Abstract
The cell cycle regulatory protein cyclin D1 is essential for G1-S phase transition in several epithelial and mesenchymal tissues but is apparently not essential in normal mature B cells. An overexpression of cyclin D1 is induced by the chromosomal translocation t(11; 14)(q13; q32), which characterizes non-Hodgkin's lymphomas (NHLs) of mantle cell type. We studied 26 cases of mantle cell lymphoma (MCL) for the expression of cyclins D1 and D3. A total of 23 lymphomas showed a nuclear staining for cyclin D1, whereas reactive B cells of residual germinal centers were constantly negative. When compared with cyclin D3, an inverse staining pattern emerged. Whereas the B cells of residual germinal centers reacted strongly positive for cyclin D3, there was low or missing expression of cyclin D3 in MCL cells. In other B-cell lymphomas (n = 55), including chronic lymphocytic leukemia, low-grade lymphomas of mucosa-associated lymphatic tissue, follicular lymphomas, and diffuse large B-cell lymphomas, no cyclin D1 expression could be detected and 89% of these cases displayed cyclin D3 positivity. Lymphoma cell lines harboring the t(11; 14) showed cyclin D1 protein but no or very low levels of cyclin D3; three other B-cell lines, a T-cell line, and peripheral blood lymphocytes strongly expressed cyclin D3 and reacted negatively for cyclin D1. We conclude that the chromosomal translocation t(11; 14) leads to an abnormal protein expression of cyclin D1 in the tumor cells of MCL and induces a consecutive downregulation of cyclin D3. In contrast to other B-NHLs, cyclin D1 and D3 expression in MCL is not related to the growth fraction.
AN IMPORTANT GROUP of the proteins involved in cell cycle regulation is constituted by the so-called cyclins of D-type, which share both structural homologies and several functional features. These cyclins play a major role in the regulation of G1-S phase transition during the cell cycle. Three types of D-cyclins have been identified that show tissue-specific patterns of expression.1 Whereas cyclin D1 is known to be expressed mainly by epithelial cells or in some mesenchymal cells,2 cyclins D2 and D3 are expressed by myelomonocytic and lymphoid cells.3,4 An overexpression of cyclin D1 can be induced either by amplification of the chromosomal region of the cyclin D1 gene at 11q13 as described in breast cancer,5 or by the chromosomal translocation t(11; 14)(q13; q32). The latter is characteristically found in non-Hodgkin's lymphoma (NHL) of mantle cell lymphoma type (MCL; centrocytic lymphoma).6,7 As a consequence, an overexpression of cyclin D1 mRNA as well as protein can be detected in most tumors of this entity.8-12
In this study we point out that, as an important further consequence of the chromosomal translocation, a downregulation of cyclin D3 unrelated to proliferative activity occurs in MCL, a feature that separates this entity from other B-cell lymphomas.
MATERIALS AND METHODS
Tissue samples. A total of 81 cases of NHL of B-cell lineage were selected from the files of the lymph node registry at the Institute of Pathology at the University of Würzburg (Würzburg, Germany). Sixty-six of these cases had already been included in a previous study on the expression of cyclin D1.12 All cases were diagnosed independently by two of us (H.K. and H.K.M.-H.). Diagnoses were made after the criteria of the Revised European American Lymphoma classification.13 In particular, 26 cases of MCL, 12 of chronic lymphocytic leukemia (CLL), 11 of low-grade lymphoma of mucosa-associated lymphatic tissue (MALT), 20 of follicle center cell lymphoma, and 12 of diffuse large B-cell lymphoma (DLBL) were included in the study.
Immunohistochemistry. The expression of CD3, CD20, CD43, CD45RO, IgM, κ, and λ was studied on paraffin sections by using a peroxidase antiperoxidase method. Ki-67 expression was investigated by using the antibody MIB1 after antigen retrieval treatment with microwave irradiation. In a similar way, the expression of cyclins D1 and D3 was investigated by using two monoclonal murine antibodies.
The DCS-6 antibody specific for cyclin D1 and the antibody DCS-22 specific for cyclin D3 are mouse monoclonal antibodies (MoAbs) applicable for immunostaining, immunoblotting, and immunoprecipitation.14-16
Immunohistochemical staining was performed by using the streptavidin-biotin-complex-method combined with antigen retrieval by microwave heating as described earlier.12,17 In detail, paraffin sections of 4 to 6 μm were mounted on silan-coated slides, deparaffinized, and rehydrated in decreasing ethanol concentrations. The slides were treated for 30 minutes in a microwave oven (750 W) in a citrate solution (2.1 g citrate/L H2O; pH 6.0). After 20 minutes of cooling, the slides were washed three times in phosphate buffered saline (PBS). Endogenous peroxidase was blocked by treatment with a peroxidase-blocking solution (430 μL, 30% H2O2 , and H2O to a final volume of 4,000 μL) for 10 minutes. After washing in PBS, the antibody DCS-6 or DCS-22, respectively, was applied (dilution 1:500, 100 μL per slide) and was allowed to incubate for 30 to 45 minutes at room temperature. The slides were then washed in PBS three times. The secondary antibody (“link-antibody”) and the peroxidase-streptavidin-complex were obtained from Biogenex (Neufahrn, Germany). The slides were incubated with the secondary antibody for 20 minutes and washed in PBS; in the same way, the peroxidase-streptavidin-complex was applied. The color reaction was performed by using a Diamino-Benzidin/Peroxide solution (DAB) from Biogenex (0.5 mL substrate buffer and 4.5 mL H2O were mixed with 4 drops DAB and 2 drops of peroxide solution). The slides were incubated with this solution for 5 to 10 minutes and washed again in PBS. Finally, after 5 minutes in H2O, the slides were stained for 1 to 3 minutes in hematoxylin. The staining pattern on paraffin sections of formalin-fixed specimens was controlled in tonsillary tissue and lymph nodes with reactive changes and mantle zone lymphomas expressing cyclin D1. The staining quality of cyclin D1 was noted to be sufficient when endothelial cells and fibroblasts were found to exhibit nuclear positivity. The percentage of cells exhibiting positive nuclear staining reactions was determined for Ki-67, cyclin D1, and cyclin D3 for each case. The number of positive cell nuclei in a total of 500 cells was counted in two to four high-power-fields in areas of intense immunostaining. The results were independently controlled by a second observer in a semiquantitative fashion by using a visual estimate as described for Ki-67.18 Discrepant cases were recounted by using the formal counting method. The mean of the obtained values was determined for each case and rounded to 5% steps. Finally, the mean value and standard deviation for the percentage of positive cells was calculated for each lymphoma entity and each parameter.
Double stainings for cyclin D3 and CD20 or CD45R0 were performed in a similar way. The primary antibody DCS-22 for cyclin D3 was used in a dilution of 1:100 and the reaction was detected by the peroxidase technique as described previously. After the DAB reaction and washing in PBS, the second primary antibody, L26 for CD20 or UCHL1 for CD45R0, respectively, was applied. The secondary antibody (link-antibody) for this reaction, as well as the alkaline phosphatase-streptavidin-complex, were also achieved from Biogenex. The slides were incubated with the secondary link-antibody for 20 minutes and washed in PBS before the alkaline-phosphatase-streptavidin complex was applied. The color reaction was performed with a solution of naphthol phosphate in Tris-HCl buffer after adding one tablet of Fast red chromogen (Biojenex, Neufahrn, Germany) to 5 mL substrate. After washing in PBS and H2O, the slides were counterstained in hematoxylin.
Cell lines and culture conditions. Human peripheral blood lymphocytes were isolated, cultured, and stimulated by phytohemagglutinin (PHA) as described previously.19 All other cell lines, including WI38 diploid fibroblasts; the T-cell leukemia cell line, MOLT-4; and the human B-cell lymphoma-derived cell lines, SP-49, NCEB-1, K620, JVM-2, Ramos, Daudi, and Namalwa were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 10 U/mL penicillin, and 10 U/mL streptomycin. The cell lines had been described earlier in more detail.19-21
Gel electrophoresis and immunoblotting. Total cell extracts were prepared by direct lysis of cell monolayers or series of cryostat sections from frozen biopsy specimens with hot Laemmli sample buffer. Extracted proteins were electrophoretically separated on either 10% or 12% polyacrylamide gels in the presence of sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were either stained with Coomassie blue to control balanced loading or blotted onto nitrocellulose (ECL grade; Amersham, Arlington Heights, IL) by a semi-dry transfer method.20 Membranes were subjected to immunoblotting with the enhanced chemiluminescence detection system (Amersham) according to the manufacturer's instructions.
RESULTS
All cases studied showed a positive reaction of the tumor cells for the B-cell antigen, CD20, and were negative for CD3 and CD45R0. A light chain restriction was found in the majority of cases. All cases of MCL showed positive reactions for CD43 as well as 10 of 12 cases of CLL, whereas all other tumors reacted negatively for CD43.
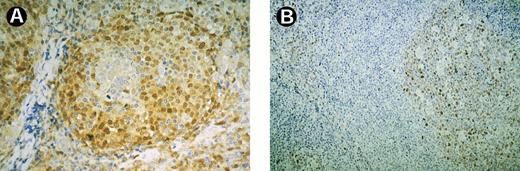
A total of 23 of 26 cases of MCL were found to express cyclin D1. The nuclear staining reaction was present in up to 80% of the tumor cells, although the staining intensity varied among the cells of each tumor (Fig 1A). Mitotic figures were negative. As reported earlier,12 staining for cyclin D1 resulted in an impressive accentuation of the mantle zone (perifollicular) growth pattern in several cases, because reactive B cells of residual germinal centers were found to be negative. Moreover, a difference in the overall staining intensity was noted between the single cases as described earlier.12 Cyclin D1 expression was not detected in 55 cases of B-cell lymphoma other than MCL.
Expression of cyclin D1 and cyclin D3 in a case of MCL. (A) MCL cells staining positively for cyclin D1. Note negative staining reaction in the residual germinal center. (B) Cyclin D3 is strongly expressed by B cells of the residual germinal center whereas MCL cells react negatively.
Expression of cyclin D1 and cyclin D3 in a case of MCL. (A) MCL cells staining positively for cyclin D1. Note negative staining reaction in the residual germinal center. (B) Cyclin D3 is strongly expressed by B cells of the residual germinal center whereas MCL cells react negatively.
Immunohistochemical staining with MoAb DCS-22 for cyclin D3 resulted in a completely negative reaction in seven cases of MCL. In the remaining 19 lymphomas, none or less than 5% of the cells within the tumor infiltrate expressed cyclin D3. Double staining showed that DCS-22–expressing cells reacted primarily positively for CD45R0 but not for CD20 (Fig 2A and B). The three cases of MCL staining negatively for Cyclin D1 did not express cyclin D3 either. Two of these had been found to have a bcl-1 rearrangement by Southern blotting in a previous study.12 However, residual B-cells in preserved germinal centers reacted strongly positively for cyclin D3, resulting in an inverse staining pattern compared with cyclin D1 (Fig 1B).
(A) Double staining of CD20 (red) and cyclin D3 (brown) in a case of MCL. Lymphoma cells exhibit a B-cell phenotype with red membrane staining for CD20, whereas the nuclei are negative for cyclin D3. By contrast there are a number of cyclin-D3–positive brown labeled nuclei in the periphery of the tumor nodule, most probably belonging to reactive T cells that are CD20 negative. (A) Double staining of CD45R0 (red) and cyclin D3 (brown) in a case of MCL shows that most of the cyclin-D3–positive cells within this tumor display simultaneously T-cell properties (arrow). (C) Double staining of CD20 (red) and cyclin D3 (brown) in a lymphoma of MALT. There are few cells labeled for cyclin D3 in their nuclei. These cells also exhibit CD20 staining with one double labeled cell within the epithelium of a gastric gland (arrow). (D) Double staining of CD20 (red) and cyclin D3 (brown) of a large B-cell lymphoma. There are a number of cyclin-D3–positive cycling cells that also show CD20 expression.
(A) Double staining of CD20 (red) and cyclin D3 (brown) in a case of MCL. Lymphoma cells exhibit a B-cell phenotype with red membrane staining for CD20, whereas the nuclei are negative for cyclin D3. By contrast there are a number of cyclin-D3–positive brown labeled nuclei in the periphery of the tumor nodule, most probably belonging to reactive T cells that are CD20 negative. (A) Double staining of CD45R0 (red) and cyclin D3 (brown) in a case of MCL shows that most of the cyclin-D3–positive cells within this tumor display simultaneously T-cell properties (arrow). (C) Double staining of CD20 (red) and cyclin D3 (brown) in a lymphoma of MALT. There are few cells labeled for cyclin D3 in their nuclei. These cells also exhibit CD20 staining with one double labeled cell within the epithelium of a gastric gland (arrow). (D) Double staining of CD20 (red) and cyclin D3 (brown) of a large B-cell lymphoma. There are a number of cyclin-D3–positive cycling cells that also show CD20 expression.
On the other hand, we found cyclin D3 expression in 12 of 12 cases of CLL, in 9 of 11 cases of low-grade lymphoma of MALT, in 17 of 20 cases of follicular cell lymphoma, and in 11 of 12 cases of DLBL. As a whole, 49 of 55 cases of B-NHL other than MCL were shown to express cyclin D3 (89.1%; Table 1). Cyclin D3 expressing cells in lymphomas other than MCL could be shown to express simultaneously CD20, proving their B-cell lineage (Fig 2C and D). In cases of CLL and low-grade lymphoma of MALT, residual reactive B cells of remnant follicles also exhibited a positive double staining reaction. Therefore, the counting was carefully performed, excluding areas of residual B cells as far as possible.
Expression of Cyclin D1 and Cyclin D3 in Cases of Non-Hodgkin's Lymphomas of B-Cell Lineage
| . | Cyclin D3 . | Cyclin D1 . |
|---|---|---|
| MCL | 0/26* | 23/26 (88.5%) |
| CLL | 12/12 (100.0%) | 0/12 |
| MALT | 9/11 (81.2%) | 0/11 |
| FCL | 17/20 (85.0%) | 0/20 |
| DLBL | 11/12 (91.7%) | 0/12 |
| . | Cyclin D3 . | Cyclin D1 . |
|---|---|---|
| MCL | 0/26* | 23/26 (88.5%) |
| CLL | 12/12 (100.0%) | 0/12 |
| MALT | 9/11 (81.2%) | 0/11 |
| FCL | 17/20 (85.0%) | 0/20 |
| DLBL | 11/12 (91.7%) | 0/12 |
Abbreviations: MCL, mantle cell lymphoma; CLL, chronic lymphocytic lymphoma; MALT, mucosa-associated lymphatic tissue; FCL, follicular cell lymphoma; DLBL, diffuse large B-cell lymphoma.
Seven cases showed a completely negative reaction; less than 5% of cells within the tumor cell infiltrate were positive in the remaining 19 cases.
We studied the proliferative activity by determining the Ki-67 labeling index and set up the mean values for the proliferation index as defined by the percentage of Ki-67–positive cell nuclei for each lymphoma entity. Detailed data of the immunohistochemical investigations are given in Table 2. Ki-67 and cyclin D3 labeling indices for each entity of B-NHL, with the exception of MCL, were found to be positively correlated (correlation coefficient, 0.969). No correlation was found between Ki-67 and cyclin D1 nor cyclin D3 in MCL regarding the amount of cyclin D1-positive cells or regarding the staining intensity.
Expression of Cyclins D1 and D3 and Ki-67 in Non-Hodgkin's Lymphomas of B-cell Lineage
| . | Ki-67 . | Cyclin D3 . | Cyclin D1 . |
|---|---|---|---|
| MCL | 38.2 ± 21.7 | <5%* | 42.7 ± 32.0 |
| CLL | 16.9 ± 10.5 | 9.0 ± 4.9 | negative |
| MALT | 20.0 ± 27.6 | 5.9 ± 4.9 | negative |
| FCL | 36.5 ± 18.1 | 12.1 ± 7.3 | negative |
| DLBL | 69.8 ± 21.1 | 38.1 ± 29.8 | negative |
| . | Ki-67 . | Cyclin D3 . | Cyclin D1 . |
|---|---|---|---|
| MCL | 38.2 ± 21.7 | <5%* | 42.7 ± 32.0 |
| CLL | 16.9 ± 10.5 | 9.0 ± 4.9 | negative |
| MALT | 20.0 ± 27.6 | 5.9 ± 4.9 | negative |
| FCL | 36.5 ± 18.1 | 12.1 ± 7.3 | negative |
| DLBL | 69.8 ± 21.1 | 38.1 ± 29.8 | negative |
Values represent mean percentage of labeled cells and standard deviation. Ki-67 and cyclin D3 are positively correlated (0.969).
Abbreviations: MCL, mantle cell lymphoma; CLL, chronic lymphocyte lymphoma; MALT, mucosa-associated lymphatic tissue; FCL, follicular cell lymphoma; DLBL, diffuse large B-cell lymphoma.
Only CD45R0-positive T cells and/or reactive B cells of germinal centers expressed cyclin D3.
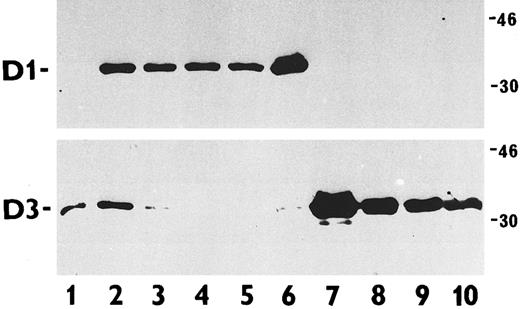
The results of immunoblotting are shown in Fig 3. Whereas normal peripheral blood lymphocytes exclusively expressed cyclin D3, WI38 diploid fibroblasts showed coexpression of cyclin D1 and cyclin D3 (positive control). The B-cell lymphoma cell lines, Ramos, Daudi, and Namalwa, all lacking the chromosomal translocation t(11; 14)(q13; q32), expressed cyclin D3 exclusively. The same reaction was present in the T-cell leukemia cell line MOLT-4. All B-cell lymphoma cell lines harboring the t(11; 14) were positive for cyclin D1, whereas cyclin D3 could not, or only weakly, be detected.
Immunoblotting analysis of cyclin D1 and D3 protein abundance in total cell extracts of various exponentially proliferating human cells. Upper panel, cyclin D1 detection with the MoAb DCS-6; lower panel, cyclin D3 detected with the MoAb DCS-22. Cell types examined were PHA-stimulated normal lymphocytes (lane 1); WI38 diploid fibroblasts (positive control, lane 2); B-cell lymphoma lines harboring the t(11; 14) translocations JVM-2 (lane 3), K620 (lane 4), NCEB-1 (lane 5), and SP-49 (lane 6); MOLT-4 T-cell leukemia line (lane 7); and B-cell lymphoma lines lacking the t(11; 14) translocations Ramos (lane 8), Daudi (lane 9), and Namalwa (lane 10). Note the reciprocal expression of cyclins D1 and D3 among the lymphoma/leukemia-derived cell lines (lanes 3 to 6 versus lanes 7 to 10). The molecular weight of the markers is given in kilodaltons.
Immunoblotting analysis of cyclin D1 and D3 protein abundance in total cell extracts of various exponentially proliferating human cells. Upper panel, cyclin D1 detection with the MoAb DCS-6; lower panel, cyclin D3 detected with the MoAb DCS-22. Cell types examined were PHA-stimulated normal lymphocytes (lane 1); WI38 diploid fibroblasts (positive control, lane 2); B-cell lymphoma lines harboring the t(11; 14) translocations JVM-2 (lane 3), K620 (lane 4), NCEB-1 (lane 5), and SP-49 (lane 6); MOLT-4 T-cell leukemia line (lane 7); and B-cell lymphoma lines lacking the t(11; 14) translocations Ramos (lane 8), Daudi (lane 9), and Namalwa (lane 10). Note the reciprocal expression of cyclins D1 and D3 among the lymphoma/leukemia-derived cell lines (lanes 3 to 6 versus lanes 7 to 10). The molecular weight of the markers is given in kilodaltons.
DISCUSSION
Since it had become evident that the candidate oncogene associated with the bcl-1 region at the chromosome band 11q13 is identical with cyclin D1,22 several studies have been addressed to the overexpression of cyclin D1 mRNA as well as of the protein cyclin D1 in mantle cell lymphoma.8-12,23 Obviously, the expression of cyclin D1 is a fairly unique feature of MCLs compared with other types of B-cell lymphomas, and the immunohistochemical detection of cyclin D1 protein can be even of diagnostic value.9,11,12 The overexpression of cyclin D1 mRNA in cases of hairy cell leukemia is obviously not related to a t(11; 14), and the over expression of cyclin D1 mRNA or protein is because of a yet unknown, different mechanism.24,25 Therefore, this special feature of MCL seems to be even more significant when taking into account the broad range of physiological cyclin D1 expression in normal human tissues. Cyclin D1 has been shown to be expressed at its peak during the G1 phase in the nuclei of several epithelial and mesenchymal cells. Cell lines of diverse epithelial and mesenchymal tumors have been found to express cyclin D1 in comparable amounts with respect to their normal tissue counterparts.2,14,15 Furthermore, it became evident that cell lines harboring the chromosomal translocation t(11; 14) show cyclin D1 protein expression oscillating during the cell cycle.20 This is in contrast to the findings in normal lymphoid tissues or cell lines of B-cell lymphomas without t(11; 14), in which no cyclin D1 expression was detected.2,20 The G1-S phase transition in hematopoietic cells is mainly regulated by cyclins D2 and D3 but not by cyclin D1.3 26
By using the antibody DCS-22 for the detection of cyclin D3 in our present series, we failed to observe positive reactions in the tumor cells of MCL. Double staining immunohistochemistry confirmed that, not the CD20-positive tumor cells, but primarily CD45R0-positive intermingled T cells expressed cyclin D3. The reactive CD20-positive B cells of residual germinal centers also expressed cyclin D3, leading to an inverse staining pattern when compared with cyclin D1. A total of 55 B-cell lymphomas other than MCL were found to be completely negative for cyclin D1. Most of the B-cell lymphomas in the non-MCL group showed nuclear expression of cyclin D3 as well as a membrane positivity for CD20. The exact determination of the percentage of cyclin D3-positive tumor cell fraction was hampered in low proliferating B-cell lymphomas by the admixture of cyclin D3/CD20-positive reactive B cells, eg, derived from residual germinal centers. Nevertheless, the fraction of cyclin-D3–positive tumor cells as determined by two independent investigators was found to be positively correlated to that of Ki-67–expressing cells, as could have been expected. The fraction of cyclin-D3–labeled cells was lower than the Ki-67 index because Ki-67 is present throughout the whole cell cycle from G1- to M-phase in cycling cells. In MCL there was no correlation of cyclin D1 and D3 expression with the Ki-67 determined growth fraction (neither with regard to the percentage of labeled cells nor the staining intensity). Whether the failure to detect cyclin D3 in a minor portion of non-MCL B-cell lymphomas had technical reasons, as reflects a very low or missing level of expression, cannot be determined with certainty at present. Negative staining of bystander cells also argues in favor of artificial antigen degradation.
Immunoblotting investigation of lymphoid cell lines confirmed the results found in tissue sections. The cell lines containing a t(11; 14) were all found to express cyclin D1 but not cyclin D3. On the other hand, B-cell lymphoma cell lines lacking the t(11; 14) did not express cyclin D1 but were positive for cyclin D3 in agreement with the immunohistochemical findings.
The reduced or negative expression of cyclin D3 in t(11; 14)-positive cells with cyclin D1 expression suggests that a downregulation of the physiological cyclin D3 is induced by overexpression of cyclin D1. In the normal cell cycle, cyclins of D-type have been shown to be expressed at their highest peaks at different times: Cyclin D1 is at its maximum during G1, whereas cyclin D3 is regulated posttranscriptionally and maximally expressed at the G1/S interface.27 The protein overexpression of cyclin D1 in the nuclei of B cells with t(11; 14) might be responsible for the absence of a cyclin D3 peak at G1/S phase transition.
The expression of D-type cyclins in different entities of NHL has not been studied in detail so far. An overexpression of cyclin D2 mRNA in cases of CLL has been reported recently, and no expression of cyclin D3 mRNA was detected in this study.28 However, the expression of cyclin D3 on the protein level in our cases of B-NHL, including cases of CLL, was distinctive, and we were able to observe a positive correlation of Ki-67 and cyclin-D3–positive fractions in all lymphoma entities other than MCL. A cross reactivity of the MoAb DCS-22 for cyclin D2 can definitely be ruled out by the primary characterizing experiments on this MoAb.16 As described in this report more in detail, the MoAb DCS-22 was shown to exclusively recognize cyclin D3 but not cyclin D1 or cyclin D2. However, the percentage of cyclin-D3–positive cells in our cases of CLL was small (mean value 9%) and obviously corresponds to the low growth fraction, which might explain why no mRNA for cyclin D3 was detected in cases of CLL in the study of Delmer et al.28
The informative report of Tanguay and Chiles29 has provided even more insight into the role of D-type cyclins and the expression of the cyclin-dependent kinase 4 (cdk4) in mature B lymphocytes. These authors observed expression of cyclin D2 but not of cyclin D1 and cyclin D3 in murine primary mature B cells from the spleen. Their immunoprecipitation experiments were performed by using polyclonal antibodies directed against individual D-type cyclin-glutathione S-transferase fusion proteins. However, Tanguay and Chiles29 mentioned the unpublished findings of Solvason and Howard, who could confirm the observations on cdk4 expression but also noted the expression of cyclin D3 after treatment of B cells with interleukin-4 and anti-Ig.
Miyatake et al30 recently reported on their observations on the induction of G1 arrest by downregulation of cyclin D3 in T-cell hybridomas. Activation of T-cell hybridomas by anti–T-cell receptor antibody induced growth arrest at the G1 phase of the cell cycle and was shown to result in activation-driven cell death. The activity of cyclin-D3–dependent kinase was found to be severely impaired. The investigators did not observe a similar crucial role for cyclin D2 in their experiments, and they suggested that similar mechanisms exist also in normal T cells. Regarding the expression of cyclin D3 in normal tonsillary tissues,16 as well as in the residual germinal center cells in the cases of our study, it is suggestive that similar mechanisms of activation like those described in T-cell hybridomas may play a role in the induction of G1 arrest in B cells. Therefore, in B cells carrying a t(11; 14) resulting in an overexpression of cyclin D1 and a lack of cyclin D3, B-cell activation would not result in a growth arrest because the physiological regulation would not influence the overexpression of cyclin D1. As a consequence, the affected cells would be equipped with a capacity of escape from activation-driven cell death.
A minor portion of MCL cases did not show cyclin D1 expression, including two cases with bcl-1 rearrangement. Interestingly, these cases did not show enhanced levels of cyclin D2 (data not shown) or D3. Because deregulation of G1/S-phase transition can be caused either by cyclin D1 overexpression or by deletion of the cyclin D1 target retinoblastoma gene (Rb) we looked for expression of the latter in cyclin-D1–negative MCL. Immunohistochemically, all three cases were positive for Rb (data not shown). Therefore, the cyclin D1 negativity in some MCL cases cannot be explained at present. However, it can be speculated that clonal evolution of the lymphomas could have led to additional defects concerning other regulators of the G1/S-phase transition, eg, cyclin inhibitors such as p16, abrogating the need for high-level cyclin D1 expression for sustained growth.
Our group recently showed that a tetraploidization of the karyotype is a frequent finding in MCLs and especially found in over 50% of large-cell or blastoid variants. This particular feature is observed in other types of B-cell lymphomas to a far lesser extent.31 Permanent and unphysiological cyclin D1 expression without sufficient downregulation during S/G2 phase might be responsible for a subsequent reentry into a second S phase without mitotic disjunction of the sister chromatids. The reduced or missing expression of cyclin D3 that is also involved in G1/S-phase transition could contribute to the disturbed cell cycle regulation in the pathogenesis of MCL.
ACKNOWLEDGMENT
The help of Dr T. Fekete in performing statistical evaluation is gratefully acknowledged. We are indebted to Mrs Sabine Roth for the excellent technical assistance in performing double staining experiments.
Supported by the Deutsche Forschungs-gemeinschaft, Grant No. DFG Kr 849/4-1 to H.K. and Grant No. Ot 168/1-1 to M.M.O.
Address reprint requests to Hans Kreipe, MD, Institute of Pathology, University of Würzburg, Josef-Schneider-Straβe 2, D-97080 Würzburg, Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal