Abstract
Thrombopoietin (TPO) was evaluated for efficacy in a placebo-controlled study in rhesus monkeys with concurrent administration of either granulocyte/macrophage colony-stimulating factor (GM-CSF) or granulocyte CSF, (G-CSF). Rhesus monkeys were subjected to 5 Gy total-body irradiation (TBI), resulting in 3 weeks of profound pancytopenia, and received either TPO 5 μg/kg intravenously (IV) at day 1 (n = 4), GM-CSF 25 μg/kg subcutaneously (SC) for 14 days (n = 4), TPO and GM-CSF (n = 4), G-CSF 10 μg/kg/d SC for 14 days (n = 3), TPO and G-CSF (n = 4), or placebo (carrier, n = 4; historical controls, n = 8). Single-dose IV treatment with TPO 1 day after TBI effectively counteracted the need for thrombocyte transfusions (provided whenever thrombocyte levels were <40 × 109/L) and accelerated platelet reconstitution to normal levels 2 weeks earlier than placebo controls. TPO/GM-CSF was more effective than single-dose TPO alone in stimulating thrombocyte regeneration, with a less profound nadir and a further accelerated recovery to normal thrombocyte counts, as well as a slight overshoot to supranormal levels of thrombocytes. Monkeys treated with TPO/GM-CSF uniformly did not require thrombocyte transfusions, whereas those treated with GM-CSF alone needed two to three transfusions, similar to the placebo-treated monkeys, which required, on average, three transfusions. Also, reticulocyte production was stimulated by TPO and further augmented in monkeys treated with TPO/GM-CSF. TPO alone did not stimulate neutrophil regeneration, whereas GM-CSF shortened the period of neutrophil counts less than 0.5 × 109/L by approximately 1 week; TPO/GM-CSF treatment elevated the neutrophil nadir, but did not further accelerate recovery to normal values. TPO also augemented the neturophil response to G-CSF, resulting in similar patterns of reconstitution following TPO/G-CSF and TPO/GM-CSF treatment. TPO/GM-CSF resulted in significantly increased reconstitution of CD34+ bone marrow cells and progenitor cells such as GM-CFU and BFU-E. Adverse effects of combining TPO with the CSFs were not observed. It is concluded that (1) a single IV administration of TPO is sufficient to prevent severe thrombocytopenia following myelosuppression, (2) TPO/G-CSF and TPO/GM-CSF treatment result in distinct response patterns, with TPO/GM-CSF being superior to TPO/G-CSF in stimulating thrombocyte and erythrocyte recovery while being equivalent in stimulating neutrophil recovery; and (3) TPO significantly improves the performance of CSFs in alleviating severe neutropenia.
IDENTIFICATION of thrombopoietin (TPO)1-4 as the major regulator of thrombocyte production5,6 has resulted in novel insights into the regulation of immature hematopoietic cell differentiation,7-9 and has potentially provided a therapeutic approach to counteract thrombocytopenic states, particularly those associated with intensive cytoreductive treatment of malignancies. Its pharmaceutical development for the latter application requires demonstration of efficacy in experimental animal models alone and in conjunction with other cytokines. In view of the generally complex receptor distribution patterns of growth factors,10-12 interactions resulting from concurrent administration of the growth factors are difficult to predict by any approach other than detailed experimental animal in vivo studies. We have presently focused on growth factors that are likely to be used clinically with TPO, ie, granulocyte/macrophage colony-stimulating factor (GM-CSF) and granulocyte CSF (G-CSF).
In previous studies in myelosuppressed mice and rhesus monkeys, a supraoptimal dose of human TPO with or without concurrent administration of G-CSF was found to prevent thrombocytopenia, accelerate platelet and red blood cell reconstitution, alleviate neutropenia, and promote recovery of immature bone marrow cells.13-18 The latter observation was unexpected, but was consistent with the demonstration of TPO receptors on immature hematopoietic cells7 and with more recent reports on stimulation of immature cells by TPO.8 9 TPO also effectively promoted the neutrophil response to G-CSF, an effect thought to be mediated by TPO-stimulated bone marrow progenitor cell expansion.
Myelosuppression is a serious complication of current chemotherapy regimens, resulting in life-threatening neutropenia and thrombocytopenia and hampering full deployment of anticancer therapy. Both G-CSF and GM-CSF treatment have become established therapy19-23 to alleviate the cytopenia, particularly the neutropenia resulting from intensive cytoreductive treatment.21 Although G-CSF and GM-CSF are grossly similar in the pharmaceutical profile,24,25 GM-CSF has advantages in that it also stimulates megakaryocytopoiesis and monocyte differentiation.26,27 In addition, G-CSF was found to dampen thrombocyte production. This was also observed in a transplant model in rhesus monkeys,28 as well as after TPO and G-CSF treatment of irradiated mice.16 The beneficial effects of both GM-CSF and G-CSF on neutropenia following cytoreductive treatment are in most studies restricted to approximately a 5-day earlier recovery, or less in dose-intensified chemotherapy,21,23 29 for a total median neutropenia of about 20 to 25 days. It is therefore of considerable importance to select combinations of growth factors that provide optimal costimulatory efficacy.
The study was undertaken (1) to explore the option of limiting the total dose of TPO, based on previous studies in mice30 showing that a single administration of TPO might be sufficient to prevent thrombocytopenia following cytoreductive treatment; and (2) to compare concurrent administration of TPO and GM-CSF versus TPO and G-CSF, and to identify optimal growth factor therapy to counteract both neutropenia and thrombocytopenia. The study involved rhesus monkeys exposed to 5 Gy total-body irradiation (TBI), which results in a profound pancytopenia for 3 weeks, and use of an optimal dose of TPO on the first day and G-CSF or GM-CSF treatment for the first 14 consecutive days after TBI. On-study parameters included, apart from blood cell counts, assessment of immature bone marrow cells and monitoring of adverse effects.
MATERIALS AND METHODS
Animals.Purpose-bred male rhesus monkeys (Macaca mulatta ) weighing 2.5 to 4.0 kg and aged 2 to 3 years were used. The monkeys were housed in groups of 4 to 6 in stainless steel cages in rooms with a reverse-filtered air barrier, normal daylight rhythm, and conditioned to 20°C with a relative humidity of 70%. Animals were fed ad libitum with commercial primate chow and fresh fruits and received acidified drinking water. All animals were free of intestinal parasites and were seronegative for herpes B, simian T-lymphotropic viruses and simian immunodeficiency virus. The animal housing, experiments, and all other conditions were approved by an ethics committee in conformity with legal regulations in The Netherlands.
TBI.Monkeys were irradiated with a single dose of 5 Gy TBI delivered by two opposing x-ray generators operating at a tube voltage of 300 kV and a current of 10 mA. The half-layer thickness was 3 mm Cu. The focus skin distance was 0.8 m and the average dose rate 0.20 to 0.22 Gy/min. During TBI, the animals were placed in a cylindrical polycarbonate cage that rotated slowly (three times per minute) around its vertical axis.
Supportive care.Two weeks before TBI, the monkeys were placed in a laminar-flow cabinet, and the gastrointestinal tract was selectively decontaminated by administering oral ciprofloxacin (Bayer, Mijdrecht, The Netherlands), nystatin (Sanofi, Maassluis, The Netherlands), and polymyxin B (Pfizer, New York, NY). This regimen was supplemented with systemic antibiotics, in most cases ticarcillin (Beecham Pharma, Amstelveen, The Netherlands) and cefuroxim (Glaxo, Zeist, The Netherlands), when leukocyte counts were less than 109/L. Guided by fecal bacteriograms, antibiotics were continued until leukocyte counts increased to more than 109/L. Dehydration and electrolyte disturbances were treated by appropriate fluid and electrolyte administration subcutaneously (SC). The monkeys received irradiated (15 Gy γ-irradiation) platelet transfusions whenever thrombocyte counts were less than 40 × 109/L, packed red blood cells whenever hematocrits were less than 20%, and, occasionally, whole-blood transfusions in case of coincidence of both transfusion criteria. The criterion of transfusion of thrombocytes at counts less than 40 × 109/L was chosen because monkeys already develop a propensity to petechiae and other hemorrhages at this level. These are associated with mortality at the midlethal dose of radiation used.31-33
Test drugs.Recombinant full-length rhesus monkey TPO produced by Chinese hamster ovary cells was supplied by Genentech Inc (South San Francisco, CA). The dose used was 0.5, 5, or 50 μg/kg IV on day 1 after TBI. The dose was diluted to a volume of 1 mL with phosphate-buffered saline (PBS)/0.01% Tween 20 before administration. Placebo-treated monkeys were only given the same volume of diluent. Recombinant human G-CSF (Neupogen; Amgen Inc, Thousand Oaks, CA) was administered at a dose of 10 μg/kg/d SC once daily during days 1 to 14 after TBI. Recombinant human GM-CSF (Leukine; Immunex Corp, Seattle, WA) was given at a dose of 25 μg/kg/d SC once daily during days 1 to 14 after TBI. The daily doses were diluted to a volume of 1 mL in the solution indicated by the suppliers.
TPO levels.Serum for measurement of TPO levels was sampled from the monkeys 24 hours after cytokine administration and stored at −20°C. A full description of the TPO enzyme-linked immunosorbent assay (ELISA) has been reported elsewhere.34,35 Briefly, ELISA plates were incubated overnight at 4°C with 2 μg/mL rabbit F(ab′)2 to human IgG Fc (Jackson ImmunoResearch, West Grove, PA) and 2 hours at room temperature with conditioned medium containing 100 ng/mL mpl-IgG.1 Twofold serial dilutions of samples (starting at 1:10) and standards (recombinant full-length human and/or rhesus TPO) were added to wells and incubated for 1 hour. Bound TPO was detected using biotinylated rabbit antibody to full-length human TPO (Genentech), followed by peroxidase-labeled streptavidin. The range of the assay for rhesus serum samples is 0.32 to 10 ng/mL TPO. The assay preferentially detects active full-length TPO, rhesus TPO equally as well as human TPO, and correlates well with a bioassay using the megakaryoblastic HU-3 cell line.
Study groups.Monkeys were randomly assigned to the treatment groups and received either rhesus TPO IV on day 1 after TBI at a dose of 5 μg/kg (n = 4), 0.5 μg/kg (n = 2), or 50 μg/kg (n = 1), GM-CSF at a dose of 25 μg/kg/d SC from days 1 to 14 (n = 4), TPO and GM-CSF (TPO/GM-CSF, n = 4), G-CSF at a dose of 10 μg/kg/d SC (n = 3), TPO and G-CSF (TPO/G-CSF, n = 4), or placebo (n = 4; historical controls, n = 8). The TPO dose of 5 μg/kg on day 1 after TBI was administered to the monkeys that also received GM-CSF or G-CSF SC for 14 consecutive days.
Bone marrow aspirates.Bone marrow was aspirated under neuroleptic anesthesia using Ketalar (Apharmo, Arnhem, The Netherlands) and Vetranquil (Sanofi, Maassluis, The Netherlands). Small bone marrow aspirates for analytical purposes were taken from the shaft of the humerus using pediatric spinal needles and collected in bottles containing 2 mL Hanks buffered HEPES solution (HHBS) with sodium heparin 200 IU mL (Leo Pharmaceutical Products, Weesp, The Netherlands). Low-density cells were isolated using Ficoll separation (density = 1.077; Nycomed Pharma, Oslo, Norway).
Colony assays.Cells were plated in 35-mm dishes (Becton Dickinson, Leiden, The Netherlands) in 1 mL α-DMEM (GIBCO, Gaithersburg, MD) containing 0.8% methylcellulose, 5% fetal calf serum (FCS), and additives as described previously.36-38 For burst-forming units–erythroid (BFU-E), cultures were supplemented with hemin (2 × 10−4 mol/L), human recombinant erythropoietin (4 U/ml; Behring, Germany), and Kit ligand ([KL] 100 ng/mL; kindly provided by Dr S. Gillis, Immunex, Seattle, WA). For granulocyte/macrophage colony-forming units (GM-CFU), cultures were supplemented with recombinant human GM-CSF (5 ng/mL; Behring), recombinant rhesus monkey interleukin-3 (30 ng/mL) produced in Bacillus licheniformis and purified as described previously,39 40 and KL. Low-density cells were plated at 5 × 104 per dish in duplicate. Colony counts were calculated per milliliter of bone marrow aspirated using the recovery of cells over the Ficoll density gradient. Colony numbers represent the mean ± SD of bone marrow samples of individual monkeys.
Hematologic examinations.Complete blood cell counts were measured daily using a Sysmex F-800 hematology analyzer (Toa Medical Electronics Co, Kobe, Japan). The differential of the nucleated cells was determined by standard counting after May-Grünwald-Giemsa staining. For reticulocyte measurements, 5 μL EDTA blood was diluted in 1 mL PBS/EDTA/azide and 1 mL thiazole orange dilution was added, using thiazole at a final concentration of 0.5 μg/mL. Measurements were made on a FACScan (Becton Dickinson, Leiden, The Netherlands) and analyzed using the Reticount software.
Measurement of surface antigens.Once weekly, a FACScan analysis was made on peripheral blood and bone marrow samples on the following surface antigens: CD8, CD4, CD20, CD11b, CD56, CD16, and CD34. Directly labeled monoclonal antibodies (MoAbs) were used for CD8, CD4, CD20, CD56, and CD16 (Leu 2a-FITC, Leu 3a-PE, Leu 16-PE, Leu 19-PE, and Leu 11aFITC [Becton Dickinson], respectively). For CD11b, the MoAb MO1-FITC (Coulter Immunology, Hialeah, FL) was used, and for CD34, an MoAb against human CD34 (MoAb 566) that had been fluoresceinated with FITC (Sigma, St Louis, MO) according to standard procedures. Whole blood or bone marrow (0.5 mL) was lysed in 10 mL lysing solution (8.26 g ammonium chloride, 1.0 g potassium bicarbonate, and 0.037 g EDTA per liter) for 10 minutes at 4°C. After lysis, the cells were washed twice with HHBS containing 2% FCS and 0.05% (wt/vol) sodium azide (HFN). The cells were resuspended in 100 μL HFN containing 2% normal monkey serum to prevent aspecific binding of the MoAbs. MoAbs were added in a volume of 5 μL and incubated for 30 minutes on ice. After two washes, the cells were measured on the flow cytometer. Ungated list-mode data were collected for 10,000 events and analyzed using the Lysis II software (Becton Dickinson).
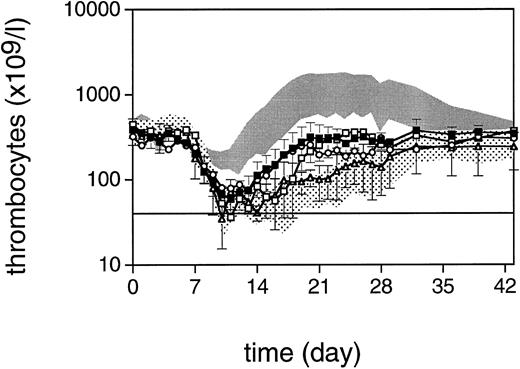
Thrombocyte counts after 5 Gy TBI (day 0) for monkeys treated with TPO 5 μg (▪, n = 4), TPO 50 μg (□), TPO 0.5 μg (○, n = 2), and the concurrent placebo treatment (▵, n = 4). The lower shaded area represents the mean ± SD of 12 control monkeys; the upper shaded area is the mean ± SD of 4 monkeys treated with human TPO for 21 days after irradiation. Data represent the arithmetic mean ± SD of the various treatment groups. The horizontal line defines the level of thrombocytopenia (40 × 109/L) below which thrombocyte transfusions are given.
Thrombocyte counts after 5 Gy TBI (day 0) for monkeys treated with TPO 5 μg (▪, n = 4), TPO 50 μg (□), TPO 0.5 μg (○, n = 2), and the concurrent placebo treatment (▵, n = 4). The lower shaded area represents the mean ± SD of 12 control monkeys; the upper shaded area is the mean ± SD of 4 monkeys treated with human TPO for 21 days after irradiation. Data represent the arithmetic mean ± SD of the various treatment groups. The horizontal line defines the level of thrombocytopenia (40 × 109/L) below which thrombocyte transfusions are given.
Transfusion Requirements and Blood Cell Regeneration After 5 Gy TBI and Growth Factor Treatment
| Treatment . | No. of Monkeys . | No. of Transfusions . | Thrombocytes . | Reticulocytes . | Neutrophils . |
|---|---|---|---|---|---|
| . | . | . | >40 × 109/L (d) . | >1% (d) . | >0.5 × 109/L (d) . |
| TPO | 4 | 0/0/1/1* | NA | 14.2 ± 3.6* | 21.5 ± 2.4 |
| TPO + GM-CSF | 4 | 0/0/0/0* | NA | 10.5 ± 1.7* | 14.5 ± 2.6* |
| TPO + G-CSF | 4 | 0/0/1/0* | NA | 13.2 ± 2.6* | 14.2 ± 1.2* |
| GM-CSF | 4 | 3/2/2/2 | 13.2 ± 1.7 | 16.2 ± 2.5* | 17.7 ± 2.2* |
| G-CSF | 3 | 1/2/1 | 13.3 ± 1.5 | 17.3 ± 0.6 | 19.7 ± 3.2 |
| Placebo | 4 | 3/6/3/3 | 19.5 ± 7.0 | 20.5 ± 2.1 | 22.5 ± 2.4 |
| Treatment . | No. of Monkeys . | No. of Transfusions . | Thrombocytes . | Reticulocytes . | Neutrophils . |
|---|---|---|---|---|---|
| . | . | . | >40 × 109/L (d) . | >1% (d) . | >0.5 × 109/L (d) . |
| TPO | 4 | 0/0/1/1* | NA | 14.2 ± 3.6* | 21.5 ± 2.4 |
| TPO + GM-CSF | 4 | 0/0/0/0* | NA | 10.5 ± 1.7* | 14.5 ± 2.6* |
| TPO + G-CSF | 4 | 0/0/1/0* | NA | 13.2 ± 2.6* | 14.2 ± 1.2* |
| GM-CSF | 4 | 3/2/2/2 | 13.2 ± 1.7 | 16.2 ± 2.5* | 17.7 ± 2.2* |
| G-CSF | 3 | 1/2/1 | 13.3 ± 1.5 | 17.3 ± 0.6 | 19.7 ± 3.2 |
| Placebo | 4 | 3/6/3/3 | 19.5 ± 7.0 | 20.5 ± 2.1 | 22.5 ± 2.4 |
Data for individual monkeys or the mean ± SD are shown.
Abbreviation: NA, not applicable, ie, level <40 × 109/L not reached in the majority of monkeys.
Statistically significantly different from placebo-treated monkeys (P < .05).
Statistics.Standard deviations were calculated and are presented in the text and figures on the assumption of a normal distribution. The significance of differences was calculated by Fisher's exact test for categorical data, and for continuous data by a one-way analysis of variance followed by a nonpaired Student's t-test.
RESULTS
Dose of rhesus monkey TPO and pattern of thrombocyte reconstitution.Based on data in normal monkeys41 and extrapolation of doses used in mice,30 as well as the supraoptimal dose of 10 μg/kg human TPO in a previous study,13 a single IV dose of 5 μg/kg rhesus monkey TPO at day 1 after TBI was considered optimal. The thrombocyte regeneration of four monkeys treated in this way is shown in Fig 1 in comparison to placebo controls and the previous data on human TPO administered for 21 consecutive days at a dose of 10 μg/kg/d. The data for the four concurrent control monkeys have been included in Fig 1 in addition to the eight historical controls; significant differences were not observed in the regeneration patterns of any of the blood cell lineages between these groups of controls. The single-dose treatment was effective in that the four TPO-treated monkeys needed only two thrombocyte transfusions (one in each of two monkeys), as opposed to the four concurrent placebo controls, which needed a total of 13 transfusions (mean, 3; range, 1 to 6) since their thrombocytes had decreased to less than 40 × 109/L; this is statistically significant (P < .04; Table 1). In addition, TPO-treated monkeys displayed a clearly accelerated thrombocyte reconstitution and reached normal thrombocyte levels 2 weeks before the placebo-treated controls (P < .03).
To validate the choice of the dose of 5 μg/kg IV further, one monkey was treated with a 10-fold higher dose and two monkeys with a 10-fold lower dose. The results are also presented in Fig 1. Rhesus monkey TPO at the single dose of 50 μg/kg IV was effective in preventing thrombocyte transfusions, with a nadir for thrombocytes of 75 × 109/L at day 13. Thrombocyte reconstitution following this dose precisely coincided with the mean values for monkeys treated with the 5-μg/kg dose, on which basis we considered that the latter dose provided the maximal stimulation to be expected from a single administration of TPO 1 day after TBI. Monkeys treated with 0.5 μg/kg both had nadirs lower than 40 × 109/L, and needed one and two thrombocyte transfusions, respectively, providing a further validation of the dose of 5 μg/kg as being close to the minimum required for a maximal response.
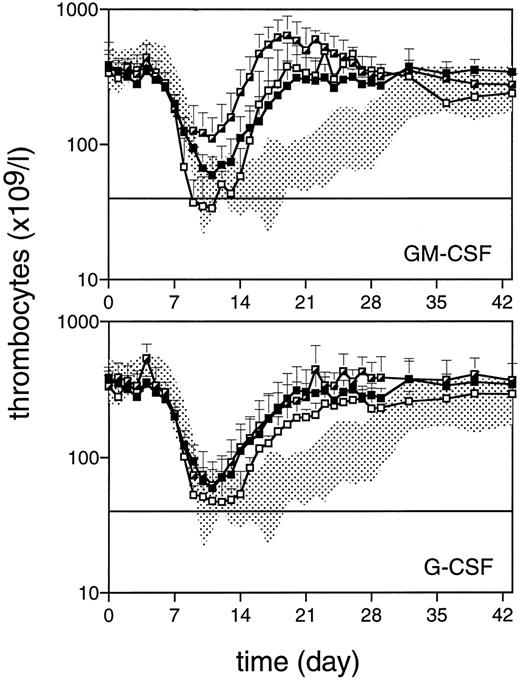
Thrombocyte counts after 5 Gy TBI (day 0) for monkeys treated with TPO (▪, n = 4), TPO/GM-CSF (┌, n = 4), or GM-CSF (□, n = 4) in the upper panel and TPO/G-CSF (┌, n = 4) or G-CSF (□, n = 3) in the lower panel. Data represent the arithmetic mean ± SD of the various treatment groups. The horizontal line defines the level of thrombocytopenia (40 × 109/L) below which thrombocyte transfusions are given.
Thrombocyte counts after 5 Gy TBI (day 0) for monkeys treated with TPO (▪, n = 4), TPO/GM-CSF (┌, n = 4), or GM-CSF (□, n = 4) in the upper panel and TPO/G-CSF (┌, n = 4) or G-CSF (□, n = 3) in the lower panel. Data represent the arithmetic mean ± SD of the various treatment groups. The horizontal line defines the level of thrombocytopenia (40 × 109/L) below which thrombocyte transfusions are given.
TPO levels.TPO levels were measured in serum collected 24 hours after cytokine administration (day 2 after irradiation), and for some monkeys also in samples taken later in the first week after irradiation. Levels measured were 1.9 ± 0.5 ng/mL for TPO-treated monkeys and 2.5 ± 0.4 for monkeys treated with TPO and G-CSF or GM-CSF; this difference is not significant. TPO levels were not different for monkeys treated simultaneously with either TPO/G-CSF or TPO/GM-CSF. Levels at day 3 or 4 were just above the detection limit of the assay (31.2 pg/mL in a 1:10 dilution) at about 0.4 ng/mL in some monkeys, and below the detection limit in others.
Comparison of peripheral blood cell counts in monkeys treated with TPO/GM-CSF and TPO/G-CSF.In contrast to monkeys treated with TPO alone, of which two needed a single thrombocyte transfusion, monkeys treated with TPO/GM-CSF remained completely transfusion-free and showed an even more elevated nadir, with two of four monkeys never reaching thrombocyte levels less than 100 × 109/L. Recovery to normal values was accelerated by 23 days compared with placebo-treated monkeys (P < .003), and an overshoot to supranormal values was apparent, which returned to normal values after cessation of GM-CSF treatment (Fig 2). Monkeys treated with TPO/G-CSF had a thrombocyte regeneration pattern identical to that of the monkeys treated with TPO alone, and one of the four monkeys needed a thrombocyte transfusion. Monkeys treated with GM-CSF or G-CSF alone all needed one to three transfusions. Although there was no significant difference versus the placebo-treated controls in the nadir and transfusion requirement, the subsequent thrombocyte regeneration was faster than that of the placebo controls for both G-CSF– and GM-CSF–treated monkeys (Fig 2).
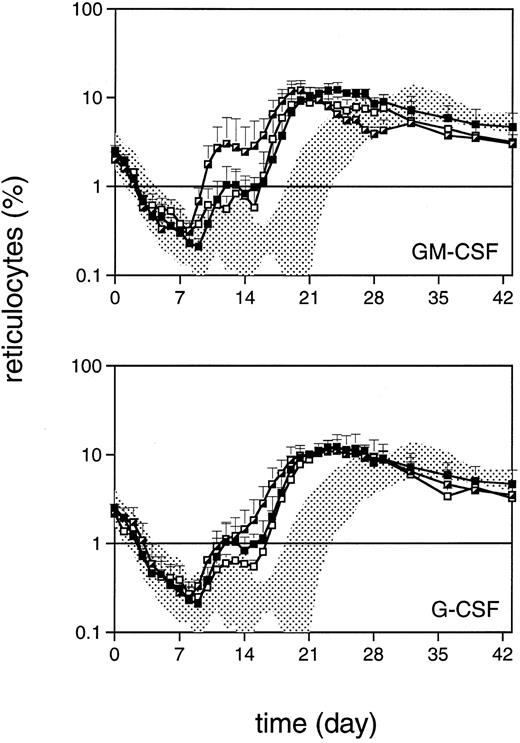
Reticulocyte regeneration to values more than 1% occurred approximately 1 week earlier in TPO-treated monkeys than in placebo-treated controls (P < .03) and showed a biphasic response (Fig 3). Again, TPO/G-CSF was similar to TPO alone, whereas TPO/GM-CSF was more effective, further accelerating the reconstitution by 4 days. GM-CSF alone also slightly accelerated reticulocyte regeneration by 4 days (P < .04) and G-CSF alone by 3 days (Fig 3). This pattern was also reflected in the recovery of red blood cell counts after the initial decline, which is largely due to diagnostic bleeding, with TPO/GM-CSF monkeys being the fastest to recover (data not shown).
Reticulocyte regeneration after 5 Gy TBI (day 0). Symbols are as in Fig 2. The horizontal line defines the level of 1%, a regeneration marker.
Reticulocyte regeneration after 5 Gy TBI (day 0). Symbols are as in Fig 2. The horizontal line defines the level of 1%, a regeneration marker.
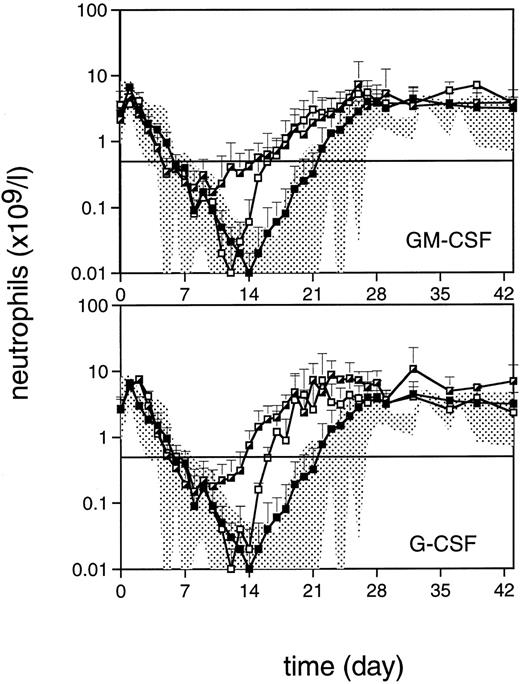
Neutrophil reconstitution for TPO-treated monkeys was within the range of that for placebo-treated monkeys, with recovery to levels more than 0.5 109/L occurring at about day 21 (Fig 4). Both TPO/GM-CSF and TPO/G-CSF treatment elevated the neutrophil nadir, reflecting an accelerated recovery to reach levels more than 0.5 × 109/L 1 week earlier than TPO and placebo treatment. Recovery to normal values was slightly more pronounced in monkeys treated with TPO/G-CSF versus TPO/GM-CSF, but the difference did not reach statistical significance. Monkeys treated with G-CSF alone and GM-CSF alone had similar neutrophil recovery patterns, reaching levels more than 0.5 × 109/L 5 days before placebo-treated monkeys (P < .03, Fig 4). Treatment with CSFs resulted in a slightly earlier but not detectably less profound neutrophil nadir.
Neutrophil regeneration after 5 Gy TBI (day 0). Symbols are as in Fig 2. The horizontal line defines the level of 0.5 × 109/L.
Neutrophil regeneration after 5 Gy TBI (day 0). Symbols are as in Fig 2. The horizontal line defines the level of 0.5 × 109/L.
Transfusion data and regeneration parameters are summarized in Table 1, which shows that the TPO, TPO/GM-CSF, and TPO/G-CSF monkey groups are all significantly different in transfusion requirements versus the placebo controls. In addition, the TPO/GM-CSF group differed significantly from the GM-CSF group in transfusion requirement (P < .003), but not from the TPO-treated group (P = .13).
White blood cell subsets measured by flow cytometry.Flow cytometry was performed on lysed peripheral blood cell samples to assess regeneration of several subsets of white blood cells. CD11b+ cells, representing granulocytes and monocytes, showed a pattern similar to neutrophil regeneration, in which both TPO/G-CSF– and TPO/GM-CSF–treated monkeys had a higher nadir and an accelerated recovery to normal values. Placebo-treated monkeys recovered to the lower range of baseline levels at day 28, TPO-treated monkeys at day 21, TPO/G-CSF–treated monkeys at day 14, and TPO/GM-CSF–treated monkeys at day 17. G-CSF-alone– and GM-CSF-alone–treated monkeys recovered similarly to TPO/GM-CSF–treated monkeys, but had a more profound nadir. The difference between TPO-alone and placebo-treated monkeys could be attributed to a more rapid monocyte regeneration (data not shown) and not to differences in neutrophil regeneration (Fig 4).
CD8+ lymphocytes recovered to normal levels within the observation period of 6 weeks in all treatment groups without significant differences. All animals had subnormal levels of CD4+ T lymphocytes and CD20+ B lymphocytes at the end of the observation period. These levels were not significantly different between the various treatment groups (data not shown).
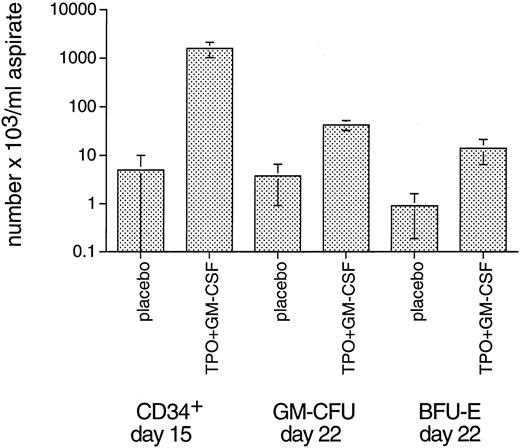
Bone marrow cellularity and progenitor cell content.Bone marrow aspirations were performed before TBI and once weekly thereafter. Cellularity was very low in the first week after TBI in all monkeys, not yielding sufficient numbers of cells to perform colony assays. Two weeks after irradiation, both TPO/G-CSF and TPO/GM-CSF monkeys started to show repopulation of the bone marrow. Three weeks after TBI, all treatment groups except for placebo-treated monkeys had normal bone marrow cellularity. Placebo-treated monkeys did not have near-normal cell numbers until week 4 after TBI. The stimulating effect of the single administration of 5 μg/kg TPO on bone marrow cellularity and CD34+ cell and clonogenic progenitor cell recovery was considerably less prominent than reported in the previous TPO/G-CSF study using the same myelosuppression model13 for the human TPO dose of 10 μg/kg/d administered for 21 consecutive days after TBI. This is understandable, since the total TPO dose is much lower. Since the variance of this type of data is inevitably large due to variations of bone marrow content at the puncture site and admixture with peripheral blood cells, we present only the most relevant data, ie, those obtained in the TPO/GM-CSF group of monkeys for CD34+ cells at day 15 and for GM-CFU and BFU-E at day 22 after TBI (Fig 5). At day 15 after TBI, CD34+ cell numbers significantly exceeded those of placebo controls in the TPO/GM-CSF (P < .03; Fig 5) and G-CSF (P < .01; not shown) treatment groups. For GM-CFU, only the TPO/GM-CSF group significantly exceeded (P < .01) the placebo controls by approximately 1 log at day 22 after TBI (Fig 5). The TPO/GM-CSF group was also richer in GM-CFU than animals treated with GM-CSF alone (P < .04), underscoring the significant impact of TPO if combined with GM-CSF. As for BFU-E, similar patterns were observed, with only the TPO/GM-CSF treatment group doing significantly (P < .02) better than the placebo controls (Fig 5).
Bone marrow CD34+ cells and GM-CFU 2 and 3 weeks after 5 Gy TBI, respectively (arithmetic mean ± SE). Since the variance of this type of datum is inevitably large due to variations of bone marrow content at the puncture site and admixture with peripheral blood cells, in addition to possible variations in radiation sensitivity and exponential reconstitution, the other groups did not show significant differences versus the placebo controls.
Bone marrow CD34+ cells and GM-CFU 2 and 3 weeks after 5 Gy TBI, respectively (arithmetic mean ± SE). Since the variance of this type of datum is inevitably large due to variations of bone marrow content at the puncture site and admixture with peripheral blood cells, in addition to possible variations in radiation sensitivity and exponential reconstitution, the other groups did not show significant differences versus the placebo controls.
Other study parameters and febrile episodes.No abnormalities were observed for most of the serum clinical chemistry. Albumin concentrations were stable at about 40 g/L, except for GM-CSF monkeys, which developed edema (vide infra). Electrolyte disturbances due to gastrointestinal radiation damage were minor during the first week after TBI. The liver enzymes alkaline phosphatase, ALAT, and ASAT also showed only minor changes. The number of febrile episodes (axillary temperature >40°C) was 5.3 ± 3.4 days, with no significant differences between the various treatment groups, although TPO/GM-CSF and TPO/G-CSF monkeys tended to have more febrile days. There was no association between febrile episodes and C reactive protein (CRP) levels.
Adverse effects of growth factor treatment.Two of four GM-CSF–treated monkeys developed generalized edema ascribed to capillary leakage, which resolved following discontinuation of GM-CSF. In comparison to the other 26 consecutive monkeys that did not receive GM-CSF, the phenomenon proved statistically significant (P = .02, Fisher's exact test). None of four TPO/GM-CSF–treated monkeys developed edema; the difference with the GM-CSF–treated monkeys is statistically nonsignificant. Apart from slight local irritation at the injection site of GM-CSF and to a lesser extent at that of G-CSF, other adverse effects were not observed.
DISCUSSION
The present preclinical evaluation demonstrates that a single IV dose of TPO administered 1 day after a myelosuppressive dose of radiation that results in 3 weeks of cytopenia in placebo-treated controls is sufficient to virtually prevent the need for thrombocyte transfusions and to accelerate thrombocyte reconstitution to normal levels by 2 weeks. Furthermore, the results demonstrate that TPO/GM-CSF and TPO/G-CSF treatment display distinct response patterns among three major peripheral blood cell types. Coadministration of TPO/GM-CSF augmented thrombocyte, red blood cell, and neutrophil production over either of the individual growth factors alone, whereas in the TPO/G-CSF group only neutrophil reconstitution appeared to benefit from the combination of growth factors.
The observation that a single IV dose of TPO shortly after intensive cytoreductive treatment is sufficient to significantly alleviate the course of thrombocytopenia is of considerable practical and clinical importance. The finding is consistent with the data in mice,30 with the kinetics of TPO-stimulated thrombocyte reconstitution in nonhuman primates indicating a very early action of TPO,13,14 and with the decline in response when TPO administration is delayed.42 These studies all point to a critical time phase early after TBI during which TPO has to be administered to achieve an optimal response. This could be explained by a decline in the number of bone marrow TPO-responsive target cells as a function of time after irradiation, possibly due to the absence of sufficiently high concentrations of TPO. This may be due to either prevention of physiologic death or apoptosis in the presence of TPO similar to that identified for erythropoietin and red blood cell progenitors,43 44 or protection of TPO-responsive progenitors from radiation-induced cell death by TPO. Elucidation of such mechanisms not only will provide further insight into the physiologic function of TPO, but also will be of considerable importance to achieve the maximum clinical benefit of its therapeutic use. Although the data show that a single IV dose of 5 μg/kg TPO is sufficient to prevent thrombocytopenia at the level of myelosuppression chosen, this finding does not preclude that clinical cytoreductive therapy requires a more intensive TPO treatment regimen.
A central issue of TPO treatment is prevention of bleeding as a consequence of myelosuppression. Our policy to transfuse donor thrombocytes at a level of 40 × 109/L coincides with the first appearance of petechiae and other bleeding, and was chosen to prevent undue deaths due to hemorrhage. For obvious reasons, this level is higher than used for human patients where instructions can be given to the patients and sensitization to alloantigens should be avoided. However, as can be extrapolated from the postirradiation decrease in thrombocyte counts after the first week and the first ascending counts in the third week after TBI in placebo-treated monkeys, without transfusions, the thrombocytes would have decreased to levels less than 10 × 109/L within 2 days after the first transfusion (Fig 1). It can thus be concluded that TPO treatment that prevented the decline of thrombocyte counts to levels less than 40 × 109/L early in the second week after irradiation also effectively prevented the propensity to bleeding (Fig 1).
Administration of TPO/GM-CSF proved to be superior to all other growth factors or combinations of growth factors studied for stimulating thrombocyte reconstitution. GM-CSF alone did not influence the thrombocyte nadir or significantly reduce the need for thrombocyte transfusions. However, it was as effective as the single administration of TPO alone in stimulating post nadir thrombocyte production. The augmented thrombocyte reconstitution of the TPO/GM-CSF monkeys may reflect synergism of the two growth factors, since the initial recovery of both thrombocytes (Fig 2) and reticulocytes (Fig 3) in the TPO/GM-CSF group exceeded the sum of those in the monkeys treated with either of the growth factors alone. A stimulatory action of GM-CSF on megakaryocytes resulting in increased thrombocyte production has been described in normal nonhuman primates,26,45 although the results of in vivo treatment with GM-CSF on thrombocyte reconstitution after cytotoxic insult to the bone marrow have always been heterogenous46-48 and, in retrospect, may have been codependent on variations in endogenous TPO levels. From TPO levels measured 24 hours after injection, we could not conclude that there was a major change in TPO pharmacokinetics due to coadministration of GM-CSF as a basis for the augmented recovery of thrombocytes. The same observation was made for G-CSF. Surprisingly, the 10-μg/kg/d dose (for 2 weeks) of G-CSF also appeared to stimulate thrombocyte production to a certain extent, in contrast to the 5-μg/kg/d dose (for 3 weeks), which was used in a previous study in the same nonhuman primate model,13 but the result is consistent with that in a similar model in which 10 μg/kg/d was also used.14 We previously observed, in the same myelosuppression model as used here, a dampening effect of G-CSF on TPO-stimulated supranormal thrombocyte production.13 This was also seen in mice.16 Meanwhile, in a transplant model in rhesus monkeys involving 8 Gy TBI followed by infusion of highly purified stem cells, protracted thrombocytopenia significantly related to G-CSF treatment was encountered in a few cases.28 The reported effects of G-CSF on thrombocyte production are ambiguous: most reports mention no effects at all,19,49-51 some a positive effect,52 and others a negative effect.53-55 The causes of the variable reaction of thrombocytes to G-CSF treatment are not elucidated and may be governed by complex mechanisms; it should be noted that G-CSF receptors are present on thrombocytes.56 We conclude provisionally that combining TPO and G-CSF treatment may have a variable, as-yet-unpredictable, and occasionally adverse outcome.
TPO augmented the neutrophil reconstitution stimulated by GM-CSF as well as G-CSF, which might be of considerable clinical significance, since in the reported clinical trials the effects of the CSFs in general have been modest,22,23,29 similar to the results presented here in a preclinical nonhuman primate model. In particular, the neutrophil nadir appeared to be greatly improved in TPO/CSF-treated animals. G-CSF or GM-CSF treatment alone did not appreciably influence the neutrophil nadir, but accelerated regeneration afterward with a time course similar to that for clinically acceptable neutrophil counts. We attributed the advantageous effect of TPO to expansion of immature cells along multiple hematopoietic lineages,7-9,13 57 thus making more G- or GM-CSF target cells available for myelopoiesis. From the kinetics of neutrophil reconstitution, it is also apparent that the effect originates from stimulation at an early stage after TBI (Fig 4).
The slightly more rapid reconstitution of neutrophils following TPO/G-CSF administration should be weighed against the reported dampening effect of G-CSF treatment on thrombocyte recovery,13,15 although this effect was not apparent in the present study. However, the greater target cell range of the TPO/GM-CSF combination should be balanced against the slightly higher incidence of reported adverse effects of GM-CSF,22,23 as also seen in the present study. The development of edema in two monkeys treated with GM-CSF was an unwanted adverse effect of the cytokine treatment, a rare event that has been previously reported, albeit only at higher doses of GM-CSF.22,23,58 For as-yet-unexplained reasons, edema was not observed in monkeys treated with TPO/GM-CSF; however, this difference is not statistically significant. Apart from stimulation of thrombocyte production, a prominent feature of TPO administration is the accelerated reconstitution of immature bone marrow cells, consistent with the presence of TPO receptors and stimulatory effects in vitro.7-9 Accelerated recovery of bone marrow cellularity, CD34+ cells, and clonogenic progenitor cells such as GM-CFU and BFU-E following supraoptimal TPO treatment has been reported previously.13 14 The present study with a much more limited TPO dose confirmed these data and demonstrated that the effect is reinforced by combining TPO with GM-CSF. This places TPO among the few growth factors that can presently be safely used clinically to promote the recovery of immature bone marrow cells in an early stage after cytoreductive therapy without notable adverse effects. As a direct implication, the future development of TPO treatment regimens in the clinic should be directed not only at optimal thrombocyte recovery but also at the most optimal reconstitution of immature bone marrow cells.
ACKNOWLEDGMENT
The authors acknowledge the significant contributions of Dr F. de Sauvage and Dr Jeff Gorell, Genentech Inc, during the cloning, expression, and purification of recombinant rhesus TPO; the advice of Dr Mary Napier and Dr Robert Cohen; the technical assistance of Trudy Visser, Hannie Busking-van der Lelie, and Dorinde Kieboom-Pluimes; the care of the animals by Albert Kloosterman and Ron Briegoos; and Dr Albertus W. Wognum for assistance with flow cytometric analysis and a critical reading of the manuscript.
Supported in part by The Netherlands Cancer Foundation Koningin Wilhemina Fonds, the Dutch Organization for Scientific Research NWO, the Royal Netherlands Academy of Arts and Sciences, and Contracts of the Commission of the European Communities.
Address reprint requests to Gerard Wagemaker, PhD, Institute of Hematology, H Ee 1314, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal