Abstract
Basic helix-loop-helix proteins, which are tissue specific (SCL) or broadly expressed (E proteins), interact positively to regulate erythroid specific genes. Here, expression of SCL and two broadly expressed E proteins, E47 and HEB, was high early in erythroid differentiation and declined during maturation. Stimulation of erythroid progenitors/precursors with stem cell factor (SCF ) enhanced SCL and E protein levels, one mechanism by which SCF may increase erythroid proliferation. Interactions between SCL and E proteins are competed by Id2, which binds and sequesters E proteins. Upregulation of Id2, demonstrated here late in erythroid differentiation, may downregulate genes involved in erythroid proliferation/differentiation. We examined expression of bHLH proteins in transfusion-dependent patients with Diamond-Blackfan anemia (DBA) to determine if these interactions are disrupted. In erythroblasts from patients, expression of SCL protein and mRNA was normal and SCL increased in response to SCF. However, E47 and HEB protein levels were significantly decreased. Id2 was strongly expressed in patients. Through reduction of SCL/E protein heterodimer formation, abnormal levels of bHLH transcription factors may affect expression of erythroid specific genes, such as β globin. Stimulation of Diamond-Blackfan cells with SCF partially compensated for this defect, enhancing expression of E47, HEB, and SCL. SCF may function to increase SCL/E protein heterodimer formation, which may be one of the mechanisms through which SCF stimulates erythroid proliferation/ differentiation in DBA.
TRANSCRIPTION FACTORS that have specificity in erythroid proliferation and differentiation have recently been described.1-7 An example is the gene for SCL (also known as TCL5 or tal1), which was first discovered due to its disruption in a stem cell leukemia.8 High levels of the SCL gene are expressed in erythroid, megakaryocyte, mast cell, and early myeloid cell lines and its protein contains a basic helix-loop-helix (bHLH) motif which binds DNA.3,4,9 SCL functions as a regulator of erythroid proliferation. The highest levels of SCL protein are expressed early in erythropoiesis,10-12 and antisense SCL suppresses self-renewal in K562 cells9 and the proliferation of normal erythroid progenitor cells.12 In addition, targeted SCL gene disruption in mice results in a complete block in embryonic erythropoiesis13 as well as other hematopoietic lineages.14 SCL may also be an important regulator of erythroid differentiation. SCL expression persists during this process,4,12,15 unlike myeloid and monocytic differentiation where its expression is downregulated.12,15 16
bHLH proteins are important modulators of tissue specific gene expression. Murre et al17 divided them into categories to differentiate the broadly expressed class A proteins (E12, E47, E2-2, and HEB) from the tissue specific class B proteins (MyoD, SCL). SCL interacts with class A E proteins to form heterodimers which bind to specific DNA sequences.18 This heterodimer formation is in competition with interactions with Id proteins, which retain the dimerization domain but lack the basic region and thus are unable to bind DNA.19 Id/bHLH heterodimers form with specific bHLH proteins and impede bHLH/bHLH homodimer or heterodimer formation between SCL and Class A proteins.19-21 Recently published data suggest that downmodulation of Id2 early in erythroid development allows SCL and E2A heterodimer formation, which subsequently bind DNA and activate erythroid specific genes.12 In terminal erythroid maturation, Id2 is again expressed, impeding SCL/E2A heterodimer formation and suppressing erythroid specific gene expression.12
The ligand for the c-kit receptor protein, called stem cell factor (SCF ), mast cell growth factor, or Steel factor,22-24 stimulates hematopoietic proliferation25,26 and is required in early erythropoiesis.27 SCL protein levels are increased in early erythroid precursors stimulated with SCF, suggesting this is a mechanism through which SCF enhances erythroid proliferation.10
A defect in erythroid proliferation and/or differentiation is observed in Diamond-Blackfan anemia (DBA), a severe congenital hypoplastic anemia characterized by macrocytic anemia developing early in childhood, sporadic, autosomal dominant or recessive inheritance, and associated physical abnormalities.28 The molecular basis of this disorder has not been identified, but presumably results from several different mutations based on the heterogeneous inheritance and presentation. Exposure of erythroid progenitors from patients with DBA to SCF results in significant enhancement of erythroid proliferation.29-31 However, no defects in the SCF or c-kit genes have been observed,31 32 suggesting defects of other genes are involved. Because of the importance of bHLH proteins in erythroid proliferation, we examined burst-forming unit-erythroid (BFU-E) derived erythroblasts from patients with transfusion-dependent DBA for defects in the expression of SCL, E-proteins and Id2, and the response to SCF exposure.
MATERIALS AND METHODS
Preparation of BFU-derived erythroblasts.Peripheral blood (PB) was obtained from normal volunteer donors and patients with steroid-unresponsive transfusion dependent DBA at The Milton S. Hershey Medical Center and the Hospital for Sick Children (Toronto, Canada) under protocols approved by the Institution's Clinical Investigation Committee. PB mononuclear cells (MNC) were separated on Ficoll-Paque (Pharmacia, Piscataway, NJ). MNC were cultured in 0.9% methylcellulose media containing 30% fetal calf serum, 9.0 mg/mL deionized bovine serum albumin (Cohn fraction V; Sigma, St Louis, MO), 1.4 × 10−4 mol/L β-mercaptoethanol, 2 U/mL erythropoietin (recombinant Epo > 100,000 U/mg; Amgen, Thousand Oaks, CA), 0 or 100 ng/mL recombinant human SCF (gift of D.E. Williams, S.D. Lyman, Immunex Corp, Seattle, WA). Doses were on plateau for maximal stimulation of colony growth.10
Cells were plated at 1 to 1.5 × 105 cells/mL, and cultures were incubated in humidified 4% CO2 at 37°C. Erythroid colonies were counted and harvested at day 7, 10, or 14 of culture. One hundred to 1,000 (day 7) BFU-E–derived colonies were plucked and pooled on each day and the average number of erythroid cells per colony was determined.
For patients with DBA, obtaining sufficient erythroblasts for study from BFU-E cultured without SCF was a significant problem. When 20 mL of patient blood was separated on Ficoll-Paque, 75% of mononuclear cells were cultured without SCF and 25% with SCF. When all cells were removed at day 10, the yield of plucked day 10 erythroblasts, cultured without SCF, ranged from 4 × 104 in one patient to a peak yield of 5 × 105 in another (median 1 × 105). To obtain sufficient material for cytocentrifuge preparations and immunoblotting experiments with SCL, E47, and HEB antibodies at day 10 and 14 of culture with and without SCF, each patient was cultured repeatedly before transfusion at monthly intervals.
Immunoblotting.BFU-E–derived cells cultured with or without 100 ng/mL human SCF factor (Immunex Corp) were procured at day 7, 10, or 14 and washed twice with phosphate-buffered saline. Cells per sample (1 to 2 × 105), were pelleted and resuspended in 40 μL boiling sodium dodecyl sulfate (SDS) sample buffer (10% glycerol, 0.7 mol/L β-mercaptoethanol, 3% SDS, 62 mmol/L Tris pH 6.8). Samples were boiled for 5 minutes and then centrifuged at 10,000g for 10 minutes at 4°C to remove debris. Supernatants containing the protein content of 1 to 2 × 105 erythroblasts were loaded into each lane and fractionated by gel electrophoresis on 7% (E47, HEB) or 10% (SCL) polyacrylamide gels. Proteins were electroblotted onto Immobilon membranes (Millipore, Bedford, MA) or Hybond-ECL (nitrocellulose). Membranes were blocked with 5% nonfat dry milk in TTBS (20 mmol/L TRIS HCl, pH 7.5, 500 mmol/L NaCl, 0.05% Tween-20; BioRad, Hercules, CA) overnight at 4°C. Membranes were then washed with TTBS. They were incubated with anti-SCL (diluted 1:100),33 anti-E47 (Santa Cruz Biotechnology, Santa Cruz, CA, diluted 1:250), or anti-HEB (Santa Cruz, diluted 1:150) for 3 hours at room temperature. All antibodies were polyclonal rabbit antisera. Detection was with an amplified alkaline phosphatase Immuno-Blot Assay (Biorad, Richmond, CA) for anti-SCL10 or with ECL (Amersham Life Sciences, Buckinghamshire, UK) for other antibodies.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.Total RNA was isolated from 1 × 106 to 1 × 107 BFU-E derived cells by RNeasy Total RNA Kits (QIAGEN, Chatsworth, CA). RT-RNA (cDNA) was made by Reverse Transcription System (Promega, Madison WI), and PCR reaction was performed using the Elmer-Perkins Gene Amp PCR Reagent Kit (Perkin Elmer, Roche Molecular Systems, Branchburg, NJ).
Dose-response curve and cycle number for each target gene were first determined to establish the optimal amount of total cellular RNA required for RT-PCR detection. To construct the standard dose response curve, 0 to 1,000 ng of total cellular RNA from normal BFU-E derived cells at day 10 of culture was used to make 10 μL of RT reaction mixture. Two microliters of such cDNA was then amplified in 25 μL of PCR reaction mixture. Based on standard-dose response results, the linear range of cycle number was determined using a quantity of RNA on the slope of the dose-response curve. 2 μCi of (α-32p)deoxyadenosine 5′-triphosphate (dATP) was added to each PCR reaction for further kinetic analysis. After PCR, 10-μL aliquots were electrophoresed on 1.2% agarose gel and the amount of radioactivity incorporated into each band was measured by phosphoimager analysis.
18S rRNA was similarly amplified for standardization. A small quantity (one-fifth of RT reaction or 0.1 ng) of 18S rRNA RT-RNA (cDNA) was amplified for 22 cycles (denaturation at 95°C for 30 seconds, annealing at 52°C for 30 seconds and extention at 72°C for 45 seconds). The same amount of RT-RNA and the same PCR procedure was used to analyze β-globin gene expression. For SCL, a larger aliquot of RT-RNA (2 ng) was amplified for 28 cycles. For PCR of E2A RT-RNA, 50 ng was used. The amplification procedure for E2A involved denaturation at 95°C for 30 seconds, annealing at 59°C and extension at 72°C for 45 seconds. Twenty nanograms of Id2 RT-RNA was amplified. Since Id2 PCR showed high nonspecific amplification, Taq start antibody (Clontech, Palo Alto, CA) was used to enhance the specificity. Equal amounts of Taq Start antibody were incubated with Taq DNA polymerase for 5 minutes before addition to PCR reaction mixture. This was followed by denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds, and extention at 72°C for 45 seconds. The following 5′ and 3′ primers were used in RT-PCR: 18S rRNA: 5′ primer, 5′-GAAAGTCGGAGGTTCGAAGA-3′; 3′ primer, 5′-ACCAACTAAGAACGGCCATG-3′34; β globin: 5′ primer, 5′-ATGGTGCACCTGACTCCTGA-3′, 3′ primer, 5′-GCTTAGTGATACTTGTGGGC-3′35; SCL: 5′ primer, 5′-TTCACCACCAACAATCGAGTG-3′; 3′ primer, 5′-ATTGAGCAGCTTGGCCAAGAA-3′36; E2A: 5′ primer, 5′-CCTGCACCAGCACGAGCGTATGGG-3′; 3′ primer, 5′-TGGTTGTGCATGAGGCTG-3′37; Id2: 5′ primer, 5′-CCTGTGGACGACCCGATGAGC-3′; 3′ primer, 5′-GCCACACAGTGCTTTGCTGTC-3′.21
RESULTS
SCF stimulates proliferation of normal and DBA erythroid progenitors.BFU-E from the PB of 16 normal donors and six patients with transfusion-dependent DBA were cultured in vitro with or without 100 ng/mL human SCF. The number of BFU-E–derived colonies and number of cells/BFU-E–derived colony were assessed to quantitate the influence of SCF on BFU-E proliferation (Table 1). As previously shown, SCF significantly increased colony size but not the number of BFU-E–derived colonies in normal donors.10 For the six patients with DBA studied here, BFU-E–derived colony number was significantly less than normal and did not increase in response to SCF. Colony size at day 7 was not assessed in patients with DBA because colonies were barely detectable. Colony size at day 10 and 14 was smaller than normal, but did significantly increase in response to SCF stimulation (Table 1).
Influence of Stem Cell Factor on BFU-E Proliferation
| SCF . | No. of BFU-E Derived Colonies . | No. of Cells × 10−4/BFU-E Derived Colony . | ||
|---|---|---|---|---|
| . | − . | + . | − . | + . |
| Normal | ||||
| Day 7 | 40 ± 4 | 52 ± 5 | 0.11 ± 0.03 | 0.29 ± 0.05* |
| 10 | 57 ± 5 | 62 ± 6 | 0.54 ± 0.05 | 2.45 ± 0.18* |
| 14 | 79 ± 8 | 79 ± 7 | 1.96 ± 0.23 | 3.96 ± 0.50* |
| Diamond-Blackfan | ||||
| Day 10 | 6 ± 2 | 10 ± 2 | 0.22 ± 0.05 | 1.22 ± 0.23* |
| 14 | 8 ± 2 | 12 ± 4 | 0.51 ± 0.17 | 3.93 ± 0.94* |
| SCF . | No. of BFU-E Derived Colonies . | No. of Cells × 10−4/BFU-E Derived Colony . | ||
|---|---|---|---|---|
| . | − . | + . | − . | + . |
| Normal | ||||
| Day 7 | 40 ± 4 | 52 ± 5 | 0.11 ± 0.03 | 0.29 ± 0.05* |
| 10 | 57 ± 5 | 62 ± 6 | 0.54 ± 0.05 | 2.45 ± 0.18* |
| 14 | 79 ± 8 | 79 ± 7 | 1.96 ± 0.23 | 3.96 ± 0.50* |
| Diamond-Blackfan | ||||
| Day 10 | 6 ± 2 | 10 ± 2 | 0.22 ± 0.05 | 1.22 ± 0.23* |
| 14 | 8 ± 2 | 12 ± 4 | 0.51 ± 0.17 | 3.93 ± 0.94* |
Mean number of BFU-E ± SEM per 2 × 105 PB MNC from 16 normal volunteer donors or 6 patients with DBA plated with 2 U/mL erythropoietin with or without 100 ng/mL human SCF. Mean number of cells × 104 ± SEM per BFU-E derived colony counted on days 7 to 14 of culture are shown.
Indicates significant increase from control cultured without SCF (P < .05).
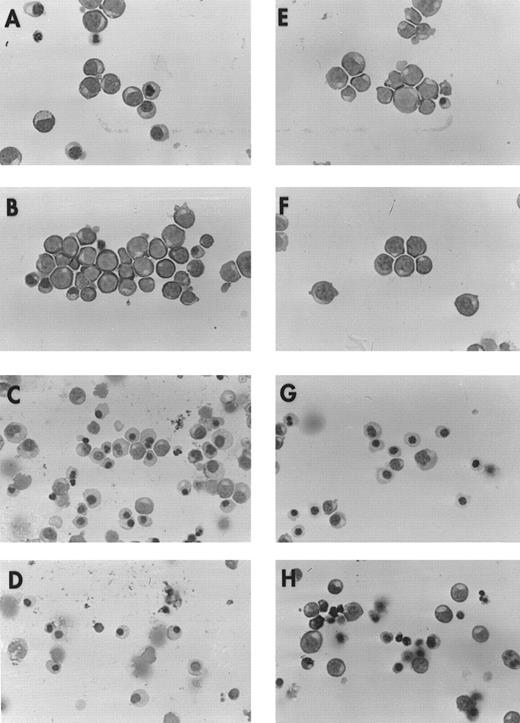
To determine whether differences in maturation exist between cultured precursors from normal donors and patients with DBA, we prepared cytocentrifuged slides of BFU-E–derived erythroblasts removed from culture on day 10 or 14 (Fig 1). Erythroblasts at day 10 were predominantly proerythroblasts and basophilic normoblasts, and at day 14 were largely polychromatophilic or orthochromatic normoblasts, although some heterogeneity existed. No detectable maturational differences were observed between cells from patients and normal controls, except that day 14 erythroblasts from patients appeared to be less well hemoglobinized.
Morphology of erythroblasts from normal donors and patients with DBA. Cytocentrifuge preparations were prepared from day 10 and 14 BFU-E–derived erythroblasts cultured with or without 100 ng/mL SCF. BFU-E–derived erythroblasts from a representative normal donor are shown at day 10 (A, −SCF; B, +SCF ), and day 14 (C, −SCF; D, +SCF ) and from a patient with transfusion-dependent DBA at day 10 (E, −SCF; F, +SCF ) and 14 (G, −SCF; H, +SCF ) of maturation. Preparations made from three additional patients and normal donors showed similar results.
Morphology of erythroblasts from normal donors and patients with DBA. Cytocentrifuge preparations were prepared from day 10 and 14 BFU-E–derived erythroblasts cultured with or without 100 ng/mL SCF. BFU-E–derived erythroblasts from a representative normal donor are shown at day 10 (A, −SCF; B, +SCF ), and day 14 (C, −SCF; D, +SCF ) and from a patient with transfusion-dependent DBA at day 10 (E, −SCF; F, +SCF ) and 14 (G, −SCF; H, +SCF ) of maturation. Preparations made from three additional patients and normal donors showed similar results.
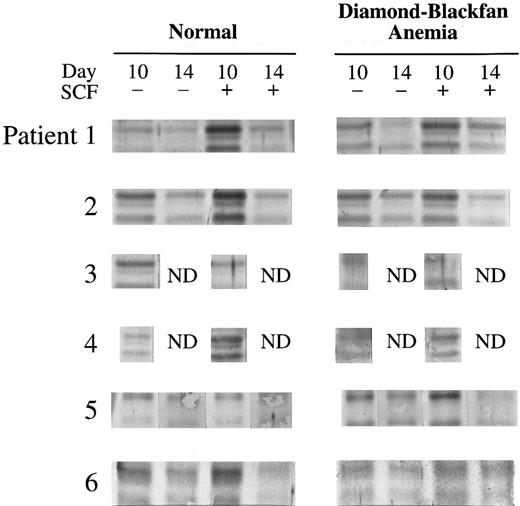
SCL expression is normal in DBA.BFU-E–derived cells were removed from cultures of six patients with DBA at day 10 and 14 and expression of SCL protein was compared to normal controls (Table 2). In normal donors, SCL was greater in day 10 compared to day 14 cells, and SCL expression was significantly stimulated in day 10 cells by SCF, as previously shown.10 In all patients with DBA, the quantity and size of SCL protein at days 10 and 14 were normal (Fig 2). SCF stimulated an increase in SCL in DBA samples which was similar to that observed in normal donors (Table 2, Fig 2).
SCL Expression During Erythroid Differentiation in Normal Donors and DBA
| SCF . | Quantity (OD × mm) . | |||||
|---|---|---|---|---|---|---|
| . | Day 10 . | Day 14 . | n . | . | ||
| . | − . | + . | − . | + . | . | . |
| Normal donors | 0.084 ± 0.013 | 0.137 ± 0.021* | 0.055 ± 0.012 | 0.055 ± 0.007 | 6 | |
| DBA | 0.080 ± 0.012 | 0.122 ± 0.019* | 0.044 ± 0.010 | 0.058 ± 0.008 | 6 | |
| SCF . | Quantity (OD × mm) . | |||||
|---|---|---|---|---|---|---|
| . | Day 10 . | Day 14 . | n . | . | ||
| . | − . | + . | − . | + . | . | . |
| Normal donors | 0.084 ± 0.013 | 0.137 ± 0.021* | 0.055 ± 0.012 | 0.055 ± 0.007 | 6 | |
| DBA | 0.080 ± 0.012 | 0.122 ± 0.019* | 0.044 ± 0.010 | 0.058 ± 0.008 | 6 | |
PB BFU-E from normal donors or patients with DBA were cultured with or without 100 ng/mL human SCF and colonies removed from culture on day 10 and 14. The cell lysate from 1 × 105 BFU-E derived cells was loaded onto each lane of a 10% polyacrylamide gel and immunoblotting performed with anti-SCL antibody. The mean ± SEM density of bands at 45 kD and 47 kD quantitated with a Molecular Dynamics Densitometer and Quantity One Software (Huntington, NY) is shown here. n = number of individuals studied.
A significant increase in SCL expression was observed in response to stem cell factor stimulation at day 10 (P < .05).
SCL expression in DBA. Immunoblots of SCL in day 10 and 14 BFU-E– derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL SCF are shown here. The cell lysate from 1 × 105 BFU-E–derived cells was loaded onto each lane of a 10% polyacrylamide gel and immunoblotting performed with anti-SCL antibody as described in Materials and Methods.
SCL expression in DBA. Immunoblots of SCL in day 10 and 14 BFU-E– derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL SCF are shown here. The cell lysate from 1 × 105 BFU-E–derived cells was loaded onto each lane of a 10% polyacrylamide gel and immunoblotting performed with anti-SCL antibody as described in Materials and Methods.
E protein expression during erythroid differentiation in normal donors and patients with DBA.Since SCL/E protein dimer formation is required for normal erythroid proliferation and differentiation to occur,12,13 the expression of two E proteins, E47 and HEB, was determined during normal erythroid differentiation and compared to that in patients with transfusion-dependent DBA. These two E proteins were chosen for study since their DNA-binding domains differ. Immunoblot analyses were performed on cell lysates from day 7, 10, and 14 normal erythroblasts cultured with or without SCF (Table 3). E47 and HEB were strongly expressed in day 7 and 10 cells and declined by day 14 as cells approached terminal differentiation. SCF stimulated a significant increase in these E protein levels early in normal erythroid differentiation (days 7 and 10, Table 3), similar to the pattern observed for SCL.10
E Protein Expression During Erythroid Differentiation in Normal Donors and DBA
| Transcription . | Quantity (OD × mm) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor . | . | Day 7 . | Day 10 . | Day 14 . | n . | . | |||
| . | . | SCF − . | + . | − . | + . | − . | + . | . | . |
| A. Normal donor | 3.26 ± 0.78 | 6.07 ± 0.893-150 | 3.14 ± 0.52 | 4.66 ± 0.873-150 | 1.15 ± 0.36 | 0.47 ± 0.14 | 11 | ||
| E47 | |||||||||
| HEB | 1.38 ± 0.23 | 2.75 ± 0.373-150 | 1.65 ± 0.46 | 3.22 ± 1.073-150 | 0.78 ± 0.36 | 0.53 ± 0.17 | 7 | ||
| B. Diamond-Blackfan | — | — | 0.77 ± 0.213-151 | 1.94 ± 0.54*† | 0.27 ± 0.093-151 | 0.51 ± 0.15 | 6 | ||
| E47 | |||||||||
| HEB | — | — | 0.50 ± 0.213-151 | 1.31 ± 0.243-150 | 0.31 ± 0.11 | 0.23 ± 0.16 | 6 | ||
| Transcription . | Quantity (OD × mm) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor . | . | Day 7 . | Day 10 . | Day 14 . | n . | . | |||
| . | . | SCF − . | + . | − . | + . | − . | + . | . | . |
| A. Normal donor | 3.26 ± 0.78 | 6.07 ± 0.893-150 | 3.14 ± 0.52 | 4.66 ± 0.873-150 | 1.15 ± 0.36 | 0.47 ± 0.14 | 11 | ||
| E47 | |||||||||
| HEB | 1.38 ± 0.23 | 2.75 ± 0.373-150 | 1.65 ± 0.46 | 3.22 ± 1.073-150 | 0.78 ± 0.36 | 0.53 ± 0.17 | 7 | ||
| B. Diamond-Blackfan | — | — | 0.77 ± 0.213-151 | 1.94 ± 0.54*† | 0.27 ± 0.093-151 | 0.51 ± 0.15 | 6 | ||
| E47 | |||||||||
| HEB | — | — | 0.50 ± 0.213-151 | 1.31 ± 0.243-150 | 0.31 ± 0.11 | 0.23 ± 0.16 | 6 | ||
PB BFU-E from normal donors and patients with DBA were cultured with or without 100 ng/mL human SCF and removed from culture on day 7, 10, or 14. The cell lysate from 2 × 105 BFU-E derived cells was loaded onto each lane and immunoblotting performed with anti-E47 or anti-HEB antibodies. The densities of bands were measured and the mean ± SEM is shown. n = number of individuals studied.
Significantly increased E protein expression in response to SCF (P < .05).
Significantly decreased E protein expression compared to normal donors (P < .05).
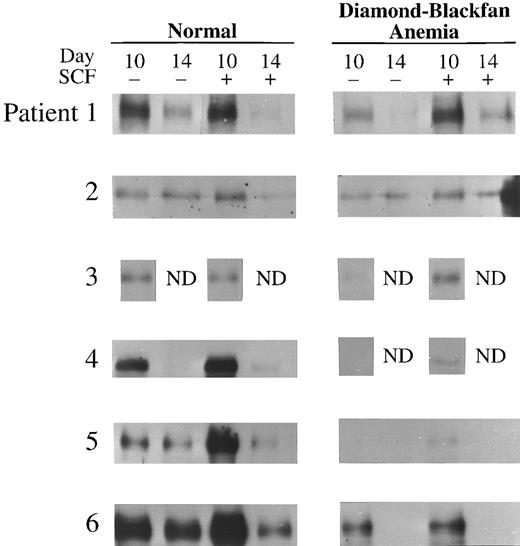
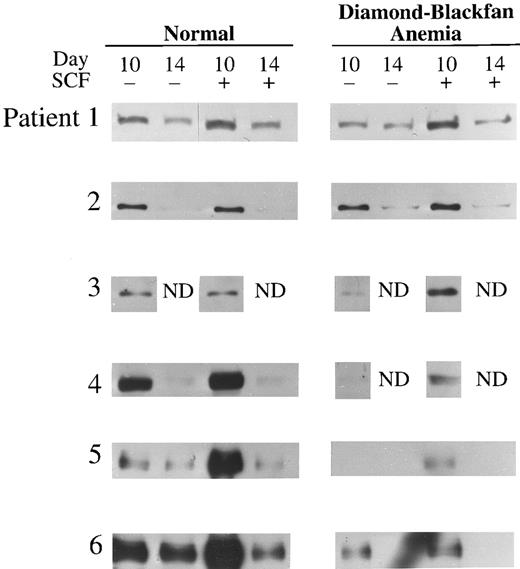
E protein expression was also determined in day 10 and 14 BFU-E–derived cells of six patients with transfusion-dependent DBA (Table 3). Day 10 was the earliest day of culture from which sufficient number of cells could be obtained from patients with DBA for immunoblotting; poor cell yield in the absence of SCF also restricted the number of experiments which could be performed. E47 expression in DBA was significantly decreased at day 10 and 14 compared to normal cells (P < .001 and P < .05, respectively; Table 3 and Fig 3). Likewise, expression of HEB was significantly decreased in day 10 erythroblasts from DBA patients compared to normal (P < .05; Table 3 and Fig 4). As observed with normal donors, expression of both E47 and HEB significantly increased in erythroblasts from DBA patients stimulated with SCF (Table 3, Figs 3 and 4).
E47 Expression in transfusion-dependent DBA. Western blot analysis of E47 in day 10 or 14 BFU-E–derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL human SCF is shown here. The cell lysate from 2 × 105 BFU-E–derived cells was loaded onto each lane of a 7% polyacrylamide gel and immunoblotting performed as described in Materials and Methods.
E47 Expression in transfusion-dependent DBA. Western blot analysis of E47 in day 10 or 14 BFU-E–derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL human SCF is shown here. The cell lysate from 2 × 105 BFU-E–derived cells was loaded onto each lane of a 7% polyacrylamide gel and immunoblotting performed as described in Materials and Methods.
HEB Expression in transfusion-dependent DBA. Western blot analysis of HEB in day 10 or 14 BFU-E–derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL human SCF is shown here. The cell lysate from 2 × 105 BFU-E–derived cells was loaded into each lane of a 7% polyacrylamide gel and immunoblotting performed as described in Materials and Methods.
HEB Expression in transfusion-dependent DBA. Western blot analysis of HEB in day 10 or 14 BFU-E–derived cells from six normal donors and six patients with DBA cultured with or without 100 ng/mL human SCF is shown here. The cell lysate from 2 × 105 BFU-E–derived cells was loaded into each lane of a 7% polyacrylamide gel and immunoblotting performed as described in Materials and Methods.
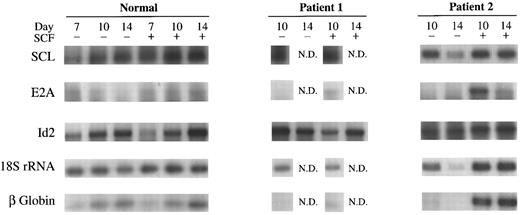
SCL, E2A, and Id2 mRNA expression in normal erythroid precursors at different stages of differentiation and in DBA.RT-PCR was used to quantitate specific mRNA transcripts in BFU-E derived normal cells at days 7, 10, and 14 of culture and in DBA cells at day 10 and 14. Dose-response curves were performed for each transcript using different quantities of mRNA template and varying cycle number to determine conditions on the linear slope for reasonable quantitation (data not shown). The expression of SCL, E2A, Id2, 18S rRNA and β-globin mRNA, was examined in preparations from four normal donors after culture with or without 100 ng/mL SCF. Representative results are shown in Fig 5. SCL, E2A, Id2, 18S rRNA and β-globin mRNA levels were also measured in two of the transfusion-dependent patients, who were repetitively cultured to obtain sufficient material (Fig 5).
RT-PCR of SCL, E2A, Id2, 18S rRNA, and β-globin. Results of studies on day 7, 10, and 14 BFU-E–derived cells from a representative normal donor and from day 10 and 14 cells from two patients with transfusion-dependent DBA. BFU-E were stimulated with or without 100 ng/mL SCF. The same patients (1 and 2) studied in Figs 2, 3, and 4 are shown for comparison of protein and mRNA levels.
RT-PCR of SCL, E2A, Id2, 18S rRNA, and β-globin. Results of studies on day 7, 10, and 14 BFU-E–derived cells from a representative normal donor and from day 10 and 14 cells from two patients with transfusion-dependent DBA. BFU-E were stimulated with or without 100 ng/mL SCF. The same patients (1 and 2) studied in Figs 2, 3, and 4 are shown for comparison of protein and mRNA levels.
SCL mRNA levels were maintained in normal cells from day 7 to 14 of culture and did not increase in response to SCF, consistent with results obtained with Northern blot analysis.10 In cells from patients with DBA, SCL mRNA levels were similar to normal (Fig 5), consistent with immunoblot protein data for these patients shown in Fig 2. E2A mRNA levels declined in normal cells from day 7 to 14 of differentiation, consistent with previous observations.12 In DBA, E2A mRNA was greatly decreased in day 10 cells compared to normal and increased in response to SCF stimulation (Fig 5), consistent with immunoblot results (Fig 3). In fact, E2A mRNA levels were barely detectable in day 10 cells cultured without SCF, probably because the amount of E2A mRNA was in the low range for amplication in these patients under conditions used. Id2 mRNA levels progressively increased in normal donors from day 7 to terminal differentiation, confirming previous results.12 Unlike E proteins, Id2 was strongly expressed in day 10 and 14 erythroblasts in DBA.
18S rRNA and β-globin mRNA were also studied for comparative purposes. 18S rRNA was expressed at a uniform level in day 7 to 14 normal cells. Results were similar in patients with DBA. β-Globin mRNA levels increased steadily from day 7 to 14 of differentiation in normal donors and this was not influenced by SCF stimulation. In contrast, patients with transfusion-dependent DBA had greatly reduced β-globin mRNA levels in day 10 and 14 erythroid precursors, but the levels increased in response to SCF stimulation (Fig 5).
DISCUSSION
Basic helix-loop-helix transcription factors are regulators of cell proliferation and differentiation. SCL, E2A, and Id2 have all been shown to have an important role in erythroid progenitor proliferation and differentiation.4,9,12-15 SCL mRNA is expressed early in erythroid differentiation and continues to be expressed at high levels until the late erythroblast stage,10,15,38 unlike the myeloid lineage where SCL mRNA is low or undetectable in granulocyte and monocyte progenitors and progeny.15,16 High levels of SCL mRNA measured by Northern blotting and RT-PCR in day 14 erythroblasts, in which SCL protein levels are low, shows the role of posttranscriptional mechanisms in regulation of SCL.10 As shown here and elsewhere,12 E47 and HEB are also strongly expressed during erythroid differentiation with a significant decline at terminal maturation on day 14. Protein dimerization required for DNA recognition by bHLH proteins can occur as homodimer formation for each of the four E proteins (E12, E47, E2-2, and HEB), as heterodimer formation between E proteins, or between E protein and tissue specific bHLH proteins.18,39,40 Homodimers and heterodimers of these proteins distinguish between closely related E box sequences,39 although the precise role of each of the different dimers in regulating gene expression is not yet clear. SCL forms heterodimers to bind DNA with E12, E47, E2-2, and HEB,18 but SCL does not homodimerize.12 In the presence of SCL, E-proteins preferentially bind DNA as heterodimers and SCL/E2A heterodimers are present in normal erythroid cells.12 These proteins are thought to be of importance in regulation of gene expression during erythroid differentiation. Erythroid genes with SCL/E protein binding sequences include the erythroid promoters of the carbonic anhydrase II, band 3 and GATA-1 genes, the hypersensivity site 2 of the β-globin locus control region, a site near the 3′ enhancer of the human Aγ-globin gene and the serum response element of c-fos.12 18
Id proteins belong to a class of helix-loop-helix proteins, which lack the basic amino acid domain necessary for DNA binding. Id proteins do not homodimerze or bind to SCL efficiently.20 Id inhibits differentiation by forming heterodimers with and inhibiting DNA binding of the available pool of E proteins including E47.20 The predominant Id protein expressed in erythroid differentiation is Id2.12 Data suggests that when the level of Id2 is high in early erythroid proliferation, Id2 is complexed with the ubiquitous bHLH proteins to inhibit differentiation, as well as to the retinoblastoma protein to allow cellular proliferation.12,41 Downregulation of Id2 expression enables bHLH factors to dimerize and bind DNA, resulting in erythroid differentiation.12 The reexpression of Id2 late in normal erythroid differentiation may result in terminal inhibition of remaining SCL and E2A DNA binding.12
Since SCL, E protein, and Id2 interactions play an important role in erythroid proliferation and differentiation, we examined expression of E proteins in normal erythropoiesis and studied these bHLH proteins in DBA, a congenital hypoplastic anemia in which both erythroid proliferation and differentiation are abnormal. The disease appears to be heterogeneous on a molecular basis,31 and we thus selected a restricted group of patients to study who were transfusion-dependent, since they had failed steroids. All of the patients we studied showed an increase in BFU-E derived colony size in response to SCF stimulation. In BFU-E derived erythroblasts from DBA patients, no quantitative or qualitative deficiency in SCL protein or mRNA was detected. SCL protein was significantly increased by stimulation with SCF, showing that mechanisms controlling SCL expression in response to growth factor stimulation, which are largely posttranscriptional, seem to be intact.
In contrast, levels of both E47 and HEB protein were significantly decreased in unstimulated cells from patients with transfusion-dependent DBA. This appeared to be on a transcriptional basis for E47, since E2A mRNA levels were decreased. The basic underlying molecular defect responsible for this deficiency in the hierarchy of transcription factors has not been identified here. Work by others has shown that mutations in the erythropoietin receptor gene42 or c-kit31,32 are not responsible. The decrease in E47 and HEB may contribute to inhibition of erythroid differentiation through decreased heterodimer formation needed to stimulate erythroid gene expression. In DBA, a significant amount of Id2 was present in day 10 cells, which may compete effectively for the decreased E47 and HEB available. An example of diminished expression of an erythroid specific gene is the low level of β-globin mRNA observed in day 10 erythroblasts in DBA. In erythroblasts from Diamond-Blackfan patients, exposure to SCF enhances SCL expression. SCL overexpression alone has been shown to increase erythroid differentiation and hemoglobinization in TF-1 and Mel cells.4 15 SCF stimulation also increased E47 and HEB levels, making it more likely that SCL/E protein heterodimerization occurs. This increase in SCL and E proteins is presumably a factor resulting in enhanced β-globin mRNA expression in SCF stimulated cells and in promoting further erythroid proliferation and differentiation.
ACKNOWLEDGMENT
The authors thank Ilan R. Kirsch, Cornelis Murre, Robert Kingston, and Mark Israel for cDNA probes used to confirm PCR bands. We also thank D.E. Williams and S.D. Lyman for the gift of SCF. We are grateful to Tina M. Eberly for careful preparation of the manuscript and to Andrew Freiberg, Debra Shade, Karen Gee, Judy Weigel, and the nurses in Pediatric Hematology/Oncology Clinic for assistance in obtaining blood samples.
Supported by National Institutes of Health (Bethesda, MD) Grants DK 46778 and M01 RR10732-02, the Four Diamonds Fund (Hershey, PA), and an American Cancer Society Faculty Award (Atlanta, GA) (to B.A.M.).
Address reprint requests to Barbara A. Miller, MD, Department of Pediatrics, The Milton S. Hershey Medical Center, PO Box 850, Hershey, PA 17033-0850.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal