Abstract
Immunizing bone marrow donors prior to bone marrow transplant (BMT) has the potential for adoptively transferring specific immunity against opportunistic pathogens. Studies have shown that long-term antibody production occurs in the bone marrow and that specific humoral immunity may be transferred from donor to recipient following BMT. However, the magnitude and duration of T-cell memory in the bone marrow compartment has not been adequately investigated. In this study, virus-specific CD8+ T-cell responses in the bone marrow were compared with those observed in the spleen of mice acutely infected with lymphocytic choriomeningitis virus (LCMV). During the acute stages of infection, most CD8+ T cells in the spleen and bone marrow showed upregulated surface expression of the activation/memory marker, LFA-1 (LFA-1hi). After clearing LCMV infection, the antiviral immune response subsided to homeostatic levels and the ratio of CD8+/LFA-1hi to CD8+/LFA-1lo T cells in the spleen and bone marrow of LCMV immune mice returned to the value observed in naive mice. Virus-specific ex vivo effector cytotoxic T-lymphocyte (CTL) responses could be identified in both spleen and bone marrow compartments at 8 days postinfection. LCMV-specific CTL precursor (CTLp) frequencies peaked in the bone marrow at 8 days postinfection and averaged one in 200 to one in 650 CD8+ T cells, a frequency similar to that observed in the spleen. After clearing the acute infection, potent LCMV-specific CTL memory responses could be demonstrated in the bone marrow for at least 325 days postinfection, indicating long-term persistence of antiviral T cells at this site. Adoptive transfer of LCMV-immune bone marrow into severe combined immunodeficiency (SCID) mice provided protection against viral challenge, whereas SCID mice that received naive bone marrow became chronically infected upon challenge with LCMV. These results indicate that after acute viral infection, virus-specific memory T cells can be found in the bone marrow compartment and are maintained for an extended period, and when adoptively transferred into an immunodeficient host, they are capable of conferring protection against chronic viral infection.
BONE MARROW TRANSPLANT (BMT) patients are severely immunosuppressed for the first several months after transplantation, with many long-term survivors remaining immunocompromised for 1 to 2 years or longer.1-3 It is therefore not surprising that vaccination of BMT recipients often fails to elicit an effective immune response whether given immediately before or after BMT.4 This is perplexing, because these patients are at high risk for developing potentially lethal viral and bacterial infections.1 Recent studies have shown that by immunizing bone marrow donors before BMT, antigen-specific humoral and cell-mediated immunity can be transferred to bone marrow recipients. Adoptive transfers of specific immunity to pseudomonas, hepatitis, varicella, cytomegalovirus, tetanus toxoid, and diphtheria toxoid have all been demonstrated following human BMT.5-9 Several studies have characterized virus-specific antibody production in bone marrow following acute viral infection,10-12 but little is known about the kinetics or relative magnitude of T-cell memory in the bone marrow compartment.
This study provides a comparison of virus-specific CD8+ T-cell memory in the bone marrow in relation to the spleen following acute infection with lymphocytic choriomeningitis virus (LCMV). Acute LCMV infection is resolved within approximately 2 weeks postinfection, and it is well documented that clearance of LCMV is mediated by CD8+ cytotoxic T lymphocytes.13-25 Following acute LCMV infection of adult BALB/c mice, CD8+ T cells in both spleen and bone marrow showed similar upregulation of the activation/memory marker, LFA-1. Virus-specific effector and memory cytotoxic T-lymphocyte (CTL) responses were identified in the bone marrow compartment and found to be comparable to the spleen after correction for the number of CD8+ T cells present in each location. In addition, adoptive transfer of LCMV-immune bone marrow conferred protection against challenge with LCMV, whereas mice receiving naive bone marrow became chronically infected. These results indicate that the bone marrow of LCMV-immune mice contained functional virus-specific CTLs capable of conferring protection upon viral challenge.
MATERIALS AND METHODS
Mice.BALB/cByj and severe combined immunodeficiency (SCID) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The congenital BALB/c carrier colony was derived from neonatally infected mice and bred at Emory University.
Virus.The Armstrong CA 1371 strain of LCMV was used in this study. LCMV-immune mice were obtained by injecting 5- to 8-week-old mice intraperitoneally with 2 × 105 PFU of LCMV-Armstrong. For protection studies, SCID mice were challenged intraperitoneally with 2 × 105 PFU LCMV-Armstrong within 12 hours of adoptive BMT.
Determination of viral titers.Infectious LCMV was quantified by plaque assay on Vero cell monolayers as previously described.13
Ex vivo CTL assay.MHC class I–restricted LCMV-specific CTL activity was determined using BALB clone 7 cells as targets in a 6-hour 51Cr-release assay.13
Limiting dilution analysis.Splenocytes were cultured in RPMI supplemented with 10% fetal calf serum (FCS) (Hyclone, Logan, UT), L-glutamine, antibiotics (penicillin, streptomycin, and amphotericin B), and 5 × 10−5 mol/L β-mercaptoethanol (BME) (Sigma, St Louis, MO). Spleen cells were cultured in graded doses (12 to 24 wells per dose) with 8 × 105 syngeneic spleen cell feeders (1,200 rad) from uninfected mice and 2 × 105 syngeneic spleen stimulator splenocytes (1,200 rad) from LCMV-carrier mice in 96-well flat-bottom plates. Recombinant human interleukin-2 (Cetus Corp, Emeryville, CA) was added at 50 U/mL final concentration. After 8 days, the contents from each well were split to test CTL activity against LCMV-infected and uninfected targets in a 6-hour 51Cr-release assay.14 26
Determination of lytic units.Spleen or bone marrow cells (8 × 106 per well) were cultured in 24-well plates with 2 × 106 LCMV-infected spleen cells in a total volume of 2 mL RPMI supplemented with 10% FCS and 5 × 10−5 mol/L BME. After 5 days of in vitro stimulation, cells were resuspended, counted, and seeded onto LCMV-infected and uninfected targets at effector to target (E:T) ratios of 30:1, 10:1, 3:1, and 1:1 in a 6-hour 51Cr-release assay. Lytic units (LU) were determined as the number of effector cells required to exhibit 20% lysis of infected targets. To estimate total bone marrow, the number of cells extracted from two femurs combined was multiplied by a coefficient of 7.9.27
Flow cytometry.Monoclonal antibodies used to stain cell surface markers included phycoerythrin-conjugated anti-CD8a (Ly-2) and fluorescein isothiocyanate–conjugated anti-CD18 (stains the β chain of the LFA-1 family) (Pharmingen, San Diego, CA). Fluorescent-activated cell sorter (FACS) analysis was performed as previously described.15
Isolation of peripheral blood mononuclear cells.Pooled blood was collected into heparinized tubes and diluted 1:3 with RPMI containing 1% FCS. Cells were isolated by layering this suspension onto a cushion of Histopaque-1077 (Sigma) followed by centrifugation at 2,000 rpm for 20 minutes at room temperature in an IEC Centra 7R table-top centrifuge (International Equipment Co, Needham Heights, MA). Cells were collected from the interphase, washed once in 15 mL medium, resuspended, and counted. Typical recovery was 3 to 8 × 106 cells/mL blood. For estimating total cells in circulation, 2.5 mL blood per adult mouse was assumed.
BMT.After euthanization, femurs were removed and placed into small petri dishes containing RPMI and 1% FCS, and any remaining muscle tissue was removed. Femoral bone marrow was extracted by inserting a syringe equipped with a 26-gauge needle into one end of the bone and flushing with 3 to 4 mL RPMI containing 1% FCS into a 15-mL conical tube. Cells were pelleted, and contaminating erythrocytes were cleared by a single round of 0.83% NH4Cl and resuspended in RPMI supplemented with 1% FCS. On average, two adult femurs yield between 2.0 and 2.5 × 107 total bone marrow cells. SCID mice at 6 to 8 weeks of age received 2 × 107 total bone marrow cells from either naive BALB/c or LCMV-immune BALB/c mice (>90 days postinfection) by intravenous injection through the lateral tail vein in 0.5 mL RPMI containing 1% FCS.
RESULTS
Virus-specific primary ex vivo CTL responses.The peak effector CTL response following acute LCMV infection occurs in the spleen at about 8 days postinfection.14 Ex vivo CTL assays were performed at this time point to compare the direct antiviral cytolytic activity of spleen cells, peripheral blood mononuclear cells (PBMCs), and bone marrow cells against LCMV-infected targets (Table 1). Virus-specific CTL activity of the spleen and PBMC samples was comparable and demonstrated about 70% to 80% specific lysis of virus-infected targets at an E:T ratio of 30:1. The direct ex vivo CTL response of bone marrow cells at 8 days postinfection showed a highly reproducible 20% specific lysis of infected targets at an E:T ratio of 100:1. One major difference between CTL activity of the bone marrow compared with the spleen or peripheral blood was the relatively low number of CD8+ T cells in the bone marrow compartment (Fig 1). After normalizing for the total number of CD8+ T cells present during the ex vivo CTL assay, there was only about a twofold to threefold difference in LU per 106 CD8+ T cells between these three anatomic sites (Table 1). However, if total virus-specific CTL are taken into consideration, the spleen accounted for 80% of the effector CTL response, followed by CTLs found in the bloodstream (16%) and the bone marrow (4%). These results indicate that virus-specific effector CTLs can readily be detected in the bone marrow during the acute phase of viral infection, but the bone marrow is not a major reservoir of active cytolytic activity.
Primary LCMV-Specific Effector CTL Response at 8 Days Postinfection
| Cell Sample . | Ex Vivo Virus-Specific CTLs (% specific 51Cr release)* . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Infected Targets . | Uninfected Targets . | LU† . | . | . | . | . | |||||||
| . | 100:1 . | 30:1 . | 10:1 . | 3:1 . | 100:1 . | 30:1 . | 10:1 . | Per 106 Cells . | Per 106 CD8+ Cells . | Per Organ or in Circulation . | . | . | . | . |
| Spleen | ND | 82 ± 1 | 50 ± 6 | 19 ± 2 | ND | 3 ± 1 | 0 | 24 ± 8 | 96 ± 30 | 2,928 ± 612 | ||||
| PBMCs‡ | 72 | 69 | 53 | 30 | 0 | 0 | 0 | 25 | 72 | 579 | ||||
| Bone marrow | 19 ± 1 | 8 ± 1 | 3 ± 1 | 1 ± 1 | 0 | 0 | 0 | 0.71 ± 0.08 | 36 ± 4 | 162 ± 27 | ||||
| Cell Sample . | Ex Vivo Virus-Specific CTLs (% specific 51Cr release)* . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Infected Targets . | Uninfected Targets . | LU† . | . | . | . | . | |||||||
| . | 100:1 . | 30:1 . | 10:1 . | 3:1 . | 100:1 . | 30:1 . | 10:1 . | Per 106 Cells . | Per 106 CD8+ Cells . | Per Organ or in Circulation . | . | . | . | . |
| Spleen | ND | 82 ± 1 | 50 ± 6 | 19 ± 2 | ND | 3 ± 1 | 0 | 24 ± 8 | 96 ± 30 | 2,928 ± 612 | ||||
| PBMCs‡ | 72 | 69 | 53 | 30 | 0 | 0 | 0 | 25 | 72 | 579 | ||||
| Bone marrow | 19 ± 1 | 8 ± 1 | 3 ± 1 | 1 ± 1 | 0 | 0 | 0 | 0.71 ± 0.08 | 36 ± 4 | 162 ± 27 | ||||
Adult BALB/c mice were infected intraperitoneally with 2 × 105 PFU LCMV-Armstrong, and cytolytic virus-specific CTL activity was determined in the spleen, peripheral blood, and bone marrow at 8 days postinfection.
Abbreviation: ND, not determined.
Without any in vitro stimulation, antiviral CTL activity exhibited by spleen, PBMCs, and bone marrow cells was determined by measuring specific 51Cr release from either uninfected or LCMV-infected targets at the indicated effector:target (E:T) ratios.
Lytic units (LU) ar defined as the number of effector cells required to exhibit 20% lysis of infected targets. The spleen and PBMCs contained approximately 30% CD8+ T cells, and CD8+ T cells accounted for about 2.0% of total cells in the bone marrow. LU per total organ were calculated based on total cells recovered from spleen, were estimated for bone marrow from 2 femurs (∼12% of total bone marrow27 ), and were estimated for PBMCs assuming 2.5 mL circulating blood per adult mouse.
Data pooled from 3 mice.
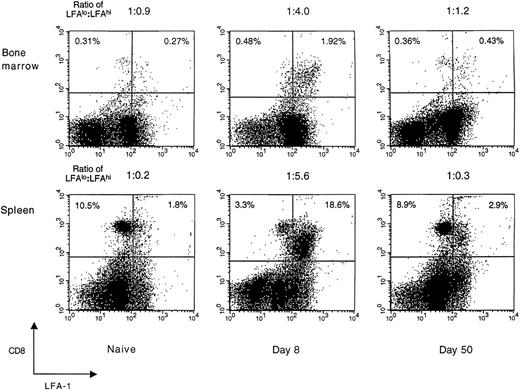
LFA-1 expression on CD8+ T cells during acute LCMV infection. Expression of the activation/memory marker, LFA-1 (CD18), on CD8+ T cells in the spleen and femoral bone marrow of naive and LCMV-infected BALB/c mice was studied using FACS analysis. Splenic and bone marrow–localized CD8+ T cells exhibited similar patterns of LFA-1 surface expression that peaked at day 8 and then declined to homeostatic levels by 50 days postinfection. Data shown are representative of ≥3 independent experiments, each with similar results. CD8+ cells are shown on the vertical axis and LFA-1+ cells on the horizontal axis, with the ratio of LFAlo:LFAhi CD8+ T cells shown above each panel. Each dot plot represents 30,000 acquired events.
LFA-1 expression on CD8+ T cells during acute LCMV infection. Expression of the activation/memory marker, LFA-1 (CD18), on CD8+ T cells in the spleen and femoral bone marrow of naive and LCMV-infected BALB/c mice was studied using FACS analysis. Splenic and bone marrow–localized CD8+ T cells exhibited similar patterns of LFA-1 surface expression that peaked at day 8 and then declined to homeostatic levels by 50 days postinfection. Data shown are representative of ≥3 independent experiments, each with similar results. CD8+ cells are shown on the vertical axis and LFA-1+ cells on the horizontal axis, with the ratio of LFAlo:LFAhi CD8+ T cells shown above each panel. Each dot plot represents 30,000 acquired events.
LFA-1 expression on CD8+ T cells in the spleen and bone marrow.Compared with naive T cells, activated T cells generally express higher levels of adhesion molecules.28 To determine if these phenotypic changes are evident on both splenic and bone marrow–localized T cells during acute viral infection, CD8+ T cells were stained for expression of the activation/memory marker, LFA-1. In naive mice, the majority of splenic CD8+ T cells expressed low levels of LFA-1 (LFA-1lo). At 8 days after LCMV infection, the massive expansion and activation of CD8+ T cells in the spleen changed this cell population to mostly LFA-1hi (Fig 1). After resolving the acute infection over the next 1 to 2 weeks, the actively cytolytic immune response to LCMV declined rapidly, and by 50 days postinfection, the relative surface expression of LFA-1 on CD8+ T cells in the spleen returned to levels similar to those observed in uninfected mice.
In the bone marrow of naive mice, there was a nearly equal distribution of LFA-1lo to LFA-1hi CD8+ T cells (ratio, 1:0.9). However, at 8 days postinfection, the bone marrow was similar to the spleen in having a heavily skewed distribution of LFA-1lo to LFA-1hi CD8+ T cells (ratio, 1:4). This striking difference in the ratio of LFA-1lo to LFA-1hi CD8+ T cells in the bone marrow of LCMV-infected mice indicated that most of the CD8+ T cells in the bone marrow were in an activated state. By 50 days postinfection, the ratio of LFA-1lo to LFA-1hi CD8+ T cells in the bone marrow returned to the homeostatic levels observed in uninfected mice. These results show that during acute viral infection, the kinetics of LFA-1 surface expression on CD8+ T cells in the bone marrow are similar to that observed in the spleen.
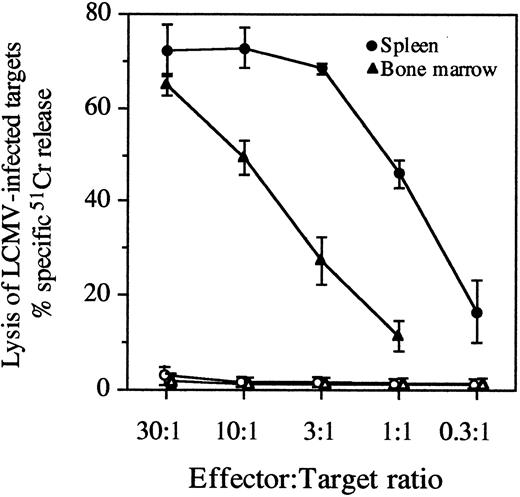
Comparison of antiviral CTL memory in the spleen and bone marrow.To determine if virus-specific CTL memory in the bone marrow of LCMV-immune mice was as long-lived as that observed in the spleen,14 we compared CTL memory responses of these two anatomic sites following acute LCMV infection. At 325 days postinfection, virus-specific CTL memory was demonstrated by potent antiviral CTL responses elicited from both spleen and bone marrow cultures after in vitro stimulation with LCMV (Fig 2). To quantitatively estimate antiviral CTL memory in the spleen and bone marrow compartments, LCMV-specific LU and CTLp frequencies were determined (Table 2). Virus-specific CTL responses in the spleen and bone marrow peaked at 8 days postinfection, but after declining about 10-fold, they were maintained from 57 to 325 days postinfection. The magnitude of the antiviral CTL response in the bone marrow was about 10-fold lower than in the spleen if calculated per total spleen or bone marrow cells. However, after normalizing for the percentage of CD8+ T cells present in each of these anatomic locations, the virus-specific CTLp frequency (per CD8+ T cell) was nearly equal in both the spleen and bone marrow compartments (Table 2). These results show that the virus-specific CTL memory in the bone marrow was as long-lasting as that observed in the spleen and remarkably similar in magnitude if CTLp frequencies were calculated per CD8+ T cell.
Persistence of virus-specific CTL responses in the spleen and bone marrow. At 325 days postinfection, LCMV-immune mice were euthanized, and spleen and bone marrow cells were stimulated in vitro with virus for 5 days. Following stimulation, the antiviral CTL activity of spleen and bone marrow samples was determined by measuring specific 51Cr release from the lysis of LCMV-infected targets. Data represent the mean ±SD of 4 mice. Lysis of LCMV-infected targets is shown with closed symbols and lysis of uninfected targets is shown with open symbols at the indicated E:T ratios.
Persistence of virus-specific CTL responses in the spleen and bone marrow. At 325 days postinfection, LCMV-immune mice were euthanized, and spleen and bone marrow cells were stimulated in vitro with virus for 5 days. Following stimulation, the antiviral CTL activity of spleen and bone marrow samples was determined by measuring specific 51Cr release from the lysis of LCMV-infected targets. Data represent the mean ±SD of 4 mice. Lysis of LCMV-infected targets is shown with closed symbols and lysis of uninfected targets is shown with open symbols at the indicated E:T ratios.
Acute LCMV Infection Results in Virus-Specific CTL Memory in the Spleen and Bone Marrow
| Days Postinfection . | LU/106 Total Cells (range)* . | LU/106 CD8+ T Cells (range)* . | ||
|---|---|---|---|---|
| . | Spleen . | Bone Marrow . | Spleen . | Bone Marrow . |
| 85 | 140 (108-171) | 10 (7-14) | 873 (675-1,070) | 690 (450-930) |
| 230 | 117 (92-146) | 17 (12-23) | 1,314 (1,028-1,635) | 1,223 (888-1,731) |
| 325 | 173 (141-200) | 23 (19-29) | 1,131 (975-1,247) | 1,166 (914-1,392) |
| CTLp Frequency/Total Cells† | CTLp Frequency/CD8+ T Cells† | |||
| Early | ||||
| 8 | ||||
| 1 | 1/4.7 × 103 | 1/4.3 × 104 | 1/6.6 × 102 | 1/6.5 × 102 |
| 2 | 1/9.3 × 102 | 1/1.5 × 104 | 1/1.4 × 102 | 1/2.1 × 102 |
| 3 | 1/1.6 × 103 | 1/1.4 × 104 | 1/2.2 × 102 | 1/2.0 × 102 |
| Late | ||||
| 57‡ | 1/1.8 × 104 | 1/1.3 × 105 | 1/2.8 × 103 | 1/2.0 × 103 |
| 230 | ||||
| 1 | 1/2.1 × 104 | 1/2.2 × 105 | 1/1.9 × 103 | 1/2.9 × 103 |
| 2 | 1/2.1 × 104 | 1/2.1 × 105 | 1/1.9 × 103 | 1/2.7 × 103 |
| Days Postinfection . | LU/106 Total Cells (range)* . | LU/106 CD8+ T Cells (range)* . | ||
|---|---|---|---|---|
| . | Spleen . | Bone Marrow . | Spleen . | Bone Marrow . |
| 85 | 140 (108-171) | 10 (7-14) | 873 (675-1,070) | 690 (450-930) |
| 230 | 117 (92-146) | 17 (12-23) | 1,314 (1,028-1,635) | 1,223 (888-1,731) |
| 325 | 173 (141-200) | 23 (19-29) | 1,131 (975-1,247) | 1,166 (914-1,392) |
| CTLp Frequency/Total Cells† | CTLp Frequency/CD8+ T Cells† | |||
| Early | ||||
| 8 | ||||
| 1 | 1/4.7 × 103 | 1/4.3 × 104 | 1/6.6 × 102 | 1/6.5 × 102 |
| 2 | 1/9.3 × 102 | 1/1.5 × 104 | 1/1.4 × 102 | 1/2.1 × 102 |
| 3 | 1/1.6 × 103 | 1/1.4 × 104 | 1/2.2 × 102 | 1/2.0 × 102 |
| Late | ||||
| 57‡ | 1/1.8 × 104 | 1/1.3 × 105 | 1/2.8 × 103 | 1/2.0 × 103 |
| 230 | ||||
| 1 | 1/2.1 × 104 | 1/2.2 × 105 | 1/1.9 × 103 | 1/2.9 × 103 |
| 2 | 1/2.1 × 104 | 1/2.1 × 105 | 1/1.9 × 103 | 1/2.7 × 103 |
Adult BALB/c mice were infected intraperitoneally with 2 × 105 PFU LCMV-Armstrong, and virus-specific CTL activity was determined after in vitro restimulation with virus for 5 days at the indicated times postinfection.
Defined as the number of effector cells required to exhibit 20% lysis of infected targets. The percentage of CD8+ T cells in the spleen and bone marrow was calculated by FACS prior to in vitro stimulation, and the range is given for 3 to 4 mice per group.
Determined by limiting dilution analysis as previously described.14
CTLp frequency at 57 days postinfection was calculated from a pool of 20 LCMV-immune mice.
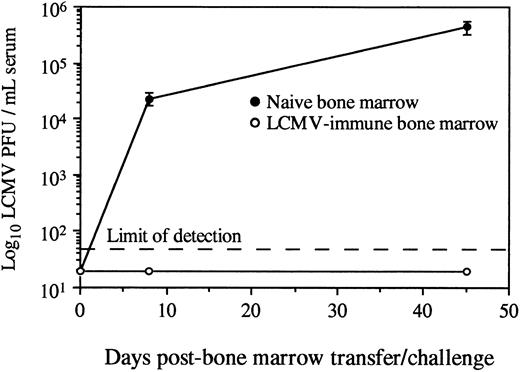
Adoptive transfer of virus-specific T-cell memory results in protection against viral challenge.Protection against LCMV infection absolutely requires an effective CD8+ T-cell–mediated immune response.13-25 To determine if the virus-specific CD8+ T cells found in the bone marrow of LCMV-immune mice could provide antiviral immunity in an otherwise naive and immunocompromised host, we transferred either naive or LCMV-immune bone marrow into SCID recipients and then challenged with infectious virus. Since SCID mice have no functional T cells, they were totally dependent on the mature T cells received during BMT for eliciting a protective antiviral response. SCID mice receiving naive bone marrow were susceptible to LCMV infection and became chronically infected, as demonstrated by high titers of infectious virus in the serum (Fig 3). In contrast, SCID mice receiving LCMV-immune bone marrow were resistant to chronic viral infection and cleared infectious virus from the serum within 8 days. These data show that adoptive transfer of bone marrow containing virus-specific memory CD8+ T cells confers protection against challenge with a viral pathogen.
Adoptive transfer of LCMV-immune bone marrow protects against viral infection. Total femoral bone marrow from naive or LCMV-immune donors (<90 days postinfection) was transferred intravenously into SCID recipients (2 × 107 bone marrow cells per mouse). Mice (2 to 4 per group) were challenged intraperitoneally with 2 × 105 PFU LCMV-Armstrong, and serum titers of infectious virus were monitored by plaque assay as previously described.13
Adoptive transfer of LCMV-immune bone marrow protects against viral infection. Total femoral bone marrow from naive or LCMV-immune donors (<90 days postinfection) was transferred intravenously into SCID recipients (2 × 107 bone marrow cells per mouse). Mice (2 to 4 per group) were challenged intraperitoneally with 2 × 105 PFU LCMV-Armstrong, and serum titers of infectious virus were monitored by plaque assay as previously described.13
DISCUSSION
Following acute LCMV infection, virus-specific CTLs can be identified in the bone marrow compartment. CD8+ T cells in the bone marrow follow the same pattern of activation as observed in the spleen with regard to surface expression of LFA-1, ex vivo CTL activity, and memory CTLp frequencies. Adoptive transfer of LCMV-immune bone marrow into naive recipients conferred protection against viral infection. This shows that prior immunization of bone marrow donors with a specific viral pathogen allows BMT recipients to respond to viral challenge with a protective cell-mediated immune response.
During acute LCMV infection, effector CTL activity was found in the spleen, blood, and bone marrow (Table 1). Since LCMV spreads systemically, it is not unusual to find virus-specific CTL responses throughout the periphery as the host resolves the infection. LCMV may be found in the bone marrow as early as 3 to 5 days postinfection, but infectious virus or viral genomic RNA is not detected in the bone marrow by 8 days postinfection.12 Ex vivo effector (ie, directly cytolytic) CTL activity was identified in the bone marrow at day 8 postinfection and may have been involved in the viral clearance of the bone marrow compartment. Since there was approximately twofold less ex vivo CTL activity per CD8+ T cell in the bone marrow than in PBMCs, it seems unlikely that the bone marrow was a primary site of virus-specific CTL generation and expansion, suggesting that antiviral CTLs probably migrated to the bone marrow from other sites.
Memory T cells may be distinguished from naive T cells based on the expression of various adhesion/activation markers on the cell surface.28-31 During acute LCMV infection, we studied the expression of LFA-1 on CD8+ cells in the spleen and bone marrow. LFA-1 is an activation/memory marker, and surface expression of this molecule is important to CTL activity by contributing to the avidity of a CTL for its target cell.32 The results presented here (Fig 1) show that CD8+ cells in the bone marrow followed a comparable pattern of LFA-1 surface expression as observed in the spleen. This indicated that during acute viral infection, CD8+ T cells in both locations appeared phenotypically similar with respect to the kinetics of activation and the mechanisms of homeostatic control.
Quantitation of antiviral CTLp populations in the spleen and bone marrow showed that virus-specific CTL memory in the bone marrow was as long-lived as in the spleen (Table 2). Even though LCMV is cleared from the host within 1 to 2 weeks, it is noteworthy that virus-specific CTL memory may persist for more than 10 months in the bone marrow compartment. Virus-specific CTL memory was also maintained after adoptive transfer of LCMV-immune bone marrow into SCID mice, with LCMV-specific CTLp numbers sustained for longer than 7 months posttransfer (M.K. Slifka and R. Ahmed, unpublished results, October 1994). This indicates that virus-specific T cells isolated from bone marrow are similar to splenic T cells14 in the capacity to maintain stable antiviral memory T-cell populations after adoptive transfer into uninfected hosts.
T-cell depletion of donor bone marrow has been commonly performed to reduce the incidence and severity of graft-versus-host disease.33-35 However, a recent clinical trend is not to deplete T cells from bone marrow in cases of matched allogeneic or autologous BMT. In cases where T cells are not depleted, specific immunization before bone marrow extraction may provide a valuable source of immunologic memory for the bone marrow recipient. In this regard, there is growing interest in developing immunization strategies for bone marrow donors and recipients36 and using adoptive T-cell therapy for treatment of a variety of human viral diseases.37 Since adoptive transfer of bone marrow from LCMV-immune mice into SCID mice conferred protection against chronic LCMV infection (Fig 3), this indicates that immunization of bone marrow donors may be an effective means of generating protective immune responses in naive and/or immunocompromised hosts. Vaccination of bone marrow donors therefore has the potential to provide adoptive cell-mediated (as well as humoral) immunity against the many opportunistic infections that endanger the lives of BMT recipients.
Supported by Grant No. AI30048 from the National Institutes of Health.
Address reprint requests to Rafi Ahmed, PhD, Emory Vaccine Center, Emory University, School of Medicine, G211 Rollins Research Bldg, 1510 Clifton Rd, Atlanta, GA 30307.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal