP with NSF is measured by determining the amount of radiolabeled α-SNAP that is coimmunoprecipitated with NSF. The resulting complex (SNAP/NSF/SNARE) has been called the “20 S fusion complex” and has been shown, using neuronal microsomes, to contain both vesicle and target-membrane–derived SNAREs.9 As shown in Fig 4, α-SNAP associates with NSF in a platelet-membrane extract-dependent manner (complete reaction). The association is competed for by an excess of unlabeled α-SNAP (+ α-SNAP), but not by γ-SNAP (+ γ-SNAP ). As has been reported,10 when ATP hydrolysis is allowed by the addition of Mg2+ (+MgCl2 ), the association between NSF, α-SNAP, and the SNAREs is disrupted, and the 20 S fusion complex is disassembled, resulting in release of the radiolabeled α-SNAP from the immunoprecipitated NSF. These data clearly indicate that platelet membranes contain SNARE activity as defined by Wilson et al10 and that the platelet SNAREs are able to participate in ATP-hydrolysis–dependent disassembly of the 20 S complex.
SNAP receptor activity of platelet membrane extract. Detergent solubilized membrane extract from human platelets (HPE) was tested for SNARE activity using the assay of Wilson et al.10 In the complete reaction, radiolabeled α-SNAP was incubated for 2 hours at 4°C with NSFmyc (12 μg), HPE (40 μg), and beads with a covalently coupled anti-myc antibody. The buffer contained 0.5 mmol/L ATP, 2 mmol/L EDTA, and 1% Triton X-100. After incubation, the beads were captured on a glass fiber filter and the [35S] α-SNAP associated with the anti-myc antibody beads was determined by scintillation counting. To determine the specificity of the interaction, unlabeled α-(63 μg, + α-SNAP ) and γ- (5 μg, + γ-SNAP ) SNAP was added to the initial incubation. To determine the effect of ATP hydrolysis, 6 mmol/L MgCl2 (+MgCl2 ) was included. SNARE activity is defined as that which induced the association of [35S] α-SNAP with NSF. The data presented is representative of three separate experiments.
SNAP receptor activity of platelet membrane extract. Detergent solubilized membrane extract from human platelets (HPE) was tested for SNARE activity using the assay of Wilson et al.10 In the complete reaction, radiolabeled α-SNAP was incubated for 2 hours at 4°C with NSFmyc (12 μg), HPE (40 μg), and beads with a covalently coupled anti-myc antibody. The buffer contained 0.5 mmol/L ATP, 2 mmol/L EDTA, and 1% Triton X-100. After incubation, the beads were captured on a glass fiber filter and the [35S] α-SNAP associated with the anti-myc antibody beads was determined by scintillation counting. To determine the specificity of the interaction, unlabeled α-(63 μg, + α-SNAP ) and γ- (5 μg, + γ-SNAP ) SNAP was added to the initial incubation. To determine the effect of ATP hydrolysis, 6 mmol/L MgCl2 (+MgCl2 ) was included. SNARE activity is defined as that which induced the association of [35S] α-SNAP with NSF. The data presented is representative of three separate experiments.
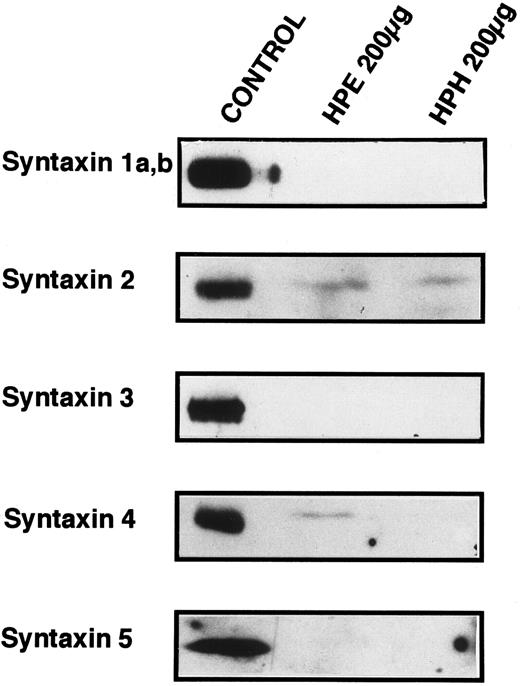
Known t-SNARES that are present in platelets.Because Fig 4 shows that platelet membranes contain SNARE activity, the next series of experiments was designed to determine if any of the previously identified SNARE proteins are present in the platelet. Figure 5 shows the results of Western blots of total platelet homogenate (HPH) and detergent-solubilized, salt-washed membranes (HPE) using antiserum to five members of the syntaxin family of t-SNAREs. Syntaxin 2 and 4 are detected in platelet membranes, but 1, 3, and 5 are not, although the antibodies to syntaxin 1, 3, and 5 readily cross-react with human proteins (data not shown and syntaxin 5 control). Syntaxin 2 has been shown to be localized to the plasma membranes and to be expressed in numerous tissues suggesting that it could be involved in constitutive secretion.15 Syntaxin 4, which has been localized to the plasma membrane, is expressed only in a distinct subset of tissues: spleen, heart, skeletal muscle and kidney.15 Syntaxin 4 is especially interesting as it has been implicated in the secretion of lysosomal enzymes from various hematopoetic cell types such as neutrophils.44,45 Given the low levels of the syntaxin proteins detected (see below), it is a formal possibility that the presence of syntaxin 2 and 4 in our samples is due to contaminating cell types. Of the leukocytes that could be contaminating our preparations, it would appear that neutrophils are not present because we do not detect VAMP/synaptobrevin-2 (see below), and monocytes/macrophages are not present because we do not detect syntaxin 3. In both cases, these proteins have been readily detected in lower amounts of total cellular protein from neutrophils45 and monocytes/macrophages.46 Of the other possible contaminants, no nonplatelet cells were evident on microscopic examination of our preparations (see Materials and Methods).
Syntaxin 2 and syntaxin 4 are present in platelets. SDS-PAGE and Western blotting analyses were employed to determine if previously identified syntaxins are expressed in platelets. Total homogenates (HPH) and membrane extracts (HPE) from platelets were separated by SDS-PAGE and then electroblotted onto nitrocellulose. These blots were probed with the specific antibodies HPC-1 (syntaxin 1a,b), antisyntaxin 2 (syntaxin 2), antisyntaxin 3 (syntaxin 3), antisyntaxin 4 (syntaxin 4), and antisyntaxin 5 (syntaxin 5). The following samples (Control) were used as positive controls for the specific antisera: HPC-1, detergent-solubilized bovine brain membrane extract (BBE, 0.5 μg); antisyntaxin 2, recombinant syntaxin 2 (2.5 ng); antisyntaxin 3, recombinant syntaxin 3 (5 ng); antisyntaxin 4, recombinant syntaxin 4, (2.5 ng); antisyntaxin 5, HepG2 cell detergent-solubilized membrane extract (200 μg).
Syntaxin 2 and syntaxin 4 are present in platelets. SDS-PAGE and Western blotting analyses were employed to determine if previously identified syntaxins are expressed in platelets. Total homogenates (HPH) and membrane extracts (HPE) from platelets were separated by SDS-PAGE and then electroblotted onto nitrocellulose. These blots were probed with the specific antibodies HPC-1 (syntaxin 1a,b), antisyntaxin 2 (syntaxin 2), antisyntaxin 3 (syntaxin 3), antisyntaxin 4 (syntaxin 4), and antisyntaxin 5 (syntaxin 5). The following samples (Control) were used as positive controls for the specific antisera: HPC-1, detergent-solubilized bovine brain membrane extract (BBE, 0.5 μg); antisyntaxin 2, recombinant syntaxin 2 (2.5 ng); antisyntaxin 3, recombinant syntaxin 3 (5 ng); antisyntaxin 4, recombinant syntaxin 4, (2.5 ng); antisyntaxin 5, HepG2 cell detergent-solubilized membrane extract (200 μg).
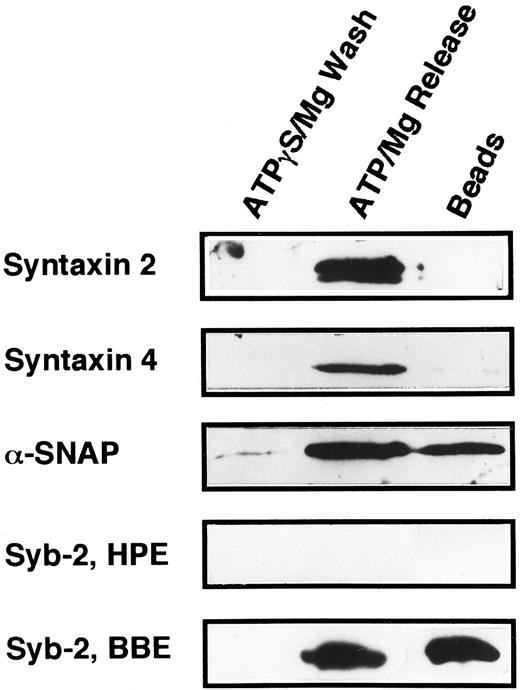
The technique of Söllner et al9 was used to determine if any of the known syntaxin molecules were present in platelets at quantities below our original detection limits in Fig 5 (Fig 6). Using this method, it is possible to purify SNARE proteins from fairly large amounts of crude membrane extracts (3.8 mg) and, in effect, concentrate these proteins. 20 S fusion complex was first formed using recombinant NSFmyc and α-SNAP and detergent-solubilized platelet membrane proteins. The complexes were captured on protein G beads to which the anti-myc antibody, 9E10, had been covalently coupled. The complexes were washed in binding buffer and then incubated under ATP hydrolysis conditions to disassemble the 20 S fusion complex. The wash (ATPγgS/Mg Wash), material released by ATP hydrolysis (ATP/Mg Release), and immunobead-bound material (Beads) were then analyzed by Western blotting using the same antibodies as in Fig 5. Figure 6 shows that syntaxin 2 and 4 can participate in 20S fusion complex formation and that both proteins are released on ATP hydrolysis as is α-SNAP. Syntaxin 1, 3, and 5 were not detected in these experiments (data not shown), strongly suggesting that they are absent from the platelet preparations. As a control, when α-SNAP was not included in the initial complex formation step, no syntaxins or VAMP/synaptobrevins were found associated with the immunobeads (data not shown). The greater intensity of the bands for syntaxin 2 and 4 in Fig 6, when compared with those detected in Fig 5, show the power of this method to concentrate the SNARE proteins. Also, it should be noted that there is a doublet in the syntaxin 2 Western blot. In Fig 5, the band intensity was not great enough to detect this doublet. At this point, it is not clear whether the doublet represents proteolysis or alternative splicing variants, which have been reported for syntaxin 2 from other tissues.15
Platelet syntaxin 2 and syntaxin 4 participate in 20S complex formation. The 20S particle formation assay9 was used to show that syntaxin 2 and 4 behave as SNAREs in platelets. HPE, α-, γ-SNAP, and NSFmyc were incubated for 30 minutes at 4°C. The binding buffer contained 0.5 mmol/L ATPγS and 1 mmol/L EDTA. The NSFmyc was then immunoprecipitated with an excess of anti-myc, 9E10, antibody coupled to Protein G-Superose beads. Following immunoprecipitation, the beads were washed first in binding buffer and then with ATPγS/Mg (ATPγS/Mg Wash). The beads were specifically eluted with ATP/Mg (ATP/Mg Release). Material remaining on the beads was harvested by treatment with 200 mmol/L glycine/HCl, pH 2.7, O.5% Triton X-100 (Beads). All eluted proteins were analyzed by SDS-PAGE followed by Western blotting. Blots were probed with antisera directed against syntaxin 2, syntaxin 4, α-SNAP, and synaptobrevin 2. In Syb-2, BBE, detergent-solubilized bovine brain membrane extract was used in place of the HPE used in Syb-2, HPE.
Platelet syntaxin 2 and syntaxin 4 participate in 20S complex formation. The 20S particle formation assay9 was used to show that syntaxin 2 and 4 behave as SNAREs in platelets. HPE, α-, γ-SNAP, and NSFmyc were incubated for 30 minutes at 4°C. The binding buffer contained 0.5 mmol/L ATPγS and 1 mmol/L EDTA. The NSFmyc was then immunoprecipitated with an excess of anti-myc, 9E10, antibody coupled to Protein G-Superose beads. Following immunoprecipitation, the beads were washed first in binding buffer and then with ATPγS/Mg (ATPγS/Mg Wash). The beads were specifically eluted with ATP/Mg (ATP/Mg Release). Material remaining on the beads was harvested by treatment with 200 mmol/L glycine/HCl, pH 2.7, O.5% Triton X-100 (Beads). All eluted proteins were analyzed by SDS-PAGE followed by Western blotting. Blots were probed with antisera directed against syntaxin 2, syntaxin 4, α-SNAP, and synaptobrevin 2. In Syb-2, BBE, detergent-solubilized bovine brain membrane extract was used in place of the HPE used in Syb-2, HPE.
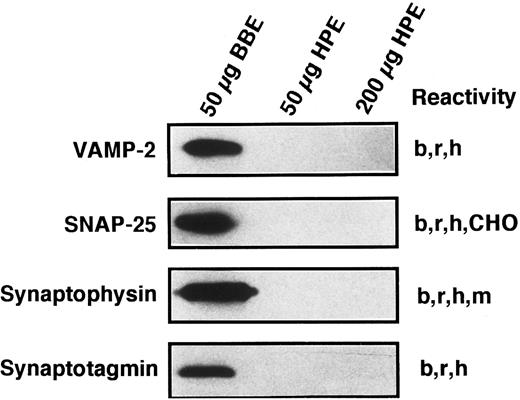
Known secretory machine proteins not detected in platelets.In addition to the t-SNARE syntaxins that have been detected in the platelet, we sought to determine if other known SNARE-like or SNARE-associated proteins are present. Although each of the antibodies used in the Western blotting experiments of Fig 7 has been shown to cross-react with the human forms of these proteins29,47 (and data not shown), we were unable to detect SNAP-25, VAMP/synaptobrevin 2, synaptophysin, or synaptotagmin I. Because the syntaxin proteins were detectable in 200 μg of platelet proteins and at least the SNAP-25 and VAMP/synaptobrevin 2 proteins would be expected to be present in similar amounts, it would seem that these proteins are not present in platelets or are present in very low amounts. The lack of synaptophysin has been partially addressed by other investigators, and it appears that there is a synaptophysin homologue called granulophysin that is present on dense core granules.48 The antibody used in this study would not recognize the granulophysin protein.49 The lack of synaptotagmin poses a more interesting situation, as platelet exocytosis is thought, at least in part, to be controlled by increases in intracellular calcium levels. We have only tested for one of the 10 forms of synaptotagmin.50 Therefore, our search is incomplete at this point.
Known secretory machine proteins that have not been detected in platelets. Platelet membrane extract (HPE) was immunochemically tested for the presence of known SNARE and SNARE-associated integral membrane proteins. HPE in the amounts of 50 and 200 μg was examined by SDS-PAGE, Western blotting, and probing with antibodies against VAMP-2, SNAP-25, synaptophysin, and synaptotagmin (see Materials and Methods). Fifty micrograms of detergent-solubilized bovine brain membrane extract (BBE) were analyzed in parallel as a positive control for these antisera. The immunoreactivities of these antibodies are given to the right of each blot: bovine (b), CHO cells (CHO), human (h), mouse (m) rat (r).
Known secretory machine proteins that have not been detected in platelets. Platelet membrane extract (HPE) was immunochemically tested for the presence of known SNARE and SNARE-associated integral membrane proteins. HPE in the amounts of 50 and 200 μg was examined by SDS-PAGE, Western blotting, and probing with antibodies against VAMP-2, SNAP-25, synaptophysin, and synaptotagmin (see Materials and Methods). Fifty micrograms of detergent-solubilized bovine brain membrane extract (BBE) were analyzed in parallel as a positive control for these antisera. The immunoreactivities of these antibodies are given to the right of each blot: bovine (b), CHO cells (CHO), human (h), mouse (m) rat (r).
Because it has been suggested that syntaxin 4 and VAMP/synaptobrevin 2 might participate in the same membrane transport events,51 we examined the 20 S complex shown in Fig 6 for the presence of VAMP/synaptobrevin 2. As can be seen for the bovine brain extract control (Syb-2, BBE), VAMP/synaptobrevin 2 can be readily detected and behaves as would be expected for a SNARE protein. No VAMP/synaptobrevin 2 could be seen in the 20 S complex formed from platelet membrane extracts. Because the formation of the 20 S complex represents a partial purification and concentration of SNAREs and the antibodies used can recognize human VAMP/synaptobrevin 2 (data not shown), these data strongly suggest that the VAMP/synaptobrevin 2 protein is not present in platelets.
Quantification of exocytosis machinery proteins in platelets.With the ultimate goal of determining the molecular mechanisms behind regulated exocytosis in platelets, we sought to measure the cellular concentrations of the proteins of the exocytosis machinery. Given that some of the binding constants for the various protein-protein interactions have been estimated,34,52,53 it would seem possible that by determining the cellular concentrations of the machinery proteins, it might be possible to gain insight into what reactions are possible. To this end, we have attempted to determine the cellular concentrations of α-SNAP, γ-SNAP, and NSF (see Table 1) and compare them with estimates of the concentrations of syntaxin 2 and 4. Quantitative Western blotting was used for these experiments, employing recombinant proteins as standards and polyclonal antiserum for detection. Due to the high amino acid sequence identities between the human and recombinant proteins (see Materials and Methods), it seems reasonable to expect that the specific polyclonal antiserum used would recognize the platelet and recombinant proteins equally. Understanding that there are limitations to this analysis, care was taken to assure that the measurements were as accurate as possible given the antibodies and recombinant proteins available. Alpha SNAP is present in platelets in the amount of 3.1 ng/107 cells, which converts to approximately 5,600 molecules per cell. Gamma SNAP is present in platelets at lower amounts, 1.7 ng/107 cells, which converts into approximately 2,950 molecules per cell. This is consistent with the amount of NSF present in platelets, 7.0 ng/107 cells, which converts to 1,700 molecules per cell and suggests that the intracellular ratio of α-SNAP to γ-SNAP to NSF trimer is 3/1.7/1. Using 9 fL as the volume of a platelet,54,55 the concentrations of the SNAPs and NSF, are well above the kd that have been estimated for the SNAP-SNARE (14 nmol/L)34 and NSF-SNAP–SNARE (≈60 pmol/L)52 interactions. The low level of the syntaxin 2 and 4 proteins in platelets have made accurate determination of their amounts per cell difficult. The concentrations of syntaxin 2 and 4 were estimated to be at least two orders of magnitude lower on a molecule per cell basis than shown for SNAPs and NSF. We find this quite striking because syntaxin 1 in the brain appears, on a per protein basis, to be a fairly abundant membrane protein.56 Syntaxin 2 and 4 also appear to be fairly abundant in other cell types such as monocytes/macrophages46 and neutrophils,45 requiring only 20 μg of total cellular protein to obtain a robust signal by Western blotting (as opposed to the 200 μg used in Fig 5). Additionally, it has been estimated that v-SNAREs, such as synaptobrevin molecules, are present in micromolar amounts in the synaptosome.57 The quantitative data presented are consistent with the general proteins (SNAPs and NSF ) of the exocytosis machinery being in excess relative to the components (t-SNAREs) that are predicted to mediate the specific steps of granule docking.
Quantification of Secretory Machinery in the Platelet
| Protein . | Calculated MW* (g/mol) . | ng/107 Cells . | Molecules/cell . | Concentration (μmol/L)† . |
|---|---|---|---|---|
| NSF | 247.9 × 103‡ | 7.0 ± 1.3 | 1,700 | 0.32 |
| (nρ = 11) | ||||
| α-SNAP | 33.2 × 103 | 3.1 ± 0.66 | 5,600 | 1.0 |
| (n = 10) | ||||
| γ-SNAP | 34.7 × 103 | 1.7 ± 0.55 | 2,950 | 0.54 |
| (n = 7) |
| Protein . | Calculated MW* (g/mol) . | ng/107 Cells . | Molecules/cell . | Concentration (μmol/L)† . |
|---|---|---|---|---|
| NSF | 247.9 × 103‡ | 7.0 ± 1.3 | 1,700 | 0.32 |
| (nρ = 11) | ||||
| α-SNAP | 33.2 × 103 | 3.1 ± 0.66 | 5,600 | 1.0 |
| (n = 10) | ||||
| γ-SNAP | 34.7 × 103 | 1.7 ± 0.55 | 2,950 | 0.54 |
| (n = 7) |
Cells were counted by hemocytometer and were solubilized in SDS-sample buffer before SDS-PAGE and Western blotting. The antigens were detected by chemiluminescence and the images were quantified by digital scanning (see Materials and Methods for details). Integration of the platelet proteins were compared with that of a standard curve of recombinant proteins to determine the amount of antigen per platelet.
Molecular weights were calculated based on the amino acid sequences.
The calculated molecular weight for a homo-trimeric NSF of 82.6 × 103 g/mol subunits.
ρ Represents the number of independent measurements made.
DISCUSSION
The goal of this work was to determine what proteins could potentially play a role in platelet exocytosis. To this end, we have shown that platelets contain some of the same proteins (α-SNAP, γ-SNAP, p115/TAP, and NSF ), which are used in many vesicular transport processes in other cells types.7 This data suggests that the molecular mechanisms used in platelets are similar to those described in other cells, most notably neurons.6,8 cDNAs encoding two of these proteins, α- and γ-SNAP were cloned from a platelet-derived cDNA library. α-SNAP showed high sequence conservation from yeast to squid to human; however, γ-SNAP homologues were not identified in any other species except bovine. SNARE activity was detected in platelet membrane extracts and shown to respond to ATP hydrolysis as previously shown for neuronal SNARE activity. In addition to reporting the presence of SNARE activity, we have shown that platelets contain two previously identified plasma membrane t-SNAREs, syntaxin 2 and 4.15 Syntaxin 2 is generally expressed in a number of tissues, and because of this, it has been suggested to participate in constitutive secretion.15 Syntaxin 4 is more specifically expressed and has been suggested to play a role in secretion of lysosomal contents by hematopoetic cells44,45 and glucose transporter mobilization in adipocytes.58 The two syntaxins, syntaxin 2 and 4, could be sufficient for platelet exocytosis; however, at this stage, we do not know if other as-yet–uncharacterized syntaxins are present in platelets. Finally, by determining the subcellular concentrations of these potential vesicular transport proteins, we can suggest a mechanistic model as to how platelet exocytosis might be regulated (see below).
Our data indicates that it is possible to use the neuro-secretory process as a conceptual framework in the study of platelet exocytosis. From the work on neuro-transmission, one would expect that platelets should contain several SNARE and SNARE-associated proteins that have not been detected in our experiments. Of special interest is the apparent lack of VAMP/synaptobrevin 2, which until this report, had usually been associated with syntaxin 4.51 The fact that VAMP/synaptobrevin 2 was not found in the 20S complex formed from 3.8 mg of platelet membrane proteins strongly suggests that it is not present in platelets. To add context to this result, a novel synaptobrevin, recently cloned from a megakaryocyte-like cell line cDNA library, was readily detected in a similar experiment using platelet membrane extracts (Bernstein and Whiteheart, in preparation). It is quite possible that other synaptobrevin-like molecules are yet to be identified. The absence of the t-SNARE, SNAP-25, is not surprising due to its restricted tissue distribution.59 However, platelets do express an homologous protein, SNAP-23,17 which does have SNARE activity (Chen and Whiteheart, in preparation). As for the regulatory proteins, the absence of synaptophysin is partially explained by the presence of a homologous molecule in dense core granules called granulophysin.48 Syntaxin-regulating n-Sec1 proteins are present in platelets and to date a cDNA clone encoding one distinct n-Sec1 isoform has been isolated from the platelet cDNA library (Lemons and Whiteheart, unpublished). The apparent absence of synaptotagmin represents a more interesting case because platelet exocytosis is, in part, regulated by changes in intracellular calcium concentrations. At this stage, we cannot make a definitive conclusion, as there are at least 10 isoforms of synaptotagmin50 and our search has only dealt with synaptotagmin 1. In summary, this data shows that while platelet exocytosis may be mechanistically similar to neural transmission, the proteins used by the platelet and their modes of regulation may be quite different.
The data presented in this report are consistent with a mode of exocytic regulation in which the granule-plasma membrane docking step is the rate-limiting step. The intracellular concentrations of the general transport proteins (NSF and SNAPs) are sufficiently high (>10-fold over kd) as to be saturating based on the kd measured for their various interactions. The estimates of the cellular amounts of the two syntaxin proteins suggest that the intracellular concentrations of these t-SNAREs are much lower than the binding constants estimated for the binding of syntaxin 1 and VAMP-2/synaptobrevin 2 (4.7 μmol/L).53 The low concentration of SNARE proteins also appears to be true for the one v-SNARE that we have recently identified (Bernstein and Whiteheart, in preparation). We propose that the low concentration of the identified v- and t-SNAREs suggests that the docking of the granule to the target membrane is rate limiting. This model is consistent with what has been proposed to occur once a platelet is stimulated. When platelets are activated, the secretory granules are concentrated into the center of the cell by cytoskeletal rearrangements.2-4 This physical concentration together with the close proximity of the SCCS would serve to increase the local concentrations of the v- and t-SNAREs thereby increasing the likelihood of their mutual recognition and thus promoting docking and membrane fusion. In contrast, in the neuron, the concentration of at least the v-SNAREs is estimated to be relatively high (10 μmol/L).57 In this system, the synaptic vesicles are predocked, which insures that the vesicles can respond rapidly (milliseconds) to exocytic stimulus. Platelet granules, however, do not appear to be predocked, and there is a significant time lag (seconds) between cell surface activation and secretion.60 We propose that the key regulatory step in platelet exocytosis is not at the membrane fusion step, as it appears in the rapid secretion of the neuron, but instead is at the granule docking step. By controlling an early step in the membrane fusion pathway (ie, docking), it would be possible to more effectively limit the possibility of spurious exocytic events.
The goal of this study was to understand the molecular events that culminate in the release of platelet granule contents. The SNARE hypothesis holds that there will be a specific set of SNARE proteins for each type of membrane fusion event. We feel that the platelet offers a unique system to test this, as platelets carry out three distinct regulated fusion events. The evidence presented in this report shows the use of platelets as a model system to test the SNARE hypothesis. However, despite the identification of several of the potential SNAREs and SNARE-associated proteins, it is obvious that more proteins are yet to be identified in platelets.
ACKNOWLEDGMENT
The authors thank the following for their generous gifts of antibodies and reagents: Dr Reinhard Jahn, Dr Pietro DeCamilli, Dr Mark Bennett, Dr James Rothman, Dr Thomas Söllner, and Dr M.G. Waters. We thank Mochida Pharmaceuticals, Dr Takeyuki Sato, Dr Gerald Roth, and Dr Si Lok for the generous gift of their cDNA libraries. We also thank H.P. Shiao, Elzbieta Nagiec, Ping He, and Chen Xi for technical assistance, Dr S.A. Buhrow for critical reading of the manuscript, and Jim Smith for photographic services. We especially thank Katherine Wells of the Central Kentucky Blood Center. We thank Dr Gerald Roth for his invaluable discussions during the course of this work.
P.P.L. and D.C. contributed equally to this work.
Supported by an American Heart Association-Sanofi Winthrop Grant-in-Aid (95006590), the American Cancer Society (CB-153), and National Institutes of Health Grant No. HL 56652 (to S.W.W.).
Address reprint requests to S.W. Whiteheart, PhD, Department of Biochemistry, University of Kentucky College of Medicine, 800 Rose St, Lexington, KY 40536.

![Fig. 4. SNAP receptor activity of platelet membrane extract. Detergent solubilized membrane extract from human platelets (HPE) was tested for SNARE activity using the assay of Wilson et al.10 In the complete reaction, radiolabeled α-SNAP was incubated for 2 hours at 4°C with NSFmyc (12 μg), HPE (40 μg), and beads with a covalently coupled anti-myc antibody. The buffer contained 0.5 mmol/L ATP, 2 mmol/L EDTA, and 1% Triton X-100. After incubation, the beads were captured on a glass fiber filter and the [35S] α-SNAP associated with the anti-myc antibody beads was determined by scintillation counting. To determine the specificity of the interaction, unlabeled α-(63 μg, + α-SNAP ) and γ- (5 μg, + γ-SNAP ) SNAP was added to the initial incubation. To determine the effect of ATP hydrolysis, 6 mmol/L MgCl2 (+MgCl2 ) was included. SNARE activity is defined as that which induced the association of [35S] α-SNAP with NSF. The data presented is representative of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1490/3/m_bl_0001f4.jpeg?Expires=1765943815&Signature=wAwrZGHJ6IsugGH26EUUKiyaE-ERbwTzU5ITJQIXvkZ0JNoTq2fhwMtY8oxI4~auPLbvIm0yFbBzCXaGcE17ZdFrXX9FeO8NmnkxHwV0hRLWD8le~~NBJMYEVxoHfptije5G9Jwdn5MWTpRifanOMALD3IYGSEM4ZAPO0YMZB6Amy1AXXGOLDCUi0mLWfNobfGB2upQA34sYwPH--R79dlhapzrhhqLHhQEKjLArTVHEJa83-BjhDSlwLjAkYNYOzpPVIFR4wsBvesQBcGlwN8Sfz66SfeTZpznqNsPlr8B5s5YQ~46dg4kgTVqMpG-IUPbHCSxao08kdRwC87uc6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal