Abstract
The CD34 antigen is thought to be expressed by hematopoietic stem cells in adult humans and nonhuman primates. We present data that baboons transplanted with highly purified allogeneic CD34+ marrow cells devoid of detectable mature and immature T and B lymphocytes and myeloid cells, isolated from sex-mismatched mixed lymphocyte culture (MLC) nonreactive siblings, have maintained stable lymphohematopoietic engraftment with donor cells for greater than 4.9, greater than 6.0, and 5.0 years. Cytogenetic analysis of unfractionated marrow and peripheral blood cells at multiple time points after transplantation show virtually all donor cells in two animals and stable mixed chimerism in the third. We used polymerase chain reaction to show that colony-forming unit–granulocyte-macrophage, burst-forming unit-erythroid, and high proliferative potential colony-forming cells (HPP-CFC) were virtually all of donor origin in two animals and present at lower levels in the stable mixed chimera. CD20+ B-lymphoblastoid cell lines derived by Herpesvirus Papio transformation of peripheral blood cells were virtually all donor in two animals and 50% donor in the mixed chimera. CD4+ and CD8+ T cells and neutrophils purified from the peripheral blood of the two female animals also were all donor-derived. To assess immunologic function after transplantation, we immunized the three long-term chimeric animals and two normal control animals with bacteriophage ΦX-174, a neoantigen that requires the interaction of antigen-presenting cells, T lymphocytes, and B lymphocytes to mount a normal antibody response. Experimental and control animals, when immunized with bacteriophage, had similar serum Ig levels. The experimental and control animals generated similar titers of antibacteriophage antibodies after primary and secondary immunizations with evidence of amplification and class switching. These findings further support the hypothesis that the CD34+ antigen is expressed on hematopoietic stem cells that can mediate stable long-term lymphohematopoiesis in vivo and, importantly, that normal immunologic function can be reconstituted in vivo after transplantation of the highly purified CD34+ Lin− cells alone.
A HALLMARK OF hematopoietic stem cells is the ability to produce normal numbers of functioning myeloid and lymphoid cells for at least the lifespan of the animal. Hematopoietic progenitor and stem cells can be defined based on expression of specific cell surface antigens, as well as by specific cell cycle, metabolic, and physical properties.1-3 Evidence supporting the expression of CD34 by marrow repopulating hematopoietic stem cells has been derived from transplantation studies in nonhuman primates and humans.4-6 Xenotransplant models in which SCID mice and fetal sheep have been transplanted with purified human CD34+ cells and subsets of CD34+ cells have provided further evidence that CD34+ hematopoietic cells give rise to mature blood elements of both the lymphoid and myeloid lineages.7-9 To date, there has been no evidence that normal in vivo immunologic function has been reconstituted by the progeny of transplanted CD34+ cells or that the CD34+ cells provided stable long-term (>1 year) engraftment when transplanted in a large animal model as the sole source of marrow repopulating cells.
After marrow transplantation, immunologic function has been assessed by both in vitro and in vivo assays; however, no single in vitro assay can assess all aspects of immunologic function. Immunization with neo-antigens that require antigen processing, T-cell help, and antigen-specific B cells to mount an Ig response have been used to test the fundamental integrity of the immune system. Abnormalities in antibody response after immunization with the bacteriophage (phage) ΦX174 have been shown to be a sensitive, albeit nonspecific, indicator of immunologic dysfunction in humans with various congenital immunodeficiency syndromes as well as with graft-versus-host disease after bone marrow transplantation.10-21 Therefore, in the present study, we used immunization with phage ΦX174 to evaluate the immunologic function of three baboons 2 to 3 years after they were transplanted with highly purified allogeneic, marrow-derived, CD34+, lymphocyte-depleted cells. We present evidence that donor-derived lymphohematopoiesis has remained stable in these animals for more than 5 years and that normal immunologic function has been reconstituted.
MATERIALS AND METHODS
Animals.Healthy juvenile baboons (Papio cynocephalus) were housed at the University of Washington Regional Primate Research Center, under American Association for Accreditation of Laboratory Animal Care approved conditions as previously described.5 Studies were conducted under Institutional Review Board and Animal Care and Use Committee approved protocols. All animals were provided with water, biscuits, and fruit ad libitum throughout the study. Mixed lymphocyte culture reactivity of peripheral blood cells was used to identify sex-mismatched sibling baboons that were histocompatible as described.5 Immediately before transplantation, the animals reported here received a single dose of 920 or 1,020 cGy total body irradiation from two opposing 60Cobalt sources at a rate of 7 cGy per minute. Animals were transfused with irradiated (2,000 to 3,000 cGy) fresh whole blood for treatment of thrombocytopenia and anemia and received broad spectrum antibiotic prophylaxis until stable engraftment had occurred, as previously described.5
Colony-forming cell assays.Unfractionated and isolated marrow cells were cultured in a double-layer agar culture system, as previously described.22 Briefly, isolated cells were cultured in α medium supplemented with 25% fetal bovine serum (FBS; Hyclone, Logan, UT), 0.1% bovine serum albumin (Fraction V; Sigma, St Louis, MO), 0.3% (wt/vol) agar (SeaPlaque; FMC, Bioproducts, Rockland, ME), overlaid on medium with 0.5% agar (wt/vol) containing recombinant human stem cell factor (SCF ), interleukin-3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor, and granulocyte colony-stimulating factor each at 100 ng/mL, and erythropoietin at 4 U/mL (growth factors were kindly provided by Dr Ian McNiece, Amgen, Inc, Thousand Oaks, CA). Cells were plated at 100 to 50,000 cells per dish, depending on the expected enrichment of the sample. Cultures were incubated at 37°C in 5% O2 , 5% CO2 , 90% N2 humidified atmosphere in polystyrene boxes sealed with gas impermeable tape (3M, St Paul, MN). All cultures were performed in triplicate, unless otherwise indicated. At day 14 of culture, colonies were enumerated using an inverted microscope.
Monoclonal antibodies and cell separation.Monoclonal antibodies 9.6 (CD2), 51.1 (CD 8), and 24.1 (CD10) were provided by Dr P. Martin (Fred Hutchinson Cancer Research Center, Seattle, WA). Antibodies G17.2 (CD 4) and G28.4 (CD40) were provided by Dr J. Ledbetter (Bristol-Meyers-Squibb, Seattle, WA). Dr E. Clark (University of Washington, Seattle, WA) provided antibody 1F5 (CD20). Antibody 5B12 to a molecular weight 40-kD antigen on baboon neutrophils was generated as previously described.5 Marrow cells were first depleted of T, B, and myeloid cells by negative selection using immunoadsorption to magnetic beads (Dynal, Oslo, Norway), followed by positive selection using two-color fluorescence-activated cell sorting (FACS) as previously described.5 These depleted cells were labeled with the anti-CD34 antibody 12.8 (mouse monoclonal IgM) and again labeled with the IgG antibodies used for negative selection (see above). These cells were then stained with phycoerythrin-conjugated antimouse IgM-specific antiserum (Cal Biochem [La Jolla, CA] or Biomeda [Foster City, CA]) and fluorescein isothiocyanate (FITC)-conjugated antimouse IgG-specific antiserum (Kirkegaarde and Perry, Gaithersberg, MD). As controls, unseparated marrow buffy coat cells were labeled with the IgM antibody H12C12 (antimouse Thy 1.2) and the IgG antibodies 31.A and 1A14 (antimouse Thy 1.1) and then stained with the anti–IgM-specific and anti–IgG-specific antisera. Cells expressing CD34 and not binding antibodies used for negative selection were separated by FACS as previously described.5
Phenotypic analysis of peripheral blood cells from transplanted baboons.Peripheral blood buffy coat cells were labeled with murine monoclonal (IgG) antibodies 9.6 (CD2), G17.2 (CD4), 51.1 (CD8), or 1F5 (CD20), after which they were stained with the FITC-conjugated antimouse IgG-specific antiserum, as described above. As controls, cells were labeled with the irrelevant murine monoclonal antibody (IgG) 31.A (antimouse Thy 1.1). Cells were analyzed and sorted as described.5 CD4+ and CD8+ cells isolated by two cycles of cell sorting had fluorescent staining intensity that was greater than 99% of the cells stained with the isotype control antibodies, and sorted cells were greater than 99% of the desired phenotype.
Transformed B-cell lines.The S394 baboon lymphoblastoid cell line23 producing the Herpesvirus Papio (HVP) was obtained from Dr Philip Greenberg (University of Washington School of Medicine, Seattle, WA). S394 was grown in Iscove's modified Dulbecco's medium (IMDM) supplemented with 20% FBS and 2 mmol/L glutamine. Virus-containing supernatants were harvested from cultures that had been allowed to grow until medium became acidic and extensive syncytial formation was present. Cellular debris was removed by centrifugation. The clarified supernatant was then used fresh or frozen at −70°C for later use.
Fresh peripheral blood buffy coat cells were suspended at 1 × 106/mL in IMDM and 20% FBS and plated in 100-μL aliquots per well of 96-well plates. An equal volume of fresh or freshly thawed S394 supernatant containing HVP was added to each well. Plates were incubated in a 37°C, 5% CO2 , humidified incubator. Half of the medium in each well was replaced with fresh IMDM and 20% FBS after 72 hours and then at weekly intervals and incubation was continued. Cultures were scored weekly using an inverted microscope for the presence of cell proliferation. Wells containing proliferating lymphoblastoid cells were expanded and then formally cloned by limiting dilution (1 cell per 3 wells) twice. The transformed B-cell lines obtained all expressed CD20, as shown by flow cytometry.
Amplification of male-specific sequence using polymerase chain reaction (PCR).PCR was used to amplify a 174-bp male-specific sequence found in baboons and macaques, but not in humans, as described by Reitsma et al.24 As a control, PCR was used to amplify a 300-bp β2-microglobulin sequence. A sample was interpreted as containing only female, and not male, cells if the β2-microglobulin but not the male-specific sequence was amplified. If neither the β2-microglobulin nor the male-specific sequence amplified from the sample DNA, then the sample was considered to not be evaluable. Briefly, 10 to 105 cells of unfractionated marrow and peripheral blood as well as cells purified by flow cytometry or from cell lines were placed in 90 μL sterile water, heated to 100°C for 10 minutes, cooled on wet ice for 5 minutes, and centrifuged at 14,000g for 10 minutes, and 60 μL was removed for PCR analysis (50 μL for male PCR and 10 μL for β2 microglobulin). Colonies grown in agar from purified CD34+ cells, cultured at 100 to 500 cells per 35-mm plate, were picked using a dissecting microscope, placed in 90 μL sterile water, heated at 100°C for 10 minutes, cooled at 4°C for 5 minutes, treated with proteinase K (Boehringer Mannheim, Indianapolis, IN) at 200 μg/mL for 2 hours at 55°C, cooled at 4°C for 5 minutes, mixed, and then centrifuged to sediment debris, and 60 μL was removed and dried in a speed-vac. The dried material was dissolved in 30 μL sterile water for use in PCR. The PCR reaction mixture (total volume, 30 μL) contained 2.5 mmol/L MgCl2 , 200 μmol/L of each dTNP, 0.75 U Taq Polymerase (Perkin-Elmer, Branchburg, NJ), and 200 nmol/L of each primer. Amplifications for both the male and the β2-microglobulin sequences were run in the thermal cycler (MJ Research, Watertown, MA) with cycles 1 and 2 having a denaturation step at 94°C for 2 minutes, annealing at 52°C for 1 minute, and extension at 72° for 1 minute. For cycles 3 through 40, the denaturation was 93°C for 1 minute, annealing 52°C for 1 minute, and amplification 72°C for 1 minute. PCR products (20 μL) are analyzed by ethidium bromide agarose gel electrophoresis in 3% agarose gel. As positive controls in each amplification, we used DNA extracted from 105 peripheral blood leukocytes (PBL) from normal male baboons and DNA from single colony-forming unit–granulocyte-macrophage (CFU-GM) grown in agar from marrow of normal male baboons. Samples containing no DNA and DNA from 105 PBL from normal female baboons were used as negative controls for each amplification.
The primer pairs used for the male-specific sequence were designated RhM3 (5′ GAA AGA ACA TAA AGG ACC TA 3′ ) and RhM4 (5′ GGT AGA ATT AAT ATG ACC 3′ ), as described by Reitsma et al,24 with each primer at 200 nmol/L per reaction. The primers used for the β2-microglobulin sequence were β2MG-A (5′ ATG TCT CGC TCC GTG GCC TTA GCT 3′ ) and β2MB-B (5′ CCT CCA TGA TGC TGC TTA CAT GTC 3′; provided by Dr L. Milner, Fred Hutchinson Cancer Research Center, Seattle, WA, unpublished data).
Bacteriophage φX174 immunization protocol.Bacteriophage φX174 was grown, harvested, and purified as previously described.10 The final concentration of the phage preparation used was 1 × 1011 plaque-forming units (PFU)/mL. Phage was administered intravenously at a dose of 2 × 1010 PFU/kg of body weight.25 The secondary immunization was administered intravenously 6 weeks later using the same dose. Serum was collected at 15 minutes before the primary immunization and 1 week, 2 weeks, and 4 weeks after both the primary and secondary immunizations. Antibody activity was determined by a sensitive phage neutralizing assay and expressed as the rate of phage inactivation (Kvalue [Kv]). Susceptibility of phage-neutralizing antibody to 2-ME was determined by the method of Grubb and Swahn.26 Antibody resistant to 2-ME is considered to be of the IgG isotype.
Cytogenetic analysis.Chromosomes from unfractionated bone marrow were prepared from direct and 24-hour unstimulated cultures. Chromosomes from unfractionated peripheral blood cells were prepared from 96-hour cultures stimulated with phytohemagglutinin and IL-2, as previously described.5 Cells from male (42,XY) and female (42,XX) baboons were distinguished by analysis of G-banded metaphase chromosome spreads consisting of chromosome count and identification of sex chromosome complement.
RESULTS
Long-term lymphohematopoietic engraftment of baboons transplanted with purified CD34+ marrow cells depleted of T and B lymphocytes.Three baboons transplanted with purified allogeneic CD34+ Lin− marrow cells from their HLA-matched and sex-mismatched siblings, two of which were previously reported in studies of short term engraftment,5 were observed for long-term stability of engraftment with donor-derived lymphohematopoietic cells. Two animals remain alive with normal blood counts more than 4.9 and 6.0 years after transplantation and the third animal died at 5 years with stable mixed chimerism (see the Materials and Methods, Table 1, and Fig 1). One month after transplant, cytogenetic analysis of unfractionated marrow and peripheral blood cells showed that all three animals had engrafted with donor cells (Table 1). Cytogenetic analysis of unfractionated marrow and blood cells at multiple time points thereafter has shown that virtually all metaphases were of donor type in two female animals. The third animal, a male, had evidence of autologous marrow recovery between 3 and 12 months after transplantation and subsequently remained a stable mixed chimera with a predominance of host cells (Fig 2). At 5 years after transplantation, the male developed pneumonia as a complication of disseminated SA8-like herpesvirus infection and died of complications associated with treating the infection. Subsequent serologic studies showed this animal to have been STLV-1 seropositive. The other two long-term survivors are STLV-1 seronegative.
Engraftment of Purified Allogeneic CD34+ Lymphocyte-Depleted Marrow Cells
| Animal . | CD34+/kg . | Days After Transplant . | Survival (yr) . | ||||
|---|---|---|---|---|---|---|---|
| . | (×10−6) . | WBC* . | ANC† >500 . | PLTS‡ >20,000 . | % Donorρ on d 28 . | . | |
| . | . | >500 . | >1,000 . | . | . | . | . |
| A89164 | 1.8 | 20 | 24 | 28 | 23 | 90% | 5 |
| A89163 | 2.1 | 17 | 20 | 22 | 28 | 50% | >6.0 |
| A91382 | 1.9 | 14 | 17 | 17 | 14 | 80% | >4.9 |
| Animal . | CD34+/kg . | Days After Transplant . | Survival (yr) . | ||||
|---|---|---|---|---|---|---|---|
| . | (×10−6) . | WBC* . | ANC† >500 . | PLTS‡ >20,000 . | % Donorρ on d 28 . | . | |
| . | . | >500 . | >1,000 . | . | . | . | . |
| A89164 | 1.8 | 20 | 24 | 28 | 23 | 90% | 5 |
| A89163 | 2.1 | 17 | 20 | 22 | 28 | 50% | >6.0 |
| A91382 | 1.9 | 14 | 17 | 17 | 14 | 80% | >4.9 |
The purified allogeneic CD34+ marrow cells devoid of detectable mature and immature lymphocytes and myeloid cells were greater than 95%, greater than 97%, and greater than 98% CD34+ at the time of transplantation for animals A89164, A89163, and A91382, respectively.
Day after which total white blood cell count was maintained above 500/μL and 1,000/μL.
Day after which absolute neutrophil count was maintained above 500/μL.
Day after which platelet count was maintained above 20,000/μl without transfusion.
ρ Karyotyping was performed on bone marrow cells from each animal on day +28. For A89164, 18 of 20 metaphases were female (donor); for A89163, 8 or 16 metaphases were male (donor); and for A91382, 16 of 20 metaphases were male (donor).
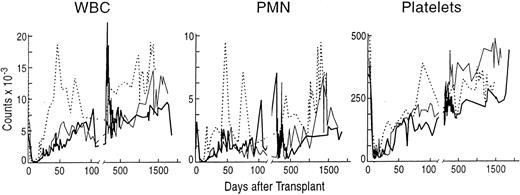
Stable peripheral blood counts after engraftment with purified allogeneic CD34+ lymphocyte-depeleted marrow cells from sex-mismatched, MLC-identical sibling baboons. (A) The total white blood cell count (WBC × 10−9/L), (B) the absolute neutrophil count (ANC × 10−9/L), and (C) the platelet count (PLT × 10−9/L) for each of the animals after transplantation. (━) A98164; (─) A89163; (⋅⋅⋅⋅) A91382
Stable peripheral blood counts after engraftment with purified allogeneic CD34+ lymphocyte-depeleted marrow cells from sex-mismatched, MLC-identical sibling baboons. (A) The total white blood cell count (WBC × 10−9/L), (B) the absolute neutrophil count (ANC × 10−9/L), and (C) the platelet count (PLT × 10−9/L) for each of the animals after transplantation. (━) A98164; (─) A89163; (⋅⋅⋅⋅) A91382
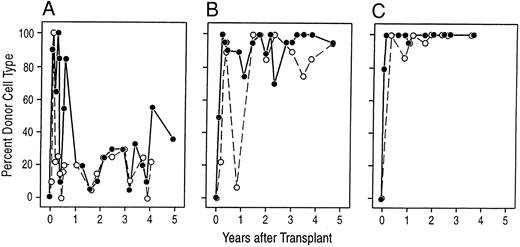
Donor-derived cells are responsible for the stable engraftment. The panels show the proportion of donor karyotypes observed at various times after transplantation in preparations of unstimulated bone marrow cells (•) and PHA-stimulated peripheral blood cells (○). In general, 20 metaphases were examined at each time point, unless indicated. (A) A98164; (B) A89163; (C) A91382.
Donor-derived cells are responsible for the stable engraftment. The panels show the proportion of donor karyotypes observed at various times after transplantation in preparations of unstimulated bone marrow cells (•) and PHA-stimulated peripheral blood cells (○). In general, 20 metaphases were examined at each time point, unless indicated. (A) A98164; (B) A89163; (C) A91382.
We asked to what hematopoietic lineages were the donor CD34+ cells contributing progeny. Cytogenetic analysis of unfractionated marrow and blood cells does not allow the types of cells dividing to be specifically identified. Therefore, we used PCR for a male-specific sequence to examine the progeny of individual progenitor cells as well as cells of specific lineages purified by flow cytometry as a means of determining donor origin.
Donor origin of hematopoietic progenitor cells in marrow.We asked what proportion of marrow-derived myeloid and erythroid progenitor cells are derived from donor cells. CFU-GM, burst-forming unit-erythroid (BFU-E), and high proliferative potential colony-forming cells (HPP-CFC) grown from either purified CD34+ or unfractionated marrow cells were picked individually and analyzed by PCR for the presence of a male-specific 174-bp sequence (Table 2). This was informative, because each recipient and donor pair were sex-mismatched. In the two female animals, CFU-GM and HPP-CFC were 88% to 100% PCR+, whereas BFU-E ranged from 38% to 100% donor. Neutrophils purified by flow cytometry from the peripheral blood of A89163 and A91382 were shown to be male-derived using PCR (data not shown). The male animal, A89164, had low frequencies of PCR− (donor) CFU-GM, BFU-E, and HPP-CFC detected 2, 3, and 5 years after transplantation, consistent with the mixed chimerism by karyotyping (Fig 2). Thus, the transplanted CD34+ lymphocyte-depleted marrow cells produced stable reconstitution of granulopoiesis and erythropoiesis in vivo.
Progenitor Cells in Animals Transplanted With Purified Allogeneic CD34+ Lymphocyte-Depleted Marrow Cells Are Donor-Derived
| Animal . | Day After Transplant . | % Donor by PCR . | ||
|---|---|---|---|---|
| . | . | CFU-GM . | BFU-E . | HPP-CEC . |
| A89164 | 807 | 0/10 (0%) | 4/8 (50%) | ND |
| 1,094 | 0/21 (0%) | 3/29 (10%) | 1/2 (50%) | |
| 1,825 | 4/10 (40%) | 4/10 (40%) | ND | |
| 4/41 (13%) | 11/47 (23%) | 1/2 (50%) | ||
| A89163 | 723 | 10/10 (100%) | 4/4 (100%) | ND |
| 786 | 16/18 (89%) | 6/13 (46%) | 3/3 (100%) | |
| 819 | 9/10 (90%) | 3/4 (75%) | ND | |
| 1,010 | 30/30 (100%) | 27/27 (100%) | 3/3 (100%) | |
| 1,755 | 19/19 (100%) | 18/20 (90%) | ND | |
| 1,888 | ND | ND | 13/13 (100%) | |
| 84/87 (97%) | 58/68 (85%) | 19/19 (100%) | ||
| A91382 | 336 | 10/10 (100%) | 9/10 (90%) | ND |
| 399 | 7/8 (88%) | 6/16 (38%) | ND | |
| 432 | 12/13 (92%) | 7/12 (58%) | ND | |
| 459 | 6/6 (100%) | 5/8 (63%) | ND | |
| 636 | 30/30 (100%) | 8/12 (67%) | 1/1 (100%) | |
| 1,399 | 19/19 (100%) | 20/20 (100%) | ND | |
| 1,532 | ND | ND | 8/8 (100%) | |
| 84/86 (98%) | 55/78 (83%) | 9/9 (100%) | ||
| Animal . | Day After Transplant . | % Donor by PCR . | ||
|---|---|---|---|---|
| . | . | CFU-GM . | BFU-E . | HPP-CEC . |
| A89164 | 807 | 0/10 (0%) | 4/8 (50%) | ND |
| 1,094 | 0/21 (0%) | 3/29 (10%) | 1/2 (50%) | |
| 1,825 | 4/10 (40%) | 4/10 (40%) | ND | |
| 4/41 (13%) | 11/47 (23%) | 1/2 (50%) | ||
| A89163 | 723 | 10/10 (100%) | 4/4 (100%) | ND |
| 786 | 16/18 (89%) | 6/13 (46%) | 3/3 (100%) | |
| 819 | 9/10 (90%) | 3/4 (75%) | ND | |
| 1,010 | 30/30 (100%) | 27/27 (100%) | 3/3 (100%) | |
| 1,755 | 19/19 (100%) | 18/20 (90%) | ND | |
| 1,888 | ND | ND | 13/13 (100%) | |
| 84/87 (97%) | 58/68 (85%) | 19/19 (100%) | ||
| A91382 | 336 | 10/10 (100%) | 9/10 (90%) | ND |
| 399 | 7/8 (88%) | 6/16 (38%) | ND | |
| 432 | 12/13 (92%) | 7/12 (58%) | ND | |
| 459 | 6/6 (100%) | 5/8 (63%) | ND | |
| 636 | 30/30 (100%) | 8/12 (67%) | 1/1 (100%) | |
| 1,399 | 19/19 (100%) | 20/20 (100%) | ND | |
| 1,532 | ND | ND | 8/8 (100%) | |
| 84/86 (98%) | 55/78 (83%) | 9/9 (100%) | ||
Data represent the number of colonies of donor type by PCR over the number of colonies of each type tested. A colony from which the male-specific sequence did not amplify was defined as negative only if the DNA sample was considered intact or not degraded as determined by the ability to amplify a 300-bp β2-microglobulin sequence from the sample. A sample from which we failed to amplify both the male and β2-microglobulin products was defined as nonevaluable and was not included in the analysis. Of a total of 63 male-negative colonies, 46 (73%) had intact DNA as judged by PCR for the β2-microglobulin sequence. More than half of the samples that had degraded DNA had been stored at −70°C for more than 18 months.
Donor origin of B and T lymphocytes in blood.We next asked if both the B- and T-lymphocyte compartments had been reconstituted by the donor CD34+ cells. To determine the contribution of donor cells to the B-lymphocyte compartment, we isolated transformed B-cell lines using HVP23 that has been shown to transform B cells from baboons and macaques. The CD20+ B-cell clones derived by this approach were then analyzed by cytogenetics and by PCR for the male-specific sequence (Table 3). In the two female animals, virtually all B-cell clones isolated were male or donor-derived. Of interest, more than half of the B-cell clones isolated from the male animal that was a mixed chimera were female and thus of donor origin.
HVP-Transformed CD20+ Clonal B-Cell Lines Isolated From Animals After Transplantation Are Donor-Derived
| Animal . | Cytogenetic Analysis . | PCR Analysis . |
|---|---|---|
| A89164 | 12 XX (Donor)/8 XY | 11 PCR− (Donor)/9 PCR+ |
| A89163 | 12 XY (Donor)/2 XX | 12 PCR+ (Donor)/2 PCR− |
| A91382 | 22 XY (Donor)/0 XX | 21 PCR+ (Donor)/1 PCR− |
| Animal . | Cytogenetic Analysis . | PCR Analysis . |
|---|---|---|
| A89164 | 12 XX (Donor)/8 XY | 11 PCR− (Donor)/9 PCR+ |
| A89163 | 12 XY (Donor)/2 XX | 12 PCR+ (Donor)/2 PCR− |
| A91382 | 22 XY (Donor)/0 XX | 21 PCR+ (Donor)/1 PCR− |
Karyotyping of unfractionated peripheral blood cells stimulated with phytohemaglutinin and IL-2 showed that, for A89163 and A91382, virtually all metaphases were donor (male), whereas for A89164, the proportion of donor (female) metaphases ranged between 5% and 25% after 1 year. To confirm that T lymphocytes were donor-derived in the two female animals, we sorted CD4+ and CD8+ T cells to greater than 99% purity from the peripheral blood. PCR analysis of samples of 1,000, 100, and 10 purified T cells was uniformly positive for the male-specific sequence (Table 4 and Fig 3).
Peripheral Blood CD4+ and CD8+ T Cells Are Donor (Male)-Derived as Determined by PCR
| Animal . | CD4+ . | CD8+ . |
|---|---|---|
| A89163 (female with male donor) | ||
| cells per PCR reaction | ||
| 1,000 | 6/6 | 5/5 |
| 100 | 12/12 | 12/12 |
| 10 | 12/12 | 11/11 |
| A91382 (female with male donor) | ||
| cells per PCR reaction | ||
| 1,000 | 6/6 | 6/6 |
| 100 | 6/6 | 6/6 |
| 10 | 6/6 | 5/5 |
| Animal . | CD4+ . | CD8+ . |
|---|---|---|
| A89163 (female with male donor) | ||
| cells per PCR reaction | ||
| 1,000 | 6/6 | 5/5 |
| 100 | 12/12 | 12/12 |
| 10 | 12/12 | 11/11 |
| A91382 (female with male donor) | ||
| cells per PCR reaction | ||
| 1,000 | 6/6 | 6/6 |
| 100 | 6/6 | 6/6 |
| 10 | 6/6 | 5/5 |
Values are the number of positive PCRs/the number of samples tested. The cells were isolated from blood of A89163 5.3 years after transplantation and from A91382 4.2 years after transplantation. Cells were purified by two cycles of FACS. The purified cell populations were ≥99% of the desired phenotype. Samples were assayed by PCR for the 174-bp male-specific sequence.
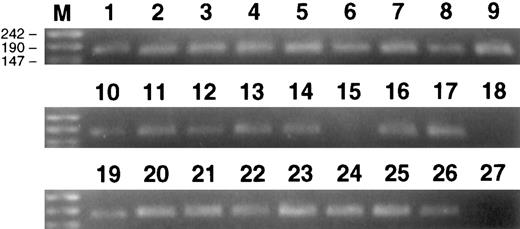
Ethidium bromide-stained gels showing 174-bp male-specific product from PCR amplification of DNA from CD4+ and CD8+ cells purified from the blood of female animals A89163 and A91382 that had been transplanted with purified CD34+ Lin− marrow cells from their male siblings. Positive controls, 105 PBL from male baboon (lanes 1, 10, and 19); negative controls, 105 PBL from female baboon (lane 15); water, no DNA, control (lanes 18 and 27); 100 CD8+ PBL from A89163 (lanes 2 through 5); 100 CD4+ PBL from A91382 (lanes 6 through 9); 10 CD8+ PBL from A89163 (lanes 11 through 14); 10 CD4+ PBL from A91382 (lanes 16 and 17); 10 CD4+ PBL from A91382 (lanes 20 through 22); 10 CD8+ PBL from A91382 (lanes 23 through 26). Lane M contains DNA molecular weight markers (molecular weight markers VIII; Boehringer Mannheim).
Ethidium bromide-stained gels showing 174-bp male-specific product from PCR amplification of DNA from CD4+ and CD8+ cells purified from the blood of female animals A89163 and A91382 that had been transplanted with purified CD34+ Lin− marrow cells from their male siblings. Positive controls, 105 PBL from male baboon (lanes 1, 10, and 19); negative controls, 105 PBL from female baboon (lane 15); water, no DNA, control (lanes 18 and 27); 100 CD8+ PBL from A89163 (lanes 2 through 5); 100 CD4+ PBL from A91382 (lanes 6 through 9); 10 CD8+ PBL from A89163 (lanes 11 through 14); 10 CD4+ PBL from A91382 (lanes 16 and 17); 10 CD4+ PBL from A91382 (lanes 20 through 22); 10 CD8+ PBL from A91382 (lanes 23 through 26). Lane M contains DNA molecular weight markers (molecular weight markers VIII; Boehringer Mannheim).
Antibody response to immunization with bacteriophage φX174.Because the transplanted CD34+ Lin− marrow cells had produced stable lymphohematopoietic reconstitution, we then wanted to know if normal immunologic function had been restored in these animals. To study the in vivo antibody response to a T-dependent neoantigen, we immunized the three animals with bacteriophage φX174. This approach, although not useful for specifying the nature of an immunologic abnormality, if present, is extremely sensitive to even minor defects.10-21,25 At the time of immunization with φX174, animals A89164, A89163, and A91382 were 3.6, 3.3, and 2.3 years after transplantation, respectively. All three experimental animals had serum IgM, IgG, and IgA levels comparable to those of the two control animals (Table 5), and the frequency of T and B cells in peripheral blood was similar to normal controls (data not shown). As determined in previous experiments using nonhuman primates, the dose of φX174 selected for administration was 10-fold greater (2 × 1010 PFU/kg body weight) than that used in studies of humans.25 As controls, we immunized two normal adult baboons. As shown in Fig 4, all of the animals produced neutralizing anti-φX174 antibodies after immunization. The antibody titers produced after primary and secondary immunization were similar in both the control and transplanted baboons. Importantly, the experimental animals showed evidence of class switch, from IgM to IgG, after the secondary immunization that was similar to controls. Thus, these animals with stable lymphohematopoietic reconstitution after transplantation have an antibody response to a T-cell–dependent neo-antigen that is similar to that of normal control animals.
Serum Ig Levels in Animals Transplanted With Allogeneic CD34+ Lymphocyte-Depleted Marrow Cells
| Animal . | IgM (mg/100 mL) . | IgG (mg/100 mL) . | IgA (mg/100 mL) . |
|---|---|---|---|
| A89164 | 61 | 1,498 | 208 |
| A89163 | 31 | 1,362 | 110 |
| A91382 | 33 | 838 | 64 |
| Control no. 1 | 91 | 1,645 | 219 |
| Control no. 2 | 73 | 1,252 | 149 |
| Animal . | IgM (mg/100 mL) . | IgG (mg/100 mL) . | IgA (mg/100 mL) . |
|---|---|---|---|
| A89164 | 61 | 1,498 | 208 |
| A89163 | 31 | 1,362 | 110 |
| A91382 | 33 | 838 | 64 |
| Control no. 1 | 91 | 1,645 | 219 |
| Control no. 2 | 73 | 1,252 | 149 |
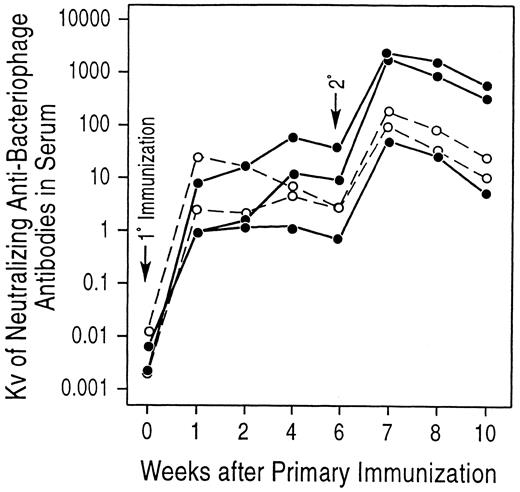
Stable hematopoietic chimeras immunized with bacteriophage φX174 have primary and secondary antibody responses with Ig class switching similar to that of control animals. At the time of 1° immunization, animals A89164, A89163, and A91382 were 1,324, 1,212, and 835 days posttranplantation, respectively. (•) Experimental animals; (○) control animals.
Stable hematopoietic chimeras immunized with bacteriophage φX174 have primary and secondary antibody responses with Ig class switching similar to that of control animals. At the time of 1° immunization, animals A89164, A89163, and A91382 were 1,324, 1,212, and 835 days posttranplantation, respectively. (•) Experimental animals; (○) control animals.
DISCUSSION
CD34, a member of the sialomucin family, was initially identified as an antigen that is restricted in its expression on normal cells to vascular endothelial cells and a population of immature hematopoietic cells that includes progenitors and their precursors in long-term culture.27-30 The anti-CD34 antibody 12.831 immunoprecipitates antigens of the same molecular size from both human and baboon marrow cells (unpublished observations) and reacts with virtually all baboon marrow and peripheral blood hematopoietic progenitor cells identified by in vitro colony-forming cell assays and long-term marrow cultures. Studies in primates showed that marrow cells expressing CD34 could engraft lethally irradiated animals, whereas marrow depleted of these cells failed to rescue animals.4,5 CD34+ cells enriched from human marrow6 and blood32 have been shown to engraft patients treated with myeloablative therapies. Using an allogeneic model in baboons, we showed that CD34+ marrow cell populations that have been depleted of all detectable mature and immature lymphocytes and myeloid cells can engraft and give rise to myeloid and lymphoid progeny in vivo.5 However, the long-term stability of grafts from highly purified CD34+ cells and the ability of such grafts to differentiate and restore normal immunologic function after transplantation remained undetermined. In this report, we provide evidence that animals transplanted with highly purified CD34+ marrow cells that do not contain mature T and B lymphocytes can reconstitute a normally functioning immune system in vivo. Furthermore, these grafts appear to be stable for periods in excess of 5 years.
To test immunologic competence, we immunized transplanted animals and normal controls with the T-dependent neoantigen bacteriophage φX174. To produce a normal antibody response to this antigen, intact functioning of antigen-presenting cells, T lymphocytes, and B lymphocytes is required.18,33 An abnormality in antiphage antibody response is a sensitive indicator of defects involving B cells,19,20 T cells,20,21 complement,11 and adhesion molecules.11 For this reason, immunization with phage has been used as a tool in evaluating immunologic function in patients with suspected primary or secondary immunodeficiency states,10,11,14-24,34-36 leukocyte adhesion defects,37,38 and after bone marrow transplantation.13-17 39-41 We hypothesized that, if reconstitution of lymphoid compartments from only primitive stem cells resulted in a limited T- and B-cell repertoire, the transplanted animals would fail to respond with a proper antibody response to phage, and this approach would provide a sensitive screening assay.
The three long-term chimeras were studied 3.6, 3.3, and 2.3 years after transplantation of matched purified CD34+ marrow cells devoid of any detectable mature lymphocytes and myeloid cells. All showed normal responses to immunization with phage, as assessed by the production of normal quantities of neutralizing antibody and switch from IgM to IgG isotype. Two of these animals, both female, have virtually all lymphoid and myeloid cells being of donor origin, whereas one, a male, was a stable mixed chimera. Thus, in two of these animals, the immunologic response measured the function of lymphocytes that were derived solely from donor CD34+ stem cells after transplantation. This demonstrates that not only are normal numbers of phenotypically normal lymphoid cells produced after transplantation with purified CD34+ cells devoid of lymphocytes, but that the cells produced are capable of normal in vivo function.
In addition to evaluating the immunologic function of these long-term chimeras, we have shown that donor-derived lymphohematopoiesis has remained stable in these animals for more than 5 years. Because these animals were transplanted with purified CD34+ marrow cells from their sex-mismatched siblings, it was possible to use cytogenetics as well as PCR for a male-specific sequence to determine the origin of cells in marrow and peripheral blood. Of interest is that, whereas all three engrafted rapidly with donor cells present in marrow and blood, two remain engrafted with entirely donor cells greater than 4.9 and 5.8 years. One of the two animals treated with 920 cGy total body irradiation in preparation for the transplant underwent recovery of autologous hematopoietic cells after 6 months. That animal remained a stable mixed chimera until its death, 5 years after transplantation, secondary to complications of treating a disseminated SA8-like herpes virus infection.42 At the time of death, that animal was discovered to be STLV-1 seropositive,43 although the role of STLV-1 in this animal's death remains unclear. At the time of death, bone marrow and peripheral lymph node morphology and cellularity were normal. The other two survivors are STLV-1 seronegative.
The CD34 genes in mice (mCD34)28,44-47 and dogs (cCD34)48 have been identified and cloned, and antibodies have been raised against both antigens.45-48 In mice, mCD34 is expressed on vascular endothelium and some in vitro colony-forming progenitors and spleen colony-forming cells.45-47 However, the expression of mCD34 by murine hematopoietic stem cells is controversial. Studies by Krause et al45 suggest that mCD34 is expressed by murine marrow repopulating cells. In contrast, Osawa et al47 suggest that long-term marrow repopulating cells in mice express low to undetectable levels of mCD34, whereas only short-term repopulating cells in vivo express mCD34. It is unclear if these conflicting findings represent the use of antibodies to different isoforms or glycoforms of mCD34. It is of interest that Osawa et al47 report that mCD34 is only expressed at detectable levels by a subpopulation of in vitro colony-forming cells that proliferate to a single growth factor, whereas the mCD34− population contains most colony-forming cells that grow in the presence of multiple hematopoietic growth factors. This is distinctly different from the reported expression of CD34 by human and nonhuman primate progenitor cells assayed in vitro.4,5,31 In these species, highly purified CD34− cells are profoundly depleted of progenitors and their precursors detectable in long-term culture initiating cells (LTC-IC). In dogs, cCD34 is expressed by most in vitro progenitor cells48 and also appears to be expressed by canine marrow repopulating cells (McSweeney et al, unpublished observations).
CD34 is expressed by hematopoietic progenitor cells in multiple species. We have shown that purified CD34+ cells devoid of any mature or immature lymphoid cells can fully reconstitute lymphohematopoiesis in vivo in baboons that is morphologically and functionally normal and stable in excess of 5 years. Further dissection of this population will be necessary to understand the contributions of specific CD34+ cell types to early and late marrow reconstitution after transplantation. Such information would allow more direct targeting of the desired stem cells for gene transfer as a means of treating heritable genetic defects of the immune and hematopoietic system.
ACKNOWLEDGMENT
The authors thank Mike Gough, Ray Colby, Gary Millen, and the staff of the University of Washington Regional Primate Research Center for their excellent support. We also thank the staff of the Clinical Hematology Laboratory at the Fred Hutchinson Cancer Research Center, who performed most of the complete blood counts.
Supported in part by Grants and Contracts No. AI35191, HL54881, HD17427, and NIHRR00166.
Presented in part at the American Society of Hematology meeting, December 1-5, 1995, Seattle, WA.
Address reprint requests to Robert G. Andrews, MD, Pediatric Oncology Program, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave No, Cl-169 PO Box 19024 Seattle, WA 98109-1024.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal