Abstract
Phorbol myristate acetate (PMA) treatment of U937 human leukemic cells results in late G1 cell cycle arrest and terminal monocyte/macrophage-like differentiation. The PMA-induced G1 arrest involves a marked decrease in cdk2 activity, which correlates with total cdk2 dephosphorylation. Here, we show that the levels of cyclin A mRNA and protein markedly decrease during PMA-induced differentiation of U937 cells. In contrast, the level of cyclin E protein remains unchanged and in a complex with cdk2 during the entire course of PMA treatment. During the PMA-induced differentiation, cyclin E-associated cdk2 activity drops markedly. Furthermore, the amount of p27Kip1 protein associated with cyclin E/cdk2 greatly increases 24 to 72 hours after PMA treatment. The absence of changes in p27Kip1 mRNA levels by Northern blot suggest that the levels of this protein are controlled by posttranscriptional or posttranslational mechanism(s). These results show that the mechanisms mediating PMA-induced G1 arrest are complex. The inhibition of cdk2 activity is associated with (1) a decrease in cyclin A protein levels, (2) inactivation of cdk2 complexes, and (3) upregulation of p27Kip1 protein.
PROGRESSION THROUGH the eukaryotic cell cycle is driven by the activation of cyclin-dependent kinases (cdks). The G1/S transition is under the control of several cdks, including cdk2.1 cdk2 is activated by binding of cyclins E and A, and phosphorylation of threonine-160 residue by the cdk-activating kinase (CAK), and inactivated by phosphorylation of tyrosine-15 or association with proteins designated cdk inhibitors, eg, p21, p27Kip1.1-3
Phorbol myristate acetate (PMA), a strong activator of protein kinase C (PKC), induces the macrophage/monocyte-like differentiation of the human myeloid leukemic cell line, U937, accompanied by growth arrest in late G1 phase of the cell cycle.4-7 PMA induces the synthesis of the cdk2 inhibitor p21 (WAF1, Cip1, SDI1) which is maximally expressed after 8 hours of treatment.8-10 However, maximal p21 levels correlated with only 50% to 60% inhibition of cdk2 activity.8 After 24 to 72 hours of PMA treatment, when cdk2 was totally inactive, p21 was undetectable in cdk2 immunoprecipitates.8
Phosphate labeling studies show that cdk2 inactivation following PMA treatment correlated directly with cdk2 dephosphorylation.8 Dephosphorylation cdc2, a cyclin-dependent kinase that regulates the G2/M transition, on threonine-161 follows cyclin degradation and subsequent release of monomeric cdc2.11 Recently, a cdk-associated phosphatase, KAP/Cdi1, was shown to dephosphorylate native monomeric cdk2, but not cdk2 associated with cyclin A.12,13 In addition, the threonine-160 dephosphorylated cdk2 was catalytically inactive when associated with cyclin A.13
To understand the biochemical mechanism regulating PMA-induced cdk2 dephosphorylation, we examined whether this dephosphorylation is preceded or accompanied by changes in the level of specific cyclins. Here we report that 24 to 72 hours after the initiation of PMA treatment, the level of cyclin A mRNA and protein are markedly decreased. In contrast, cyclin E protein levels remain unchanged in response to PMA treatment, and cyclin E remains associated with cdk2 during the differentiation of U937 cells. However, cyclin E-associated cdk2 activity is abolished after 24 to 72 hours of PMA treatment without disruption of the cyclin E/cdk2 complexes. After 24 to 72 hours, PMA induces the expression of the cdk inhibitor p27Kip1 and p27Kip1 accumulates in cyclin E/cdk2 complexes. These results show that during phorbol ester-induced differentiation of hematopoietic cells the marked decrease in cdk2 activity is mediated by a complex series of transcriptional and posttranscriptional events.
MATERIALS AND METHODS
Immunoprecipitation kinase assays. U937 human myeloid leukemic cells were cultured as described previously.8 PMA-treated U937 cells were lysed in 0.5% NP-40 lysis buffer containing protease and phosphatase inhibitors.8 Immunoprecipitation kinase assays were performed as described previously.8 The anti-cdk2 polyclonal antibody (sc-163, used at a dilution of 1:100), and cyclin E monoclonal antibody conjugated to agarose (sc-248AC) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoprecipitation and Western blotting. Exponentially growing U937 cells (5.0 × 107) were treated with 200 nmol/L PMA for various times and labeled for 4 to 6 hours with [32P]orthophosphate (1 mCi per mL) in a total of 10 mL as described previously.8 Cell lysates containing equivalent counts per minute (cpm) were immunoprecipitated with cyclin E monoclonal antibody conjugated to agarose or cdk2 polyclonal antibody essentially as described previously.8 The cyclin E immunoprecipitates were washed with 0.5% NP-40 lysis buffer supplemented with 0.1% sodium dodecyl sulfate (SDS). Proteins in the immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography. Protein extracts from PMA-treated cells were analyzed by Western blotting as described previously.8 Cyclin A monoclonal antibody, cyclin E antibodies, and cdk2 polyclonal antibody for Western blotting were purchased from Pharmingen (San Diego, CA), Santa Cruz Biotechnology and Pharmingen, and UBI (Lake Placid, NY), respectively.
For p27 immunoprecipitates, 107 U937 cells were treated with 200 nmol/L PMA for 48 hours and then lysed in NP-40 lysis buffer plus inhibitors. Anti-p27 antibodies (Santa Cruz Biotechnologies) prebound to protein A agarose were added to 400 μL of lysate (approximately 400 μg of total protein) and incubated for 1 hour at 4°C. The immunoprecipitate was pelleted, and the procedure repeated two times. Immunoprecipitates were denatured in SDS sample buffer and run on SDS-PAGE along with samples of cell lysate taken before and after the immunoprecipitations. The proteins were transferred to nitrocellulose for Western blot analysis with anti-cdk2 antibodies; the filters were then stripped and incubated with anti-p27 antibodies.
Effect of PMA on cdk2 activity and phosphorylation. (A) PMA treatment induces inhibition of cdk2 activity. U937 cells were treated with 200 nmol/L PMA for the indicated times. cdk2 was immunoprecipitated from whole cell lysates (400 μg total protein) and analyzed for histone H1 kinase activity. (B) PMA treatment stimulates the dephosphorylation of cdk2. U937 cells were treated with PMA for the indicated times and labeled with [32P]orthophosphate. Cell lysates containing equivalent cpm were immunoprecipitated with anti-cdk2 antibody. Proteins present in the cdk2 immunoprecipitates were resolved by SDS-PAGE and visualized by autoradiography.
Effect of PMA on cdk2 activity and phosphorylation. (A) PMA treatment induces inhibition of cdk2 activity. U937 cells were treated with 200 nmol/L PMA for the indicated times. cdk2 was immunoprecipitated from whole cell lysates (400 μg total protein) and analyzed for histone H1 kinase activity. (B) PMA treatment stimulates the dephosphorylation of cdk2. U937 cells were treated with PMA for the indicated times and labeled with [32P]orthophosphate. Cell lysates containing equivalent cpm were immunoprecipitated with anti-cdk2 antibody. Proteins present in the cdk2 immunoprecipitates were resolved by SDS-PAGE and visualized by autoradiography.
Expression of cyclins A and E in PMA-treated U937 cells. (A) Treatment of U937 cells downregulates cyclin A. Cells were treated with PMA for the indicated times and cell extracts (300 μg total protein) were subjected to Western blotting with an anticyclin A antibody (1:333 dilution). (B) Cyclin E levels are sustained in PMA-treated U937 cells. Cell extracts (250 μg total protein) were subjected to Western blotting with anticyclin E antibody (1:1,000 dilution).
Expression of cyclins A and E in PMA-treated U937 cells. (A) Treatment of U937 cells downregulates cyclin A. Cells were treated with PMA for the indicated times and cell extracts (300 μg total protein) were subjected to Western blotting with an anticyclin A antibody (1:333 dilution). (B) Cyclin E levels are sustained in PMA-treated U937 cells. Cell extracts (250 μg total protein) were subjected to Western blotting with anticyclin E antibody (1:1,000 dilution).
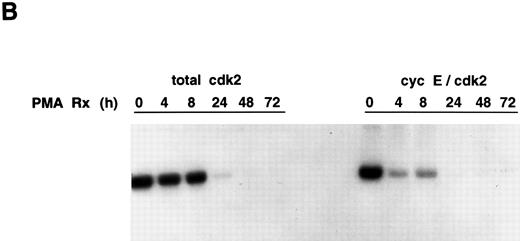
Cyclin E-associated cdk2 in PMA-treated cells. (A) Cyclin E/cdk2 complexes are present in U937 cells during PMA-induced differentiation. Cells were treated with PMA for the indicated times and cyclin E immunoprecipitated from cell extract (1.4 mg total protein). An aliquot of the identical extract was also immunoprecipitated with cdk2 antibody. The immunoprecipitates were run on an SDS-PAGE, transferred to nitrocellulose, and on a Western blot probed with a cdk2 polyclonal antibody (1:3750 dilution). (B) PMA inhibits cyclin E-associated cdk2 activity. Cyclin E or cdk2 was immunoprecipitated from PMA-treated cell lysates (500 μg and 200 μg total protein, respectively) and analyzed for histone H1 activity as described in Materials and Methods.
Cyclin E-associated cdk2 in PMA-treated cells. (A) Cyclin E/cdk2 complexes are present in U937 cells during PMA-induced differentiation. Cells were treated with PMA for the indicated times and cyclin E immunoprecipitated from cell extract (1.4 mg total protein). An aliquot of the identical extract was also immunoprecipitated with cdk2 antibody. The immunoprecipitates were run on an SDS-PAGE, transferred to nitrocellulose, and on a Western blot probed with a cdk2 polyclonal antibody (1:3750 dilution). (B) PMA inhibits cyclin E-associated cdk2 activity. Cyclin E or cdk2 was immunoprecipitated from PMA-treated cell lysates (500 μg and 200 μg total protein, respectively) and analyzed for histone H1 activity as described in Materials and Methods.
PMA treatment upregulates the levels of p27Kip1 protein in U937 cells. (A) p27Kip1 Northern blot. Total RNA was isolated from U937 cells treated with PMA for the indicated times and 20 μg/lane was probed with a 32P-labeled p27Kip1 cDNA. The same gel was stained with ethidium bromide and photographed to show equal loading of RNA. (B) PMA upregulates the total cellular levels of p27Kip1 protein. Cell lysates (260 μg total protein) were resolved by SDS-PAGE and subjected to Western blotting with p27Kip1 antibody (1:5,000 dilution). (C) Cyclin E-associated p27Kip1 protein increases during PMA-induced differentiation of U937 cells. PMA-treated cell lysates (1.4 mg total protein) were immunoprecipitated with cyclin E monoclonal antibody agarose conjugate, and the associated proteins were subjected to Western blotting with a p27Kip1 antibody (1:5,000 dilution).
PMA treatment upregulates the levels of p27Kip1 protein in U937 cells. (A) p27Kip1 Northern blot. Total RNA was isolated from U937 cells treated with PMA for the indicated times and 20 μg/lane was probed with a 32P-labeled p27Kip1 cDNA. The same gel was stained with ethidium bromide and photographed to show equal loading of RNA. (B) PMA upregulates the total cellular levels of p27Kip1 protein. Cell lysates (260 μg total protein) were resolved by SDS-PAGE and subjected to Western blotting with p27Kip1 antibody (1:5,000 dilution). (C) Cyclin E-associated p27Kip1 protein increases during PMA-induced differentiation of U937 cells. PMA-treated cell lysates (1.4 mg total protein) were immunoprecipitated with cyclin E monoclonal antibody agarose conjugate, and the associated proteins were subjected to Western blotting with a p27Kip1 antibody (1:5,000 dilution).
Coimmunoprecipitation of p27Kip1 and cdk2. 107 U937 cells were treated with PMA for 48 hours. p27Kip1 was immunoprecipitated (IP-1, IP-2, and IP-3) three times from the same extract. Both the immunoprecipitate and the supernatant either before (Pre-IP supernatant [sup]) or after the immunoprecipitate (Post-IP sup) were run on an SDS polyacrylamide gel and Western blotted with p27 antibody. The Western blot was then stripped and reprobed with cdk2 antibody.
Coimmunoprecipitation of p27Kip1 and cdk2. 107 U937 cells were treated with PMA for 48 hours. p27Kip1 was immunoprecipitated (IP-1, IP-2, and IP-3) three times from the same extract. Both the immunoprecipitate and the supernatant either before (Pre-IP supernatant [sup]) or after the immunoprecipitate (Post-IP sup) were run on an SDS polyacrylamide gel and Western blotted with p27 antibody. The Western blot was then stripped and reprobed with cdk2 antibody.
Total RNA isolation and Northern blot analysis. Total cellular RNA was extracted by the guanidium thiocyanate/CsCl ultracentrifugation method and subjected to Northern blot analysis as described previously.8 The probes used were p27Kip1 (1.5-kb EcoRI fragment from pBS/p27Kip1, a generous gift of Dr Joan Massagué, Memorial Sloan-Kettering Cancer Center, New York) and an alpha tubulin (1.5-kb Pst I fragment) cDNA.
RESULTS
PMA treatment results in cdk2 dephosphorylation and inactivation. Treatment of hematopoietic cells with PMA blocks their progression through the cell cycle at the G1/S transition phase.5-7 To examine the effects of PMA on the activity of cdk2, which regulates the G1/S transition, cdk2 was immunoprecipitated from PMA-treated U937 cells, and cdk2 activity was assessed by an immune kinase assay.8 As reported previously,8 PMA treatment completely abolished cdk2 activity by 24 hours (Fig 1A). To directly evaluate the phosphorylation state of cdk2 during PMA-induced U937 cell differentiation, cdk2 was immunoprecipitated from cells treated with PMA and labeled with [32P]orthophosphate. The levels of cdk2 phosphorylation decreased by 24 hours of treatment and no phosphorylation was apparent after 48 and 72 hours of treatment (Fig 1B). This experiment directly demonstrates the dephosphorylation of cdk2 after PMA treatment. The lack of cdk2 phosphorylation is not the result of cdk2 protein degradation, as cdk2 protein is detectable even after 72 hours of PMA treatment (see Fig 3A).8 In addition, pulse-chase experiments showed that PMA treatment does not alter cdk2 half-life (data not shown). Therefore, cdk2 inactivation during PMA-induced differentiation of U937 cells correlates with the dephosphorylation of cdk2.
Effect of PMA treatment on cyclin A and cyclin E levels. To determine whether the PMA-induced cdk2 dephosphorylation was accompanied or preceded by changes in the levels of cyclin A and or cyclin E, the steady state levels of these cyclins was investigated by Western blotting. Cyclin A levels decreased markedly by 48 hours of PMA treatment (Fig 2A). Northern blot analysis showed that a decrease in cyclin A mRNA was apparent by 2 hours (data not shown), suggesting that PMA regulation of cyclin A levels is at least partly controlled by a transcriptional mechanism(s). In contrast to cyclin A, the steady state level of cyclin E protein increased slightly following PMA treatment (Fig 2B). Thus, cyclin A levels decrease and cyclin E levels increase slightly during PMA-induced differentiation of U937 cells.
Cyclin E/cdk2 complexes persist in PMA-treated U937 cells. To examine whether cyclin E is complexed with cdk2 during the course of PMA-induced U937 cell differentiation and whether the cyclin E/cdk2 complex is active during the differentiation process, cyclin E was first immunoprecipitated from PMA-treated cells and then the associated cdk2 was detected by Western blotting using a cdk2 polyclonal antibody. As a control, a portion of extract from some of the time points was immunoprecipitated with cdk2 antibody and run on the identical Western blot. As previously reported,8 at zero time, the control cdk2 Western blot shows a cdk2 doublet, with the phosphorylated form running much faster, but by 72 hours, the amount of the lower (phosphorylated) band is markedly decreased. The amount of cyclin E-associated cdk2 remained unchanged throughout the PMA time course (0 to 72 hours) (Fig 3A). However, because of the small amounts of cdk2 that bind cyclin E (approximately 5% by densitometry), after PMA treatment, it has not been possible to observe the shift of cdk2 in this complex to the higher migrating form.
Cyclin E/cdk2 complexes are present in proliferating and PMA-treated growth-arrested U937 cells. We next evaluated the kinase activity of cyclin E-associated cdk2 by immunoprecipitation using a cyclin E antibody. Cyclin E-associated cdk2 kinase activity was inhibited completely 24 to 72 hours after PMA treatment with kinetics similar to that observed for total cdk2 activity (Fig 3B). The early drop in cyclin E-cdk2 activity may be caused by increases in the p21 protein that we have previously observed.8 Thus, cyclin E/cdk2 complexes are present, but inactive, during PMA-induced differentiation of U937 cells.
PMA treatment upregulates p27Kip1 by posttranscriptional mechanisms. The binding of p21 and p27Kip1 to cdk2/cyclin complexes prevents their phosphorylation and activation by CAK in vitro.14,15 p27Kip1 blocks CAK from phosphorylating and activating cyclin D1-associated cdk4 in macrophages treated with cyclic adenosine monophosphate (cAMP) inducers or analogs.16 Relevant to this work, it was reported that in normal fibroblasts forced to grow in suspension, cyclin E/cdk2 complexes are inactivated by dephosphorylation of cdk2 on threonine-160.17 This cdk2 dephosphorylation in these fibroblasts was thought to be caused by a blocking effect of increased levels of p21 and p27Kip1.17 However, in U937 cells treated with PMA, cdk2 dephosphorylation is maximal at a time when p21 protein is undetectable in cdk2 immunoprecipitates (data not shown).8 To examine whether the observed PMA-induced dephosphorylation of cdk2 was mediated by an increase in p27Kip1, U937 cells were treated with PMA, and p27Kip1 mRNA was analyzed by Northern blotting. However, no increase in p27Kip1 mRNA levels was seen after PMA treatment (Fig 4A). Western blots showed that small amounts of p27Kip1 protein were present in untreated proliferating U937 cells (Fig 4B). In contrast to p27Kip1 mRNA, p27Kip1 protein levels increased 24 to 72 hours after PMA treatment (Fig 4B). Immunoprecipitation of cyclin E followed by Western blotting with p27Kip1 antibody shows that the amount of p27Kip1 protein associated with cyclin E/cdk2 increases 24 to 72 hours after PMA treatment (Fig 4C).
To determine whether all of the p27 was bound to cdk2, U937 cells were treated with PMA for 48 hours, and the extract immunoprecipitated with p27 antibody three times (Fig 5). The supernatant before and after immunoprecipitation was Western blotted with anti-cdk2 antisera, showing that p27 is bound to only a small percentage of cdk2 (approximately 15% by densitometry) in PMA-treated cells. When this immunoblot was stripped and probed with antibody to cyclin E, small amounts of cyclin E were identified in the pre-immunoprecipitation (IP) supernatant. Following immunoprecipitation with p27, cyclin E could no longer be detected in the supernatant (data not shown). Thus, our results indicate that p27Kip1 protein levels are increased by PMA treatment, and this protein directly associates with a portion of the total cellular cdk-2, which is bound to cyclin E.
DISCUSSION
The addition of PMA to U937 cells inhibits the growth of these leukemic cells, as well as inducing differentiation. To understand the mechanism by which growth is inhibited we have studied the regulation of cdk2 activity during this process. These studies show that PMA-induced inactivation of cdk-2 is complex, involving marked decreases in cyclin A levels, dephosphorylation of cdk2/cyclin E complexes, and increases in the p27 protein.
Cyclin A levels have been shown to be decreased during the differentiation of murine erythroleukemia cells, myocytes, and PC-12 cells.18-21 The cyclin A promoter has a transcription factor ATF site that is important for regulation of cyclin A gene expression.22 PMA may downregulate cyclin A by modulating the levels or activity of specific ATF binding proteins. In the absence of cyclin A, monomeric cdk2 can be dephosphorylated.13
However, cyclin A levels are only 50% decreased by 24 hours, at which point cdk2 activity is completely decreased. When total cdk2 is analyzed by Western blotting, the faster migrating, threonine-160 phosphorylated form of cdk2 is decreased with time of PMA treatment.8 Thus, cdk2 dephosphorylation appears to precede the total decrease in cyclin A protein levels seen at 48 hours. It is therefore possible that cdk2 dephosphorylation is an independent critical event in cdk2 inactivation during phorbol ester-induced differentiation of U937 cells.
Although cyclin E is bound to only a minor portion of the total cdk2 activity, unlike the levels of cyclin A, cyclin E levels do not change during differentiation. In other cell types, the levels of cyclin E are increased during terminal differentiation, including oseoblasts, and during the senescence of fibroblasts.23,24 We show that cyclin E remains associated with cdk2 in PMA-treated U937 cells. Because of the low levels of cdk2 associated with cyclin E, we were not able to see the retarded mobility of dephosphorylated cdk2 on SDS polyacrylamide gels, and it is therefore difficult to use the mobility of cyclin E-associated cdk2 to determine its phosphorylation state. Other studies also indicate that cdk2 dephosphorylated on threonine-160 can remain associated with cyclin E.18,23,24 Transforming growth factor (TGF )-β–induced inhibition of cyclin E-associated histone H1 kinase activity correlates with cdk2 dephosphorylation on threonine-160, however, in that study, cdk2 was dissociated from cyclin E.25 Preliminary experiments show that cdk2 associated with cyclin E is also dephosphorylated after PMA treatment, suggesting an additional level of control of cdk-2 activity.
CAK phosphorylates cdk2 on threonine-160, and this phosphorylation is essential for cdk2 activation.26-28 During the PMA-induced differentiation of U937 cells, the activity and level of CAK does not change (data not shown). This result is consistent with previous findings that the level and activity of CAK remains constant throughout the cell cycle.29 Thus, the activating phosphorylation on the conserved threonine residue in cdk2 is likely to be regulated by substrate availability to the protein kinase and or to a unique phosphatase activity.
The cdk inhibitors, p21 and p27Kip1, have been shown to block CAK from phosphorylating cdk2 on threonine-160 in vitro.14,15 p27Kip1 also blocks the activating phosphorylation of cdk4 on threonine-172 by CAK in vivo.16 Reports suggest that p21 mediates the growth arrest associated with the differentiation of hematopoietic cells.9,10,30 We have previously shown that p21 associates with total cdk2 during PMA-induced differentiation of U937 cells.8 It is possible that the binding of p21 to cdk2/cyclin complexes during the first 24 hours after PMA treatment blocks the ability of CAK to phosphorylate cdk2.
Our finding that p27Kip1 is detectable in untreated proliferating U937 cells is in agreement with recently published results.31 Similarly, the cross-linking IgM on B cells also induces p27Kip1, but in this case, cyclin A kinase activity is inhibited.32 Because p27Kip1 is degraded by the ubiquitin proteasome pathway,33 PMA may enhance the stability of p27Kip1 by inhibiting some component of the ubiquitin proteasome pathway. Preliminary experiments suggests that the half-life of p27kip1 is prolonged and the level of synthesis of this protein is increased (unpublished results). PMA has been shown to induce a posttranscritional stabilization of p21 protein,34 suggesting that a number of cell cycle proteins could be regulated in this manner. This result is in agreement with previous reports that TGF-β1 and lovastatin also regulate p27Kip1 by posttranscriptional mechanisms.35,36 Recently, it was reported that cyclin E/cdk2 complexes are inactivated by dephosphorylation of cdk2 on threonine-160 in normal fibroblasts forced to grow in suspension. cdk2 dephosphorylation in these fibroblasts was thought to be caused by a blocking effect of elevated levels of p21 and p27Kip.17 It is possible that the PMA-induced dephosphorylation and inactivation of cyclin E-associated cdk2 could be mediated by both p21 and p27Kip1. Maximal levels of p21 protein peak 8 hours after the addition of PMA and then rapidly decrease to almost undetectable levels by 24 hours. In contrast, p27Kip1 levels increase at 24 hours and are further elevated at 72 hours. p27Kip1 accumulates during vitamin D3 -induced monocytic differentiation of HL-60 promyelocytic leukemic cells.36 It is also possible that specific cdk2 phosphatases are being regulated during PMA-induced differentiation. Treatment of U937 cells with PMA has been shown to induce a marked increase in synthesis of uncharacterized novel phosphatases,37 one of which might function to dephosphorylate cdk2. Initial studies (data not shown) did not show any change in the mRNA levels of KAP/Cdi1 during differentiation. This protein has been shown to dephosphorylate monomeric cdk2.
By immunoprecipitation of p27Kip1 we show that after 48 hours of PMA treatment, p27Kip1 is associated with approximately 10% to 20% of the cdk2. p27Kip1 binds more tightly to cdk2 or cdk4 in the presence of a cyclin.38 When cyclins are degraded p27Kip1 is released and binds to other cdk/cyclin complexes.39 The minimal binding of p27Kip1 to cdk2 is consistent with our observation that PMA treatment leads to marked decreases in cyclin A levels with only small amounts of the cdk2 bound to cyclin E. The observation that immunoprecipitation of p27Kip1 depletes all of the cyclin E suggests that this protein binds and inactivates the cyclin E/cdk2 complex.
Thus, we show that cdk2 activity and phosphorylation decrease markedly during PMA-induced U937 cell differentiation likely accounting for the G1 block observed in these cells. The potential mechanisms for this reduction in cdk2 activity are complex and include (1) an early, transient increase in p21 that is maximal at 8 hours, (2) increases in p27Kip1 protein level starting at 24 hours, and (3) a marked decrease in the level of cyclin A.
ACKNOWLEDGMENT
We thank Dr Vincent Kidd (St Jude Children's Hospital, Memphis, TN) for instructing the laboratory in the cdk2 kinase assays and engaging in many helpful conversations. Dr W. Harper (Baylor University College of Medicine, Houston, TX) supplied a cyclin A antibody used in a number of the experiments. Dr E. Nigg (Swiss Institute for Experimental Cancer Research, Epalinges, Switzerland) provided an antibody to CAK (cdk7), and Drs Joan Massagué and Andrew Koff provided p27Kip1 antisera and additional p27Kip1 reagents. Dr Bill Weaver was very helpful in reviewing this manuscript.
Supported by Grant No. CA 42533 from the National Institutes of Health (Bethesda, MD) and Grant No. DHP 83 from the American Cancer Society (Atlanta, GA) to A.S.K.; and a Milheim Foundation grant to J.B.
Address reprint requests to Andrew S. Kraft, MD, The Division of Medical Oncology, 4200 E 9th Ave, BRB 525, University of Colorado Health Sciences Center, Denver, CO 80262.

![Fig. 1. Effect of PMA on cdk2 activity and phosphorylation. (A) PMA treatment induces inhibition of cdk2 activity. U937 cells were treated with 200 nmol/L PMA for the indicated times. cdk2 was immunoprecipitated from whole cell lysates (400 μg total protein) and analyzed for histone H1 kinase activity. (B) PMA treatment stimulates the dephosphorylation of cdk2. U937 cells were treated with PMA for the indicated times and labeled with [32P]orthophosphate. Cell lysates containing equivalent cpm were immunoprecipitated with anti-cdk2 antibody. Proteins present in the cdk2 immunoprecipitates were resolved by SDS-PAGE and visualized by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3430/4/m_bl_0004f1.jpeg?Expires=1767710729&Signature=L~6knpEtwbwCtu1xtK7xYMSbs-D7OfPB285YpCQ0WljHSOARwBZ9OHvixzaW3aXASjz8nfpgoRCKVaWYeOzngGzqTAzXLjwf~VMOAaeACMUXyFNxQtAoDCB4GszhEyXSp4Vgsmj84CB28I1Y-HU7c2uW94ywhB1BZGBA1gg6XYg0qC3kfNi6YI5cHDwUfmGLwHOGWwIINxZvnTLR4nBl5KrYlhb1A7yXt-dm-DrJrmtJQTDmKyTBODC6EXTHOvWhmSeMd0z6oH4Z6qeK-Pm2zMEf2vMWo9MXHglRmPfBOLzB9com9F8e8XBu-GbVQjP6mG9H1Y1l11WIO1q5~HQGBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 5. Coimmunoprecipitation of p27Kip1 and cdk2. 107 U937 cells were treated with PMA for 48 hours. p27Kip1 was immunoprecipitated (IP-1, IP-2, and IP-3) three times from the same extract. Both the immunoprecipitate and the supernatant either before (Pre-IP supernatant [sup]) or after the immunoprecipitate (Post-IP sup) were run on an SDS polyacrylamide gel and Western blotted with p27 antibody. The Western blot was then stripped and reprobed with cdk2 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3430/4/m_bl_0004f5.jpeg?Expires=1767710729&Signature=FsnZ7Y7CZq--MnkNU3IT06JiXg9KlHYm~Xzwbok1v9szKthTKrH02IBzNKN~yflpSqKGWy0I2z9RY7AAswglSN1WpudT6OtQ82AQ5Cb1m1NoaQPKyBRBY2idZ2qC7QXaRMMzxZm4iyGDZXFMHEgBeo37861gToyPwhGtmEDxKSo3Wt55UV1pVPuLcFzX0C-hPVOdbIntdR1Iu~YaFuMzzRhZhyDNYFgWrscu3swz-ZeAXXacY75P3WwYPl81CF7jTClxLyncWZLJqCV1gpLr7GYMaShZfmUUu23qrEt-GdSqQW9HpDSaEX82CAdLZSkTtn1BYQzb11Oi47FNmTojfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal