Abstract
The human T-cell leukemia virus type I (HTLV-I), which infects a wide variety of mammalian cells including monocytes and macrophages, encodes a transactivating protein designated as Tax. We now report that Tax induces the human prointerleukin-1β (IL1B) gene promoter in monocytic cells. In our transient transfection assays using human THP-1 monocytic cells, a chloramphenicol acetyltransferase (CAT) construct containing the IL1B promoter sequence between positions −131 and +12 showed an approximately 90-fold increase in activity following cotransfection of a Tax expression vector. Moreover, Tax synergized with lipopolysaccharide (LPS) to induce the IL1B promoter activity. Analyses of specific nucleotide substitutions further indicated that the Tax-induced transcriptional activation requires two transcription factor binding motifs within the IL1B promoter; one is a binding site for nuclear factor (NF)-IL6 (CCAAT/enhancer binding protein β, C/EBPβ), which belongs to the basic region-leucine zipper (bZIP) family and the other for Spi-1 (PU.1), which is an Ets family protein found principally in monocytes, macrophages, and B lymphocytes. In electrophoretic mobility shift assays (EMSA) using in vivo THP-1 nuclear extracts, Tax expression in THP-1 monocytic cells significantly increased binding of the two factors to their target IL1B promoter sequences. However, in contrast to NF-IL6 and Spi-1, DNA binding activity of Oct-1, an ubiquitously expressed octamer-binding protein was not affected by Tax. Additional EMSA using in vitro translated proteins also showed that recombinant Tax enhances DNA binding of both of recombinant NF-IL6 and Spi-1 proteins. These data were supported by our glutathione S-transferase (GST)-pulldown data, which indicated that Tax physically interacts with the two proteins. Based on the results obtained from the present study, we conclude that the IL1B promoter is a Tax-responsive sequence as a result of ability of Tax to induce binding of NF-IL6 and Spi-1 to the IL1B promoter sequence through protein-protein interaction.
THE HUMAN T-CELL leukemia virus type I (HTLV-I) infection is endemic in Japan, the Caribbean, Africa, and the southeastern United States. The HTLV-I is known to be the etiologic agent of an aggressive form of human malignancy, adult T-cell leukemia/lymphoma (ATLL) and is also associated with many inflammatory diseases including myelopathy/tropical spastic paraparesis (HAM/TSP), arthropathy (HAAP), bronchopneumonopathy (HAB), uveitis, and Sjögren's syndrome. ATLL is characterized as mature CD4+ T-lymphocyte leukemia and lymphoma. However, recent in vivo and in vitro studies have demonstrated that HTLV-I infection is widely distributed among mammalian cells including synovial cells,1 B lymphocytes,2 endothelial cells,3 monocyte/macrophage cells,4 and fibroblasts.5 Monocyte/macrophage cells are known to be versatile secretory cells able to release various kinds of cytokines, which contribute substantially to host defense and inflammation by acting as systemic or local cells in their environment. Recently, HTLV-I–infected monocyte/macrophage cells from patients with ATLL or HAM/TSP have been demonstrated to express not only HTLV-I antigen, but also transcriptional activator Tax RNA, suggesting that the HTLV-I–infected monocyte/macrophage cells participate in various pathologic events in ATLL and HTLV-I–associated inflammatory diseases.6 Consistent with this argument, an animal model expressing HAM/TSP-like myelopathy has been reported to show not lymphocytic infiltration, but foamy macrophage infiltration in affected spinal cord.7 In addition, a recent report has shown that interleukin-2 (IL-2) production by primary ATLL cells is macrophage-dependent and further have demonstrated that macrophage-derived IL-1β is an important physiologic signal in vivo that induces IL-2 production by primary ATLL cells.8
Tax protein encoded by HTLV-I genome is not only critical for transformation, but also a transcriptional activator of both viral and cellular genes. Replication of HTLV-I depends on Tax protein, which activates HTLV-I long terminal repeat (LTR) transcription.9-11 Cellular genes such as IL-1α, IL-2, IL-2 receptor (IL-2R), IL-6, IL-8 c-fos, and parathyroid hormone-related protein (PTH-rP) are also activated by Tax.12-18 These gene expressions induced by Tax require specific DNA sequences including the three 21-bp Tax-response elements (TRE) of the HTLV-I gene,19 the nuclear factor (NF)-κB–binding site of the IL-2R gene20 and the serum responsive element (SRE) of c-fos.16 However, Tax induction of the genes is not due to direct binding of Tax to DNAs and has been demonstrated to result from interaction of Tax with transcription factors such as basic region-leucine zipper (bZIP) family proteins and NF-κB.21-24 Additionally, Tax has been reported to induce translocation of NF-κB p65, an active form of transcriptional factor NF-κB into the nucleus by interfering or dissociating complex of NF-κB with its inhibitory factor-κB (I-κB).25 A recent report has shown that Tax recruits the CREB coactivator CBP in a manner independent of CREB phosphorylation.26 Thus, various mechanisms by which Tax transactivates the cellular genes have been identified.
IL-1β is an important inflammatory and immunoregulatory cytokine expressed by activated monocytes/macrophages in response to a variety of stimuli including lipopolysaccharide (LPS), phorbol myristate acetate (PMA), IL-1β itself, and the other cytokines.27,28 IL-1β possesses a broad range of biological activities on various tissue types including connective tissue, bone, and nervous system. Increased levels of IL-1β have been reported in sera and peripheral blood cells of patients with HAM/TSP.29 Dhib et al30 have shown that HTLV-I Tax induces IL-1β mRNA expression in both human primary microglia and peripheral blood macrophages. The human proIL-1β gene (referred to here by its genomic locus name, IL1B) coding for the IL-1β precursor protein is normally silent, but is rapidly transcribed in monocytes/macrophage when stimulated. This observation suggests that the IL1B gene is tightly regulated by positive and negative regulatory elements.31 However, the mechanism by which HTLV-I infection activates the IL1B gene still remains unclear.
Monocyte/macrophage-specific expression of the IL1B gene depends on its promoter located between positions −131 and +12.32 In the present study, we examined the promoter activity of the IL1B gene in transiently transfected monocytic cells and showed that HTLV-I Tax induces the IL1B promoter. Interestingly, synergy between Tax and LPS in inducing the IL1B gene promoter is observed. The Tax-induced transcriptional activation requires two transcription factor binding sites in the context of the promoter. The proteins that bind to the two promoter sequences are Spi-1 (PU.1) and NF-IL6 (CCAAT/enhancer binding protein β, C/EBPβ). Our electrophoretic mobility shift assay (EMSA) data using nuclear extracts and recombinant Spi-1, NF-IL6, and Tax proteins further show that HTLV-I Tax enhances binding of both Spi-1 and NF-IL6 to the IL1B promoter. In addition, GST-pulldown assays using GST-Spi–1 and GST-NF–IL6 fusion proteins and radiolabeled Tax show that Tax physically interacts with both Spi-1 and NF-IL6. The data obtained from the present study show that the IL1B promoter is a Tax-responsive sequence as a result of protein-protein association of Tax with two transcription factors, NF-IL6 and Spi-1, which recognize the IL1B promoter sequence.
MATERIALS AND METHODS
Cell culture. The human THP-1 monocytic leukemic cell line (JCRB 0112) was purchased from Health Science Research Resources Bank (HSRRB; Osaka, Japan). THP-1 cells were carefully maintained in endotoxin-free complete RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Equitech-bio. Inc, Ingram, TX). Cells were split at 1:3 dilution every 3 or 4 days to avoid overcrowding and were further split at 1:2 on the day before transfection or preparation for the nuclear extracts.
Plasmids. Human IL1B genomic DNA fragments were derived from clone BDC454.33 We have used the identical sequence numbering as that described in our recent report.32 A construct 3MEHT contained the IL1B promoter sequence as previously described.34 The HT sequence (construct 3MEHT) located between positions −131 and +12 (see Fig 2A) was cloned into the chloramphenicol acetyltransferase (CAT) gene plasmid vector pA10CAT3ME (3ME). Mutations of the Spi-1 (NF- βA) and the −91 to −83 upstream NF-IL6 sites (see Fig 2A) were the same as those reported previously.32 These mutations are located at specific sites known to be critical for NF-IL6 and Spi-1 binding, respectively32,34 and were verified by sequencing as described previously.34 HTLV-I Tax expression vector (pcTax) was generated by inserting the Tax coding region into pcDNA1 (Invitrogen Corp, San Diego, CA). Expression vectors for the full-length NF-IL6 (pcNF-IL6) and a truncated NF-IL6 with an internal deletion of the splI-splI fragment (pcmNF-IL6[Δspl]; amino acid sequence between 41 and 205) were described previously.35 The truncated NF-IL6 still contains intact basic and leucine zipper regions. A Spi-1 expression vector for the wild-type protein (pcSpi-1) was also generated by inserting the Spi-1 coding region into pcDNA1. Glutathione S-transferase (GST)-NF–IL6 fusion protein expression vector (GST-NF–IL6) was constructed by inserting the truncated NF-IL6 coding region (Δ spl ) into pGEX, a GST gene fusion vector (Pharmacia Biotech; Uppsala, Sweden). GST-Spi–1 fusion protein expression vector (GST-Spi–1) containing the entire Spi-1 coding region was kindly provided by Dr D.G. Tenen (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA).
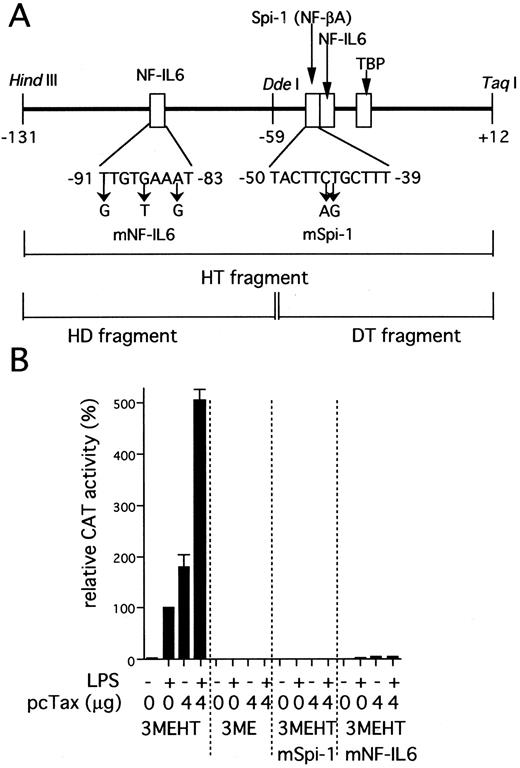
IL1B promoter activity induced by HTLV-I Tax requires two binding sites for NF-IL6 and Spi-1. (A) The scheme shows a representation of the IL1B promoter HT fragment. Specific nucleotide substitutions contained in the two distinct mutated HT fragments are indicated by an arrow. These mutations are located at specific sites known to be critical for NF-IL6 and Spi-1 binding respectively.32 34 (B) pcTax, a Tax expression vector (4 μg) was transfected as indicated along with either wild-type or mutated 3MEHT CAT reporters (10 μg). Following transfection, cells were treated with 100 ng/mL of LPS or left untreated. The CAT data were normalized to the average activity elicited by LPS-induced 3MEHT construct in the absence of expression vector cotransfection. Error bars represent SD from three independent experiments. The total amount of transfected DNA was kept constant (14 μg) by the addition of control vector.
IL1B promoter activity induced by HTLV-I Tax requires two binding sites for NF-IL6 and Spi-1. (A) The scheme shows a representation of the IL1B promoter HT fragment. Specific nucleotide substitutions contained in the two distinct mutated HT fragments are indicated by an arrow. These mutations are located at specific sites known to be critical for NF-IL6 and Spi-1 binding respectively.32 34 (B) pcTax, a Tax expression vector (4 μg) was transfected as indicated along with either wild-type or mutated 3MEHT CAT reporters (10 μg). Following transfection, cells were treated with 100 ng/mL of LPS or left untreated. The CAT data were normalized to the average activity elicited by LPS-induced 3MEHT construct in the absence of expression vector cotransfection. Error bars represent SD from three independent experiments. The total amount of transfected DNA was kept constant (14 μg) by the addition of control vector.
Transfection and CAT assay. The human THP-1 monocytic cell line was transfected by the diethyl aminoethyl (DEAE)-dextran method as described previously.34 This DEAE-dextran method, unlike electroporation, does not generate induction of the endogenous IL1B gene. THP-1 cells (1 × 107 cells per plate) were transfected with 10 to 14 μg of plasmids. At 24 hours after transfection, cells were left untreated or treated with 100 ng/mL of LPS for 24 hours before harvesting. The CAT assays were performed by a liquid scintillation method. The protein concentrations for extracts were determined by Bio-Rad Bradford protein assay kit (Melville, NY). CAT activities were determined by calculating slopes from plots of time versus cpm within a linear range of the response as described previously.34
Nuclear extracts, recombinant proteins, and EMSA. Nuclear extracts were prepared from THP-1 cells after a 45-minute incubation with LPS (100 ng/mL) as previously reported.34 In some experiments, to examine the effect of Tax protein on DNA binding activity of Spi-1 and NF-IL6, THP-1 cells were transfected with pcTax or control pcDNA1 vector by the DEAE-dextran method. At 24 hours after transfection, the cells were harvested without any additional stimulation or were induced with 100 ng/mL of LPS for an additional 45 minutes before harvest. Recombinant HTLV-I Tax, Spi-1, and NF-IL6 proteins were produced in a TNT-coupled transcription/translation reticulocyte lysate system (Promega, Madison, WI) in the presence or absence of 35S-methionine. Anti-NF–IL6 Ab was a gift from S. Akira (Osaka University, Osaka, Japan). This antibody (Ab) was raised against a synthetic peptide corresponding to the sequence of NF-IL6 between amino acids 261 and 278, SKAKKTVDKHSDEYKIRR. Anti-Oct–1 Ab were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). This Ab was raised against a peptide corresponding to amino acids 723-743 mapping at carboxy terminus of human Oct-1 protein and was not cross-reactive with other homeodomain transcription factors.
Synthetic oligonucleotide 6/IL-1, 5′ AGCTTAACTTGATTGTGAAATCAGGTAGCT 3′ (the core recognition sequence was underlined) contained the −91 to −83 NF-IL6 site from the IL1B promoter and Hind III sites on both ends for labeling. Mutated 6/IL-1 (6/IL-1mNF–IL6), 5′ AGCTTAACTTGAgTGTtAAgTCAGGTAGCT 3′ was identical to the wild-type 6/IL-1, but contained nucleotide substitutions, mNF-IL6 within the −91 to −83 NF-IL6 binding motif (see Fig 2A). The NF-IL6 site from the human IL-6 gene (oligonucleotide 6/6) was as described previously.34 The DT (−59 to +12), DTmSpi-1 and HD (−131 to −59) DNA fragments were generated by restriction endonuclease digestion. DTmSpi-1 was identical to the wild-type DT, but contained nucleotide substitutions, mSpi-1 within the −50 to −39 Spi-1 (NF-βA) site (see Fig 2A). Blunt-ended Oct-1 consensus oligonucleotide, 5′ TGTCGAATGCAAATCACTAGAA 3′ was purchased from Promega Corp.
Oligonucleotides were labeled using DNA polymerase Klenow fragment and two [α-32P] deoxynucleoside triphosphates (dNTPs; deoxycytidine 5′-triphosphate and deoxyadenosine 5′-triphosphate) at 3,000 Ci/mmol (Amersham, Buckinghamshire, UK). Blunt-ended Oct-1 oligonucleotide was labeled with T4 polynucleotide kinase and [γ-32P]ATP. Unincorporated dNTPs were removed by use of G-25 spin columns (5 Prime-3 Prime, Inc, Boulder, CO). Binding reactions were performed as previously described,32 followed by analysis on a 4% polyacrylamide gel running in 0.5× TBE buffer (89 mmol/L Tris-borate and 2.5 mmol/L EDTA).
GST pulldown assay. Overnight cultures of bacterial cells containing pGEX, GST-Spi–1, or GST-NF–IL6 plasmid were diluted 1:10 in Luria-Bertani (LB) medium with ampicillin and grown for 1 hour at 37°C. Cultures were incubated for an additional 3 hours in the presence of isopropyl-β–D-thiogalactopyranoside (IPTG; 0.1 mmol/L). Bacteria were pelleted and resuspended in 0.1 vol of NETN (20 mmol/L Tris pH 8.0, 100 mmol/L NaCl, 1 mmol/L EDTA, 0.5% nonidet P-40 [NP-40]) containing 100 μg/mL of lysozyme. After incubation at 4°C for 15 minutes, the cells were sonicated three times for 15 seconds on ice and then centrifuged at 10,000×g for 5 minutes. Glutathione-agarose was added and the samples were rocked at 4°C for 30 minutes. The beads were washed at least three times in NETN and mixed with 35S-labeled in vitro translated HTLV-I Tax protein at room temperature for 1 hour. The beads were washed five times with NETN, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer, boiled, and subjected to electrophoresis on 10% SDS polyacrylamide gels.
RESULTS
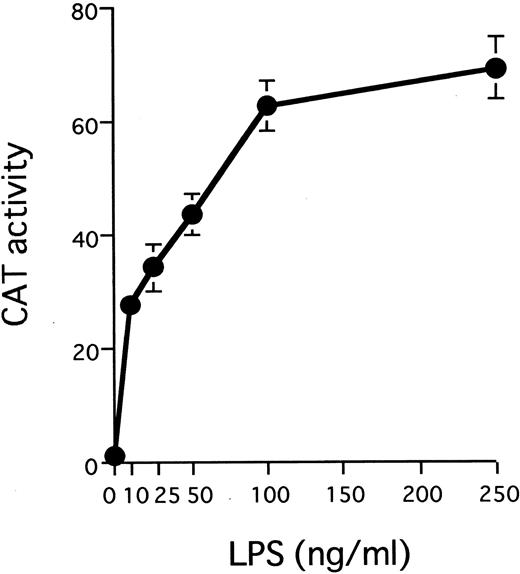
The IL1B promoter is transactivated by HTLV-I Tax. We have recently shown that monocyte/macrophage-specific expression of the IL1B gene depends on its promoter located between positions −131 and +12.32 The −131/+12 IL1B promoter sequence (HT fragment; see Fig 2A) was introduced into 3ME CAT vector and assayed for promoter activity. The 3MEHT construct showed a very low level of background CAT activity. CAT activity for 3MEHT was dose-dependently induced following treatment of THP-1 cells with LPS and reached a maximum at 100 ng/mL of LPS (Fig 1). LPS stimulation generated an approximately 15-fold increase in 3MEHT CAT activity at a concentration of 10 ng/mL. To evaluate the effect of Tax on IL1B promoter activity, 3MEHT CAT reporter was cotransfected into THP-1 cells along with pcTax, a Tax expression vector or a control plasmid. At 24 hours after transfection, the cells were either left unstimulated or stimulated with an optimal concentration of LPS (100 ng/mL). The cells were harvested after an additional 24-hour incubation. As shown in Fig 2B, treatment of THP-1 with an optimal (100 ng/mL) concentration of LPS resulted in an approximately 50-fold increase in CAT activity over control in the absence of Tax. However, when 4 μg of pcTax was cotransfected into THP-1 along with 3MEHT, a 90-fold increase in activity over control was observed, even in the absence of LPS. The CAT activity induced by Tax was significantly higher than that by stimulation of LPS. Furthermore, when THP-1 carrying pcTax were stimulated with LPS, LPS further enhanced Tax-induced promoter activity 2.8-fold. Thus, synergy between LPS and Tax in inducing the IL1B promoter acitivity in monocytic cells was observed. 3ME vector, in contrast to 3MEHT, did not show any increased CAT activity following either cotransfection of pcTax or LPS treatment (Fig 2B).
LPS stimulates transcription from the IL1B promoter in a dose-dependent manner. A total of 10 μg of 3MEHT CAT vector was transfected into THP-1 cells. After transfection, cells were treated with various concentrations of LPS or left untreated. Error bars represent standard deviation (SD) from three experiments.
LPS stimulates transcription from the IL1B promoter in a dose-dependent manner. A total of 10 μg of 3MEHT CAT vector was transfected into THP-1 cells. After transfection, cells were treated with various concentrations of LPS or left untreated. Error bars represent standard deviation (SD) from three experiments.
Two transcription factor binding elements, Spi-1 (NF-βA site) and NF-IL6 binding sites are critical for Tax induction of the IL1B promoter. The IL1B promoter contains two transcription factor binding motifs for NF-IL6 (C/EBPβ) and Spi-1 (PU.1), which have been shown to be essential for LPS induction of the IL1B gene in monocytic cells (Fig 2B).32 To determine whether both of NF-IL6 and Spi-1 play an important role in IL1B promoter induction by Tax as well as LPS, two distinct mutated CAT constructs were used instead of the wild-type 3MEHT CAT construct. Specific mutations (Fig 2A) were introduced into either the −50 to −39 Spi-1 (NF-βA site; 3MEHTmSpi-1) or the −91 to −83 NF-IL6 (upstream NF-IL6 site; 3MEHTmNF-IL6) binding site within the HT sequence. As shown in Fig 2B, when pcTax was cotransfected along with 3MEHTmSpi-1, an almost complete loss of Tax-induced CAT activity was observed. Mutation of the −91 to −83 NF-IL6 site (3MEHTmNF-IL6) also resulted in an approximately 95% decrease in Tax-induced activity. These results clearly show the importance of the two sites for Tax-induction of the IL1B promoter and suggest the possible involvement of Spi-1 and NF-IL6 proteins.
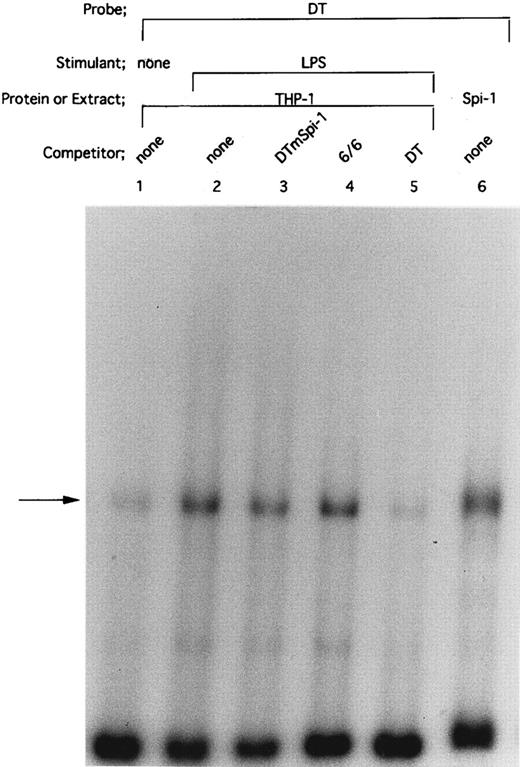
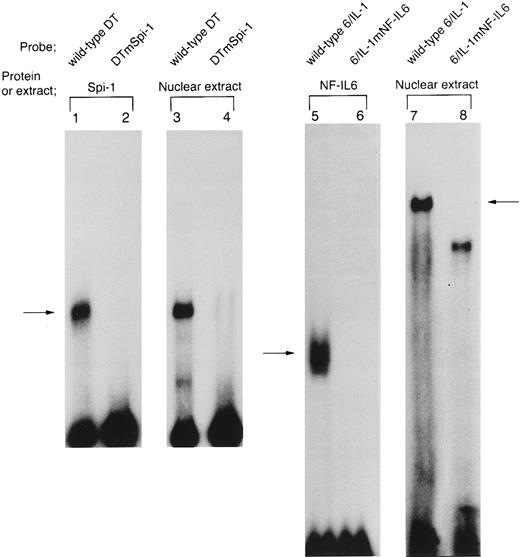
HTLV-I Tax protein enhances binding of both Spi-1 and NF-IL6 to the IL1B promoter. Tax is not a sequence-specific DNA binding protein, but has been known to associate with bZIP family proteins such as cAMP-responsive element binding protein (CREB), activating transcription factor (ATF), and C/EBP to increase their DNA binding activities.23 In the present study, our mutational CAT analysis has shown the possible involvement of Spi-1 and NF-IL6 in Tax induction of the IL1B promoter. In this regard, Zhang et al36 have reported two NF-IL6 binding motifs in the −131 to +12 IL1B promoter sequence. One is located at positions −91 to −83, mutation of which abrogated Tax induction of the IL1B promoter in the present study. Another is located at positions −41 to −33. However, the downstream NF-IL6 site at −41 to −33 overlaps the 3′ end of the binding site for Spi-1 (NF-βA site) at −50 to −39 by 3 bp, suggesting that this overlapping binding element is not shared by the two factors. This argument is supported by a recent report using RAW murine macrophage cells treated with LPS, which has shown that Spi-1, not NF-IL6 is a principal protein binding to the overlapping sequence and is responsible for transcriptional activation of the −59 to +12 IL1B proximal promoter (DT fragment).37 Thus, the function of the −41 to −33 downstream NF-IL6 binding site is poorly defined. To investigate the possible effect of Tax on DNA binding of Spi-1 and NF-IL6, EMSA studies were performed with radiolabeled IL1B promoter probes. Nuclear extracts were prepared from THP-1 monocytic cells transfected with pcTax or a control vector. Figure 3 showed a −59 to +12 DT probe containing the overlapping Spi-1/NF–IL6 site. THP-1 nuclear extracts showed formation of a DT-protein complex (arrow), migration pattern of which is indistinguishable from that formed with in vitro translated recombinant Spi-1 protein (Fig 3, lane 6). The DT-protein complex was competed for by a 50-fold molar excess of unlabeled DT fragment itself (Fig 3, lane 5). DTmSpi-1 containing site-specific mutation of the −50 to −39 Spi-1 (NF-βA) site, unlike wild-type DT, did not compete for the protein-DT complex (Fig 3, lane 3). Furthermore, the finding that an oligonucleotide 6/6 containing a strong NF-IL6 binding motif from the human IL-6 gene showed no significant competition (Fig 3, lane 4) supported a recent report of Buras et al,37 which has shown that the overlapping site predominantly binds Spi-1 protein. Based on these data, we compared Spi-1/DT complex derived from untreated THP-1 cells with that from Tax-expressing THP-1 cells. As shown in Fig 4, a significant increase in Spi-1 binding to DT fragment was observed following transfection of pcTax into THP-1 cells (Fig 4, lanes 1 and 3), indicating that Tax induces binding of Spi-1 to the DT IL1B promoter sequence. To confirm this finding, we used recombinant Spi-1 and Tax proteins with radiolabeled DT probe. As shown in Fig 4, lanes 5 to 7, recombinant Tax protein itself did not bind to DT fragment, but significantly enhanced formation of recombinant Spi-1/DT complex. These results clearly showed that Tax enhances binding of Spi-1 to the IL1B promoter. Shackelford et al38 have recently reported that LPS induces DNA binding of Spi-1 in murine tissue macrophages. We also observed a significant increase in binding of Spi-1 to the DT fragment following treatment of THP-1 monocytic cells with LPS (Fig 4, lanes 1 and 2).
Spi-1 is a predominant protein that binds to the overlapping NF-IL6/Spi–1 binding site. The −59 to +12 DT fragment was used as a radiolabeled probe. Nuclear extracts were derived from THP-1 cells untreated (lane 1) or treated with 100 ng/mL of LPS for 45 minutes (lanes 2 to 5). In lane 6, in vitro expressed recombinant Spi-1 was used. Cold competitor DNAs were described in the text and in the legend to Fig 2A. All competitors were used in a 50-fold molar excess over the radiolabeled probe. The arrow locates the mobility of Spi-1.
Spi-1 is a predominant protein that binds to the overlapping NF-IL6/Spi–1 binding site. The −59 to +12 DT fragment was used as a radiolabeled probe. Nuclear extracts were derived from THP-1 cells untreated (lane 1) or treated with 100 ng/mL of LPS for 45 minutes (lanes 2 to 5). In lane 6, in vitro expressed recombinant Spi-1 was used. Cold competitor DNAs were described in the text and in the legend to Fig 2A. All competitors were used in a 50-fold molar excess over the radiolabeled probe. The arrow locates the mobility of Spi-1.
HTLV-I Tax induces binding of Spi-1 to the IL1B promoter. The −59 to +12 DT fragment was used as a radiolabeled probe. Nuclear extracts were prepared from THP-1 cells carrying either pcTax (4 μg; lanes 3 and 4) or control pcDNA1 vector (4 μg; lanes 1 and 2). THP-1 cells were transfected with either pcTax or pcDNA1. At 24 hours after transfection, cells were treated with 100 ng/mL of LPS for 45 minutes (lanes 2 and 4) or left untreated (lanes 1 and 3). In vitro expressed recombinant Spi-1 was incubated with the radiolabeled DT probe in the presence (lane 6) or absence (lane 5) of in vitro expressed recombinant Tax. The total amount of added reticulocyte lysate in each reaction mixture was kept constant by the addition of incubated control lysate. The arrow locates the mobility of Spi-1.
HTLV-I Tax induces binding of Spi-1 to the IL1B promoter. The −59 to +12 DT fragment was used as a radiolabeled probe. Nuclear extracts were prepared from THP-1 cells carrying either pcTax (4 μg; lanes 3 and 4) or control pcDNA1 vector (4 μg; lanes 1 and 2). THP-1 cells were transfected with either pcTax or pcDNA1. At 24 hours after transfection, cells were treated with 100 ng/mL of LPS for 45 minutes (lanes 2 and 4) or left untreated (lanes 1 and 3). In vitro expressed recombinant Spi-1 was incubated with the radiolabeled DT probe in the presence (lane 6) or absence (lane 5) of in vitro expressed recombinant Tax. The total amount of added reticulocyte lysate in each reaction mixture was kept constant by the addition of incubated control lysate. The arrow locates the mobility of Spi-1.
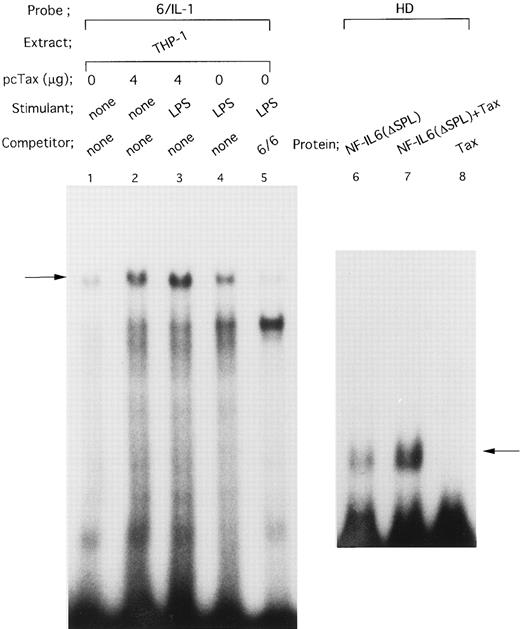
On the other hand, the −91 to −83 motif in the IL1B promoter has been reported to bind NF-IL6.39 Consistent with this report, when an oligonucleotide 6/IL-1 corresponding to the −91 to −83 NF-IL6 binding site was used as an EMSA probe (Fig 5), a complex (arrow) formed with THP-1 nuclear extract was competed for by a 50-fold molar excess of an unlabeled oligonucleotide 6/6 containing a strong NF-IL6 binding site from the human IL-6 gene (Fig 5, lanes 4 and 5), indicating binding of NF-IL6–like nuclear factor(s) to the IL1B promoter. Interestingly, the nuclear protein(s)/6/IL-1 complex formation was significantly enhanced by transfection of pcTax into THP-1 cells (Fig 5, lanes 1 and 2). In addition, when Tax-expressing THP-1 cells were stimulated with LPS, formation of the Tax-induced nuclear protein(s)/6/IL-1 complex further increased following LPS treatment (Fig 5, lanes 2 and 3). This result agrees well with our CAT data showing synergistic induction of the IL1B promoter by Tax and LPS.
HTLV-I Tax induces binding of NF-IL6 to the IL1B promoter. DNA binding reactions were performed with a radiolabeled 6/IL-1 (lanes 1 to 5) and −131 to −59 HD probe (lanes 6 to 8). The oligonucleotide 6/IL-1 sequence containing the −91 to −83 NF-IL6 site of the IL1B promoter was described in the Materials and Methods. Nuclear extracts prepared as described in the legend to Fig 4 were used. In lanes 6 to 8, recombinant truncated NF-IL6 with a deletion of the activation domain (Δspl) and/or recombinant Tax protein were incubated with a radiolabeled HD probe. An oligonucleotide 6/6 contains a strong NF-IL6 binding site from the human IL-6 gene. The 6/6 competitor was used in a 50-fold molar excess over radiolabeled probe. The arrow locates the mobility of DNA-protein complex containing NF-IL6.
HTLV-I Tax induces binding of NF-IL6 to the IL1B promoter. DNA binding reactions were performed with a radiolabeled 6/IL-1 (lanes 1 to 5) and −131 to −59 HD probe (lanes 6 to 8). The oligonucleotide 6/IL-1 sequence containing the −91 to −83 NF-IL6 site of the IL1B promoter was described in the Materials and Methods. Nuclear extracts prepared as described in the legend to Fig 4 were used. In lanes 6 to 8, recombinant truncated NF-IL6 with a deletion of the activation domain (Δspl) and/or recombinant Tax protein were incubated with a radiolabeled HD probe. An oligonucleotide 6/6 contains a strong NF-IL6 binding site from the human IL-6 gene. The 6/6 competitor was used in a 50-fold molar excess over radiolabeled probe. The arrow locates the mobility of DNA-protein complex containing NF-IL6.
The observation that Tax can induce DNA binding of NF-IL6–like factor(s), as well as Spi-1, led us to examine the effect of Tax on DNA binding activity of recombinant NF-IL6 protein. Because Tax has been recently demonstrated to associate with the conserved basic region of bZIP proteins,24 a recombinant truncated NF-IL6 protein (Δspl ) consisting of basic, leucine zipper, and a part of N terminal regions was used together with −131 to −59 HD (Fig 2A) IL1B promoter probe, which contains the −91 to −83 upstream NF-IL6 binding motif. As shown in Fig 5, lanes 6 to 8, when the truncated NF-IL6 (Δspl ) was coincubated with recombinant Tax protein, a significant increase in binding of the NF-IL6 to HD probe (arrow; Fig 5, lanes 6 and 7) was observed.
The site-specific mutations, mSpi-1 and mNF-IL6 within the IL1B promoter sequence block binding of Spi-1 and NF-IL6, respectively. In the present study, our CAT analysis using mutated constructs, 3MEHTmSpi-1 and 3MEHTmNF-IL6 showed that both the −50 to −39 Spi-1 (NF-βA site) and the −91 to −83 NF-IL6 binding sites are essential for Tax induction of the IL1B promoter. To further clarify involvement of Spi-1 and NF-IL6 in the IL1B promoter activation by HTLV-I Tax, we performed DNA/protein binding studies using mutated IL1B promoter DNA fragments (DTmSpi-1 and 6/IL-1mNF–IL6) as 32P-labeled probes (Fig 6). DTmSpi-1 DNA fragment, which was generated by restriction endonuclease digestion, was identical to the wild-type DT, but contained nucleotide substitutions, mSpi-1 within the −50 to −39 Spi-1 binding motif (Fig 2A). 6/IL-1mNF-IL6 was a synthetic oligonucleotide, which was identical to the wild-type 6/IL-1, but contained nucleotide substitutions, mNF-IL6 in the −91 to −83 NF-IL6 site (Fig 2A). As shown in Fig 6, lanes 1 to 4, when binding affinity of Spi-1 for the wild-type DT was compared with that for the DTmSpi-1 by using both THP-1 nuclear extract and recombinant Spi-1, insertion of the mutation into DT fragment significantly inhibited binding of Spi-1. Moreover, the mutated 6/IL-1 (6/IL-1mNF–IL6), in contrast to the wild-type 6/IL-1, did not bind recombinant full-length NF-IL6 (Fig 6, lanes 5 and 6). In addition, when radiolabeled 6/IL-1mNF–IL6 probe was incubated with Tax-transfected THP-1 nuclear extract, the Tax-inducible nuclear protein(s) binding to 6/IL-1 was also blocked by the mNF-IL6 mutation (arrow; Fig 6, lanes 7 and 8). These data confirm our CAT mutation results and further strongly suggest involvement of NF-IL6 and Spi-1 in the IL1B promoter induction by HTLV-I Tax. However, it may be noteworthy that in EMSA using a radiolabeled 6/IL-1 probe, the complex generated with Tax-transfected THP-1 nuclear extract appeared to migrate slower than NF-IL6 homodimer formed with recombinant full-length NF-IL6 protein (Fig 6, lanes 5 and 7). Moreover, introduction of the mNF-IL6 mutation into the −91 to −83 NF-IL6 site within the 6/IL-1 oligonucleotide caused conversion of the slow complex formed with Tax-transfected THP-1 nuclear extract to a slightly faster migrating complex (Fig 6, lanes 7 and 8).
The site-specific mutations, mSpi-1 and mNF-IL6 (Fig 2A) within the IL1B promoter block binding of Spi-1 and NF-IL6, respectively. DNA binding reactions were performed by using radiolabeled DT, DTmSpi-1, 6/IL-1, and 6/IL-1mNF-IL6 probes. DTmSpi-1 fragment containing a site-specific mutation, mSpi-1 (Fig 2A) was generated by restriction endonuclease digestion of 3MEHTmSpi-1. 6/IL-1mNF–IL6 is a synthetic oligonucleotide identical to the 6/IL-1, but possesses a site-specific mutation, mNF-IL6 described in the legend to Fig 2A. These mutations abolished IL1B promoter induction by Tax. Recombinant Spi-1 protein was incubated with either the wild-type DT (lane 1) or DTmSpi-1 (lane 2). Recombinant full-length NF-IL6 was incubated with either the wild-type 6/IL-1(lane 5) or its mutated version, 6/IL-1mNF-IL6 (lane 6). Nuclear extract prepared from THP-1 cells carrying pcTax, a Tax-expression vector was used in lanes 3, 4, 7, and 8.
The site-specific mutations, mSpi-1 and mNF-IL6 (Fig 2A) within the IL1B promoter block binding of Spi-1 and NF-IL6, respectively. DNA binding reactions were performed by using radiolabeled DT, DTmSpi-1, 6/IL-1, and 6/IL-1mNF-IL6 probes. DTmSpi-1 fragment containing a site-specific mutation, mSpi-1 (Fig 2A) was generated by restriction endonuclease digestion of 3MEHTmSpi-1. 6/IL-1mNF–IL6 is a synthetic oligonucleotide identical to the 6/IL-1, but possesses a site-specific mutation, mNF-IL6 described in the legend to Fig 2A. These mutations abolished IL1B promoter induction by Tax. Recombinant Spi-1 protein was incubated with either the wild-type DT (lane 1) or DTmSpi-1 (lane 2). Recombinant full-length NF-IL6 was incubated with either the wild-type 6/IL-1(lane 5) or its mutated version, 6/IL-1mNF-IL6 (lane 6). Nuclear extract prepared from THP-1 cells carrying pcTax, a Tax-expression vector was used in lanes 3, 4, 7, and 8.
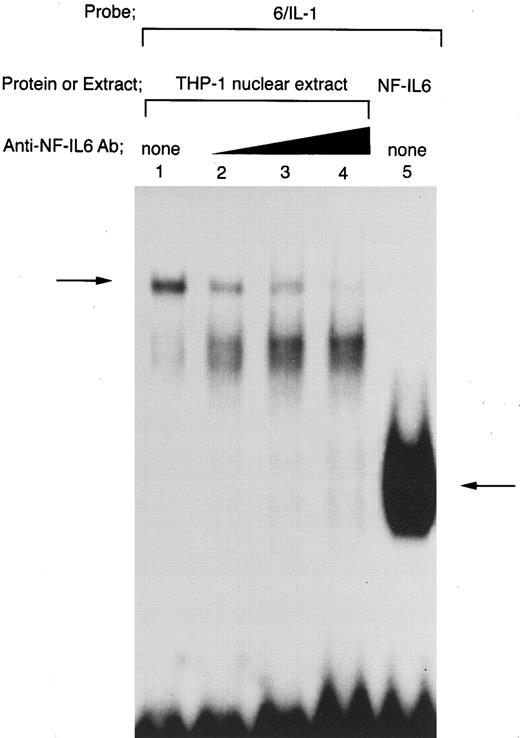
Tax-induced nuclear protein(s)/6/IL-1 complex contains not only NF-IL6, but also other protein(s) than NF-IL6. To further clarify the identity of the slow nuclear protein(s)/6/IL-1 complex formed in the presence of Tax, anti-NF–IL6 Ab was used in our EMSA analysis (Fig 7). As a result, the slow complex was eliminated by the addition of anti-NF–IL6 Ab (arrow; Fig 7, lanes 1 to 4). This result agrees well with previous reports with the same anti-NF–IL6 Ab,35,40 which have indicated that this antibody abrogates DNA/NF-IL6 complex formation. The fact that this NF-IL6 Ab was raised against a unique NF-IL6 peptide located immediately adjacent to the DNA binding domain40 may explain the abrogation of DNA/NF-IL6 complex by the antibody. In addition, this anti-NF–IL6 Ab has been shown to be not cross-reactive with other C/EBP family proteins.35,40 Akira et al40 have reported that this antibody immunoprecipitates only NF-IL6 out of nuclear extracts derived from human SK-MG–4 glioblastoma cells. Thus, these data show that the complex formed between Tax-transfected THP-1 nuclear extract and the wild-type 6/IL-1 probe involves NF-IL6 protein. However, in agreement with the data shown in Fig 6, EMSA studies using a radiolabeled 6/IL-1 probe showed that the complex formed with Tax-transfected THP-1 nuclear extract migrates significantly slower than NF-IL6 homodimer generated with recombinant full-length NF-IL6 protein (comparison of lane 1 with lane 5 in Fig 7). Moreover, our EMSA competition and antibody studies indicated that the abrogation of NF-IL6/6/IL–1 complex formation by the addition of either anti-NF–IL6 Ab (Fig 7, lanes 2 to 4) or an unlabeled 6/6 competitor (Fig 5, lane 5) leads to conversion of the Tax-inducible slow complex to a slightly faster migrating complex. These data strongly suggest that the slow complex contains not only NF-IL6, but also other protein(s) than NF-IL6. Additionally, the fact that introduction of mNF-IL6 mutation into the −91 to −83 NF-IL6 binding site, which inhibits Tax induction of the IL1B promoter in our transfection studies, also results in conversion of the slow complex to a slightly faster migrating complex in EMSA using radiolabeled 6/IL-1 and 6/IL-1mNF–IL6 probes (Fig 6, lanes 7 and 8) supports our argument and further confirms our CAT mutation results showing the importance of NF-IL6 in Tax induction of the IL1B promoter.
Tax-inducible complex formed between THP-1 nuclear extract and 6/IL-1 oligonucleotide contains not only NF-IL6, but also other protein(s) than NF-IL6. DNA binding reactions were performed with a radiolabeled 6/IL-1. Nuclear extract prepared from Tax-expressing THP-1 cells was used in lanes 1 to 4. Lane 5 shows the mobility of NF-IL6 homodimer formed with recombinant full-length NF-IL6. Nuclear extract was incubated with anti-NF–IL6 Ab at room temperature for 30 minutes and then assayed for binding activity to the radiolabeled 6/IL-1 probe. Triangle indicates increasing anti-NF–IL6 Ab concentrations. The total amount of added antibody in each reaction mixture was kept constant by the addition of a control antibody.
Tax-inducible complex formed between THP-1 nuclear extract and 6/IL-1 oligonucleotide contains not only NF-IL6, but also other protein(s) than NF-IL6. DNA binding reactions were performed with a radiolabeled 6/IL-1. Nuclear extract prepared from Tax-expressing THP-1 cells was used in lanes 1 to 4. Lane 5 shows the mobility of NF-IL6 homodimer formed with recombinant full-length NF-IL6. Nuclear extract was incubated with anti-NF–IL6 Ab at room temperature for 30 minutes and then assayed for binding activity to the radiolabeled 6/IL-1 probe. Triangle indicates increasing anti-NF–IL6 Ab concentrations. The total amount of added antibody in each reaction mixture was kept constant by the addition of a control antibody.
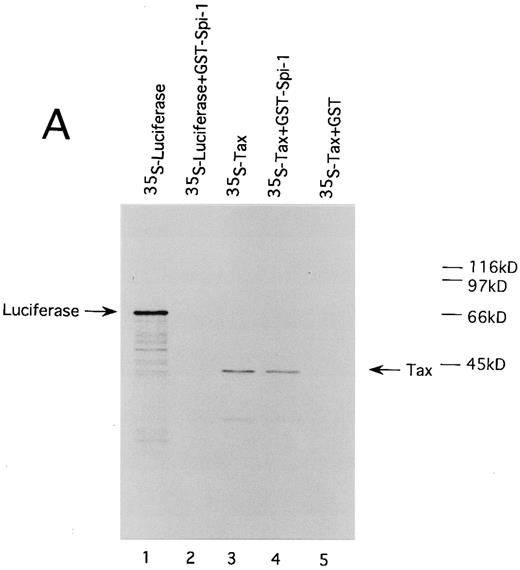
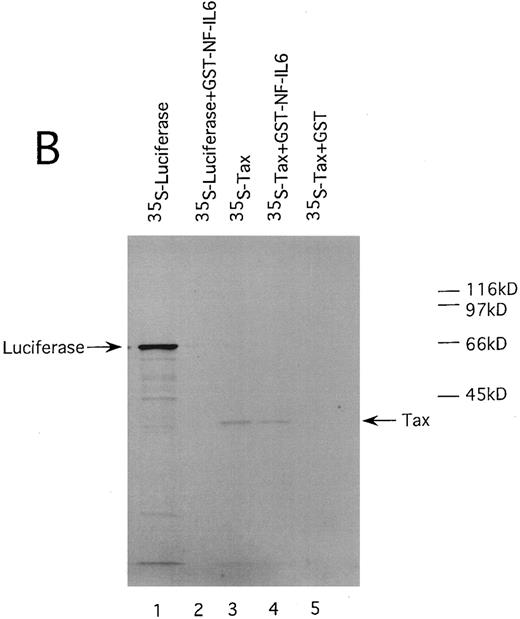
HTLV-I Tax physically interacts with both of Spi-1 and NF-IL6. We also performed GST pulldown assays to confirm the association of Tax with Spi-1 and NF-IL6. Either NF-IL6 or Spi-1 cDNA was inserted into plasmid pGEX to produce GST fusion constructs. 35S-labeled Tax protein prepared by in vitro transcription and translation was incubated with either GST, GST-NF–IL6, or GST-Spi–1 linked to glutathione-agarose beads. The beads were washed and bound proteins were eluted by boiling. The eluted proteins were subjected to SDS-PAGE. As shown in Fig 8, Tax bound directly to both GST-Spi–1 (Fig 8A, lane 4) and GST-NF–IL6 (Fig 8B, lane 4), but not to GST alone (Fig 8A and B, lanes 5). Additionally, when 35S-labeled luciferase was substituted for radiolabeled Tax, luciferase interacted with neither GST-Spi–1 (Fig 8A, lane 2) nor GST-NF–IL6 (Fig 8B, lane 2).
HTLV-I Tax physically interacts with both Spi-1 (A) and NF-IL6 (B). 35S-labeled Tax and luciferase proteins were prepared by in vitro transcription and translation. Lanes 1 and 3 of experiments A and B indicated the mobilities of these radiolabeled proteins on SDS-PAGE. In lanes 2, 4, and 5 of A and B, labeled proteins were incubated with GST, GST-Spi-1, or GST-NF-IL6 protein linked to glutathione-agarose beads. After incubation, the beads were washed, and bound proteins were eluted. The eluted proteins were analyzed on SDS-PAGE.
HTLV-I Tax physically interacts with both Spi-1 (A) and NF-IL6 (B). 35S-labeled Tax and luciferase proteins were prepared by in vitro transcription and translation. Lanes 1 and 3 of experiments A and B indicated the mobilities of these radiolabeled proteins on SDS-PAGE. In lanes 2, 4, and 5 of A and B, labeled proteins were incubated with GST, GST-Spi-1, or GST-NF-IL6 protein linked to glutathione-agarose beads. After incubation, the beads were washed, and bound proteins were eluted. The eluted proteins were analyzed on SDS-PAGE.
DNA binding of Oct-1 is enhanced by neither LPS nor HTLV-I Tax protein. To determine whether HTLV-I Tax specifically enhances DNA binding of Spi-1 and NF-IL6, we investigated the effect of Tax on DNA binding activity of Oct-1, an ubiquitously expressed octamer-binding transcription factor41 (Fig 9). When nuclear extract from untreated THP-1 cells was incubated with an Oct-1 consensus oligonucleotide containing the octamer motif ATGCAAAT, a DNA/protein complex was formed (Fig 9, lane 1; arrow). In this regard, it has been reported that Oct-1 and Oct-2 indistinguishably recognize the octamer motif.41 However, our observation that anti-Oct–1 Ab completely supershifted the complex generated with THP-1 nuclear extract and the Oct-1 consensus oligonucleotide (Fig 9, lanes 5 and 6) shows that Oct-1 is contained within the complex. The fact that Oct-2, unlike Oct-1, is a lymphoid cell-specific octamer-binding protein42 further supports our argument. Wang et al43 have recently shown that by using human peripheral blood mononuclear cells, DNA binding of Oct-1 is not affected by LPS stimulation. In the present study, we also observed that treatment of THP-1 with LPS did not enhance binding of Oct-1 to the octamer motif (Fig 9, lane 2). Additionally, when pcTax, a Tax expression vector, was introduced into THP-1 cells, Tax expression, as well as LPS, failed to affect binding of Oct-1 to its consensus oligonucleotide (Fig 9, lane 3).
Neither HTLV-I Tax nor LPS enhances binding of Oct-1 to its target octamer-binding motif. DNA binding reactions were performed with a radiolabeled Oct-1 consensus oligonucleotide. The oligonucleotide containing the octamer-binding motif was described in Materials and Methods. Nuclear extracts prepared as described in the legend to Fig 4 were used. In lanes 5 and 6, nuclear extract was incubated with anti-Oct–1 Ab at room temperature for 30 minutes. Triangle indicates increasing anti-Oct–1 Ab concentrations. In lanes 4 to 6, the total amount of added antibody in each reaction mixture was kept constant by the addition of a control antibody.
Neither HTLV-I Tax nor LPS enhances binding of Oct-1 to its target octamer-binding motif. DNA binding reactions were performed with a radiolabeled Oct-1 consensus oligonucleotide. The oligonucleotide containing the octamer-binding motif was described in Materials and Methods. Nuclear extracts prepared as described in the legend to Fig 4 were used. In lanes 5 and 6, nuclear extract was incubated with anti-Oct–1 Ab at room temperature for 30 minutes. Triangle indicates increasing anti-Oct–1 Ab concentrations. In lanes 4 to 6, the total amount of added antibody in each reaction mixture was kept constant by the addition of a control antibody.
DISCUSSION
The best characterized stimulation that triggers IL-1β release by monocytes and macrophages is LPS. On the other hand, direct action of viruses as triggering agents has not been clearly defined. In the present study, Tax expression in THP-1 monocytic cells resulted in an increased activity for the IL1B promoter. The promoter activity induced by Tax was comparable to that by LPS (Fig 2). These results clearly show that HTLV-I Tax protein transactivates the IL1B promoter in monocytic cells. Interestingly, our CAT data further show synergy between Tax and LPS in inducing the IL1B promoter. Such synergism in transcriptional activation of the IL1B gene in monocytic cells has been observed between LPS and HIV-1 tat, a major regulatory protein of HIV-1.44 Moreover, by using rat glial cells carrying a Tax expression vector, LPS-induced IL-1β production by glial cells has been reported to be enhanced by Tax expression.45
In the present study, we have shown that two transcription factor binding motifs, NF-IL6 and Spi-1 (NF-βA) sites are involved in Tax induction of the IL1B promoter in monocytic cells. Site-specific mutations of the two motifs result in an almost complete loss of Tax-inducible activity. NF-IL6 (C/EBPβ), which is a member of C/EBP family of leucine zipper transcription factors, pre-exists in cells as an inactive protein that supports transcription following induction-dependent posttranslational modification such as phosphorylation.46 47 This fact may suggest the possibility that Tax expression can activate at least one of the signal transduction pathways that phosphorylate NF-IL6 protein.
Although many cellular and viral genes including IL-1α, IL-2, IL-2 receptor (IL-2R), IL-6, IL-8, c-fos, PTH-rP, and HTLV-I have been reported to be activated by Tax, Tax is not a sequence-specific DNA-binding protein.9-18 In this regard, Tax has been shown to interact with bZIP factors, which bind to CRE within the HTLV-I promoter to increase DNA binding activity of the bZIP proteins.22-24,26 In the present study, our DNA-protein binding studies using Tax-transfected THP-1 nuclear extracts and recombinant NF-IL6 and Tax proteins show that Tax does not bind to DNAs directly, but stimulates DNA binding of NF-IL6 (Fig 5). In addition, our GST pulldown data indicate that NF-IL6 protein can physically associate with Tax protein (Fig 8). Consistent with our results, Wagner and Green23 have reported that Tax increases DNA binding of GST-C/EBP fusion protein in EMSA. bZIP domains comprise a leucine-rich dimerization motif and a basic region that mediates DNA contact. Perini et al22 have shown that through recognition of the conserved basic region within the bZIP, Tax increases DNA binding and modifies DNA site selection. We also observed that a truncated NF-IL6 containing intact basic and leucine zipper regions is still Tax-responsive (Fig 5).
Another element critical for Tax induction of the IL1B promoter is Spi-1 binding site (NF-βA site). Spi-1 is an Ets family member protein restricted in expression to monocytes, macrophages, B cells, mast cells, erythroid stem cells, and megakaryocytes and has been shown to be a major determinant in cell-type specific expressions of genes including CD11b, CSF-1 receptor, and IL1B.32,48,49 Several transcription factors such as TATA binding protein (TBP), retinoblastoma (Rb) protein, NF-IL6β, and NF-EM5 physically interact with Spi-1.50-54 However, association of Spi-1 with HTLV-I Tax has not previously been elucidated. In the present study, we have shown that in monocyte/macrophage cells, Tax stimulates binding of Spi-1 to the IL1B promoter through protein-protein interaction.
There are several reports concerning involvement of the Ets family proteins in Tax-inducible HTLV-I gene expression. The HTLV-I LTR is known to possess two Ets binding motifs, which are located between positions −117 and −160. Seeler et al55 have reported that Ets 1 transactivates the HTLV-I gene expression through recognition of the Ets site within the HTLV-I LTR. They further observed that Ets 1 increases the degree of Tax activation of the HTLV-I LTR. However, this is challenged by a report of Clark et al,56 which showed that in Jurkat T cells and peripheral blood T cells, the predominant protein binding to the HTLV-1 LTR Ets sites is Elf-1, an Ets family protein with similarity to Drosophia development factor E74, not Ets 1 and further indicated that mutations of the Ets binding sites did not inhibit response of HTLV-I LTR to Tax, suggesting that Ets family proteins are not involved in Tax activation of the HTLV-I LTR. Thus, there is disagreement concerning Tax induction of HTLV-I gene via the Ets sites. In this regard, our data obtained from EMSAs clearly show that Spi-1, an Ets family member protein, is involved in Tax induction of the IL1B promoter in monocyte/macrophage cells.
On the other hand, our binding studies using an Oct-1 consensus oligonucleotide show that in contrast to Spi-1 and NF-IL6, DNA binding of Oct-1 is not affected by either LPS stimulation or Tax (Fig 9). These results support our argument that HTLV-I Tax specifically induces DNA binding of Spi-1 and NF-IL6. Furthermore, Chen and Rothenberg57 have also reported that in IL-2 gene expression at three stages in the intrathymic development of pre-T cells, Oct-1 functions as a constitutive DNA binding factor, which does not require specific stimulation.
Although our results indicate association of Tax with both NF-IL6 and Spi-1, the combined roles of NF-IL6 and Spi-1 in Tax induction of the IL1B promoter are still unclear. The IL1B promoter contains a NF-IL6 site adjacent to a Spi-1 binding site. Potential Spi-1 and NF-IL6 sites are also present in some naturally occuring promoters such as the IL-6 gene.58 There are several reports that have indicated association of tissue-specific factors such as Ets 1 and NFATp with ubiquitous bZIP proteins.59-61 In particular, Nagulapalli et al52 have reported that Spi-1 can functionally cooperate with NF-IL6 β (C/EBPδ) to synergistically activate transcription. These observations suggest the possibility that Spi-1 cooperates with NF-IL6.
Many genes, especially inflammatory cytokine genes, harbor binding sites for NF-IL6 and NF-κB transcription factors. In addition, direct physical interaction between the two factors is known to be important in regulating the gene expressions. HTLV-I Tax associates with NF-κB to induce transcriptional activation of the genes.20,21 In this regard, the IL-8 gene promoter has binding elements for NF-IL6 and NF-κB that are directly adjacent.62 The IL-6 gene promoter also contains an NF-IL6 binding motif located approximately 70 bp upstream of an NF-κB site.58 The two promoters have been recently demonstrated to be activated by Tax through NF-κB sites.17,18 However, our observation that HTLV-I Tax associates with NF-IL6 may suggest that NF-IL6, as well as NF-κB, plays an important role in Tax induction of the IL-6 and IL-8 genes. In addition, it may be noteworthy that a region located at −300 bp upstream from the transcription start site of the IL1B gene also contains a functional NF-κB binding site, mutation of which has been reported to reduce responsiveness of the IL1B gene transcription to PMA and IL-1.63
In the present study, we have shown that Tax transactivates the IL1B promoter through association with two transcription factors, NF-IL6 and Spi-1, in monocyte/macrophage cells. IL-1β is one of the major monocyte/macrophage-specific inflammatory and immunoregulatory cytokines in chronic inflammation such as rheumatoid arthritis. IL1B gene expression has been shown to be activated at joints of HTLV-I transgenic mice, which develop inflammatory arthropathy resembling rheumatoid arthritis in humans.64 Umehara et al65 have further reported that IL-1β expression is detected on macrophages, astrocytes, and microglia in the spinal cords of patients with HAM/TSP. These observations strongly suggest that IL-1β produced by HTLV-I–infected monocyte/macrophage cells contributes to chronic inflammation or bone destruction in HTLV-I–associated diseases. Additionally, it is interesting that primary ATLL tumor cells require IL-1β derived from macrophages to produce IL-2, which is one of the well-known growth factors for ATLL.8 Thus, to understand the mechanism(s) of how expression of the IL1B gene is regulated in HTLV-I–infected cells is important for future efforts to modulate the host's response to HTLV-I infection.
Address reprint requests to Junichi Tsukada, PhD, MD, First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu, 807 Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal