Abstract
Analysis of the mitogenic activity of interleukin-3 (IL-3), Steel factor (SF ), and flt-3 ligand (FL) on acute myelogenous leukemia (AML) blasts using the short-term endpoints of proliferation in 3H-thymidine (3H-Tdr) incorporation assays or methylcellulose cultures (colony assays) showed that greater than 90% of samples contained cells that were responsive to one or more of these cytokines. With this information, culture conditions that were known to support normal long-term culture-initiating cells (LTC-IC) were tested, with or without supplements of one or more of these three growth factors, for their ability to support primitive progenitors from 10 cell samples from patients with AML. In all cases cytogenetically abnormal colony forming cells (CFC) were detected after 5 weeks when AML peripheral blood or marrow cells were cocultured on preestablished, normal human marrow feeders (HMF ) and/or Sl/Sl mouse fibroblast feeders and the number of CFC detected in these 5-week-old LTC maintained a linear relationship to the number of input AML cells. Limiting dilution analysis, performed on 6 of the 10 samples, showed the frequency of AML cells initiating LTC (AML LTC-IC) to be 5- to 300-fold lower than the frequency of AML-CFC in the same cell sample, whereas the average number of CFC produced per LTC-IC varied from 1 to 13. Surprisingly, in each case the concentration of cytogenetically normal LTC-IC detected in AML patient blood was at least 10-fold higher than that previously observed in the blood of normal individuals. “Mixed” mouse fibroblast feeders engineered to produce human G-CSF, IL-3, and SF did not enhance detection of AML LTC-IC but did increase the output of cytogenetically normal CFC from LTC of 3 of 4 patient samples. Supplementation of AML LTC with IL-3 and exogenously provided SF and/or FL increased the output of AML-CFC from 5-week-old LTC by greater than or equal to twofold with 5 of 9 patient samples, whereas in one case exogenous addition of FL reduced the output of malignant CFC from LTC. These studies show that conditions that support normal LTC-IC also allow a functionally analogous but rare AML progenitor cell type to be detected. In addition, differences in the responses of normal and leukemic cells to various cytokines active on normal LTC-IC were revealed. Further analysis of these differences may enhance our understanding of leukemogenesis and lead to observations that could be exploited therapeutically.
THE TERM ACUTE myelogenous leukemia (AML) encompasses a group of disorders characterized by the presence of malignant clones of hematopoietic cells exhibiting varying degrees of terminal differentiation.1 The facts that individual AML blasts commonly express cell surface antigens typical of more than one myeloid lineage and that cells of the erythroid, megakaryocytic, and granulocytic lineages are part of the malignant clone in some patients with AML2-4 suggest that the transforming event in these leukemias occurs in a primitive progenitor with multilineage differentiative potential. A subpopulation of the malignant cells from patients with AML gives rise to colonies of terminally differentiated blasts when plated in semisolid medium supplemented with hematopoietic growth factors (AML-CFC)5 and in liquid suspension culture some expansion of this population can be achieved.6-8 Recently it has also been possible to physically separate AML cells with different proliferative capacities in long-term suspension culture based on their cell surface phenotype.9 Taken together, these findings support the existence in AML patients of a developmental hierarchy of malignant hematopoietic cells similar to that characteristic of normal hematopoiesis.
Normal hematopoietic cells that initiate long-term hematopoiesis when placed in coculture with supportive fibroblast feeder layers (known as long-term culture-initiating cells or LTC-IC) are rare progenitors that display many of the characteristics expected of marrow repopulating “stem cells.”10,11 LTC-IC are detected and defined by their ability to give rise to committed clonogenic colony forming cells (CFC) after 5 or more weeks in LTC. Although much information about the regulation and development of primitive hematopoietic cells has been gained from the study of LTC-IC from normal individuals and patients with chronic myelogenous leukemia (CML),12-14 little is known about analogous progenitor types among the malignant cells of patients with AML. Previous efforts to characterize primitive AML progenitors in this system had suggested that they were poorly maintained under standard LTC conditions.15 However, in the intervening years several modifications that improve detection of normal LTC-IC had been made to the system that suggested that a reinvestigation of this approach was warranted. Firstly, the discovery that preestablished feeder layers from a variety of species and tissue sources can substitute for endogenous marrow stromal cells16 as support for long-term human hematopoiesis has not only allowed the standardization of LTC conditions, but it has also permitted the LTC-IC content of any cell source to be determined, including those incapable of generating stromal support such as peripheral blood (PB) cells or highly purified populations of progenitor cells.10,13,17,18 In addition, by engineering murine stromal cell lines to constitutively produce human cytokines to which normal LTC-IC were known to be responsive, it has been possible to enhance the sensitivity of detection of these progenitors and their output of CFC progeny.19 It therefore seemed possible that such feeders might also facilitate detection of a putative analogous leukemic progenitor population from patients with AML. Secondly, many hematopoietic growth factors that have activity on primitive normal progenitors and enhance their detection in colony assays also stimulate both unfractionated AML blasts and AML-CFC.20,21 The use of such recombinant cytokines might then also be expected to improve the detection of CFC produced by malignant LTC-IC. Finally, fluorescent in situ hybridization (FISH) techniques are now available that allow the recognition of chromosomal abnormalities in interphase as well as metaphase cells, enhancing the ease with which leukemic progenitors can be discriminated from their normal counterparts.22
In light of these developments experiments were initiated to determine if a sensitive and quantitative assay for “AML LTC-IC” could be developed. To determine which growth factors or combinations had the greatest activity on AML cells directly isolated from patients, a variety of cytokines were first tested in short-term proliferation studies and colony assays. Using both this information and our experience in optimizing LTC for detecting normal LTC-IC, LTC experiments were designed with a series of AML patient samples.19 Despite the anticipated heterogeneity among different AML samples in both short-term and long-term cultures, our results show that malignant progenitors that initiate LTC (AML LTC-IC) can be routinely detected and both their frequency and proliferative capacity measured using limiting dilution analysis techniques. Manipulation of the growth conditions in the LTC assay have allowed some interesting differences between the responses of normal and leukemic progenitors to be revealed.
MATERIALS AND METHODS
Patient cells.PB or bone marrow (BM) cells were obtained from patients with newly diagnosed AML undergoing marrow and blood examination for diagnostic purposes after informed consent. Mononuclear cells were separated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and either used immediately for the experiments described below or cryopreserved in DMEM with 50% fetal calf serum (FCS) and 10% dimethylsulfoxide. As part of their clinical evaluation all patient samples had morphological, histochemical, and flow cytometric studies performed on their leukemic blasts with subsequent classification according to French-American-British subtypes.1 Cytogenetic analysis was performed on the initial diagnostic BM sample. Baseline clinical and laboratory data on the patients whose cells were evaluated extensively in this study are shown in Table 1. Nine of these samples were tested under a variety of different growth conditions in LTC. In separate experiments the linearity of the LTC assay was established and limiting dilution was performed with five and six of these samples, respectively.
AML Patients Whose Blood or Marrow Samples Were Studied in LTC
| Patient Sample . | Age (yr) . | Sex . | FAB Type . | WBC (% blasts)* . | Bone Marrow Cytogenetics . | FISH Probe† . |
|---|---|---|---|---|---|---|
| . | . | . | . | (×109/L) . | [% abnormal] . | . |
| 1 | 68 | M | RAEBIT | 27 (14) | 45,X,−Y,inv(16)(p13q22) [92] | Y, 8 |
| 2 | 44 | M | M1 | 165 (95) | 45,X,−Y,t(8; 21)(q22q22) [100] | Y, 8 |
| 3 | 64 | F | M5A | 129 (95) | 47,XX,+8,rea(11)(q23) [100] | 8 |
| 4 | 69 | F | M4 | 65 (83) | 47,XX,+8 [100] | 8 |
| 5 | 59 | F | M5A | 36 (94) | 47,XX,−7,+8,+8,t(9; 11)(p22q23) [100] | 8 |
| 6 | 69 | F | M5B | 155 (95) | 46,XX,t(9; 11)(p22q23) [100] | 11q23 |
| 7 | 77 | M | M2 | 57 (51) | 47,XY,+8,t(12; 22)(p13q12)del(20)(q1.2q1.3) [100] | 8 |
| 8 | 36 | F | M4Eo | 18 (42) | 47,XX,inv(16)(p13q22),+22 [87] | 16p13 |
| 9 | 55 | M | M5 | 106 (95) | 46,XY; 48,XY,+8+8 [58] | 8 |
| 10 | 48 | F | M4Eo | 18 (52) | 46,XX,inv(16)(p13q22) [100] | 16p13 |
| Patient Sample . | Age (yr) . | Sex . | FAB Type . | WBC (% blasts)* . | Bone Marrow Cytogenetics . | FISH Probe† . |
|---|---|---|---|---|---|---|
| . | . | . | . | (×109/L) . | [% abnormal] . | . |
| 1 | 68 | M | RAEBIT | 27 (14) | 45,X,−Y,inv(16)(p13q22) [92] | Y, 8 |
| 2 | 44 | M | M1 | 165 (95) | 45,X,−Y,t(8; 21)(q22q22) [100] | Y, 8 |
| 3 | 64 | F | M5A | 129 (95) | 47,XX,+8,rea(11)(q23) [100] | 8 |
| 4 | 69 | F | M4 | 65 (83) | 47,XX,+8 [100] | 8 |
| 5 | 59 | F | M5A | 36 (94) | 47,XX,−7,+8,+8,t(9; 11)(p22q23) [100] | 8 |
| 6 | 69 | F | M5B | 155 (95) | 46,XX,t(9; 11)(p22q23) [100] | 11q23 |
| 7 | 77 | M | M2 | 57 (51) | 47,XY,+8,t(12; 22)(p13q12)del(20)(q1.2q1.3) [100] | 8 |
| 8 | 36 | F | M4Eo | 18 (42) | 47,XX,inv(16)(p13q22),+22 [87] | 16p13 |
| 9 | 55 | M | M5 | 106 (95) | 46,XY; 48,XY,+8+8 [58] | 8 |
| 10 | 48 | F | M4Eo | 18 (52) | 46,XX,inv(16)(p13q22) [100] | 16p13 |
Abbreviation: RAEBIT, refractory anemia with excess blasts in transformation.
WBC, peripheral blood total white blood cell count at diagnosis.
Probes used are described in detail in Materials and Methods.
3H-thymidine (3H-Tdr) proliferation assay.4 × 104 AML cells in 100 μL DMEM with 5% FCS were plated in round-bottom 96-well dishes (Nunc, Roskilde, Denmark). Human growth factors were added, singly or in combination, to achieve the following final concentrations: 40 ng/mL of interleukin-3 (IL-3; Sandoz International, Basel, Switzerland), granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz), granulocyte-CSF (G-CSF; Amgen, Thousand Oaks, CA), IL-6 (Terry Fox Laboratory, Vancouver, Canada), IL-11 (Genetics Institute, Cambridge, MA), or IL-1β (Biogen, Cambridge, MA); and 100 ng/mL of Steel factor (SF; purified in our center from supernatants of COS cells transfected with the cytokine cDNA), IL-2 (Biotest Pharma, Dreieich, Germany), or flt-3 ligand (FL; Immunex, Seattle, WA). After incubation for 3 days at 37°C, 1 μCi of 3H-Tdr was added to each well. After 6 hours of additional incubation, cells were obtained dried onto a glass fiber filter using an LKB Betaplate cell harvester (Wallac, Turku, Finland), and 3H-Tdr incorporation counted in a LKB Betaplate liquid scintillation counter (Wallac). All assays were set up in triplicate and the results expressed as the mean ± SEM cpm of the three replicate cultures.
Methylcellulose assays for CFC.Freshly isolated or thawed, cryopreserved light density AML cells or cells from LTC were plated at a concentration of 1 to 2 × 105 cells/mL in methylcellulose medium (0.92% methylcellulose, 30% FCS, 2 mmol/L L-glutamine, 10−4 mol/L β-mercaptoethanol, 1% bovine serum albumin, in α-medium; StemCell Technologies, Vancouver, Canada) with some or all of the following recombinant human growth factors: 3 U/mL human erythropoietin (Epo; StemCell Technologies), 10 ng/mL GM-CSF, 10 ng/mL IL-3, 50 ng/mL SF, and 50 ng/mL FL. Colonies containing more than 20 cells were scored using an inverted microscope after 14 days of incubation at 37°C in 5% CO2 .
Selection of growth conditions for testing in LTC of AML cells.The results of the short-term assays described above and experience from previous efforts to optimize LTC conditions for detection of normal LTC-IC19 were used to design the LTC conditions to be tested for their ability to support primitive AML cells (Table 2). Human marrow feeders (HMF ) were selected because they were the feeders used in the original description of the LTC-IC assay,10 and Sl/Sl mouse fibroblast feeders were chosen because they had been shown to provide support equivalent to HMF for normal LTC-IC maintenance and differentiation.23 Sl/Sl feeders had also been transfected with the human IL-3 and SF genes to derive two of the engineered feeders tested in this study and thus they also served as controls for assessing the effects of these growth factors in LTC.
Long-Term Culture Conditions
| Feeder Layer . | Soluble Cytokines Added . |
|---|---|
| (human cytokines produced, ng/mL) . | (final concentration)* . |
| HMF† | — |
| Sl/Sl‡ | — |
| Sl/Sl JIL-3ρ (IL-3, 16.5 ng/mL) | SF (50 ng/mL) |
| Sl/Sl | FL (50 ng/mL) |
| Sl/Sl JIL-3 (IL-3, 16.5 ng/mL) | FL (50 ng/mL) |
| Mixed2-155 (IL-3, 2.5 ng/mL; G-CSF, 95 ng/mL; SF, 2 ng/mL) | — |
| Mixed2-155 (IL-3, 2.5 ng/mL; G-CSF, 95 ng/mL; SF, 2 ng/mL) | FL (50 ng/mL) |
| Feeder Layer . | Soluble Cytokines Added . |
|---|---|
| (human cytokines produced, ng/mL) . | (final concentration)* . |
| HMF† | — |
| Sl/Sl‡ | — |
| Sl/Sl JIL-3ρ (IL-3, 16.5 ng/mL) | SF (50 ng/mL) |
| Sl/Sl | FL (50 ng/mL) |
| Sl/Sl JIL-3 (IL-3, 16.5 ng/mL) | FL (50 ng/mL) |
| Mixed2-155 (IL-3, 2.5 ng/mL; G-CSF, 95 ng/mL; SF, 2 ng/mL) | — |
| Mixed2-155 (IL-3, 2.5 ng/mL; G-CSF, 95 ng/mL; SF, 2 ng/mL) | FL (50 ng/mL) |
Soluble cytokines were added twice weekly to achieve final concentration in LTC medium as shown.
Feeder cells derived from the adherent layer of LTC of normal human marrow.16
Embryonic fibroblast cell line derived from Sl/Sl mice, which are defective in the production of SF.23
ρ Sl/Sl cells that have been genetically engineered to produce human IL-3.
The mixed feeders producing IL-3, G-CSF, and SF were chosen because of their demonstrated ability to enhance the detection of and CFC output from normal LTC-IC.19 The three growth conditions using Sl/Sl feeders and one or more of the growth factors IL-3, SF, and FL were selected to supplement LTC with cytokines which were found to be most active on AML cells in short-term assays. The addition of FL supplements to mixed growth factor–producing feeders was included in an effort to maximize stimulation of primitive AML progenitors based on evidence that this cytokine has potent effects on normal LTC-IC.24
Long-term cultures.HMF were obtained as previously described25 by subculturing cells from 2- to 6-week-old confluent normal BM adherent layers established in primary LTC. These were trypsinized and irradiated with 15 Gy of 250 kilovolt (peak) x-rays and then seeded into new tissue culture dishes at 3 × 104 cells/cm2. Sl/Sl feeders consist of Sl/Sl fibroblasts originally obtained from Sl/Sl mouse embryos.23 Sl/Sl-J-IL-3 fibroblasts were obtained by transduction of Sl/Sl fibroblasts with a human IL-3 cDNA-containing retrovirus as previously described.19 These cells produce bioactive human IL-3 at a concentration of 16 ng/mL as determined by the ability of their growth medium to stimulate 3H-Tdr incorporation into MO7e cells.26 Mixed feeders are a 1:1 mixture of M210B4 cells, a cloned line of mouse BM origin,27 engineered to produce human IL-3 and human G-CSF at 4 ng/mL and 190 ng/mL, respectively, and Sl/Sl cells engineered to produce human IL-3 and human SF at 1 ng/mL and 4 ng/mL, respectively.19 Sl/Sl, Sl/Sl-J-IL-3, and mixed feeders were all irradiated with 80 Gy before plating in tissue culture dishes at 3 × 104 cells/cm2 for use in LTC. AML cells were suspended in Myelocult LTC media (StemCell) with 10−6 mol/L solucortef (Sigma-Aldrich Canada Ltd, Oakville, Ontario, Canada) and seeded onto the various feeders at different cell concentrations as required by the experimental design. Cultures were maintained at 37°C in 5% CO2 and received weekly one-half–media changes. In LTCs, which were supplemented with SF or FL, the weekly half-medium change contained the cytokine of interest at twice the final concentration (50 ng/mL). In addition, a second dose of the cytokine was added midweek between feedings to yield the same final concentration. After 5 weeks both nonadherent and adherent cells were obtained from LTC (the latter by trypsinization),16 pooled, and plated in methylcellulose as described above (supplemented with 3 U/mL Epo, 10 ng/mL GM-CSF, 10 ng/mL IL-3, and 50 ng/mL SF ) to determine the clonogenic cell content of each LTC.
For limiting dilution analyses, light density AML cells were plated at four cell concentrations, ranging from 104 to 5 × 105 cells per well (25 to 30 replicate wells per concentration), in 96-well flat-bottomed Nunclon microwell plates (Nunc) containing either HMF or mixed feeders and 100 μL LTC media per well. Each well of such a microwell plate is thus a “mini LTC.” After 5 weeks of culture all nonadherent and adherent cells from each well were harvested and plated in methylcellulose assays containing 3 U/mL Epo, 10 ng/mL GM-CSF, 10 ng/mL IL-3, 50 ng/mL SF, and 50 ng/mL FL. The frequency of LTC-IC in the starting cell population and the average number of CFC generated per LTC-IC were calculated from the frequency of negative wells (wells containing no detectable CFC) in each group and the total number of CFC in the positive wells as previously described.10
FISH.Individual colonies were plucked from methylcellulose assays using finely drawn glass pipets into hypotonic (0.075 mol/L) KCl and fixed in methanol to acetic acid (3:1) on multiwell glass slides (Celline Assoc, New Field, NJ). Although some attempts were made to synchronize colony cells in metaphase as previously described,28 DNA probes were selected to recognize karyotypic abnormalities in the leukemic cells in interphase (Table 1). These included probes specific for centromeric repeat sequences; pRY3.4 for chromosome Y (gift of Dr Y.W. Kan, University of California, San Francisco, CA), and D8Z2 for chromosome 8 (ATCC, Rockville, MD), and a yeast artificial chromosome (YAC) clone containing 550 kb of human DNA encompassing the breakpoint on 16p13, which occurs in the inv(16) rearrangement (CEPHy904E02854; Max-Planck Institut, Berlin, Germany). Total plasmid DNAs containing the centromere specific DNA were labeled by nick translation with either digoxigenin (DIG; Boehringer-Mannheim, Mannheim, Germany), biotin (BRL, Gaithersburg, MD), or spectrum green (Vysis, Downers Grove, IL), using the BRL nick translation kit. The human DNA in the chromosome 16 YAC was amplified by inter-Alu polymerase chain reaction (PCR) using primers and PCR conditions described elsewhere.29 The PCR product was then labeled with spectrum green by nick translation. The 11q23 MLL probe labeled with DIG was purchased from Oncor (Gaithersburg, MD). In the case of patients 1 and 2 with the −Y abnormality (Table 1), a double hybridization was done with biotin-labeled pRY3.4 and DIG-labeled D8Z2, the latter serving as an internal control to ensure, in the absence of a Y signal, that the FISH procedure had been successful.
Microscope slide preparations of plucked colonies were pretreated in 2× SSC (NaCl, 0.3 mol/L, Na3C6H5O7 .2 H2O 0.03 mol/L, pH 7) at 37°C for 30 minutes, denatured in 70% formamide in 4× SSC at 72°C for 2 minutes and then dehydrated in ethanol. The chromosomes Y and 8 centromere probes, at a concentration of 2 ng/μL in 78% formamide, 14% wt/vol dextran sulphate, 1.4× SSC, and 100 μg/mL salmon sperm DNA, were denatured at 75°C for 5 minutes and then hybridized to slide preparations. The inversion 16 YAC probe was hybridized at a concentration of 20 ng/μL in 71% formamide, 14% wt/vol dextran sulphate, 3× SSC, 40 μg/mL human Cot-1 DNA (BRL). The probe was denatured at 75°C for 5 minutes, then incubated at 37°C for 30 minutes to allow annealing of repetitive DNA sequences before application to the slides. Denatured probes were applied to slides that were then coverslipped, sealed with rubber cement, and incubated at 37°C overnight. In the case of plasmid probes, the hybridized slides were washed for 15 minutes each in 50% formamide in 4× SSC, 2 changes of 55% formamide in 4× SSC, 2× SSC, and 2 changes of 0.1× SSC, all at 45°C with agitation. For the inversion 16 YAC probe, slides were washed in 50% formamide in 4× SSC at 43°C for 15 minutes, then in 2× SSC at 37°C for 8 minutes. The MLL probe from Oncor was hybridized and washed as instructed by the manufacturer. For spectrum green-labeled probes, the slides were immediately counterstained and viewed. DIG-labeled probes were detected by further incubation of slides with 8 μg/mL sheep anti–DIG-FITC antibody (Boehringer-Mannheim) in 1% bovine serum albumin (BSA), 4× SSC, at 37°C for 1 hour followed by three 10-minute room temperature (RT) washes in 4× SSC, 4× SSC with 0.1% Triton X-100, and PN buffer (0.1 mol/L NaH2PO4 /Na2HPO4 , pH 8.0, 0.1% Nonidet-P40). The signal was then amplified by a further 1-hour, 37°C incubation of the slide with 30 μg/mL rabbit anti–sheep-FITC (Vector Labs, Burlingame, CA) in PNM buffer (PN buffer with 5% nonfat dry milk powder) followed by four 5-minute washes, three at RT in PN buffer and one in 4× SSC with 0.1% Triton X-100 at 40°C. Biotin-labeled probes were detected by incubation of the slide for 45 minutes at 37°C with 25 μg/mL avidin-Texas Red (TR; Vector Labs) in 4× SSC, 1% BSA followed by 10-minute RT washes in 4× SSC, 4× SSC with 0.1% Triton X-100, and PN buffer. Ten micrograms per milliliter of biotin-anti-avidin (Vector Labs) in PNM was then added for 1 hour at 37°C followed by three 5-minute RT washes in PN buffer. After a second incubation with avidin-TR, slides were again washed three times in PN and finally in 4× SSC with 0.1% Triton X-100 at 40°C. Slides were counterstained either with 0.5 μg/mL propidium iodide in antifade (200 mmol/L 1,4-diazabicyclo-[2.2.2]octane, 2 mmol/L Tris, pH 8, in 90% glycerol) or by staining with 250 ng/mL 4,6-Diamidino-2-phenylindole (DAPI) for 10 minutes followed by antifade. After, coverslipping slides were viewed on a Zeiss Axioplan fluorescence microscope equipped with double and triple bandpass filters to allow simultaneous visualization of FITC and PI or FITC, TR, and DAPI signals (Omega Optical Inc, Battleboro, VT).
Stimulation of AML Blast Proliferation by Various Cytokines
| Cytokine(s)3-150 . | Proportion of AML Samples Responding . | Fold↑ 3H-Tdr Incorporation3-151 . |
|---|---|---|
| . | % (no. tested) . | Median (range) . |
| IL-3 | 79 (53) | 4 (1-126) |
| GM-CSF | 67 (49) | 5 (1-51) |
| G-CSF | 50 (50) | 1 (1-23) |
| IL-6 | 0 (7) | 1 |
| SF | 63 (48) | 2 (1-65) |
| IL-11 | 68 (44) | 1 (1-19) |
| IL-1 | 54 (41) | 2 (1-83) |
| IL-2 | 43 (35) | 1 (1-11) |
| FL | 62 (26) | 2 (1-57) |
| IL-3 + SF | 96 (46) | 11 (1-253) |
| IL-3 + FL | 96 (26) | 10 (1-99) |
| SF + FL | 91 (23) | 4 (1-118) |
| SF + IL-3 + FL | 96 (24) | 16 (1-122) |
| Cytokine(s)3-150 . | Proportion of AML Samples Responding . | Fold↑ 3H-Tdr Incorporation3-151 . |
|---|---|---|
| . | % (no. tested) . | Median (range) . |
| IL-3 | 79 (53) | 4 (1-126) |
| GM-CSF | 67 (49) | 5 (1-51) |
| G-CSF | 50 (50) | 1 (1-23) |
| IL-6 | 0 (7) | 1 |
| SF | 63 (48) | 2 (1-65) |
| IL-11 | 68 (44) | 1 (1-19) |
| IL-1 | 54 (41) | 2 (1-83) |
| IL-2 | 43 (35) | 1 (1-11) |
| FL | 62 (26) | 2 (1-57) |
| IL-3 + SF | 96 (46) | 11 (1-253) |
| IL-3 + FL | 96 (26) | 10 (1-99) |
| SF + FL | 91 (23) | 4 (1-118) |
| SF + IL-3 + FL | 96 (24) | 16 (1-122) |
Cytokines tested at final concentrations of 40 ng/mL for IL-3, GM-CSF, G-CSF, IL-6, IL-11, and IL-1 and 100 ng/mL for SF, IL-2, and FL.
As compared with control cultures without cytokines.
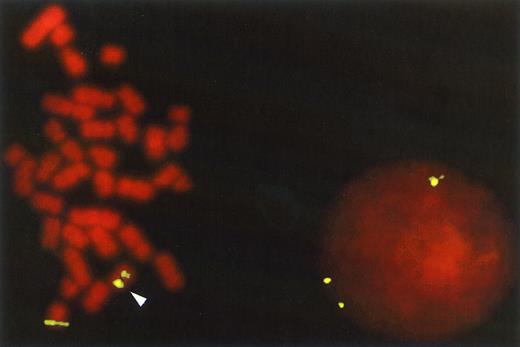
FISH with the 16p13 YAC probe on AML cells from patient 10 that contained the inv(16) (p13q22) abnormality. The chromosomal rearrangement is shown in the metaphase cell by the splitting of the hybridization signal between the p and q arm of the abnormal chromosome 16 while the normal chromosome retains only one signal. In the interphase cell this abnormality is detected by the presence of three fluorescent signals rather than the normal two. The 16p13 YAC probe was prepared and labeled with digoxigenin as described in Materials and Methods and detected with FITC-labeled anti-digoxigenin. The counterstain is propidium iodide (original magnification × 630).
FISH with the 16p13 YAC probe on AML cells from patient 10 that contained the inv(16) (p13q22) abnormality. The chromosomal rearrangement is shown in the metaphase cell by the splitting of the hybridization signal between the p and q arm of the abnormal chromosome 16 while the normal chromosome retains only one signal. In the interphase cell this abnormality is detected by the presence of three fluorescent signals rather than the normal two. The 16p13 YAC probe was prepared and labeled with digoxigenin as described in Materials and Methods and detected with FITC-labeled anti-digoxigenin. The counterstain is propidium iodide (original magnification × 630).
Cytokine-Stimulated 3H-Tdr Incorporation Into AML Blasts From Patient Samples Tested in LTC
| Patient . | CPM Without Growth Factor4-150 . | Fold-Increase in 3H-Tdr Uptake Compared With Control Without Growth Factor . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | IL-3 . | G-CSF . | SF . | FL . | IL-3 + SF . | IL-3 + FL . | IL-3 + SF + FL . |
| 1 | 88 ± 12 | 1 | 2 | 2 | 1 | 4 | 2 | 2 |
| 2 | 44 ± 4 | 126 | 2 | 24 | ND | 253 | ND | ND |
| 3 | 86 ± 5 | 30 | 2 | 5 | 2 | 56 | 65 | 85 |
| 4 | 97 ± 7 | 35 | 8 | 65 | 57 | 114 | 99 | 122 |
| 5 | 242 ± 36 | 5 | 4 | 1 | 39 | 7 | 54 | 52 |
| 6 | 85 ± 3 | 2 | 1 | 1 | 1 | 2 | 2 | 2 |
| 7 | 415 ± 78 | 16 | 3 | 3 | 3 | 20 | 22 | 24 |
| 8 | 270 ± 12 | 4 | 11 | 9 | 8 | 12 | 10 | 13 |
| 9 | 82 ± 19 | 3 | 1 | 2 | 2 | 3 | 5 | 7 |
| 10 | 339 ± 50 | 3 | 6 | 9 | 5 | 11 | 10 | 13 |
| Patient . | CPM Without Growth Factor4-150 . | Fold-Increase in 3H-Tdr Uptake Compared With Control Without Growth Factor . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | IL-3 . | G-CSF . | SF . | FL . | IL-3 + SF . | IL-3 + FL . | IL-3 + SF + FL . |
| 1 | 88 ± 12 | 1 | 2 | 2 | 1 | 4 | 2 | 2 |
| 2 | 44 ± 4 | 126 | 2 | 24 | ND | 253 | ND | ND |
| 3 | 86 ± 5 | 30 | 2 | 5 | 2 | 56 | 65 | 85 |
| 4 | 97 ± 7 | 35 | 8 | 65 | 57 | 114 | 99 | 122 |
| 5 | 242 ± 36 | 5 | 4 | 1 | 39 | 7 | 54 | 52 |
| 6 | 85 ± 3 | 2 | 1 | 1 | 1 | 2 | 2 | 2 |
| 7 | 415 ± 78 | 16 | 3 | 3 | 3 | 20 | 22 | 24 |
| 8 | 270 ± 12 | 4 | 11 | 9 | 8 | 12 | 10 | 13 |
| 9 | 82 ± 19 | 3 | 1 | 2 | 2 | 3 | 5 | 7 |
| 10 | 339 ± 50 | 3 | 6 | 9 | 5 | 11 | 10 | 13 |
Abbreviation: ND, not determined.
X̄ ± SEM.
Individual colonies were scored as either positive or negative for the particular cytogenetic abnormality. In cases where colonies were small, and therefore only a few cells could be detected on the slide, a minimum of 5 cells with a clear signal were required for that colony to be scored. In addition, in all cases the same hybridization signals had to be present in at least 80% of the cells scored from a particular colony to allow it to be classified as normal or abnormal. To determine the percentage of cytogenetically abnormal CFC shown on Tables 6 and 7, a minimum of 10 and a maximum of 80 colonies were analyzed by FISH in each experimental group. Figure 1, which shows FISH analysis of cells from patient 10, shows detection of the inv(16) abnormality in interphase as well as metaphase cells, which made the analysis of even small colonies without dividing cells possible.
CFC in Week 5 Long-Term Cultures of AML Samples Under Various Growth Conditions
| Patient . | Tissue . | CFC per 106 Cells . | Fold Change From HMF (% abnormal*) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | (% abnormal*) . | Sl/Sl . | Sl/Sl JIL3 + SF . | Sl/Sl + FL . | Sl/Sl JIL3 + FL . | Mixed . | Mixed + FL . | |
| . | . | Day 0 . | HMF . | . | . | . | . | . | . |
| . | . | . | 5 wk . | . | . | . | . | . | . |
| 1 | BM | 165 (40) | 53 (25) | 2 (6) | 3 (7)6-152 | ||||
| 2 | BM | 30 (33) | 9 (25) | 2 (0)6-152 | 6 (21) | ||||
| 3 | BM | ND | 13 (53) | ND | 8 (34) | ||||
| 3 | PB | 3,150 (90) | 15 (32) | 5 (60) | 4 (68) | 30 (5)6-152 | 12 (0)6-152 | ||
| 46-151 | PB | 485 (73) | 34 (88) | 0.4 (85) | 2 (87) | 1 (11)6-152 | 2 (23)6-152 | ||
| 55 (71) | 1 (43) | 1 (52) | 0.8 (3)6-152 | 0.7 (15)6-152 | |||||
| 5 | PB | 48,100 (100) | 307 (100) | 0.02 (92) | 0.8 (100) | 2 (100) | 0.6 (88) | ||
| 6 | PB | 158 (87) | 2,790 (80) | 0.7 (82) | 0.5 (69) | 0.6 (82) | 0.4 (82) | ||
| 7 | PB | 31,260 (95) | 129 (0) | 0.03 (29) | 0.7 (60) | 0.4 (68) | 1 (78) | 0.3 (52) | 4 (50) |
| 8 | PB | 45 (67) | 6 (65) | 0.5 (44) | 3 (33) | 3 (35) | 16 (29)6-152 | 3 (28)6-152 | 59 (38) |
| 10 | PB | 225 (50) | 3 (21) | 2 (0) | 25 (4) | 185 (13) | 547 (0)6-152 | 11 (0)6-152 | 429 (8) |
| Patient . | Tissue . | CFC per 106 Cells . | Fold Change From HMF (% abnormal*) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | (% abnormal*) . | Sl/Sl . | Sl/Sl JIL3 + SF . | Sl/Sl + FL . | Sl/Sl JIL3 + FL . | Mixed . | Mixed + FL . | |
| . | . | Day 0 . | HMF . | . | . | . | . | . | . |
| . | . | . | 5 wk . | . | . | . | . | . | . |
| 1 | BM | 165 (40) | 53 (25) | 2 (6) | 3 (7)6-152 | ||||
| 2 | BM | 30 (33) | 9 (25) | 2 (0)6-152 | 6 (21) | ||||
| 3 | BM | ND | 13 (53) | ND | 8 (34) | ||||
| 3 | PB | 3,150 (90) | 15 (32) | 5 (60) | 4 (68) | 30 (5)6-152 | 12 (0)6-152 | ||
| 46-151 | PB | 485 (73) | 34 (88) | 0.4 (85) | 2 (87) | 1 (11)6-152 | 2 (23)6-152 | ||
| 55 (71) | 1 (43) | 1 (52) | 0.8 (3)6-152 | 0.7 (15)6-152 | |||||
| 5 | PB | 48,100 (100) | 307 (100) | 0.02 (92) | 0.8 (100) | 2 (100) | 0.6 (88) | ||
| 6 | PB | 158 (87) | 2,790 (80) | 0.7 (82) | 0.5 (69) | 0.6 (82) | 0.4 (82) | ||
| 7 | PB | 31,260 (95) | 129 (0) | 0.03 (29) | 0.7 (60) | 0.4 (68) | 1 (78) | 0.3 (52) | 4 (50) |
| 8 | PB | 45 (67) | 6 (65) | 0.5 (44) | 3 (33) | 3 (35) | 16 (29)6-152 | 3 (28)6-152 | 59 (38) |
| 10 | PB | 225 (50) | 3 (21) | 2 (0) | 25 (4) | 185 (13) | 547 (0)6-152 | 11 (0)6-152 | 429 (8) |
Growth conditions as shown on Table 2.
% Abnormal, % cytogenetically abnormal colonies detected by FISH.
Data for two separate experiments in LTC.
The difference in the proportion of cytogenetically abnormal CFC between this LTC condition and that of LTC containing HMF achieved a P value <.05 by chi-square analysis.
Frequency and Proliferative Capacity of LTC-IC in AML PB
| Patient . | Day 0 CFC/106 Cells . | Feeder7-150 . | LTC-IC/106 Cells . | CFC/LTC-IC7-151 . |
|---|---|---|---|---|
| . | (% abnormal) . | . | (% abnormal) . | . |
| 3 | 3,150 (90) | HMF | 15 (32) | 1.4 |
| 4 | 485 (73) | HMF | 50 (64) | 1.8 |
| 7 | 31,260 (95) | HMF | 121 (55) | 1.2 |
| Mixed | 116 (73) | 2.7 | ||
| 8 | 45 (67) | HMF | 4 (7) | 4.4 |
| Mixed | 4 (9) | 12.8 | ||
| 9 | 1,138 (0) | HMF | 40 (0) | 1.7 |
| 10 | 225 (50) | HMF | 3 (21) | 2.1 |
| Patient . | Day 0 CFC/106 Cells . | Feeder7-150 . | LTC-IC/106 Cells . | CFC/LTC-IC7-151 . |
|---|---|---|---|---|
| . | (% abnormal) . | . | (% abnormal) . | . |
| 3 | 3,150 (90) | HMF | 15 (32) | 1.4 |
| 4 | 485 (73) | HMF | 50 (64) | 1.8 |
| 7 | 31,260 (95) | HMF | 121 (55) | 1.2 |
| Mixed | 116 (73) | 2.7 | ||
| 8 | 45 (67) | HMF | 4 (7) | 4.4 |
| Mixed | 4 (9) | 12.8 | ||
| 9 | 1,138 (0) | HMF | 40 (0) | 1.7 |
| 10 | 225 (50) | HMF | 3 (21) | 2.1 |
Frequency was determined by limiting dilution analysis as described in Materials and Methods and in Sutherland et al.10
Feeders as described in Table 2.
CFC per LTC-IC were calculated as average values by dividing the total number of CFC produced in all of the limiting dilution cultures plated by the number of calculated LTC-IC used to initiate those cultures for each culture condition tested.
Statistical analysis.Comparison of the number of cytogenetically normal and abnormal CFC recovered from different LTC conditions was performed using chi-square analysis. Statistical significance was assigned when the probability that there was no difference between two culture conditions was <.05.
RESULTS
Cytokine responsiveness of AML cells in short-term assays.In preliminary experiments nine different cytokines were tested alone and in combination for their ability to stimulate 3H-Tdr incorporation into AML blasts after 3 days in liquid suspension culture (Table 3). There was great heterogeneity both in the baseline proliferative activity in the absence of growth factors and in the extent to which this proliferation could be enhanced (up to 253-fold). Nevertheless, for the 53 different patient samples analyzed, evidence of a significant response to the single factors IL-3, SF, or FL was evident in more than 60%, and more than 90% responded to two or three of these factors in combination. The magnitude of the response was also greater with the cytokine combinations (median fold increase 3H-Tdr incorporation 2- to 4-fold for the cytokines individually versus 4- to 16-fold for the combinations). The 10 patient samples that were chosen for further evaluation in this study were selected primarily because of the presence of a chromosomal marker that would be readily detectable by FISH in interphase cells (Table 1). As shown in Table 4 the cytokine responsiveness of the cells in these samples was similar to that of the larger group. All of the cell samples showed some increased proliferation in response to single cytokines, although in the case of samples 1 and 6 this increase was modest. Combinations of two or three cytokines enhanced proliferation more than any single factor alone from every patient sample except number 6.
The growth factor responsiveness of AML progenitors was also evaluated in methylcellulose colony assays. In this case the results of the 3H-Tdr incorporation studies were used to select cytokine(s) for testing and comparison to assays using agar-stimulated leukocyte conditioned medium or medium conditioned by the 5637 cell line30 at a final concentration of 10%. Among 50 patient samples tested in preliminary studies, all showed equivalent colony growth, and 19 (38%) showed at least a twofold increase in the number of blast colonies present in assays containing SF, IL-3, and GM-CSF as compared with assays containing either of the two conditioned media. The addition of FL to this cytokine cocktail further increased the number of colonies derived from 14 (28%) of these 50 samples. Interestingly, among the 19 AML samples tested in methylcellulose assay containing serum but no other source of cytokines, 9 showed evidence of factor-independent colony growth. However, five of these also showed increased colony formation when cytokines were added. Table 5 shows the results of methylcellulose colony assays with or without the addition of recombinant cytokines from the 10 patient samples selected for further analysis. In general, the growth of AML-CFC from these samples was similar to that observed in the larger group of 50 AML specimens. Some evidence of factor-independent growth was noted in six cases, but evidence of increased colony formation in the presence of one or more cytokines was also obtained with every sample except that from patient 6, whose cells also showed a poor response to growth factors as measured by 3H-Tdr incorporation.
Blast Colony Formation per 106 AML PB Cells With Different Cytokine Combinations
| Patient . | Growth Conditions5-150 . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 1 | 70 | 125 | 10 | 0 |
| 2 | 10 | 30 | 0 | 0 |
| 3 | 1,980 | 2,350 | 1,750 | 0 |
| 4 | 320 | 740 | 1,010 | 10 |
| 5 | >10,000 | 16,500 | >10,000 | 65 |
| 6 | 215 | 240 | 180 | 155 |
| 7 | 24,250 | >24,250 | 4,220 | 2,900 |
| 8 | 15 | 45 | 5 | 10 |
| 9 | 2,050 | 2,625 | 800 | 250 |
| 10 | 170 | 215 | 20 | 0 |
| Patient . | Growth Conditions5-150 . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 1 | 70 | 125 | 10 | 0 |
| 2 | 10 | 30 | 0 | 0 |
| 3 | 1,980 | 2,350 | 1,750 | 0 |
| 4 | 320 | 740 | 1,010 | 10 |
| 5 | >10,000 | 16,500 | >10,000 | 65 |
| 6 | 215 | 240 | 180 | 155 |
| 7 | 24,250 | >24,250 | 4,220 | 2,900 |
| 8 | 15 | 45 | 5 | 10 |
| 9 | 2,050 | 2,625 | 800 | 250 |
| 10 | 170 | 215 | 20 | 0 |
Light density PB cells from patients with newly diagnosed AML were plated in methylcellulose assay containing 30% FCS and human cytokine(s) as: (1) SF (50 ng/mL) + IL-3 (10 ng/mL) + GM-CSF (10 ng/mL), (2) SF (50 ng/mL) + IL-3 (10 ng/mL) + GM-CSF (10 ng/mL) + FL (50 ng/mL), (3) FL (50 ng/mL), and (4) No added cytokines.
Detection of malignant progenitors in 5-week-old LTC containing HMF or Sl/Sl mouse fibroblast feeders.Light density PB or BM cells from nine newly diagnosed AML patients (Table 1) were cocultured with irradiated HMF or Sl/Sl fibroblasts for 5 weeks with weekly half medium changes. As shown in Table 6, FISH analysis of colonies plucked from methylcellulose assays from these 5-week-old LTC showed that, in every case, cytogenetically abnormal colonies could be detected. In colony assays from HMF cocultures, the proportion of CFC carrying the chromosomal marker was similar to that seen in colonies derived from the same cell sample plated directly into methylcellulose, except for patient 7 in whom only CFC that were karyotypically normal by FISH for trisomy 8 were seen in assays from HMF cocultures. However, colonies carrying the +8 abnormality were recovered from 5-week-old cocultures of this patient's cells with Sl/Sl feeders and also when the AML cells were cultured at lower cell densities with HMF in limiting dilution analyses described below. The frequency of CFC per 106 input light density BM or blood cells was higher in direct methylcellulose assays than from 5-week-old HMF cocultures in every case except in cultures of cells from patient 6.
Sl/Sl mouse fibroblast feeders were variable in the extent to which they supported the generation of CFC in 5-week-old cocultures with AML cells. Cocultures with cells from patients 2, 5, and 7 showed significant reductions in either the total numbers of CFC detected or the proportion that was cytogenetically abnormal as compared with HMF cocultures. However, cultures with Sl/Sl feeders and cells from the other six patients behaved similarly to cultures with HMF.
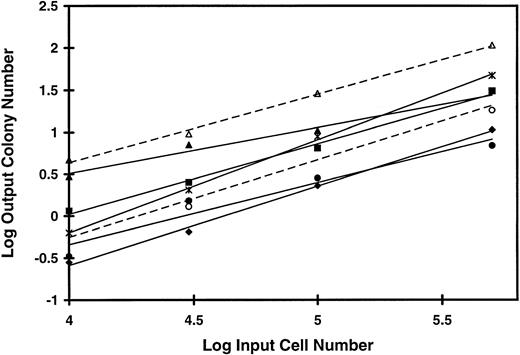
Figure 2 shows that when different concentrations of AML cells from five patients were plated on HMF and, in two cases, on the mixed feeders producing G-CSF, IL-3, and SF, the logarithm of the number of CFC recovered 5 weeks later maintained a linear relationship to the logarithm of the number of input cells. The mean (±SD) slope of the lines generated (0.85 ± 0.16) is not significantly different from one, indicative of a linear relationship between the CFC output and the inoculum input.
Linear relationship between input cell concentration and number of output colonies from LTC of AML cells. PB cells from five patients with AML were plated at concentrations varying from 5 × 106 to 1 × 105 per mL on irradiated feeders in microtitre wells. Twenty-five to 30 duplicate long-term cultures were plated at each cell concentration. After 5 weeks in culture the total cell content of each culture was obtained and plated in methylcellulose assay as described in Materials and Methods. The log of the input cell number into the duplicate long-term cultures is plotted against the log of the mean number of colonies produced per duplicate long-term culture at that cell concentration. HMF were used for long-term cultures with each of the five patient samples, patient 3 (♦), patient 4 (▪), patient 7 (▴), patient 8 (•), and patient 9 (✶). In addition, mixed feeders producing human G-CSF, IL-3, and SF were used for duplicate cultures with cells from patients 7 (▵) and 8 (○). The mean (±SD) of these slope of the graphs generated is 0.85 ± 0.16.
Linear relationship between input cell concentration and number of output colonies from LTC of AML cells. PB cells from five patients with AML were plated at concentrations varying from 5 × 106 to 1 × 105 per mL on irradiated feeders in microtitre wells. Twenty-five to 30 duplicate long-term cultures were plated at each cell concentration. After 5 weeks in culture the total cell content of each culture was obtained and plated in methylcellulose assay as described in Materials and Methods. The log of the input cell number into the duplicate long-term cultures is plotted against the log of the mean number of colonies produced per duplicate long-term culture at that cell concentration. HMF were used for long-term cultures with each of the five patient samples, patient 3 (♦), patient 4 (▪), patient 7 (▴), patient 8 (•), and patient 9 (✶). In addition, mixed feeders producing human G-CSF, IL-3, and SF were used for duplicate cultures with cells from patients 7 (▵) and 8 (○). The mean (±SD) of these slope of the graphs generated is 0.85 ± 0.16.
Analysis of cells from AML blood that initiate LTC at limiting dilution.To determine the frequency of progenitors initiating LTC among AML PB cells and the average number of CFC produced per LTC-IC, cocultures of AML cells from six different patients were established at decreasing dilutions on HMF feeders. As shown on Table 7, the frequency of LTC-IC calculated from the results of these experiments varied greatly from as low as 3 to greater than 100 per 106 cells originally placed in LTC. In every case this frequency was considerably lower than the frequency of AML-CFC in the same sample. The mean (±SD) number of CFC produced per LTC-IC in cultures containing HMF was 2.1 (±1.1), which is lower than the value of 4 to 7 CFC/LTC-IC obtained for normal blood or marrow LTC-IC analyzed under similar conditions.18 19 A proportion of the CFC detected in both direct methylcellulose assays and in assays of cells from LTC were cytogenetically abnormal by FISH for all patient cell samples except number 9. As shown on Table 1, this patient had a mixture of cytogenetically normal and abnormal cells on initial BM cytogenetic analysis. Although the morphology of colonies detected in the direct methylcellulose assays of PB cells or assays from LTC was typical of AML-CFC, none showed the +8+8 abnormality.
When less than 31% of replicate 5-week-old mini-LTC from limiting dilution analysis (plated at a given cell density in 96-well plates) contain CFC, the probability that any LTC containing CFC was initiated by a single progenitor is greater than or equal to 85% according to Poisson statistics.31 From such assays the output of CFC from an individual LTC-IC can be determined. Among the experiments with cells from patients 3, 4, 7, 8, and 10 shown on Table 7, colonies from methylcellulose assays of 43 mini-LTC plated at this limiting cell density were analyzed by FISH. Eighteen LTC (42%) produced exclusively cytogenetically abnormal CFC, whereas 23 (53%) generated only cytogenetically normal colonies and 2 (5%) showed a mixture of normal and abnormal CFC. The number of CFC produced per LTC-IC ranged from 1 to 7 and 1 to 30 for the cytogenetically abnormal and normal LTC-IC, respectively.
The concentration of cytogenetically normal and abnormal LTC-IC in AML PB was calculated using data from Tables 1 and 7 and compared with the concentration of LTC-IC in normal steady state blood that we had previously determined under the same assay conditions.18 As shown in Table 8, in each case the concentration of LTC-IC in AML blood, including those with normal cytogenetics, was at least 10-fold, and in two cases more than 100-fold higher than that detected in normal individuals.
Concentrations of Cytogenetically Normal and Abnormal LTC-IC in the Blood of AML Patients
| Patient . | LTC-IC per mL8-150 . | |
|---|---|---|
| . | Normal . | Abnormal . |
| 3 | 1,320 | 619 |
| 4 | 1,170 | 2,080 |
| 7 | 3,100 | 3,790 |
| 8 | 67 | 5 |
| 9 | 4,240 | — |
| 10 | 43 | 11 |
| Normal controls | 2.9 ± 0.58-151 | |
| n = 23 | ||
| Patient . | LTC-IC per mL8-150 . | |
|---|---|---|
| . | Normal . | Abnormal . |
| 3 | 1,320 | 619 |
| 4 | 1,170 | 2,080 |
| 7 | 3,100 | 3,790 |
| 8 | 67 | 5 |
| 9 | 4,240 | — |
| 10 | 43 | 11 |
| Normal controls | 2.9 ± 0.58-151 | |
| n = 23 | ||
As detected in cocultures with HMF.
In steady state blood from normal individuals as reported in Udomsakdi et al.18
Evaluation of the effect of cytokine supplements on AML progenitors in LTC.The data described above suggested a further series of experiments in which the growth conditions in LTC of AML cells were modified by cytokine supplements in the form of feeders genetically engineered to produce human growth factors and/or by biweekly additions of soluble cytokines over the 5-week culture period. As shown in Table 6, engineered feeders previously chosen for their ability to support the detection of normal LTC-IC (ie, mixed feeders producing IL-3, G-CSF, and SF ) produced a large increase in the output of CFC from cocultures with three of four AML samples tested. However, in all three of these cases (numbers 3, 8, and 10) the proportion of cytogenetically abnormal CFC was significantly reduced as compared with that present in cocultures with HMF. In contrast to our experience with normal LTC-IC, mixed feeders did not significantly enhance the frequency of LTC-IC detected in either patient sample where these were compared with HMF in limiting dilution experiments (Table 7). However, the CFC output per LTC-IC did increase twofold to threefold.
Cocultures with Sl/Sl feeders or mixed feeders, which were supplemented with one or more of the three factors that were most potent in stimulating colony formation or 3H-Tdr incorporation from AML blasts (SF, IL-3, or FL), showed at least a twofold increase in the number of CFC detected from 5-week-old LTC as compared with Sl/Sl or mixed feeder controls with five of the nine patient samples (numbers 2, 5, 7, 8, and 10). In cultures with Sl/Sl feeders supplemented with IL-3 and SF, the numbers of both cytogenetically normal and abnormal CFC increased with all five patient samples so that the relative proportions of each did not change, as compared with control cultures with Sl/Sl feeders alone. The response to FL supplements of Sl/Sl or mixed feeder cocultures was similar to that seen with IL-3 and SF for four patient samples (numbers 5, 7, 8, and 10). However, testing of cells from patient 4 produced a different result. Although the total number of CFC produced changed very little among the different culture conditions with Sl/Sl feeders, the addition of FL (+/− IL-3) to these cultures significantly reduced the output of cytogenetically abnormal CFC from LTC in each of two experiments. This contrasts with the apparent stimulation of AML cells from this patient by FL in short-term assays (Tables 4 and 5).
Although erythropoietin was added to both the initial methylcellulose assays of AML cells and the assays from 5-week-old LTC, few erythroid colonies were seen and their proportion of the total CFC output did not change significantly under the different culture conditions. Those BFU-E that were analyzed for chromosomal abnormalities by FISH were exclusively normal in assays of cells from patients 2, 3, 4, 6, and 8. However, in assays of cells from LTC of samples from patients 1 and 7, 7 of 23 and 1 of 6 BFU-E, respectively, were cytogenetically abnormal.
DISCUSSION
The malignancies encompassed under the term AML exhibit considerable heterogeneity in many aspects of their pathological features and clinical behavior. As shown by the data presented here (Tables 3, 4, and 5) and from a variety of other laboratories,6-8,20,21 one aspect of this heterogeneity is the variability with which leukemic cells from AML patients respond to different cytokines. However, the majority of leukemic blast cells and AML-CFC exhibit some degree of responsiveness to conditions that stimulate the growth of normal hematopoietic cells in vitro. This suggested that a more primitive type of AML precursor cell analogous to normal LTC-IC might also be supported by conditions developed for normal progenitors, as shown for Ph chromosome positive LTC-IC present in the PB and BM of patients with CML.13 14
The existence of a hierarchy of malignant progenitors in AML that might include LTC-IC had been previously suggested by the ability of some AML progenitors to undergo self-renewal to generate AML-CFC in suspension culture and by the fact that AML progenitors with differing frequency and proliferative capacity can be physically separated based on their cell surface phenotype.6-9 Other investigators have also shown that cytogenetically abnormal cells from patients with AML are present among the CD34+CD38−CD33− population as well as among cells carrying markers of committment to both myeloid and lymphoid lineages.3 Furthermore, from selected patient samples AML cells expressing a CD34+CD38− or a CD34+CD71− phenotype characteristic of primitive normal progenitors, including many LTC-IC, have been shown to engraft in NOD/SCID mice, whereas AML cells from the same sample, which were CD34−, CD34+CD38+, or CD34+CD71+, did not.32,33 The data shown in Tables 6 and 7 show that AML LTC-IC can be routinely detected in the PB and BM of AML patients at diagnosis. Because the assay is linear (Fig 2) it can be used to quantitate the numbers of such cells in different clinical samples. Although the expected heterogeneity among AML patient samples was observed in both the frequency and proliferative capacity of these cells, they could be shown to be a relatively rare cell population as compared with AML-CFC and, in most cases, to give rise to more than one CFC after 5 weeks in LTC. In two cases some malignant LTC-IC were shown to have erythroid potential. One of these samples was from a patient with myelodysplasia that had transformed to AML. Multilineage differentiative potential of the malignant clone in some patients with AML has been described previously as being associated with a “smouldering” or “preleukemic” phase and occurring more typically in elderly patients.4 Thus, it appears that the hierarchical nature of blood cell development has been retained among the malignant cells in AML and that the usual target for transformation in these diseases is at or upstream of the progenitor cell compartment that includes normal LTC-IC.
In most cases FISH analysis of CFC plated directly into methylcellulose assays from patient BM or PB samples, or CFC from 5-week-old LTC showed a mixture of cytogenetically normal and abnormal cells. In some cases the apparently high proportion of “normal” CFC may have been related to the probe that was chosen for FISH analysis, eg, the Y probe was selected for detection of cytogenetically abnormal CFC from patients 1 and 2 at a time when reliable FISH probes for the inv(16) and t(8; 21) were not available. It is possible that malignant CFC with the inversion or translocation but retaining the Y chromosome were present but scored as normal by FISH. Similarly, the absence of the +8+8 abnormality from all colonies analyzed from both direct methylcellulose assays and LTC of cells from patient 9 was surprising given the high PB blast cell count and the morphology of the colonies analyzed, which was typical of AML-CFC. It seems likely that at least some of these colonies were in fact malignant although cytogenetically normal by FISH, a possibility that has also been suggested by others.34 Thus, the percentages of cytogenetically abnormal CFC detected by FISH (shown in Tables 6 and 7) are minimal estimates of the proportion of AML progenitors present in these cultures. Nevertheless, it seems likely that residual normal hematopoietic cells coexist with AML progenitors in many of these samples. The fact that all erythroid colonies examined from five of seven patients were normal by FISH supports this possibility. Normal LTC-IC circulate in the PB at easily detectable frequencies.18 Thus, it is not surprising that they could be found in cultures from patients 8 and 10 because in these the peripheral white blood cell count and the proportion of blasts were relatively low, and the FISH probe for inv(16) would be expected to discriminate reliably between normal and malignant CFC (Fig 1). However, as shown on Table 8, the concentration of cytogenetically normal LTC-IC in the PB of these AML patients was 10- to 100-fold higher than the concentration of LTC-IC we have previously observed in the blood of normal individuals. This suggests the possibility that normal LTC-IC can be mobilized into the PB as part of the leukemic process.
Some differences were observed between the behaviour of AML progenitors in LTC and their normal counterparts characterized previously. Firstly, although Sl/Sl fibroblast feeders are equivalent to HMF for the support of normal LTC-IC,23 they did not allow the detection of AML LTC-IC from patient samples 2, 5, and 7 as well as HMF. Nevertheless, when IL-3 and SF or FL were added these patient cell samples showed an increase in the output of cytogenetically abnormal CFC to levels at least as high as those obtained with HMF, suggesting that these AML LTC-IC were particularly responsive to one or more of these cytokines and largely failed to produce AML-CFC progeny in their absence.
With four of the AML samples, growth factor supplements to LTC, either as biweekly factor additions or with the use of feeders engineered to produce human cytokines, reduced the proportion of cytogenetically abnormal CFC detected in 5-week-old cultures. In cultures of cells from patients 3, 8, and 10, the predominant effect was the stimulation of normal CFC output by mixed feeders. This finding was not unexpected as we have previously shown that these feeders producing IL-3, SF, and G-CSF enhance normal CFC output from LTC up to 20-fold as compared with control feeders. It is therefore of interest that AML LTC-IC failed to show such a response to these feeders in any of the four cases tested. One possible explanation for this finding is that high concentrations of G-CSF, which was the major difference between cocultures with mixed feeders and the other conditions tested, have little effect on the production of CFC progeny by AML LTC-IC as compared with the stimulation that is seen with normal LTC-IC. In cultures from patient 4 the presence of FL appeared to suppress the production of malignant CFC from LTC. The mechanism whereby this might occur remains to be investigated. Most AML blasts have been shown to express both flt-3 and the SF receptor, c-kit, and to respond to these cytokines in short-term assays (Tables 3, 4, and 5).21,35 36 Because LTC conditions supplemented with either of these factors, with or without IL-3 or mixed feeders, increased the output of cytogenetically abnormal CFC from cultures of five of the AML samples tested, it appears that at least some AML LTC-IC may share these characteristics.
In contrast to the results presented here, early observations showed a disappearance of AML-CFC from standard LTC.15 There are a number of possible explanations for this apparent discrepancy. Firstly, the detection of LTC-IC depends on both the sensitivity of the assay conditions and, in cultures containing a mixture of normal and malignant cells, their relative frequency. Thus, under conditions where AML LTC-IC are relatively rare in the input population, as compared with normal LTC-IC, their recognition may be difficult unless culture conditions are optimal. The use of preestablished feeders in LTC and recombinant cytokines in methylcellulose assay has improved the LTC-IC assay since the original AML LTC were performed allowing malignant LTC-IC to be observed even in the presence of residual normal hematopoiesis. However, if the detection of normal LTC-IC is substantially favored, as it appears to be in some cases when mixed feeders are used, AML LTC-IC can still be difficult to detect. The detection of AML LTC-IC is also made more difficult by their low output of CFC progeny as compared with normal LTC-IC under current assay conditions. A final possibility that would explain the reduced production of AML-CFC in previous LTC experiments, which is not addressed by the current experiments but is suggested by the results of studies in CML,14 is that the standard LTC conditions previously tested may favor the self-maintenance of normal LTC-IC whereas at least some of the conditions tested in the current experiments may provide more equivalent support for primitive normal and malignant progenitors.
In conclusion, these experiments have shown the existence of a rare progenitor among the leukemic cells of patients with AML, which will initiate long-term hematopoiesis in cocultures containing fibroblast feeder layers that support normal LTC-IC. These conditions allow such AML LTC-IC to be quantitated and characterized. Manipulation of the cytokine conditions present in LTC was shown to affect AML LTC-IC or their immediate progeny differently from coexisting normal LTC-IC. Further exploitation of this system should allow other factors important to the regulation of primitive AML progenitors to be elucidated.
Supported by a grant from the National Cancer Institute of Canada with funds from the Terry Fox Foundation. L.E.A. was a student of the Medical Research Council of Canada.
Address reprint requests to Donna E. Hogge, MD, PhD, Terry Fox Laboratory, BC Cancer Agency, 601 W 10th Ave, Vancouver, BC, Canada V5Z 1L3.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal