Abstract
Homeobox proteins comprise a major class of transcription factors, which have been implicated in normal hematopoiesis and leukemogenesis. Notable in this context is the homeobox gene HOX-B8 (formerly known as HOX-2.4), which was shown to cooperate with hematokines to induce leukemia, and to enhance self-renewal of immature myeloid progenitors when expressed alone. How HOX-B8 may affect lineage specific development of hematopoietic progenitor cells is unknown. Here it is shown that ectopic expression of HOX-B8 specifically inhibited dimethyl sulfoxide (DMSO)-induced granulocytic differentiation of autonomously proliferating HL-60 myeloid progenitor cells. HOX-B8 also inhibited the granulocyte colony-stimulating factor (G-CSF )–induced granulocytic developmental program of factor dependent 32Dcl3 hematopoietic progenitors, including survival, proliferation, and differentiation, as evident by rapid apoptosis of the cells following removal of interleukin-3 (IL-3) and addition of G-CSF. In sharp contrast, HOX-B8 had no effect on macrophage differentiation of M1 and HL-60 cells induced by IL-6 and phorbol-12-myristate-13-acetate, respectively. Moreover, HOX-B8 expression endowed the 32Dcl3 cells with the ability to be induced by granulocyte-macrophage colony-stimulating factor (GM-CSF ) for terminal differentiation exclusively along the macrophage lineage; this effect was at least partially mediated via expression of the zinc finger transcription factor Egr-1. Thus, ectopic expression of HOX-B8 in hematopoietic progenitor cells appears to differentially affect lineage specific development, negatively regulating granulocyte development and positively regulating macrophage development.
HOMEOBOX PROTEINS, sharing a common 61-amino acid homeodomain that mediates their interaction with DNA, comprise a major class of transcription factors. Originally discovered in homeotic genes responsible for segment identity in Drosophila development, they were subsequently found in a number of evolutionarily distant organisms.1,2 In addition to controlling embryonic development, evidence has been obtained that they also control ongoing differentiation processes in the adult, including normal hematopoiesis.1-5 Furthermore, a contributory role for homeobox genes in tumorigenesis and leukemogenesis is consistent with data obtained by expression analysis and genetic manipulation of various homeobox genes.3,6 7
Results from transfection and antisense experiments with various homeobox genes suggest a role for some of these genes in the regulation of blood cell differentiation and determination.4,5,8 It has been shown that the WEHI-3B myelomonocytic leukemia cell line, unlike other myeloid cell lines, expresses the HOX-B8 gene (formerly known as HOX-2.4), and it was determined that the expression is due to integration of an intracisternal A-particle (IAP) genome into the 5′ noncoding region.9,10 The observed constitutive expression of HOX-B8 may have contributed to the development of the WEHI-3B leukemia, also found to constitutively express interleukin-3 (IL-3). This hypothesis is supported by the observation that constitutive expression of both IL-3 and HOX-B8 in normal hematopoietic cells results in highly leukemic cells exhibiting retarded differentiation in vitro and generating myelomonocytic cell lines,7 whereas enforced autocrine expression only of IL-3 results in continued growth, but not tumorigenicity. More recently, Perkins and Cory11 have shown that ectopic expression of HOX-B8 in bone marrow cells enhances self-renewal of immature myeloid progenitors, but is not fully transforming. It has been suggested that HOX-B8 might be oncogenic because it mimics the action of a homeobox gene product whose function is to maintain the ability to self-renew or because it inhibits the function of genes needed for terminal differentiation.7 11
To further elucidate how deregulated HOX-B8 contributes towards oncogenicity and influences myeloid development, we investigated the effect of ectopic expression of HOX-B8 on the differentiation program of several myeloid cell lines, each with specific survival and differentiation requirements. M1 is a murine myeloblastic leukemic cell line capable of differentiating into macrophages when stimulated by various physiologic inducers such as IL-6 and leukemia inhibitory factor (LIF ).1 Unlike the monocytic lineage predisposed M1 cells, the human myeloblastic leukemia HL-60 cell line can differentiate along either the monocytic lineage following treatment with phorbol-12-myristate-13-acetate (PMA) or the granulocytic lineage following treatment with dimethyl sulfoxide (DMSO).12, 13 The hematopoietic progenitor cell line 32Dcl3, which requires IL-3 for survival and growth, undergoes limited proliferation and terminal granulocytic differentiation on removal of IL-3 and addition of granulocytic colony-stimulating factor (G-CSF ) and also exhibits limited proliferative capability, but no differentiation, in response to granulocyte-macrophage colony-stimulating factor (GM-CSF ); recently we have shown that ectopic expression of the zinc finger transcription factor Egr-1 endowed 32Dcl3 cells with the ability to be induced by GM-CSF for terminal differentiation exclusively along the macrophage lineage.14 These cell lines offer the possibility to investigate the effect of HOX-B8 on myeloid differentiation segregated from proliferation, as well as to distinguish the effects of HOX-B8 on the differentiation of each lineage.
Our data showed that ectopic expression of HOX-B8: (1) had no effect on IL-6–induced monocytic differentiation of M1 cells; (2) partially interrupted DMSO-induced granulocytic differentiation of HL-60 cells, but had no effect on PMA-induced monocytic differentiation; (3) resulted in rapid apoptosis following removal of IL-3 and addition of G-CSF to 32Dcl3 cells; and (4) endowed 32Dcl3 cells with the ability to be induced by GM-CSF for terminal differentiation exclusively along the macrophage lineage, in which this effect was at least partially mediated by expression of the zinc finger transcription factor Egr-1. Taken together, it can be concluded that inappropriate constitutive expression of the homeobox gene HOX-B8 can alter the growth, differentiation, and survival of myeloid cells, negatively regulating granulocyte development and positively regulating macrophage development; in addition, the effect is dependent on the precise developmental stage of the target cell and its requirement of factors for survival and growth.
MATERIALS AND METHODS
Cells, cell culture, and cytokines.The differentiation competent murine M1 myeloid leukemic cell line has been described previously.15 The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% heat inactivated (HA) horse serum (HS) (GIBCO-BRL) plus 1% penicillin and streptomycin (GIBCO-BRL). The 32Dcl3(G) cells were obtained from Dr Giovanni Rovera (Wistar Institute, Philadelphia, PA). The HL-60 cell line, obtained from American Type Culture Collection (ATCC; Rockville MD), was recloned in our laboratory,13 and clone 21 was used throughout the study. The 32Dcl3(G) (referred to as 32Dcl3) and HL-60 cells were cultured in RPMI 1640 (GIBCO-BRL) supplemented with 10% heat inactivated (HA) fetal bovine serum (FBS) (GIBCO-BRL), plus 1% penicillin and streptomycin (GIBCO-BRL). 32DEgr-1 cell lines were established in this laboratory.14 All cells were cultured in a humidified atmosphere with 10% CO2 at 37°C. Unless otherwise specified, the 32Dcl3 cell line, and any variants expressing Egr-1 or HOX-B8 transgenes, were grown in medium supplemented with 10% WEHI-3B conditioned medium (CM) as a source of IL-3.16 Conditions to stimulate the cells for terminal differentiation were described in detail previously for M1,15 32Dcl3,14 and HL-60 cells.13 Briefly, for inducing the M1 cells for terminal differentiation, the cells were seeded at 0.15 × l06cells/mL with or without IL-6, at 5 ng/mL or 50 ng/mL, as indicated. For RNA extractions, cell concentrations were adjusted to give a final density of > 0.25 × 106 cells/mL at the time of extraction. 32Dcl3 cells were washed twice in IL-3–free medium, followed by addition of either G-CSF (10 ng/mL) or GM-CSF (10 ng/mL). Cells were seeded at a density of 0.2 × 106 cells/mL to be maintained in IL-3 and were seeded at a density of 0.5 × 106 cells/mL when either G-CSF or GM-CSF were added to the culture medium. The culture medium, including appropriate cytokines, was changed every 48 hours. With G-CSF, following 48 hours, the cells were usually reseeded at a concentration of 0.5 × 106 cells/mL to avoid overgrowth. For inducing the HL-60 cells for monocytic differentiation, cells were seeded at 0.2 × 106 cells/mL with PMA (5 nmol/L; Sigma, St Louis, MO) and for granulocytic differentiation, cells were seeded at 0.4 × 106 cells/mL with DMSO (1.3%, Sigma). Viable cell numbers were determined by trypan blue dye exclusion, counting in a hemocytometer. In all cases, experiments were repeated at least three times.
Recombinant human IL-6 (rhuIL-6), recombinant murine granulocyte colony-stimulating factor (rmuG-CSF ), and recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF ) were generous gifts from Amgen Inc (Thousand Oaks, CA). For 32Dcl3 cells, 10% WEHI-3B conditioned medium was used as a source of IL-3.16
Assays for differentiation-associated properties and apoptosis.Morphologic differentiation was determined by counting at least 300 cells on May-Grünwald-Giemsa–stained cytospin smears and scoring the proportion of immature blast cells, cells at intermediate granulocyte or monocyte stages of differentiation, and mature granulocytes or macrophages.13,14,17 Immature blast cells are characterized by scant cytoplasm and round or oval nuclei. Granulocyte intermediates are characterized by dented, but not lobulated, nuclei and mature granulocyte-like cells are characterized by enlarged cytoplasm and lobulated nuclei. Cells at intermediate monocyte stages of differentiation are flattened, with a larger cytoplasm to nucleus ratio and contain irregularly shaped nuclei and few interspersed or no vacuoles; mature macrophage-like cells are flattened, and spread out cells are interspersed with numerous vacuoles in a greatly enlarged cytoplasm. Fc and C3 receptor assays were determined as described.17 The nonspecific esterase (NSE) assay was performed by staining cells on plates.18 Reduction of nitroblue tetrazolium (NBT) (Sigma) stain was performed as described.13 Results of all experiments represent the mean of at least three independent determinations with standard deviations up to 15% (ie, 11% = 11% + 1.6%). DNA fragmentation, indicative of apoptosis, was determined by a modification of a procedure previously described,19 20 in which after lysis of cells and treatment with proteinase K, samples were treated with RNaseA and 5 μg of the purified sample was fractionated on a 2% agarose gel.
General recombinant DNA techniques, expression vectors, and DNA probes.Plasmid pIC20H-HOX-B8 was obtained from Dr Jerry M. Adams (Walter Eliza Hall Institute of Medical Research, Victoria, Australia). The 0.82-kb murine HOX-B8 cDNA fragment was excised from the plasmid with Xba I and EcoRI, and cloned under the MLV promoter in the HindIII site of the pMLV plasmid20 by blunt end ligation. Plasmid preparations, restriction enzyme digestions, DNA fragment preparations, and agarose gel electrophoresis were as described earlier.15,21 Probes for murine MyD88, Egr-l, β-actin, junB, Ferritin, Iysozyme, and G-CSFR were the same as used previously.13,14,15,22 DNA for probes was labeled by random priming (GIBCO-BRL, RadPrime DNA labeling, catalog no. 18428-011) to a specific activity equal to or greater than 109 cpm/μg. Genomic DNA extraction and Southern blot analysis were performed as described previously.13,17 23
RNA extraction, Northern blotting, and hybridization.RNA was extracted by the method of Chomczynski and Sacchi,24 using guanidinium thiocyanate. Total RNA (10 μg/lane; equal amount of RNA in each lane was confirmed by equal intensity of ethidium bromide staining of ribosomal RNA bands) was electrophoresed on 1% agarose formaldehyde gels. Northern blots, using Duralon-UV membranes (Stratagene, La Jolla, CA), were prepared, and UV cross-linked (Stratalinker, Stratagene) before baking for 2 hours. Blots were hybridized in 50% deionized formamide, 10% dextran sulfate, 1 mol/L NaCl, 1% sodium dodecyl sulfate (SDS), and 100 mg/mL sheared salmon sperm DNA, at 42°C with 106 cpm/mL of probe for 12 to 16 hours. Blots were washed at room temperature twice for 5 minutes in 2 × SSC, 0.1% SDS, and at 60°C twice for 30 minutes in 0.1 × SSC, 1% SDS, and exposed to x-ray film at −80°C for 48 to 72 hours. Stripping blots of probe to rehybridize was performed as described previously.17
G-CSF receptor expression analysis by reverse transcriptase-polymerase chain reaction (RT-PCR).To increase the sensitivity of detection of transcripts encoding for G-CSF receptors (G-CSFR), RT-PCR was performed on aliquots of RNA, essentially as described previously.25 Briefly, 3 μg of total RNA, extracted by the method of Chomczynski and Sacchi,24 was reverse transcribed (RT) with the GIBCO-BRL Superscript preamplification system (catalog no. 180-89-011), according to manufacturer's instructions, in a final volume of 21 μL, using oligo dT as primer. For PCR, 2 μL of cDNA was taken from each RT reaction volume and samples were diluted to 50 μL with buffer (BMB 10X), yielding 0.1 mmol/L deoxynucleoside triphosphates (dNTPs), 0.5 mmol/L MgCl2 , 10 mmol/L Tris (pH 8.3), 50 mmol/L KCl; then each primer, to a final concentration of 0.1 μmol/L, and 5 U Taq DNA Polymerase (BMB) were added. Samples were covered with 50 μL mineral oil, heated at 94°C for 5 minutes, and subjected to 15 cycles of PCR in a Perkin-Elmer thermal cycler (Perkin-Elmer, Norwalk, CT), using 1 minute of denaturation at 94°C, 1 minute of annealing at 62°C, and 2 minutes of polymerization at 72°C; finally, 5 minutes of polymerization was done at 72°C. The primers used were selected with the aid of the program PCRPLAN (PCGENE; Intelligenetics, Campbell, CA). The primers corresponded to bases 307 to 326 and 1217 to 1240 of the murine G-CSFR gene. To monitor for efficiency and reproducibility of PCR amplification, β-actin transcripts were assessed, using murine β-actin amplimers (Clontech, Palo Alto, CA). After extraction with CHCl3, 40 μL of products were electrophoresed on 1% agarose gel, blotted, and hybridized with muG-CSFR probe [cDNA insert in pBluescript (gift from J. Ihle, St Jude Children's Research Hospital, Memphis, TN)]; or a β-actin probe (Clontech, catalog no. 9800-1; within the amplified PCR region). Control samples not reverse transcribed were used to monitor for possible contamination with genomic DNA.
Establishment of M1, HL-60, and 32Dcl3 cell lines that ectopically express HOX-B8 transgene.To establish M1, HL-60, and 32Dc13 cell lines that ectopically express HOX-B8 transgenes, these cell lines were transfected via electroporation (Bio-Rad Gene Pulser, Hercules, CA), as previously described,13-15 with either pMLV-HOX-B8 or pMLV (as a control, has neoR gene to confer resistance to G418). After electroporation, the cells were appropriately diluted, and after 48 hours were seeded at 5 × 104/mL in growth media containing G418 (geneticin, 400 μg/mL, GIBCO-BRL) and 1-mL aliquots were dispensed into 24-well trays. After 3 to 4 weeks, cultures from wells containing surviving cells were expanded in the presence of G418. There were fewer than 10% positive wells; therefore, the probability that cells from each positive well were derived from the same clone is greater than 95%. The transfectants were maintained in growth media containing 200 μg/mL of G418.
Antisense Egr-1 oligodeoxynucleotides in the medium.Phosphorothioate capped oligodeoxynucleotides (oligomers 21 bases in length; 5′-sGsCCGCTGCCATCCCGGACsCsA-3′ ), targeted against the 5 prime region of the murine Egr-1 mRNA,26 spanning the initiation codon AUG (oligomers corresponded to bases 250-270) were synthesized and obtained from Regional DNA Synthesis Laboratory (Calgary, Canada) and the DNA Synthesis Facility of the Fels Institute (Temple University, Philadelphia, PA). Similarly, scrambled oligodeoxynucleotides containing the same ratio of bases in random sequences and similarly modified were used as a control. Lyophilized oligomers were resuspended in phosphate-buffered saline (PBS) without Ca2+ and Mg2+ at 2 mmol/L. 32Dc13, 32DEgr-1, and 32DHOX-B8 cells were washed twice to remove serum and IL-3, and resuspended at 1 ×106 cells/mL. Aliquots of 0.5 mL were added to a 24-well Nunc dish. Oligomers were added to a final concentration of 80 umol/L, and incubated for 2 hours at 37°C in a humidified atmosphere with 10% CO2. Then, 0.5 mL medium containing serum (10%, final concentration) plus IL-3 CM (10%, final concentration), or GM-CSF (10 ng/mL, final concentration) was added. On days 3 and 6, 0.8 mL of the spent medium was removed carefully from each well and supplemented with 0.3 mL of serum-free fresh medium containing oligomers only (concentration adjusted to give 80 μmol/L final in 0.5-mL volume). After a 2-hour incubation at 37°C, 0.5 mL medium containing serum and appropriate factors (IL-3 or GM-CSF ) was added. Cells were maintained for a total of 10 days. The presence of either the antisense or control scrambled oligomers in the culture medium had no effect on cell growth and viability, determined by trypan blue dye exclusion and counting in a hemocytometer, for 32Dc13, 32Dneo, 32DEgr-1, or 32DHOX-B8 cell lines maintained in IL-3, indicative of no cytotoxic effects due to the oligomers. In the presence of GM-CSF, differentiation in culture was assessed by daily observations and by analysis of May-Grünwald-Giemsa–stained cytospin smears. Antisense oligomer concentrations used were the optima for inhibition of differentiation of 32DEgr-1 cells. In addition, indirect immunofluorescence showed that AS oligomers blocked Egr-1 expression. Results obtained (see Table 4) are representative of three independent experiments, each done in duplicate using three different batches of oligomers. Standard deviations were up to ±15% of the percentage values shown.
Effect of Blocking Egr-1 Expression on GM-CSF–Induced Differentiation of 32DHOX-B8 Cells
| Clone . | Oligos4-150 . | Cell Type (%)4-151 . | ||||
|---|---|---|---|---|---|---|
| . | . | Blast . | Granulocytes . | Macrophage . | . | |
| . | . | . | Inter . | Inter . | Mat . | . |
| 32Dneo | None | 88 | 12 | 0 | 0 | |
| Scrambled | 87 | 13 | 0 | 0 | ||
| Antisense | 89 | 11 | 0 | 0 | ||
| 32DEgr1.2 | None | 3 | 0 | 8 | 89 | |
| Scrambled | 4 | 0 | 9 | 87 | ||
| Antisense | 21 | 0 | 69 | 10 | ||
| 32DHOX-B8/6 | None | 4 | 0 | 28 | 68 | |
| Scrambled | 6 | 0 | 26 | 68 | ||
| Antisense | 41 | 0 | 49 | 10 | ||
| 32DHOX-B8/18 | None | 5 | 0 | 31 | 64 | |
| Scrambled | 7 | 0 | 30 | 62 | ||
| Antisense | 36 | 0 | 50 | 14 | ||
| Clone . | Oligos4-150 . | Cell Type (%)4-151 . | ||||
|---|---|---|---|---|---|---|
| . | . | Blast . | Granulocytes . | Macrophage . | . | |
| . | . | . | Inter . | Inter . | Mat . | . |
| 32Dneo | None | 88 | 12 | 0 | 0 | |
| Scrambled | 87 | 13 | 0 | 0 | ||
| Antisense | 89 | 11 | 0 | 0 | ||
| 32DEgr1.2 | None | 3 | 0 | 8 | 89 | |
| Scrambled | 4 | 0 | 9 | 87 | ||
| Antisense | 21 | 0 | 69 | 10 | ||
| 32DHOX-B8/6 | None | 4 | 0 | 28 | 68 | |
| Scrambled | 6 | 0 | 26 | 68 | ||
| Antisense | 41 | 0 | 49 | 10 | ||
| 32DHOX-B8/18 | None | 5 | 0 | 31 | 64 | |
| Scrambled | 7 | 0 | 30 | 62 | ||
| Antisense | 36 | 0 | 50 | 14 | ||
Cells were treated with oligomers as described in Materials and Methods.
Cell morphology was determined after 10 days treatment with indicated oligomers and GM-CSF (10 ng/mL). All values represent the mean of at least three independent experiments, as indicated in Materials and Methods.
RESULTS
Establishment of hematopoietic cell lines that ectopically express the homeobox gene HOX-B8.The homeobox gene HOX-B8 is not expressed in M1, 32Dcl3, and HL-60 cells, either untreated or following induction for differentiation with the various inducing agents. One means to better understand how HOX-B8 can alter blood cell differentiation and contribute to leukemogenesis is to examine the consequences of ectopic expression of HOX-B8 on the differentiation potential of various myeloid cell lines, including (1) M1 following stimulation with IL-6, which induces monocytic differentiation, (2) HL-60 following DMSO-induced granulocytic differentiation or PMA-induced monocytic differentiation, and (3) 32Dcl3 following G-CSF–induced granulocytic differentiation or GM-CSF treatment, which results in limited proliferation and no differentiation.14
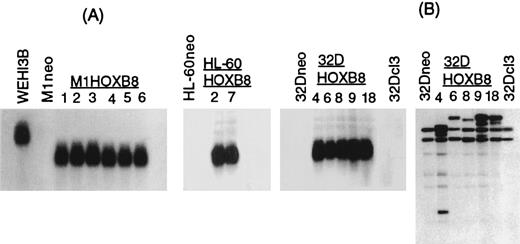
M1, HL-60, and 32Dc13 cell lines ectopically expressing HOX-B8 were established via electroporation, using the expression vector pMLV-HOX-B8, where the coding region of HOX-B8 is under control of the MLV promoter (see Materials and Methods). The level of steady-state HOX-B8 transcripts in the various transfectants, determined by Northern blot analysis, was comparable to the level expressed in WEHI-3B cells (Fig 1A). The observed difference in the size of the endogenous HOX-B8 transcript in WEHI-3B cells compared with the exogenously derived transcript in the different tranfectants was expected. The distinct integration sites for the HOX-B8 transgene, revealed by Southern blot analysis of EcoRI-digested genomic DNA from each of the clones tested (data shown for 32DHOX-B8 clones 4, 6, 8, 9, and 18) (Fig 1B) confirmed that each was an independent clone.
Establishment of cell lines that ectopically express HOX-B8. (A) Northern blot analysis of HOX-B8 expression in M1, HL-60, and 32Dcl3 derivatives, established following transfection with either pMLV or pMLV-HOX-B8, and WEHI-3B. 10 μg of total RNA was analyzed by Northern blots as described in Materials and Methods. (B) Southern blot analysis of genomic DNA from parental 32Dcl3, 32Dneo, and 32DHOX-B8 clones. Genomic DNA (l0 μg) was digested with EcoRI, resolved on a 1% agarose gel, transferred to Gene Screen Plus (NEN), and hybridized to a murine HOX-B8 cDNA probe.
Establishment of cell lines that ectopically express HOX-B8. (A) Northern blot analysis of HOX-B8 expression in M1, HL-60, and 32Dcl3 derivatives, established following transfection with either pMLV or pMLV-HOX-B8, and WEHI-3B. 10 μg of total RNA was analyzed by Northern blots as described in Materials and Methods. (B) Southern blot analysis of genomic DNA from parental 32Dcl3, 32Dneo, and 32DHOX-B8 clones. Genomic DNA (l0 μg) was digested with EcoRI, resolved on a 1% agarose gel, transferred to Gene Screen Plus (NEN), and hybridized to a murine HOX-B8 cDNA probe.
Effect of ectopic expression of HOX-B8 on differentiation of M1 and HL-60 cells.The consequences of ectopic expression of HOX-B8 on IL-6–induced monocytic differentiation of the myeloid leukemic M1 cell line was investigated. Four clones of Mlneo, six clones of MlHOX-B8, and parental M1 cells were analyzed. All cells proliferated similarly in the absence of IL-6. In addition, HOX-B8 expression had no effect on IL-6–induced changes in cell morphology, growth arrest, and induction of Fc and C3 receptors (Table 1). This was the case for M1 cells treated with either suboptimal (5 ng/mL) or optimal (50 ng/mL) levels of the differentiation factor IL-6. In addition, expression analysis of both early (MyD88, junB) and late (Ferritin, lysozyme) differentiation markers,15 using Northern blot analysis (data not shown), showed that HOX-B8 expression also had no effect on these IL-6–induced changes in gene expression. These data are consistent with the conclusion that ectopic expression of the homeobox gene HOX-B8 had no detectable influence on monocytic differentiation and growth arrest of M1 cells following IL-6 treatment.
IL-6–Induced Differentiation of M1, M1neo, and M1HOX-B8
| Clone . | IL-6 . | Cell Type (%)* . | Fc* . | C3* . | Cell No.* . | ||
|---|---|---|---|---|---|---|---|
| . | (ng/mL) . | Blast . | Inter . | Mat . | (%) . | (%) . | (106/mL) . |
| M1 | — | 99 | 1 | 0 | <1 | <1 | 1.93 |
| 5 | 46 | 52 | 2 | 20 | 10 | 1.29 | |
| 50 | 0 | 29 | 71 | 49 | 72 | 0.37 | |
| M1neo-9 | — | 99 | 1 | 0 | <1 | <1 | 2.01 |
| 5 | 52 | 45 | 3 | 21 | 12 | 1.39 | |
| 50 | 0 | 32 | 68 | 52 | 73 | 0.34 | |
| M1HOX-B8/2 | — | 99 | 1 | 0 | <1 | <1 | 2.01 |
| 5 | 47 | 49 | 4 | 19 | 13 | 1.32 | |
| 50 | 0 | 28 | 72 | 54 | 72 | 0.30 | |
| M1HOX-B8/3 | — | 99 | 1 | 0 | <1 | <1 | 1.91 |
| 5 | 51 | 46 | 3 | 22 | 10 | 1.35 | |
| 50 | 0 | 33 | 67 | 54 | 64 | 0.29 | |
| Clone . | IL-6 . | Cell Type (%)* . | Fc* . | C3* . | Cell No.* . | ||
|---|---|---|---|---|---|---|---|
| . | (ng/mL) . | Blast . | Inter . | Mat . | (%) . | (%) . | (106/mL) . |
| M1 | — | 99 | 1 | 0 | <1 | <1 | 1.93 |
| 5 | 46 | 52 | 2 | 20 | 10 | 1.29 | |
| 50 | 0 | 29 | 71 | 49 | 72 | 0.37 | |
| M1neo-9 | — | 99 | 1 | 0 | <1 | <1 | 2.01 |
| 5 | 52 | 45 | 3 | 21 | 12 | 1.39 | |
| 50 | 0 | 32 | 68 | 52 | 73 | 0.34 | |
| M1HOX-B8/2 | — | 99 | 1 | 0 | <1 | <1 | 2.01 |
| 5 | 47 | 49 | 4 | 19 | 13 | 1.32 | |
| 50 | 0 | 28 | 72 | 54 | 72 | 0.30 | |
| M1HOX-B8/3 | — | 99 | 1 | 0 | <1 | <1 | 1.91 |
| 5 | 51 | 46 | 3 | 22 | 10 | 1.35 | |
| 50 | 0 | 33 | 67 | 54 | 64 | 0.29 | |
Abbreviations: inter, intermediate; mat, mature.
Determined 3 days after the cells were seeded at a density of 0.15 × 106. Four M1neo clones and six M1HOX-B8 clones were analyzed, and data is presented for representative clones. All values represent the mean of three independent determinations with standard deviations up to 15% (ie, 11% ± 1.6%).
Because HOX-B8 expression exerted no discernable effect on IL-6–induced monocytic differentiation of M1 cells, it was of interest to ascertain the effect of ectopic HOX-B8 expression on the human myeloblastic leukemia HL-60 cell line, which can differentiate along either the monocytic or granulocytic lineage, depending on the stimulus. Two HL-60neo and four HL-60HOX-B8 cell lines were used for this analysis. Ectopic expression of Hox-B8 had no discernable effect on proliferating HL-60 cells. HL-60HOX-B8 cell lines treated with PMA underwent monocytic differentiation comparable to parental HL-60 and control HL-60neo cell lines, assessed by cell morphology and NSE staining (Table 2). Therefore, similar to monocytic differentiation of M1 cells, HOX-B8 had no detectable effect on PMA-induced monocytic differentiation of HL-60 cells.
Effect of Ectopic HOX-B8 Expression on HL-60 Induced to Undergo Monocytic or Granulocytic Differentiation
| Clone . | Inducer* . | Cell Type (%)† . | Positive (%)† . | Cell No.† . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blast . | Inter . | Mat . | NSE . | NBT . | 106/mL . |
| HL-60 | None | 100 | 0 | 0 | 1 | 1 | 2.53 |
| PMA | 0 | 13 | 87 | 90 | 0 | 0.35 | |
| DMSO | 0 | 4 | 96 | 0 | 76 | 1.32 | |
| HL-60neo5 | None | 100 | 0 | 0 | 1 | 1 | 2.41 |
| PMA | 0 | 16 | 84 | 92 | 0 | 0.36 | |
| DMSO | 0 | 8 | 92 | 0 | 71 | 1.25 | |
| HL-60HOX-B8/2 | None | 100 | 0 | 0 | 1 | 1 | 2.64 |
| PMA | 0 | 15 | 85 | 95 | 0 | 0.32 | |
| DMSO | 2 | 79 | 19 | 0 | 55 | 1.27 | |
| HL-60HOX-B8/7 | None | 100 | 0 | 0 | 1 | 1 | 2.71 |
| PMA | 0 | 14 | 86 | 92 | 0 | 0.38 | |
| DMSO | 8 | 82 | 10 | 0 | 53 | 1.33 | |
| Clone . | Inducer* . | Cell Type (%)† . | Positive (%)† . | Cell No.† . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blast . | Inter . | Mat . | NSE . | NBT . | 106/mL . |
| HL-60 | None | 100 | 0 | 0 | 1 | 1 | 2.53 |
| PMA | 0 | 13 | 87 | 90 | 0 | 0.35 | |
| DMSO | 0 | 4 | 96 | 0 | 76 | 1.32 | |
| HL-60neo5 | None | 100 | 0 | 0 | 1 | 1 | 2.41 |
| PMA | 0 | 16 | 84 | 92 | 0 | 0.36 | |
| DMSO | 0 | 8 | 92 | 0 | 71 | 1.25 | |
| HL-60HOX-B8/2 | None | 100 | 0 | 0 | 1 | 1 | 2.64 |
| PMA | 0 | 15 | 85 | 95 | 0 | 0.32 | |
| DMSO | 2 | 79 | 19 | 0 | 55 | 1.27 | |
| HL-60HOX-B8/7 | None | 100 | 0 | 0 | 1 | 1 | 2.71 |
| PMA | 0 | 14 | 86 | 92 | 0 | 0.38 | |
| DMSO | 8 | 82 | 10 | 0 | 53 | 1.33 | |
Cells were seeded at 0.2 × 106 cells/mL with PMA (5 nmol/L), or at 0.4 × 106/mL with DMSO (1.3%). Two HL-60neo and four HL-60HOX-B8 cell lines were analyzed, and data from representative clones is shown.
Cell number and morphology were determined after 3 days for untreated cells and cells treated with PMA and after 5 days for cells treated with DMSO. NSE and NBT reduction were determined after 5 days in culture, and positive cells contain red (NSE) or blue (NBT) granules in the cytoplasm. Results represent the mean of three independent determinations with standard deviations up to 15% (ie, 11% ± 1.6%).
In contrast to monocytic differentiation, it can be seen that terminal granulocytic differentiation of HL-60 cells induced by DMSO is inhibited by HOX-B8 expression (Table 2). After 5 days in the presence of DMSO, HL-60 and control HL-60neo cell lines differentiated primarily into mature granulocytes, with few intermediate type granuloctyes and no blast cells, whereas HL-60HOX-B8 cell lines were observed to be induced to predominantly intermediate granulocytes, with fewer than 20% mature cells (Table 2). The inhibition of mature granulocytic differentiation also was reflected by the presence of fewer NBT positive cells. However, there was no difference in cell numbers of DMSO-treated cells ectopically expressing HOX-B8 compared with parental HL-60 and HL-60neo control cells (Table 2). Finally, HL-60Hox-B8 cells did not undergo further differentiation when maintained in DMSO for 7 days (data not shown), supporting the notion that HOX-B8 expression blocks, rather than slows down, granulocytic differentiation.
Effect of ectopic expression of HOX-B8 on G-CSF–treated 32D cells.That HOX-B8 expression blocked terminal granulocytic differentiation in HL-60 cells prompted us to investigate the effect of ectopic expression of HOX-B8 on G-CSF–induced growth and differentiation along the granulocytic lineage of the factor dependent hematopoietic progenitor cell line 32Dcl3. In all cases, three 32Dneo clones (clones 3, 7, and 11) and five 32DHOX-B8 clones (clones 4, 6, 8, 9, and 18) were examined; data are presented for representative clones 32Dneo clone 3 and 32DHOX-B8 clone 18, unless otherwise indicated. Parental 32Dc13 and all control 32Dneo clones behaved similarly.
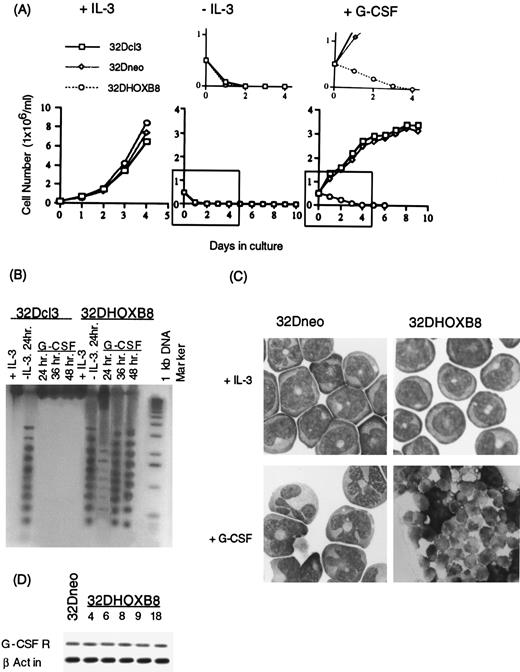
Parental 32Dc13, all 32Dneo clones, and all 32DHOX-B8 clones behaved similarly when grown in the presence of IL-3, as well as after removal of IL-3 from the culture medium (Fig 2A). However, the expression of ectopic HOX-B8 resulted in an altered response in the presence of G-CSF. In contrast to parental 32Dc13 and 32Dneo clones, in which the cells were observed to proliferate for several days and to undergo terminal granulocytic differentiation associated with growth arrest, 32DHOX-B8 cell lines did not proliferate or differentiate (Fig 2A and C). 32DHOX-B8 cell lines were observed to undergo rapid loss of viability, with no viable cells detected by 3 days. G-CSF–treated 32DHOX-B8 cell lines underwent programmed cell death, as seen by distinct cell morphology, including chromatin condensation and cytoplasmic blebbing (Fig 2C), and DNA fragmentation (Fig 2B), resulting from cleavage of nuclear DNA in internucleosomal regions, characteristic of apoptotic cells.20 Two possible explanations for this response are that ectopic Hox-B8 expression blocked the normal response to G-CSF, with the cells undergoing apoptosis, and, alternatively, that HOX-B8 expression directly activates the apoptotic pathway. An elaboration of these possibilities will be presented in the Discussion.
Growth and differentiation characteristics of parental 32Dcl3, 32Dneo, and 32DHOX-B8 cells in the presence of IL-3 or G-CSF. (A) Growth kinetics in culture medium supplemented with either IL-3 (10% WEHI-3B conditioned medium), no supplements (−IL-3), or G-CSF (10 ng/mL). Cells were seeded as indicated in Materials and Methods, and viable cell numbers were determined by trypan blue dye exclusion, with counting in a hemocytometer. An enlarged copy of each of the regions demarcated by a box is shown above the appropriate curve for −IL-3 and +G-CSF. (B) DNA fragmentation analysis in parental 32Dcl3 and 32DHOX-B8 in the presence of IL-3, after IL-3 withdrawal for 24 hours, and after treatment with G-CSF for indicated times. (C) Photomicrographs (original magnification × 400) of 32Dneo and 32DHOX-B8 cells stained with May-Grünwald-Giemsa in the presence of IL-3, or following treatment with G-CSF (9 days for 32Dneo and 2 days for 32DHOX-B8). (D) G-CSFR expression in 32Dneo and 32DHOX-B8 clones. Analysis was done by RT-PCR as described in Materials and Methods. In all cases, three 32Dneo clones (clones 3, 7, and 11) and five 32DHOX-B8 clones (clones 4, 6, 8, 9, and 18) were examined, in which data are presented for 32Dneo clone 3 and 32DHOX-B8 clone 18, unless otherwise indicated. Parental and all 32Dneo clones examined behaved similarly.
Growth and differentiation characteristics of parental 32Dcl3, 32Dneo, and 32DHOX-B8 cells in the presence of IL-3 or G-CSF. (A) Growth kinetics in culture medium supplemented with either IL-3 (10% WEHI-3B conditioned medium), no supplements (−IL-3), or G-CSF (10 ng/mL). Cells were seeded as indicated in Materials and Methods, and viable cell numbers were determined by trypan blue dye exclusion, with counting in a hemocytometer. An enlarged copy of each of the regions demarcated by a box is shown above the appropriate curve for −IL-3 and +G-CSF. (B) DNA fragmentation analysis in parental 32Dcl3 and 32DHOX-B8 in the presence of IL-3, after IL-3 withdrawal for 24 hours, and after treatment with G-CSF for indicated times. (C) Photomicrographs (original magnification × 400) of 32Dneo and 32DHOX-B8 cells stained with May-Grünwald-Giemsa in the presence of IL-3, or following treatment with G-CSF (9 days for 32Dneo and 2 days for 32DHOX-B8). (D) G-CSFR expression in 32Dneo and 32DHOX-B8 clones. Analysis was done by RT-PCR as described in Materials and Methods. In all cases, three 32Dneo clones (clones 3, 7, and 11) and five 32DHOX-B8 clones (clones 4, 6, 8, 9, and 18) were examined, in which data are presented for 32Dneo clone 3 and 32DHOX-B8 clone 18, unless otherwise indicated. Parental and all 32Dneo clones examined behaved similarly.
Removal of IL-3 from factor-dependent 32Dcl3 cells results in withdrawal from the cell cycle and loss of viability through the induction of apoptosis.27 One possible explanation for the observed response of 32DHOX-B8 cells to G-CSF is that these cells no longer express G-CSF receptors (G-CSFR), and when IL-3 is removed and G-CSF added, the cells respond as though they are IL-3 deprived. To ascertain if this is the case, the level of G-CSFR transcripts in the 32Dcl3, 32Dneo, and 32DHOX-B8 cell lines was assessed by RT-PCR. As seen in Fig 2D, G-CSFR transcripts were expressed at comparable levels for 32Dneo, which undergo differentiation following treatment with G-CSF and 32DHOX-B8 cells, which undergo apoptosis. Thus, the effect of HOX-B8 on the G-CSF response was not related to alterations in the level of G-CSFR expression. Consistent with this observation, the loss of viability was more rapid following IL-3 deprivation of 32DHOX-B8 cell lines than following treatment with G-CSF (Fig 2A, compare −IL-3 with +G-CSF ); this is further substantiated by the intensity of the DNA ladders (Fig 2B), an indicator of apoptosis, in which more of the DNA is cleaved 24 hours following IL-3 removal than following treatment with G-CSF. These data suggested that 32DHOX-B8 cells are not inert to G-CSF, although they failed to differentiate and eventually underwent apoptosis.
Effect of ectopic expression of HOX-B8 on GM-CSF–treated 32D cells.Previously, we have observed that 32Dcl3 cells, in addition to undergoing granulocytic differentiation in response to G-CSF, are induced for limited proliferation, but not differentiation, by GM-CSF.14 Ascertaining the response of 32DHOX-B8 cells to GM-CSF might shed some light on the apoptotic response elicited following treatment with G-CSF. With this in mind, 32DHOX-B8 cells were treated with GM-CSF, and their response was compared with control 32Dneo and parental 32Dcl3 cells.
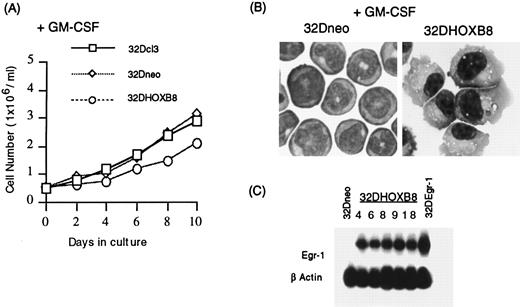
As seen in Fig 3A, all GM-CSF–treated 32D cell lines examined exhibited limited proliferation. In contrast to parental 32Dcl3 and control 32Dneo cell lines, 32DHOX-B8 cell lines underwent monocytic differentiation following treatment with GM-CSF (Table 3 and Fig 3B). This was evident from the characteristic macrophage morphology of 32DHOX-B8 cells, as seen by May-Grünwald-Giemsa staining (Fig 3B). The cells first became adherent and later flattened and spread out on the surface of the culture dish, acquiring a macrophage-like morphology. No evidence of granulocytic differentiation was observed. As noted previously, neither the parental 32Dcl3 nor 32Dneo cells underwent morphologic differentiation to monocytes following stimulation with GM-CSF11 (Fig 3B and Table 3).
Growth and differentiation characteristics of parental 32Dcl3, 32Dneo, and 32DHOX-B8 cells in the presence of GM-CSF. (A) Growth kinetics in culture medium supplemented with GM-CSF (10 ng/mL). Cells were seeded as indicated in Materials and Methods, and viable cell numbers were determined by trypan blue dye exclusion, with counting in a hemocytometer. (B) Photomicrographs (original magnification × 400) of 32Dneo and 32DHOX-B8 in the presence of GM-CSF for 10 days. (C) Egr-1 expression in 32Dneo, 32DHOX-B8, and 32DEgr-1 (clone 2) cells. Analysis was performed by Northern blots, using 10 μg of total RNA per lane. In all cases, three 32Dneo clones (clones 3, 7, and 11) and five 32DHOX-B8 clones (clones 4, 6, 8, 9, and 18) were examined; data are presented for 32Dneo clone 3 and 32DHOX-B8 clone 18, unless otherwise indicated. Parental and all 32Dneo clones examined behaved similarly.
Growth and differentiation characteristics of parental 32Dcl3, 32Dneo, and 32DHOX-B8 cells in the presence of GM-CSF. (A) Growth kinetics in culture medium supplemented with GM-CSF (10 ng/mL). Cells were seeded as indicated in Materials and Methods, and viable cell numbers were determined by trypan blue dye exclusion, with counting in a hemocytometer. (B) Photomicrographs (original magnification × 400) of 32Dneo and 32DHOX-B8 in the presence of GM-CSF for 10 days. (C) Egr-1 expression in 32Dneo, 32DHOX-B8, and 32DEgr-1 (clone 2) cells. Analysis was performed by Northern blots, using 10 μg of total RNA per lane. In all cases, three 32Dneo clones (clones 3, 7, and 11) and five 32DHOX-B8 clones (clones 4, 6, 8, 9, and 18) were examined; data are presented for 32Dneo clone 3 and 32DHOX-B8 clone 18, unless otherwise indicated. Parental and all 32Dneo clones examined behaved similarly.
Effect of Ectopic HOX-B8 Expression on 32D Cells Treated With GM-CSF
| Clone . | Treatment3-150 . | Cell Type (%)3-150 . | ||||
|---|---|---|---|---|---|---|
| . | . | Blast . | Granulocytes . | Macrophage . | NSE Pos (%)3-150 . | |
| . | . | . | Inter . | Inter . | Mat . | . |
| 32Dcl3 | IL-3 | 92 | 8 | 0 | 0 | 1 |
| GM-CSF | 84 | 16 | 0 | 0 | 3 | |
| 32Dneo | IL-3 | 89 | 11 | 0 | 0 | 1 |
| GM-CSF | 87 | 13 | 0 | 0 | 6 | |
| 32DHOX-B8/6 | IL-3 | 88 | 12 | 0 | 0 | 30 |
| GM-CSF | 4 | 0 | 30 | 66 | 95 | |
| 32DHOX-B8/9 | IL-3 | 86 | 14 | 0 | 0 | 8 |
| GM-CSF | 14 | 0 | 32 | 54 | 95 | |
| 32DHOX-B8/18 | IL-3 | 85 | 15 | 0 | 0 | 36 |
| GM-CSF | 5 | 0 | 31 | 64 | 97 | |
| Clone . | Treatment3-150 . | Cell Type (%)3-150 . | ||||
|---|---|---|---|---|---|---|
| . | . | Blast . | Granulocytes . | Macrophage . | NSE Pos (%)3-150 . | |
| . | . | . | Inter . | Inter . | Mat . | . |
| 32Dcl3 | IL-3 | 92 | 8 | 0 | 0 | 1 |
| GM-CSF | 84 | 16 | 0 | 0 | 3 | |
| 32Dneo | IL-3 | 89 | 11 | 0 | 0 | 1 |
| GM-CSF | 87 | 13 | 0 | 0 | 6 | |
| 32DHOX-B8/6 | IL-3 | 88 | 12 | 0 | 0 | 30 |
| GM-CSF | 4 | 0 | 30 | 66 | 95 | |
| 32DHOX-B8/9 | IL-3 | 86 | 14 | 0 | 0 | 8 |
| GM-CSF | 14 | 0 | 32 | 54 | 95 | |
| 32DHOX-B8/18 | IL-3 | 85 | 15 | 0 | 0 | 36 |
| GM-CSF | 5 | 0 | 31 | 64 | 97 | |
Cells were seeded as indicated in Materials and Methods. Cell morphology and NSE staining were determined after 4 days with IL-3 (10% WEHI-3 CM) or 10 days with GM-CSF (10 ng/mL).
In addition to morphology, we assayed the terminal macrophage-specific marker NSE (Table 3). When stimulated for 10 days with GM-CSF, 94% to 99% of the 32DHOX-B8 cells stained positive for NSE, whereas 6%, or fewer, of the parental and 32Dneo cells stained for NSE (Table 3). In addition, ectopic expression of HOX-B8 resulted in elevated expression of NSE in the absence of morphologic differentiation in unstimulated cells grown in IL-3–containing media.
It has been shown recently in this laboratory that ectopic expression of the zinc finger transcription factor Egr-1 potentiates macrophage differentiation; 32DEgr-1 cells treated with GM-CSF underwent monocytic differentiation, and the expression of NSE was similar to the observed expression in 32DHOX-B8 cells. Therefore, it was asked if 32DHOX-B8 cells expressed Egr-l. As seen in Fig 3C, Egr-1 transcripts were expressed by all the established 32DHOX-B8 cell lines.
Experiments were performed to ascertain if the expression of Egr-1 is necessary for GM-CSF–induced macrophage differentiation of 32DHOX-B8 cells. To do this, Egr-1 expression was blocked by antisense oligodeoxynucleotides in the culture medium. Because it has been established that expression of the Egr-1 transgene in 32DEgr-1 cells is responsible for GM-CSF–induced monocytic differentiation, the effect of Egr-1 antisense oligomers on GM-CSF–treated 32DEgr-1 cells was incorporated into the experiment to monitor for the effectiveness of blocking Egr-1 expression. In addition, indirect immunofluorescence showed that AS oligomers against Egr-1 blocked Egr-1 expression, whereas scrambled oligomers had no effect13 (data not shown).
The presence of either the antisense or control scrambled oligomers in the culture medium did not have any effect on 32Dcl3, 32Dneo, 32DEgr-1, or 32DHOX-B8 cell lines maintained in IL-3, using either morphology or cell growth as criteria (data not shown), indicative of no cytotoxic effects due to the oligomers. 32Dneo, 32DEgr-1, and 32DHOX-B8 cells were treated with either antisense or scrambled oligomers in the presence of GM-CSF and, as expected, neither scrambled nor antisense oligomers had any effect on 32Dneo cells (Table 4). In the case of 32DEgr-1 and 32DHOX-B8 cells, scrambled oligomers exerted no effect on GM-CSF–induced macrophage differentiation; however, antisense oligomers significantly reduced the number of mature macrophages, with a concomitant increase in the percentage of both blast and intermediate macrophages (Table 4). These data are consistent with the notion that the effect of the HOX-B8 transgene on GM-CSF–induced monocytic differentiation of 32D cells was mediated through Egr-1.
DISCUSSION
In this work, we have shown that the homeobox gene HOX-B8 can alter the differentiation and survival of myeloid cells; the effect is dependent on the precise stage of development of the cells, thereby demonstrating how inappropriate expression of a homeobox gene can influence the hematopoietic developmental program. Using the human leukemic cell line HL-60, the murine leukemic cell line M1, and the factor-dependent hematopoietic cell line 32Dcl3, it has been shown that ectopic expression of HOX-B8: (1) had no effect on monocytic differentiation induced by either IL-6 in M1 cells or PMA in HL-60 cells; (2) partially interrupted DMSO-induced granulocytic differentiation of HL-60 cells and blocked the G-CSF–induced granulocytic developmental program, including survival, growth, and differentiation of 32Dcl3 cells, such that the 32Dcl3 cells underwent rapid apoptosis; and (3) endowed 32Dcl3 cells with the ability to be induced by GM-CSF for terminal differentiation exclusively along the macrophage lineage, where this effect was at least partially mediated by expression of the zinc finger transcription factor Egr-1.
Egr-1 was found by us to be a macrophage differentiation primary response gene, which is essential for and restricts differentiation of hematopoietic cells along the macrophage lineage.13 More recently, we reported that ectopic expression of Egr-1 endowed 32Dcl3 cells with the ability to be induced by GM-CSF for terminal differentiation exclusively along the macrophage lineage,14 thus providing evidence that Egr-1 can potentiate terminal macrophage differentiation and leading to the suggestion that Egr-1 plays a deterministic role in governing the development of hematopoietic cells along the macrophage lineage. There are several possible explanations, carefully detailed in Lee et al,28 for reconciling these data with the recently published observations that mice with disrupted Egr-1 genes undergo normal macrophage development and function,28 including adaptation to Egr-1 deficiency during development. That all 32DHOX-B8 cell lines express Egr-1 and differentiate in response to GM-CSF, similar to 32DEgr-1, and that terminal macrophage differentiation in 32D cell lines containing either the HOX-B8 or Egr-1 transgene is inhibited in the presence of antisense Egr-1 oligomers in the culture medium, is consistent with a role for Egr-1 in the HOX-B8–mediated response of 32D cells to GM-CSF.
Both 32DHOX-B8 and 32DEgr-1 cell lines are blocked for G-CSF–induced terminal granulocytic differentiation; however, 32DEgr-1 cells are blocked at an intermediate stage and the cells are still viable by 10 days after treatment, whereas 32DHOX-B8 cells undergo rapid apoptosis after treatment with G-CSF, in which no viable cells are detected by 3 days. Thus, it is clear that the effect of ectopic HOX-B8 expression in 32D cells is not mediated solely by activation of Egr-1 expression.
The observed phenotype of 32DHOX-B8 cells following treatment with G-CSF may be due to positive activation of the apoptotic pathway by HOX-B8 or its downstream effectors. An alternative explanation is that the G-CSF–induced developmental program of granulocytic differentiation is blocked at an early stage such that the cells are still dependent on IL-3 for survival. That 32Dcl3 and 32DHOX-B8 cells display similar kinetics of loss of viability after removal of IL-3 from the culture medium is consistent with no role for HOX-B8 as a positive regulator of apoptosis. This observation lends support to the notion that HOX-B8 blocks G-CSF–induced differentiation of 32D cells at a very early point, allowing the apoptotic pathway to prevail. Consistent with this idea, we have shown that progression of the M1 myeloid differentiation program is dominant to transformation growth factor-β (TGF-β)–induced apoptosis and that when the differentiation program is blocked by deregulated expression of c-myc or c-myb, the TGF-β–mediated apoptotic response proceeds.21
That normal levels of GCSF-R are present in 32DHOX-B8 cells and that the kinetics of G-CSF–induced cell death is less rapid than after removal of IL-3, indicates that some G-CSF–induced changes do occur in 32DHOX-B8 cells. 32D cell lines ectopically expressing either the Evi-1 zinc finger gene or the Id gene, encoding a helix-loop-helix protein, are blocked for G-CSF–induced differentiation, and there is rapid loss of viability, similar to HOX-B8 expressing cells.29 30 Assessing the effect of GM-CSF treatment on these cells would further our understanding about regulation of myeloid cell differentiation.
Ectopic HOX-B8 expression in M1 and HL-60 cells did not result in activation of Egr-1 expression (data not shown), demonstrating that HOX-B8 activates different genes in different cell types or in a particular cell type at different developmental stages. No phenotypic effect was detected for monocytic differentiation induced by either IL-6 in M1 cells, in contrast to the observations report by Blatt et al31 for which we have no explanation or PMA in HL-60 cells. In addition, HOX-B8 blocks DMSO-induced granulocytic differentiation of HL-60 cells at an intermediate state, and no early cell death is observed, in contrast to 32DHOX-B8 cells treated with the granulocytic inducer G-CSF. If, as discussed above, the apoptotic response elicited by G-CSF treatment of 32DHOX-B8 cells is due to an early block in differentiation, then the observed difference in the response of 32D and HL-60 cells to a granulocytic inducer (G-CSF and DMSO, respectively) is not related to cell survival, but rather to differentiation. Because 32Dcl3 is a factor-dependent cell line, in contrast to factor independent HL-60, it is conceivable that early blocks in differentiation can lead to apoptosis. The difference in the timing of the differentiation block may be accounted for by HOX-B8–induced Egr-1 expression, in combination with other HOX-B8–mediated changes in gene expression, in 32DHOX-B8 cells, or by subtle differences in the developmental program of granulocytic differentiation induced by DMSO versus G-CSF, which is the physiologic inducer.
HOX-B8 is not expressed in normal bone marrow, and a survey of 31 leukemia cell lines of the myeloid, lymphoid, and erythroid lineages showed that HOX-B8 was expressed in only two of the cell lines analyzed, including WEHI-3B.9,10 The effect of ectopic expression of HOX-B8 in normal hematopoietic cells, alone and in combination with IL-3, demonstrated the ability of the HOX-B8 gene product to participate in self-renewal of immature myeloid progenitors, as well as to alter hematopoietic differentiation,11,24 and demonstrated the oncogenic potential of HOX-B8. It has been suggested that HOX-B8 may exert its effects on hematopoietic cells by mimicking the action of another homeobox gene product, which normally functions in hematopoietic cells11,24; another possibility is that it can override the effects of other homeobox genes whose expression is correctly regulated.8 Deregulated expression of the homeobox gene Hox-B7 (Hox-2.3) in HL-60 cells exerts an effect similar to HOX-B8, and blocking its expression inhibits GM-CSF–induced colony formation of bone marrow8; towards understanding how the different homeobox genes regulate hematopoiesis, it would be informative to see the effect of HOX-B7 in 32Dcl3 cells.
Altering expression of the HOX-B8 gene can have a profound effect on hematopoiesis, with the outcome depending on the cell type and stage of maturation of the cell. Inappropriate constitutive expression of the homeobox gene HOX-B8 can alter the growth, differentiation, and survival of myeloid cells. Continuing studies on homeobox genes in hematopoietic cells should contribute toward understanding the molecular mechanisms regulating development and differentiation of the hematopoietic system, leukemogenesis, and the role of normal and abnormal expression of homeobox genes in these processes.
Supported by National Institutes of Health Grants No. lROlCA51162 (to B.H.), lROlCA43618 (to D.A.L.), Amgen (to B.H. and D.A.L.), and the core program on carcinogenesis (5P30CA12227).
Address reprint requests to Barbara Hoffman, PhD, Fels Institute for Cancer Research and Molecular Biology and Department of Biochemistry, Temple University School of Medicine, 3307 N Broad St, Philadelphia, PA 19140.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal