Abstract
Stem cell factor (SCF) is synthesized as both soluble (S) and membrane-associated (MA) proteins. Indirect insight into the function of MA and S isoforms of SCF has come from studies performed in Steel (Sl) mutant mice. However, the physiologic role(s) of these two isoforms remain unknown. In an attempt to better understand the in vivo role of c-kit/SCF interactions on various cell lineages, transgenic mice were generated that overexpress MA isoform of human SCF (hSCF). In murine cells, hSCF behaves as an antagonist to normal SCF function, due to interference with the interaction between endogenous murine SCF and its receptor, c-kit, encoded by the dominant white spotting (W) gene. Mice expressing the hSCF transgene display a variety of phenotypic abnormalities, which are accentuated when combined with W alleles. Here we show that mice homozygous for the hSCF transgene demonstrate a coat color deficiency seen in some mice homozygous for mild W alleles. Specifically, homozygous hSCF transgenic mice (hSCF220) display a pronounced forehead blaze, with additional white spots over the cervical region, as well as a very large belly spot. Doubly heterozygous animals that carry both a mutated W allele and the hSCF transgene also display an unusual pigment defect and a dramatic reduction in the number of dermal mast cells. Furthermore, overexpression of MA hSCF in the thymus results in abnormal thymocyte differentiation and proliferation, which is associated with reduced mitogen activated protein (MAP) kinase activation. Thus, MAP kinase activation by a receptor tyrosine kinase, such as c-kit, may be critical for the differentiation of thymocytes in vivo.
THE PHENOTYPIC DEFECTS in mice with mutations affecting the dominant white spotting (W) and Steel (Sl) loci (reduction in pigment cells, sterility, and macrocytic anemia with mast cell deficiencies) demonstrate the critical nature of the proteins encoded by these loci in the normal development of hematopoietic stem and progenitor cells, melanocytes, and germ cells.1 The W locus encodes the receptor tyrosine kinase c-kit,2,3 and the Sl locus encodes soluble (S) and membrane-associated (MA) forms of its ligand, known as stem cell factor (SCF) (also called mast cell growth factor, kit ligand, and steel factor).4-11 The two major isoforms of SCF arise in both mice and humans via alternatively spliced transcripts, which result in MA glycoproteins of 248 (SCF248) and 220 (SCF220) amino acids (aa).7,10 SCF248 is rapidly cleaved to release an S protein of 164/165 aas. SCF220, which lacks the proteolytic cleavage site encoded by the differentially spliced exon 6 sequences, remains predominantly MA, but can be slowly released from the cell surface via the use of an alternative proteolytic cleavage site in exon 7.7,10,12 Little is known regarding the physiologic role(s) of these two isoforms during embryonic development or in the postnatal biology of the mouse, although distinct tissue distribution of each isoform exists.7
Sl mutant alleles that have the most severe effects generally resulting in death in utero or shortly after birth, are due to deletions or mutations that produce complete loss of SCF function (such as mice homozygous for the Sl mutation).1,10 In contrast, mice expressing the Steel-dickie (Sld) allele are viable, despite exhibiting a moderately severe macrocytic anemia, a complete lack of skin pigmentation, a profound mast cell deficiency, and sterility.1,10 It has been shown that the Sld allele encodes a truncated transcript that lacks the coding sequence for both the transmembrane and the cytoplasmic domains of the wild-type (wt) SCF protein.13 Notably, expression of the Sld cDNA in transfected COS cells results in secretion of a biologically active truncated SCF protein that is capable of inducing mast cell proliferation, but that fails to result in the appearance of MA form of SCF.10 Although, it is not clear to what extent S SCF protein is produced by Sld mice in vivo, the phenotypic abnormalities of these mice suggest that an MA form of SCF is necessary for a normal phenotype. Despite the availability of naturally occurring Sl mutants, the physiologic role(s) of MA SCF still remains enigmatic.
It is likely that a major biologic function of SCF is to promote the survival of cellular lineages that express c-kit. For example, certain c-kit positive cells such as melanocytes and mast cells reside in the tissues for long periods of time without undergoing proliferation. Several lines of evidence indicate that in vitro SCF can promote mast cell survival.14 Also, Sld mice, which lack MA SCF, cannot support the survival of normal mast cells injected into the dermis.10 In contrast, such mast cells survive in the skin of homozygous W; viable dominant spotting (Wv) mice.10 These findings indicate that the production of MA SCF is critical for the survival of mast cells in the dermis of mice in vivo or, alternatively, that production of truncated soluble SCF in Sld mice is not sufficient to maintain the viability of normal mast cells in the skin in vivo.
The expression of c-kit in the majority of thymic subsets15,16 and its interaction with SCF have been reported to be involved in early thymocyte proliferation.15,17 c-kit positive CD3−CD4−CD8− or more primitive CD3−CD41oCD8− thymocytes can repopulate the irradiated adult thymus, and the proliferation of c-kit positive CD3−CD4−CD8− thymocytes in the presence of SCF and interleukin (IL)-7 is blocked by a neutralizing antibody against c-kit.15 In a more recent study, thymocytes undergoing positive selection were shown to be TCR1o c-kit positive with intermediate levels of CD4 and CD8.16 Additionally, transcripts for both the S and the MA isoforms of SCF have been described in adult thymus.7 These data suggest a physiologic role for c-kit and SCF in thymocyte development, however, the definitive role(s) for SCF isoforms in T-cell development in vivo remains unclear.
In an attempt to better understand the in vivo role of c-kit/SCF interactions, we generated transgenic mice that express the MA isoform of hSCF. Previously it has been shown that hSCF binds to murine c-kit with a very low affinity and stimulates the proliferation/survival of murine c-kit+ cells only at very high concentrations.9,18 We have recently demonstrated that transgenic mice hemizygous for the MA form of hSCF display a coat color phenotype indistinguishable from some alleles of W.19 Compound heterozygous mice expressing both a mutated c-kit allele and the MA hSCF transgene display a more severe coat color abnormality.19 This phenotype is apparently due to downregulation of endogenous MA SCF and occupancy of murine c-kit by MA hSCF in vivo, interfering with normal signaling events that lead to proliferation and/or survival of c-kit positive cells.19 The impact of overexpression of this transgene on various c-kit positive lineages was not previously examined. In the present study, we show that hSCF transgene expression interferes with the normal development of melanocytes, dermal mast cells, and thymocytes in vivo. We show that mice homozygous for the MA hSCF transgene are affected with a more pronounced coat color phenotype. In addition, mice doubly heterozygous for a mutated c-kit allele and the MA hSCF transgene, demonstrate a significant reduction in total dermal mast cells. Finally, thymocytes from these mice show a defect in differentiation associated with altered downstream signaling as determined by reduced mitogen activated protein (MAP) kinase activation. Together, these data show that the cellular interactions between c-kit and SCF are required in vivo for the normal development of melanocytes, mast cells, and thymocytes.
MATERIALS AND METHODS
Animals.To examine the in vivo role of the two isoforms of SCF on various lineages in naturally occurring Steel (Sl ) mutants (ie, Sl/Sl and Sl/Sld), we have recently generated transgenic mice that overexpress either the S or the MA isoforms of SCF in C3H/HeJ background (unpublished data). These mice were first crossed to WC/ReJ-Sl/+ mice to obtain Sl/+ mice that overexpress either form of SCF. These mice were identified based on their phenotype (ie, forehead blaze and diluted belly) and Southern blot analysis. Transgene positive Sl/+ male mice were further backcrossed to WC/ReJ-Sl/+ or to C57BL6-Sld/+ (Jackson Laboratory, Bar Harbor, ME) mice to obtain either Sl/Sl or Sl/Sld mice. Mice obtained from these breedings were used to study thymocyte development in the present study. All mice were maintained under specific pathogen-free conditions at Indiana University Medical School. Sl/Sl and Sl/Sld mutant mice were genotypically confirmed by Southern blot analysis (data not shown). Briefly, tail DNA from mice was digested overnight in digestion buffer (100 mmol/L NaCl, 10 mmol/L Tris base pH 8, 25 mmol/L EDTA pH 8, 1% sodium dodecyl sulfate [SDS], and 150 mg/mL proteinase K) at 50°C and extracted the next day using phenol and chloroform. The high molecular weight DNA was subsequently digested with EcoRI, electrophoresed, transferred to filters, and probed using a full-length 32P-labeled cDNA murine SCF probe.
Generation of transgenic animals.Transgenic mice overexpressing the MA form of hSCF were generated as described previously.19 Briefly, a fragment comprising the human phosphoglycerate kinase promoter (hPGK) and hSCF220 minigene was microinjected into the pronuclei of fertilized C3H/HeJ eggs and subsequently transferred to a pseudo-pregnant outbred Swiss-Webster female. Offspring were tested for the presence of the transgene by analyzing tail DNA as described above. Transgenic mice were maintained on the C3H/HeJ background.
Mast cell staining.Progeny from hemizygous hSCF220 by Sl/+ and hSCF220 by W/+ matings were killed under a CO2 atmosphere. A portion of shaved skin from pigmented and hypopigmented regions of the dorsum or belly were removed, flattened, and fixed in 10% buffered formalin. After embedding, sections were cut, deparaffinized, and mast cells selectively stained using fluorescein isothiocyanate (FITC)-conjugated avidin20 21 and enumerated in areas of skin between the epidermis and panniculus carnosus muscle. A total of 20 to 40 independent fields per sample were examined. The FITC-conjugated avidin stained cells were counted under epifluorescence microscopy at 200× magnification and the number of mast cells per square millimeter was determined using a calibrated ocular grid.
Cell preparations and hematopoietic assays.Animals were killed by cervical dislocation and thymus glands, spleens, and bone marrow were surgically excised from adult (12 weeks old) transgenic, wt and Sl/Sld mice, or 3-day old Sl/Sl and wt mice. Thymus and spleen cell suspensions were prepared and filtered through a nylon-mesh to remove debris. Bone marrow was harvested as previously described,22 and cellularity determined hematopoietic progenitor assay was performed as described.22
Antibodies and flow cytometric analysis.Phycoerythrin (PE)-conjugated monoclonal antibodies (MoAb) were directed against CD8, CD3, and T-cell receptor (TCR) α/β. FITC-conjugated MoAb was directed against CD4. All the PE and FITC-conjugated MoAbs, including the isotype control antibodies were purchased from Pharmingen (San Diego, CA). Cells (1 × 106) were incubated at 4°C for 30 minutes with 1 μg of first MoAb. Cells were washed three times with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). Cells were then incubated with 1 μg of the second MoAb for 30 minutes at 4°C, subsequently washed three times with PBS containing 0.1% BSA, and analyzed by fluorescence-activated cell sorter (FACS, Becton Dickinson, San Jose, CA).
MAP kinase assay.Total cell lysates were prepared from wt control and hSCF220 transgenic mice as previously described.23 Total protein concentration was determined using the bicinchronic acid (BCA) protein assay kit (Pierce, Rockford, IL). Immunoprecipitation (IP) was performed on whole cell lysates using Erk-1 polyclonal antibody for 2 hours at 4°C. Protein A-sepharose beads (Pharmacia Biotech, Piscataway, NJ) were used to collect the Ag-Ab complexes. Erk activity was measured by the incorporation of [γ-32 phosphate] into myelin basic protein (MBP). The MBP assay consisted of 10 mL of cell lysate with 5 mg of MBP substrate incubated with 20 mmol/L Tris (pH 7.5), 10 mmol/L MgCl2 , 20 mg of BSA, 500 mmol/L unlabeled adenosine triphosphate (ATP), and 5 μCi of [γ-32P] ATP in a total reaction volume of 30 mL. Each reaction was incubated at 30°C for 15 minutes and stopped by the addition of 7.5 mL of sample buffer and boiled for 2 minutes. The kinase reaction mixtures were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) in a 12.5% gel, stained in 0.2% coomassie brilliant blue solution, and dried on Whatman 3mm paper (Fischer Scientific, Fairfield, NJ). After autoradiography, the phosphate incorporated into substrate was quantitated by liquid scintillation counting of the MBP gel bands. The counts per minute (cpm) value was then used to calculate Erk activity in pmol phosphate incorporated into MBP/min/mg protein in the cell lysate.
Proliferation assay.In vitro proliferation assay on thymocytes and splenocytes from wt and hSCF220 mice were performed by culturing 2 × 105 cells/well in 96-well plates that had been coated with various concentrations of anti-CD3 MoAb (Pharmingen) in Dulbecco's Modified Eagle Medium (DMEM; GIBCO) with 10% calf serum. These cells were cultured for 3 days at 37°C and subsequently pulsed with 5-bromo-2-deoxyuridine (BrdU). BrdU incorporation was detected using a BrdU labeling and detection kit (Boehringer Mannheim; Indianapolis, IN) according to manufacturer's instructions.
Statistical analysis.Results are expressed as a mean ± standard error of mean (SEM). The probability of significant differences between groups in each figure was determined by a Student's t-test (two-tailed).
RESULTS
Generation of transgenic mice overexpressing the human MA isoform of SCF.To achieve overexpression of the MA isoform of hSCF throughout all stages of development, transgenic mice expressing the MA form of hSCF under the control of an hPGK promoter were generated in C3H/HeJ as described previously.19 Founder transgenic mice compared with normal C3H/HeJ mice have a large white spot on the ventral surface, diamond shaped white spot on the forehead (blaze), white feet, and a banded tail.19 This phenotype is very similar to the coat color deficiency seen in mice heterozygous for some W mutations. To determine if doubling of the transgene dose results in mice with a more severe phenotype, hemizygous hSCF220 mice were mated to generate mice homozygous for the hSCF transgene. Homozygosity was confirmed by back crossing to C3H/HeJ mice. All progeny from this back cross phenotypically appeared identical to hemizygous hSCF220 transgenic mice. Mice homozygous for the transgene demonstrated a more pronounced forehead blaze, with additional white spots over the cervical region and occasionally elsewhere, as well as a significantly larger belly spot (data not shown). Despite this more severe melanocyte deficiency, homozygous hSCF220 transgenic mice had no demonstrable effect on hematopoiesis, as peripheral blood hematocrits, red and white blood cell counts (data not shown), and bone marrow cellularity and the bone marrow content of progenitors (Table 1) were normal.
Comparison of Bone Marrow Progenitor Content Between wt and hSCF 220 Mice
| Genotype . | BMC × 106/Limb . | HPP-CFC . | CFU-GM . | CFU-Mix . |
|---|---|---|---|---|
| wt | 16.0 ± 0.6 | 53.0 ± 4.3 | 96.0 ± 5.0 | 35.0 ± 2.0 |
| hSCF 220 | 15.3 ± 0.8 | 57.0 ± 4.7 | 85.0 ± 4.7 | 38.0 ± 2.7 |
| Genotype . | BMC × 106/Limb . | HPP-CFC . | CFU-GM . | CFU-Mix . |
|---|---|---|---|---|
| wt | 16.0 ± 0.6 | 53.0 ± 4.3 | 96.0 ± 5.0 | 35.0 ± 2.0 |
| hSCF 220 | 15.3 ± 0.8 | 57.0 ± 4.7 | 85.0 ± 4.7 | 38.0 ± 2.7 |
The results show mean ± SEM of at least 10 wt mice and 7 hSCF 220 mice. None of the groups demonstrated significant statistical differences by Student's t-test.
Abbreviations: BMC, bone marrow cellularity; HPP-CFC, high proliferative potential-colony forming cells; CFU-GM, colony-forming units-granulocyte/macrophage; CFU-Mix, colony-forming unit-mix.
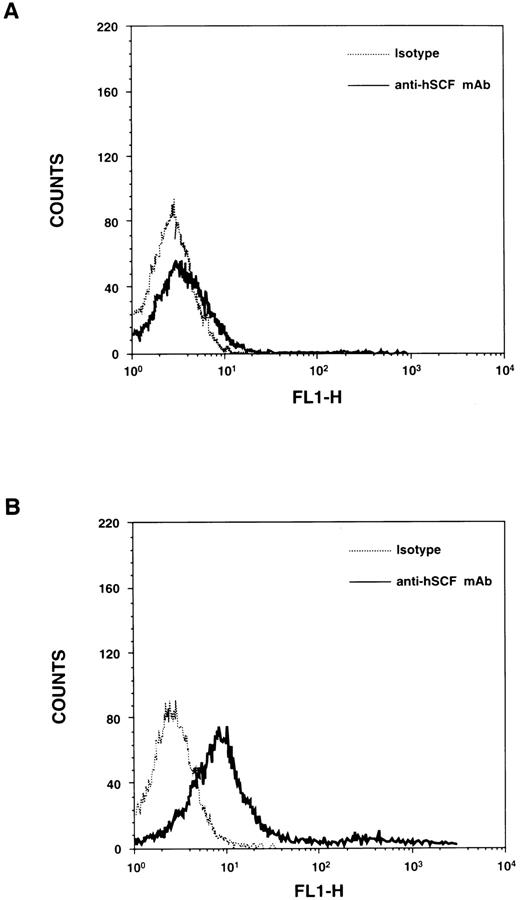
Expression of the MA form of hSCF transgene.To examine the expression of the hSCF transgene in homozygous hSCF220 mice, northern blot analysis was performed on total RNA derived from various tissues of the hSCF220 transgenic mice. Transcript for the hSCF transgene was observed in thymus, spleen, brain, bone marrow, and testes (data not shown). The transgene encodes for MA hSCF protein as demonstrated by immunofluorescence (Fig 1).
Cell surface expression of hSCF in thymocytes derived from transgenic mice. (A) Thymocytes from wt control mice were stained with either isotype control (Pharmingen) or mouse antihuman SCF MoAb (Genzyme, Cambridge, MA) and an FITC-conjugated goat F(ab′)2 antimouse IgG secondary. (B) Thymocytes from hSCF220 mice were stained with either isotype control or mouse antihuman SCF MoAb and an FITC-conjugated goat F(ab′)2 antimouse IgG secondary.
Cell surface expression of hSCF in thymocytes derived from transgenic mice. (A) Thymocytes from wt control mice were stained with either isotype control (Pharmingen) or mouse antihuman SCF MoAb (Genzyme, Cambridge, MA) and an FITC-conjugated goat F(ab′)2 antimouse IgG secondary. (B) Thymocytes from hSCF220 mice were stained with either isotype control or mouse antihuman SCF MoAb and an FITC-conjugated goat F(ab′)2 antimouse IgG secondary.
Effect of hSCF transgene expression on dermal mast cells.The coat color deficiency in homozygous hSCF220 transgenic mice suggests that the melanocyte deficiency was transgene dose-dependent. We have previously shown that matings between hemizygous hSCF220 transgenic mice and the mutant strains of W and Sl (ie, Sl/+ and W/+) produce offspring of which one-fourth have a more severe coat color deficiency than either parent.19 To determine the impact of hSCF transgene expression on connective tissue mast cells, the skin, uterus, lungs, and peripheral lymph nodes (LN) were examined. A significant decrease in the number of total dermal mast cells in comparison to normal wt, hemizygous hSCF220 (h220/+), W/+, and Sl/+ heterozygotes were observed in compound heterozygous mice (ie, hSCF220/+;W/+ (h220/W) and hSCF220/+;Sl/+ (h220/Sl) (Table 2). A systematic survey of lungs, uterus, and peripheral LN showed no apparent mast cell deficiencies (data not shown). Interestingly, peripheral LN adjacent to the panniculus carnosus muscle showed normal numbers of mast cells in the connective tissue surrounding the LN in compound heterozygous mice with epidermal mast cell deficiency (data not shown). Because mast cells arrive at LN via the blood or lymph, our data would suggest that SCF/c-kit interactions in the skin are particularly sensitive to interference by expression of hSCF transgene.
Analysis of Dermal Mast Cells in Various Compound Heterozygous Mice
| Genotype . | Mast Cells/mm2 . |
|---|---|
| +/+ | 27.14 ± 3.31 |
| hSCF 220/+ | 26.86 ± 4.26 |
| W/+ | 23.71 ± 2.62 |
| Sl/+ | 11.60 ± 2.56 |
| hSCF 220/W | 4.10 ± 1.53* |
| hSCF 220/Sl | 3.84 ± 1.40* |
| Genotype . | Mast Cells/mm2 . |
|---|---|
| +/+ | 27.14 ± 3.31 |
| hSCF 220/+ | 26.86 ± 4.26 |
| W/+ | 23.71 ± 2.62 |
| Sl/+ | 11.60 ± 2.56 |
| hSCF 220/W | 4.10 ± 1.53* |
| hSCF 220/Sl | 3.84 ± 1.40* |
wt; +/+, hemizygous hSCF 220; hSCF 220/+, W; W/+, Sl; Sl/+, and compound heterozygous mice generated by mating hSCF 220/+ by W/+ (hSCF 220/W) and hSCF 220/+ by Sl/+ (hSCF 220/Sl) were analyzed for total skin mast cells as described in the Materials and Methods section. Total mast cell number per square millimeters of skin for various mice are shown as the mean ± SEM. At least 6 mice were analyzed from each group.
P < .01 hSCF 220/Sl, hSCF 220/W v wt, hSCF 220/+, Sl/+, W/+.
Thymic cellularity and T-cell subset distribution in Sl mutant mice.To date the in vivo role of SCF isoforms in the development of thymocytes remains unknown. To determine if the lack of SCF in various Sl mutants impairs T-cell development in these mice, we sought to examine thymi of mice that are either completely devoid of SCF (Sl/Sl ) or lack MA SCF (Sl/Sld). Sl/Sl animals derived from crosses of Sl/+ × Sl/+ mice that survived up to 3 days after birth, were identified by severe paleness, runting, and significant anemia (data not shown). The genotype of these animals was confirmed by Southern blot analysis. An 80% decrease in the total thymic cellularity was observed in 3-day old Sl/Sl mutant mice when compared with wt littermates (5.88 ± 0.9 × 106v 28.38 ± 1.74 × 106, Sl/Sl v wt, [n = 10]; P = .0001).
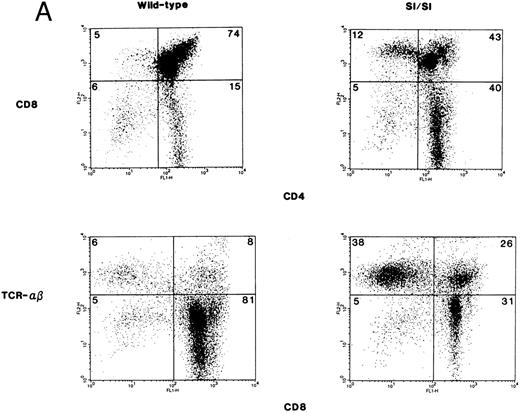
Thymocyte subsets from 3-day old Sl/Sl mutant mice and wt littermates were examined for the expression of CD4, CD8, and TCR. Shown are data from a representative group of mice (Fig 2A). In comparison to wt control mice, Sl/Sl mice exhibited a significant reduction in the percentage of immature CD8+CD4+ double-positive (DP) thymocytes (77.9 ± 0.84 v 56.2 ± 2.26, wt [n = 9] v Sl/Sl [n = 16]; P < .0001) with no significant difference in the percentage of CD4−CD8− double-negative (DN) thymocytes. In contrast, the percentage of CD4+ single-positive (SP) (10.89 ± 0.71 v 28.7 ± 1.45, wt [n = 9] v Sl/Sl [n = 16]; P < .0001) and CD8+ SP (4.9 ± 0.45 v 9.12 ± 0.9, wt [n = 9] v Sl/Sl [n = 16]; P = .009) thymocytes in Sl/Sl mice was significantly increased in comparison to wt control mice as shown here in a representative experiment (Fig 2A, upper panel). A significant reduction in the immature DP T-cell subset in Sl/Sl mice and an increase in the percentage of SP thymocytes led us to postulate that a greater percentage of thymocytes in Sl/Sl mice are of the more mature phenotype, ie, CD4+TCRhi or CD8+TCRhi SP. To test this hypothesis, we performed two-color flow cytometry on total thymocytes from Sl/Sl and wt littermate control mice, with a combination of MoAbs directed against CD8 and TCR-α/β. The anti–TCR-α/β MoAb recognizes the CD3–TCR-α/β complex. As shown in a representative experiment, in comparison to the wt control mice, thymocytes from Sl/Sl mice appear to have a higher percentage of cells that are of the more mature SP phenotype (Fig 2A, lower panel). Thus, in the absence of either isoform of SCF, thymocytes from Sl/Sl mutant mice demonstrate perturbed T-cell differentiation and a significant reduction in total thymic cellularity in comparison to wt littermate control mice.
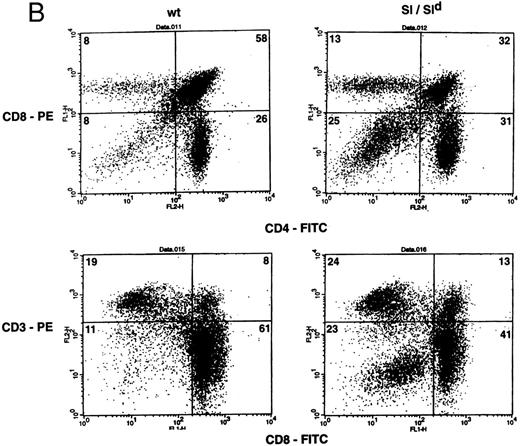
(A) Phenotypic analysis of thymocytes from Sl/Sl and wt control mice. Thymocytes were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated TCR α/β MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Nine wt control and 16 Sl/Sl mice were examined. (B) Phenotypic analysis of thymocytes from Sl/Sld mice and wt control mice. Thymocytes were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Seven wt control and 11 Sl/Sld mice were examined.
(A) Phenotypic analysis of thymocytes from Sl/Sl and wt control mice. Thymocytes were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated TCR α/β MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Nine wt control and 16 Sl/Sl mice were examined. (B) Phenotypic analysis of thymocytes from Sl/Sld mice and wt control mice. Thymocytes were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Seven wt control and 11 Sl/Sld mice were examined.
Thymic cellularity and T-cell subset distribution from Sl/Sld mice were also examined. A 70% decrease in the total thymic cellularity was observed in Sl/Sld mutants when compared with wt littermates (29.16 ± 5.5 × 106 [n = 15] v 95.8 ± 7.7 × 106, [n = 13] P = .0001). In comparison to wt littermate control mice, Sl/Sld mice also exhibited a significant reduction in the percentage of immature CD8+CD4+ DP thymocytes (53.86 ± 4.6 v 23.72 ± 4.43, wt [n = 7] v Sl/Sld [n = 11]; P = .0007) (Fig 2B, upper panel), and a significant increase in the percentage of DN thymocytes (6.71 ± 0.9 v 20.18 ± 3.2, wt [n = 7] v Sl/Sld [n = 11]; P = .01) (Fig 2B, upper panel). Shown are data from a representative group of mice (Fig 2B, upper panel). In contrast to Sl/Sl mice, the percentage of SP thymocytes in Sl/Sld mice was not significantly different than that of wt control mice. The increase in the percentage of DN thymocytes was further seen by two-color flow cytometry staining of Sl/Sld thymocytes with a combination of MoAbs against CD8 and CD3 (Fig 2B, lower panel). The percentage of T-cell subsets in the strains of mice used to generate Sl/Sl and Sl/Sld mice (ie, C3H/HeJ, WC/ReJ−Sl/+ and C57B16−Sld/+) were similar (Table 3). These findings indicate that the production of MA SCF may be essential for the proliferation and differentiation of thymocytes.
A Comparison of Thymocyte Subsets Between C3H/HeJ, WC/Rej-Sl/+, and C57BL6-Sld/+ Mice
| Genotype . | Thymocyte Subsets . | |||
|---|---|---|---|---|
| . | % CD4+CD8+ . | % CD4+CD8− . | % CD4−D8+ . | % CD4−CD8− . |
| . | (DP) . | (SP) . | (SP) . | (DN) . |
| C3H/HeJ | 76.83 | 13.40 | 3.32 | 5.55 |
| WC/ReJ-Sl/+ | 78.91 | 13.16 | 3.87 | 4.06 |
| C57BL6-Sld/+ | 78.03 | 13.70 | 4.24 | 4.03 |
| Genotype . | Thymocyte Subsets . | |||
|---|---|---|---|---|
| . | % CD4+CD8+ . | % CD4+CD8− . | % CD4−D8+ . | % CD4−CD8− . |
| . | (DP) . | (SP) . | (SP) . | (DN) . |
| C3H/HeJ | 76.83 | 13.40 | 3.32 | 5.55 |
| WC/ReJ-Sl/+ | 78.91 | 13.16 | 3.87 | 4.06 |
| C57BL6-Sld/+ | 78.03 | 13.70 | 4.24 | 4.03 |
Thymocytes from various strains of mice were harvested and stained with PE-conjugated MoAb against CD8 and FITC-conjugated MoAb against CD4 and analyzed by flow cytometry as described in the Materials and Methods.
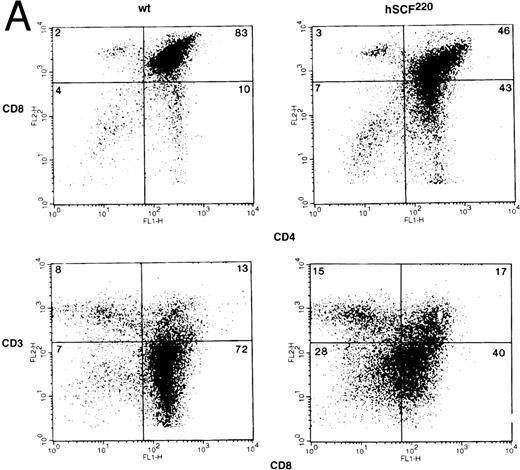
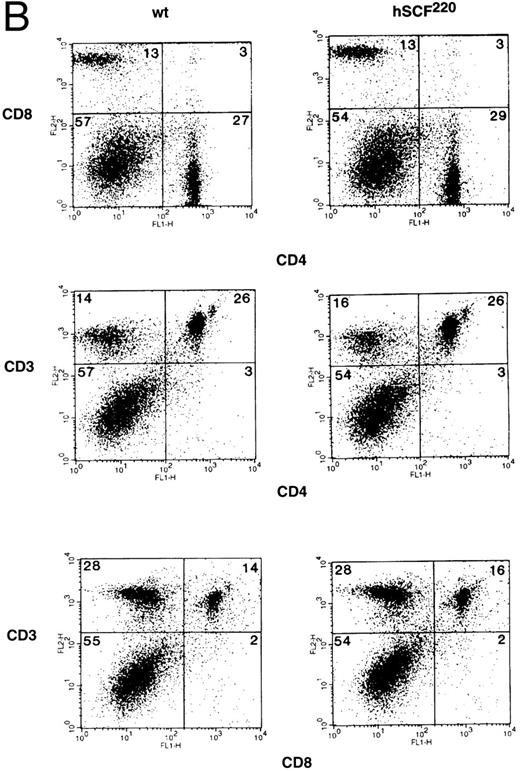
Thymic cellularity and T-cell subset distribution in hSCF transgenic mice.To further examine the role of c-kit and SCF in thymic development, we took advantage of the fact that hSCF220 transgenic mice do not display a severe hematologic phenotype. Thymocytes from hemizygous and homozygous hSCF220 transgenic mice were examined for total thymic cellularity, as well as T-cell subset distribution. Total thymic cellularity from hSCF220 transgenic mice was similar to that of wt littermate controls (106.45 ± 11.62 [n = 4] v 107.57 ± 8.84 [n = 4], no significant difference). However, flow cytometric analysis of thymocytes stained with MoAbs directed against CD4 and CD8 cell surface molecules showed differences in the distribution of T-cell subsets between homozygous hSCF220 transgenic and wt littermate control mice. Shown are data from a representative group of mice (Fig 3A, upper panel). A significant decrease in the immature DP thymocyte population was observed between wt and transgenic mice (72.3 ± 4.9 v 46.33 ± 1.3, wt [n = 3] v hSCF220 [n = 3]; P = .03) (Fig 3A, upper panel). In these experiments box grids for various T-cell subsets were based on the control MoAb staining of thymocytes and the threshold settings for both the wt and hSCF220 transgenic mice thymocyte staining were identical. The decrease in the DP thymocyte population in the transgenic mice was dependent on the hSCF transgene dosage, as hemizygous hSCF220 transgenic mice demonstrated a relatively mild decrease in the DP population as compared with homozygous hSCF220 mice (data not shown).
(A) Phenotypic analysis of thymocytes from wt control and hSCF220 transgenic mice. Total thymocytes from an hSCF220 homozygous transgenic and wt control littermates were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb, and analyzed by flow cytometry (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Three wt control and three hSCF220 mice were examined. (B) Phenotypic analysis of T-cell subsets in spleens of wt control and hSCF220 transgenic mice. Spleen cells from an hSCF220 homozygous transgenic mouse and wt control littermate were stained with FITC-conjugated CD4 MoAb (top and middle panels) and PE-conjugated CD8 and CD3 MoAb (top and middle panel) or stained with FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Numbers in quadrants indicate the percentage of the total population. Three wt control and three hSCF220 mice were examined.
(A) Phenotypic analysis of thymocytes from wt control and hSCF220 transgenic mice. Total thymocytes from an hSCF220 homozygous transgenic and wt control littermates were stained with FITC-conjugated CD4 MoAb and PE-conjugated CD8 MoAb, and analyzed by flow cytometry (upper panel) or FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Data are from a representative group of mice. Numbers in quadrants indicate the percentage of the total population from a representative group of mice. Three wt control and three hSCF220 mice were examined. (B) Phenotypic analysis of T-cell subsets in spleens of wt control and hSCF220 transgenic mice. Spleen cells from an hSCF220 homozygous transgenic mouse and wt control littermate were stained with FITC-conjugated CD4 MoAb (top and middle panels) and PE-conjugated CD8 and CD3 MoAb (top and middle panel) or stained with FITC-conjugated CD8 MoAb and PE-conjugated CD3 MoAb (lower panel), and analyzed by flow cytometry. Numbers in quadrants indicate the percentage of the total population. Three wt control and three hSCF220 mice were examined.
The altered differentiation of thymocytes in homozygous hSCF220 transgenic mice was further observed on performing two-color flow cytometry analysis using MoAbs against CD3 and CD8. Anti-CD3 MoAb recognizes the CD3-TCR-α/β complex. The data show that despite a significant decrease in the presence of DP thymocytes in hSCF220 transgenic mice, these animals still express CD3 (Fig 3A, lower panel). However, the expression of CD8 on thymocytes from transgenic mice consistently appeared to be heterogeneous when stained with a combination of MoAbs directed against CD8 and CD3 (Fig 3A, lower panel). These data suggest that SCF in vivo may play a role in the differentiation of thymocytes even in the absence of severe hematologic stress associated with the Sl/Sl or Sl/Sld genotype, and that this differentiation is perturbed in mice that overexpress the MA isoform of hSCF.
To determine if defective maturation of thymocytes in homozygous hSCF220 transgenic mice resulted in an altered phenotypic composition of mature T-cell subsets, splenocytes from homozygous hSCF220 transgenic mice and wt control littermates were stained with MoAbs directed against CD4, CD8, and CD3, and analyzed by two-color flow cytometry. The distribution of CD4, CD8, and CD3 T-cell subsets in the spleen of hSCF220 transgenic mice and wt littermate control were found to be similar (Fig 3B). Thus, despite the altered maturation in the thymus, the phenotype of the more mature T cells in the spleen was not affected by the overexpression of the hSCF transgene.
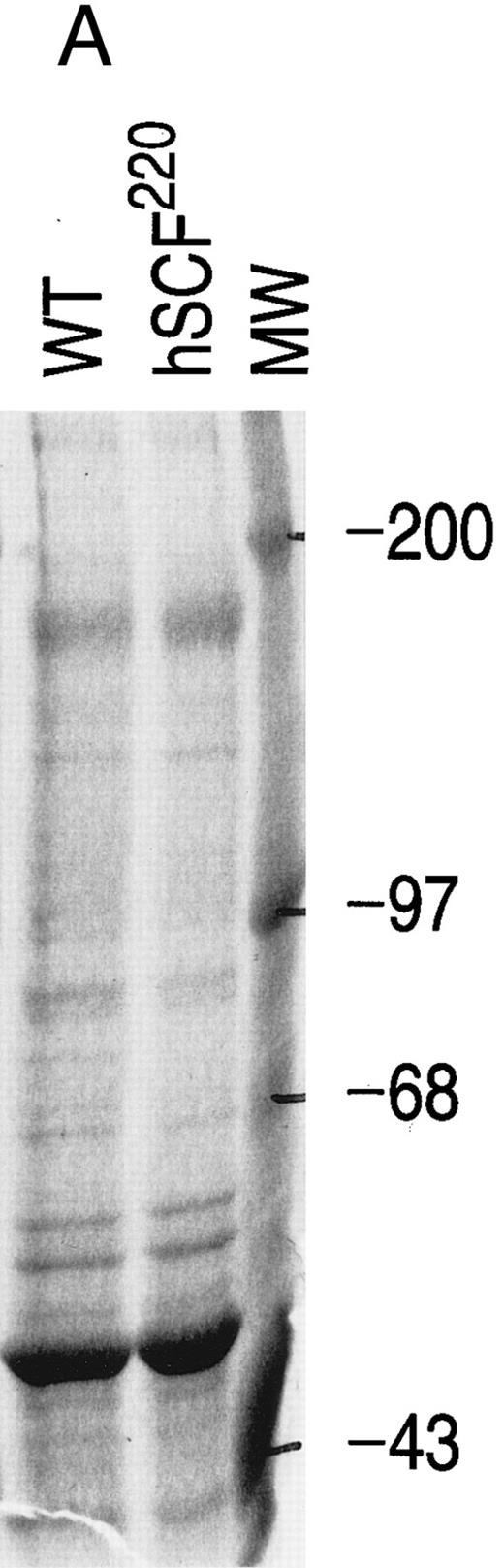
Comparison of MAP kinase activity between hSCF220 transgenic and wt control mice.Recent studies have demonstrated that thymocyte differentiation from DN to DP is coupled to MAP kinase pathway because the expression of a dominant negative MAP kinase mutant blocks the differentiation of thymocytes from DN to DP stage.24 It has been shown that activation of c-kit on SCF binding leads to receptor dimerization and autophoshorylation, with subsequent provision of docking sites for intracellular signaling molecules.18,25 One consequence of c-kit/SCF interaction is activation of the MAP kinase protein kinase cascade that mediates transfer of extracellular signals to the nucleus.26-28 To determine if the abnormalities in thymocyte differentiation seen in hSCF220 transgenic mice were associated with changes in MAP kinase activation, we measured MAP kinase activity in total thymocytes derived from hSCF220 transgenic and wt control mice. Our data consistently showed reduced MAP kinase activity in thymocytes from hSCF220 transgenic mice as compared with wt control mice. Measured as phosphate incorporation into MBP, MAP kinase was 10.6 pmol/min/mg of cell lysate (wt) versus 2.5 pmol/min/mg for hSCF220. In these experiments equal amounts of protein (Fig 4A) from wt and hSCF220 transgenic mice derived thymic whole cell lysates were immunoprecipitated (IP) with anti–Erk-1 antibody and subjected to an in vitro kinase assay (Fig 4B). Thus, we hypothesize that suboptimal activation of c-kit due to the presence of hSCF transgene results in perturbed thymocyte differentiation accompanied by altered downstream events via a pathway known to be important in c-kit receptor signaling.
Comparison of MAP kinase activity between wt and hSCF220 transgenic mice. (A) Equal amounts of wt and hSCF220 transgenic mice derived whole cell lysates were used in IP experiments, as determined by coomassie blue staining of proteins resolved on 12.5% SDS-PAGE gel. (B) Shown is an autoradiogram from a representative experiment, indicating reduced phosphate incorporation in MBP from cell lysates derived from hSCF220 transgenic mice in comparison to wt control. Two separate experiments were performed with identical results. Data from one experiment are shown.
Comparison of MAP kinase activity between wt and hSCF220 transgenic mice. (A) Equal amounts of wt and hSCF220 transgenic mice derived whole cell lysates were used in IP experiments, as determined by coomassie blue staining of proteins resolved on 12.5% SDS-PAGE gel. (B) Shown is an autoradiogram from a representative experiment, indicating reduced phosphate incorporation in MBP from cell lysates derived from hSCF220 transgenic mice in comparison to wt control. Two separate experiments were performed with identical results. Data from one experiment are shown.
Comparison of the proliferative capacity of thymocytes and mature T cells between hSCF220 transgenic and wt control mice.Perturbed thymocyte differentiation accompanied by heterogeneous expression of CD3, CD4, and CD8 cell surface molecules in hSCF220 transgenic mice led us to examine the in vitro proliferative capacity of these cells in response to anti–CD3 MoAb stimulation. Our results show that hSCF220 transgenic mice that demonstrate perturbed thymic differentiation as a result of reduced MAP kinase activation, also show significantly reduced proliferative capacity when stimulated in vitro with an optimal concentration of anti-CD3 MoAb (Table 4). In contrast, the more mature T-cell compartment in the spleen of hSCF220 transgenic mice, which contain normal T-cell subsets, also demonstrated normal proliferative responses in vitro to anti-CD3 MoAb (Table 4).
Comparison of In Vitro Proliferative Response of Thymocytes and Splenic T Cells Between wt and hSCF 220 Mice
| Genotype . | Treatment . | Thymocytes . | Splenocytes . |
|---|---|---|---|
| wt | PBS | 0.33 ± 0.03 | 0.26 ± 0.006 |
| Anti-CD3 MoAb | 0.78 ± 0.14-150 | 0.60 ± 0.064-150 | |
| hSCF 220 | PBS | 0.27 ± 0.02 | 0.36 ± 0.01 |
| Anti-CD3 MoAb | 0.39 ± 0.04 | 0.76 ± 0.14-150 |
| Genotype . | Treatment . | Thymocytes . | Splenocytes . |
|---|---|---|---|
| wt | PBS | 0.33 ± 0.03 | 0.26 ± 0.006 |
| Anti-CD3 MoAb | 0.78 ± 0.14-150 | 0.60 ± 0.064-150 | |
| hSCF 220 | PBS | 0.27 ± 0.02 | 0.36 ± 0.01 |
| Anti-CD3 MoAb | 0.39 ± 0.04 | 0.76 ± 0.14-150 |
In vitro proliferation on thymocytes and splenic T cells from wt and hSCF 220 mice was performed as described in Materials and Methods. Thymoctyes and splenocytes were cultured in the presence of 10 μg/mL of anti-CD3 MoAb, and their proliferation monitored for 3 days. Proliferation was determined by measuring the optical density (OD) at 405 nm using an Elisa plate reader. At least three mice were examined per group. Data represent the mean ± SEM for each group from two separate experiments.
P < .05 anti-CD3 v PBS control for wt thymocytes. *P < .01 anti-CD3 v PBS control for wt splenocytes and *P < .05 for hSCF 220 splenocytes. Anti-CD3 MoAb mediated proliferation of splenocytes from wt and hSCF 220 was not statistically different (P = .2).
DISCUSSION
Using transgenic mice we have attempted to dissect the in vivo role of c-kit/SCF interactions by overexpressing the MA isoform of hSCF. We have previously shown that transgenic mice hemizygous for the MA isoform of hSCF mimic coat color abnormalities observed in some alleles of W.19 We now show that mice homozygous for the MA hSCF transgene demonstrate accentuated coat color abnormalities in comparison to hemizygous hSCF220 transgenic mice. The accentuated coat color abnormalities seen in these mice may be due to increased sensitivity of melanoblasts to alterations in the presentation of endogenous MA SCF and its interaction with c-kit, as demonstrated previously.19 Mice doubly heterozygous for a mutated W or Sl allele and the hSCF transgene demonstrate significantly reduced dermal mast cells, suggesting that along with melanocytes, dermal mast cells also require MA SCF for their migration or survival/proliferation. Our data would suggest that melanocytes and mast cells are unable to migrate to the appropriate site in the skin due to the lack of MA SCF. Alternatively, on completion of migration to the appropriate site, these cells may fail to survive/proliferate due to the lack of MA SCF.
Previous in vitro experiments have implicated SCF to be a potent stimulator of proliferation of immature T cells.15 Abnormal cellularity and T-cell subset differentiation in thymuses of Sl/Sl and Sl/Sld mice presented here, also suggest an in vivo function for SCF. Because the purpose of the experiments described here was to examine the effects of transgene expression, the genetic background of these animals is not pure. Thus, although informative, the data derived from Sl/Sl and Sl/Sld mice may not represent the exact phenotype, which would be seen in inbred animals. In addition, Sl/Sl mice are extremely sick and die during gestation or within days of birth, and likely have elevated levels of circulating glucocorticoids. The thymus is particularly sensitive to stress and hormonal influences. Thus, it may not be possible to draw any meaningful conclusions regarding the observed thymic phenotype and the direct biological effects of SCF on thymocyte differentiation. In an alternative approach, we sought to examine thymuses derived from Sl/Sld mice and hSCF220 transgenic mice that have either less anemia or none at all. Sl/Sld mice are viable, however only a truncated soluble form of SCF is generated in these animals. They therefore provide a useful in vivo model to study the effect of the MA isoform in T-cell development. A dramatic reduction in total thymic cellularity was observed in these mice as compared with wt littermates. Sl/Sld mice also showed a significant increase in the percentage of DN thymocytes accompanied with a significant decrease in the DP compartment. However, no changes in the percentage of SP subset were observed. These data suggest that MA SCF may play an important role in the differentiation of DN thymocytes to more mature DP stage, or, alternatively that the truncated soluble SCF produced in Sl/Sld mice is not sufficient to induce the proliferation and differentiation of thymocytes in these mice. In this regard we have recently generated Sl/Sld mice that overexpress either the S or the MA isoform of SCF, and have observed differential affects of the two isoforms on hematopoiesis in these animals.28a
In an attempt to more clearly define the in vivo role of MA SCF in thymocyte development in mice that have no hematopoietic stress, thymuses from homozygous hSCF220 transgenic mice were analyzed for total cellularity and T-cell subset differentiation. Although these mice did not show alterations in the total thymic cellularity as compared with wt mice, overexpression of hSCF did result in significant changes in the CD4/CD8 profiles. The most profound effect in these mice is the appearance of a population of CD4+81o cells, many of which are CD31o and thus not mature SP thymocytes. One could argue that the discrepancy observed in the T-cell subset distribution between Sl/Sld and hSCF220 transgenic mice lies in the patterns of expression of endogenous MA and hSCF transgene. It is conceivable that in the thymus the expression of the endogenous MA SCF begins earlier or later or is at a higher level in the thymic maturation sequence in comparison to the hSCF transgene. This may explain, in part, the differences observed in the T-cell subset distribution between Sl/Sld and hSCF220 transgenic mice.
Previously, we have reported activation of MAP kinase in mouse c-kit+ cells treated with rat, but not human, SCF.19 Recently, the involvement of MAP kinase pathway in the differentiation of thymocytes from DN to the DP stage of development has been reported.24 These studies demonstrated that the expression of an inactive MAP kinase prevents the differentiation of thymocytes from DN to DP stage of development. To ascertain if the perturbed T-cell phenotypic abnormalities seen in hSCF220 transgenic mice were in some fashion linked to aberrant MAP kinase activation of c-kit positive thymocytes, we compared the MAP kinase activity in whole cell lysates between hSCF transgenic and wt mice. hSCF220 transgenic mice show a significant reduction in MAP kinase activation as compared with wt mice. These data suggest that the presence of an intact c-kit/SCF signaling pathway is essential for normal thymocyte differentiation. In addition, CD3-mediated proliferative signals in hSCF220 transgenic mice were also impaired in comparison to wt controls. However, from our data, we cannot conclude if the reduced proliferative capacity of thymocytes from these mice is due to reduced MAP kinase activation. Alternative signaling pathways may play a role in mediating this outcome. Recently, JNK pathway has been shown to be involved in signal transduction in mature T cells.29 It is possible that there is cross talk between MAP kinase and JNK pathways and that activation of the JNK kinase pathway in thymocytes may be important in CD3-mediated proliferation.30
Despite the significant abnormalities seen in some of the c-kit+ lineages in hSCF220 transgenic mice, the overexpression of MA hSCF did not entirely mimic the phenotype observed in Sl/Sld mice that are completely devoid of MA SCF. In this regard, the dysregulation of expression of hSCF or the level of expression of hSCF via the PGK promoter may explain the lack of abnormalities seen in other c-kit+ lineages in these animals. Three of the most dramatic phenotypic abnormalities in Sl/Sld mice, the lack of melanocytes in the skin, mast cell deficiency, and the sterility, are known to reflect, at least in part, the impaired migration of cells in these lineages during embryonic development.1 Based on our results, we can hypothesize that cells that express c-kit, such as melanocytes, mast cells, and thymocytes might respond by directed migration along a concentration gradient of MA SCF, a process designated haptotaxis.
Supported by National Institute of Diabetes, Digestive and Kidney Disease Grant No. RO1 DK 48605 to D.A.W.
Address reprint requests to David A. Williams, MD, Herman B Wells Center for Pediatric Research, Howard Hughes Medical Institute, Cancer Research Bldg, 1044 W Walnut, Room 402C, Indianapolis, IN 46202-5225.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal