Abstract
How multiple chemoattractants cooperate in directing the migration of hematopoietic progenitor cells (HPC) for homing and peripheral blood mobilization has not yet been established. We report here the behavior of HPC under the influence of two different chemoattractants, stromal cell-derived factor (SDF)-1 and steel factor (SLF), and the chemotactic nature of the bone marrow (BM) environment using a two-chamber in vitro migration system. Various formulae were adopted to quantitate these effects. Based on these quantitations, SDF-1 showed only chemotactic activity, while SLF showed both chemotactic and chemokinetic activities on factor-dependent MO7e cells. SLF, like SDF-1, attracted human HPC from a population of CD34+ cells and induced actin polymerization in MO7e cells. SLF and SDF-1 cooperated in attracting MO7e cells, as well as cord blood (CB) and BM CD34+cells. A negative concentration gradient of SLF and SDF-1, formed by the presence of chemoattractants in the upper chamber, showed potent inhibitory effects on MO7e cell migration induced by either of these chemoattractants in the lower chamber, and SDF-1 and SLF were synergistic in mobilizing cells to the lower chamber from this negative chemoattractant gradient. Plasma obtained from BM aspirates, but not CB or peripheral blood, showed strong chemotactic effects on BM and CB CD34+ cells, and an inhibitory effect in a negative gradient on SDF-1–dependent CD34+ cell migration. These in vitro migration experiments suggest that chemoattractants such as SDF-1 and SLF with other unidentified BM chemoattractants may be involved cooperatively in the migration of HPC to the BM and in preventing spontaneous mobilization of HPC out of the BM.
HEMATOPOIETIC progenitor cells (HPC) home to the extravascular compartment of the bone marrow (BM) during transplantation.1-5 Also, during fetal development, the multipotential and self-renewing hematopoietic stem cells migrate to the BM from the fetal liver. Chemoattractants may play a role in directing migration of hematopoietic stem cells and HPC to the BM, but this has not yet been clearly defined. In another direction, HPCs are mobilized from the BM to the peripheral blood (PB) in response to injected cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and Steel factor (SLF).6-9 The mechanisms involved in this mobilization of HPC from BM to PB are not known. It is possible that chemoattractants may play a direct role in mobilization of HPC, although other functions of cytokines such as proliferation, modulation of adhesion molecules, or alteration of the blood-BM barrier, may be important for these effects.4 For example, G-CSF and GM-CSF appear to have no chemotactic or chemokinetic effects on HPC, while SLF, interleukin (IL)-3, and IL-11 have been reported to chemoattract murine HPC.10
Stromal cell–derived factor-1 (SDF-1), also called pre-B–cell growth-stimulating factor (PBSF)11-14 has been reported to be a powerful chemoattractant for lymphocytes, monocytes, and primary CD34+ cells.15,16 Mice lacking SDF-1 died perinataly and had reduced numbers of B-cell progenitors in fetal liver and BM.17 These mutant mice also showed reduced numbers of myeloid progenitor cells in BM, but normal numbers in fetal liver. These results suggested that SDF-1 might be involved in the migration of hematopoietic stem cells from fetal liver to BM during fetal development. SDF-1 is a ligand for the LESTR/fusin/CXCR4 receptor and prevents T-cell line-adapted human immunodeficiency virus (HIV)-1 infection.18-22 SDF-1 mobilizes calcium and reorganizes actin structure in CXCR4-transfected Chinese hamster ovary cells.15 16
Two types of chemoattraction are distinguishable on the basis of direction of cell attraction in various chemoattractant gradients. Chemotaxis is the attraction of cells only in a positive gradient, while chemokinesis reflects activation of cell motility and an induction of cell migration in a random direction by a chemoattractant. The concepts and method of quantitation of these two characteristics of chemoattractants are only beginning to be evaluated for hematopoietic stem and progenitor cells. SLF showed chemotactic effects on mast cells and small-cell lung cancer cell lines expressing c-kit.23,24 Murine SLF has been reported to be a chemotactic and chemokinetic factor for murine HPC. However, a role for SLF as a chemoattractant for human HPC has not yet been established.15
In this study, we examined the effects of SDF-1 and SLF on migration of the growth factor-dependent human cell line MO7e and on human cord blood (CB) and BM CD34+ cells using a two-chamber in vitro migration system.25 The chemotactic and chemokinetic activities of SDF-1 and SLF, alone and in combination, and in the absence and presence of factors present in BM, CB, and PB plasma allowed us, using a newly reported method to quantitate migration, to suggest a model for HPC migration induced by gradients of chemoattractants to and mobilization from the BM microenvironment.
MATERIALS AND METHODS
Cells.
Heparinized human CB was collected from healthy, full-term neonates according to institutional guidelines immediately after vaginal delivery. Human BM was collected from healthy donors after receiving informed consent. CB and BM aspirates were diluted 1:3 with phosphate-buffered saline (PBS) containing 2 mmol/L EDTA, pH 7.4 (Sigma, St Louis, MO). Diluted CB and BM cells were separated by density gradient centrifugation on Ficoll-paque (1.077 g/mL) (Biochem KG, Berlin, Germany). Mononuclear cells were resuspended in PBS containing 0.5% bovine serum albumin (BSA) and 2 mmol/L EDTA at 3 × 108 cells/mL. These cells were further processed by magnetic cell sorting (MACS) CD34+ isolation kit (Miltenyi Biotec, Auburn, CA) to positively select CD34+ cells. The purity of isolated CD34+ cells was from 85% to 98%. The growth factor–dependent myeloid cell line, MO7e, was maintained in RPMI-1640 medium supplemented with 20% fetal calf serum (FCS) (Hyclone Laboratory, Logan, UT) and 100 U/mL GM-CSF. The biological characteristics of this cell line have previously been described.26 27 M2-10B4 (American Type Culture Collection, Rockville, MD, CRL-1972), a mouse stromal cell line, was maintained in RPMI 1640 containing 10% FCS.
Cytokines, chemokines, antibodies, and other reagents.
Chemically synthesized SDF-1 was a kind gift from Dr Ian Clark-Lewis (University of British Colombia, Vancouver, Canada). Highly purified recombinant human SLF, GM-CSF, and IL-3 were kind gifts from Immunex Corp (Seattle, WA). Erythropoietin (EPO) was purchased from Amgen Corp (Thousand Oaks, CA). Phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody was purchased from Becton Dickinson (San Jose, CA). TRI-COLOR–conjugated anti–c-kit monoclonal antibody was purchased from CALTAG Laboratory (Burlingame, CA). Antihuman SLF neutralizing antibody was purchased from R&D Systems (Minneapolis, MN). Pertussis toxin was purchased from Sigma Chemical Co.
In vitro two-chamber migration assay.
Chemotaxis and chemokinesis were assayed by a modification of checkerboard assay.25 One hundred microliters of chemotaxis buffer (RPMI 1640, 0.5% crystallized deionized bovine serum albumin [BSA; Calbiochem, San Diego, CA], and antibiotics) containing cells were added to the upper chamber of a Costar Transwell (Cambridge, MA, 6.5 mm diameter, 5 μm pore; a 5 μm pore was shown in preliminary experiments to be optimal for migration of MO7e and CD34+cells in response to chemoattractants with low background migration), and 0.6 mL of chemotaxis buffer was added to the lower chamber. 2.5 × 105 MO7e cells or 1 to 2 × 105CD34+ cells were used for each Transwell. Various amounts of chemoattractants or test plasmas were added to the chemotaxis buffer in the upper and/or lower chamber to form various chemoattractant concentration gradients. Positive gradient (0/+) was made by adding chemoattractant to the lower chamber, negative (+/0) gradient was made by adding chemoattractant to the upper chamber, and zero gradient was made by either adding chemoattractant to both chambers (+/+) or by not adding chemoattractant to either chamber (0/0). Chambers were incubated at 37°C, 5% CO2 for 4 to 5 hours, or the indicated time periods. Cells migrating into the lower chamber were counted using a FACscan (Becton Dickinson), with appropriate gating, for 20 seconds at a high flow rate. Average cell number and standard deviation was calculated from triplicated experiments. The number of events acquired for 20 seconds was approximately between 50 (medium) and 800 (SDF-1 + SLF) (CD34+ cells), and 0 (medium) and 3,000 (SDF-1+ SLF) (MO7e cells), when 2.5 × 105 MO7e cells/well or 1 to 2 × 105 CD34+ cells/well were added. Percent migration was determined by calculating percentage of input cells migrated into the lower chamber (average events for 20 seconds / average input cell events for 20 seconds × 100). For in vitro mobilization experiments, indicated amounts of SDF-1 and SLF were added with cells to the upper chamber to form a negative gradient of chemoattractants, and mobilizing chemoattractants were added to the lower chamber to mobilize cells from the upper chamber. For inhibition of SDF-1 effects, MO7e cells were pretreated for 1 hour with pertussis toxin (500 ng/mL) in the chemotaxis buffer. For blocking the effects of SLF, anti-SLF antibody (10 μg/mL, final concentration) was added in the upper and lower chambers of the migration system.
Preparation of BM, CB, and PB plasma for chemotaxis.
BM was aspirated from donors' iliac crest. BM aspirates and PB for each experiment were obtained from the same donor within minutes of each other. Heparinized BM, PB, and CB were centrifuged (1,000g, 15 minutes, 25°C). Plasma supernatants were collected and diluted twofold (final concentration) with chemotaxis buffer for the chemotaxis experiments. BM and PB plasma obtained from the same donor were used for each experiment to rule out individual differences.
Actin polymerization assay.
This assay was performed according to the study by Howard and Meyer28 with some modifications. MO7e cells were resuspended in RPMI 1640 with 0.1% BSA at 1.25 × 106cells/mL. SDF-1 (100 ng/mL) or SLF (10 μg/mL) was added to the cell solution, and 0.4 mL cell solution was transferred to 0.1 mL fluorescein isothiocyanate (FITC)-phalloidin solution (4 × 10-7 mol/L FITC-labeled phalloidin, 0.5 mg/mL 1-α–lysophosphatidylcholine, 18% formaldehyde in PBS, all from Sigma Chemical Co) to stain and fix the cells. Cells were further incubated for 10 minutes at 37°C, centrifuged, and resuspended in 0.5 mL of 1% paraformaldehyde solution. Mean fluorescence was measured by FACscan (Becton Dickinson).
Colony-forming cell assays for HPC.
Migrated or input cells were plated at concentrations not exceeding 300 CD34+ cells/mL in 35-mm plastic tissue culture dishes (Costar, Cambridge, MA) containing 1 U/mL recombinant human (rhu) EPO, 100 U/mL rhu GM-CSF, 100 U/mL rhu IL-3, with or without 50 ng/mL rhu SLF in 1.1% methylcellulose culture medium containing 30% FBS.29,30 The cultures were incubated at 37°C in a 100% humidified atmosphere of 5% CO2 at lowered (5%) O2. After 14 days of incubation, burst forming unit-erythroid (BFU-E), and colony-forming unit-granulocyte macrophage (CFU-GM) were scored from the plates containing IL-3, GM-CSF, and EPO, and mixed cell (CFU-granulocyte erythroid macrophage megakaryocyte [GEMM]) colonies were scored from the plate containing IL-3, GM-CSF, EPO, and SLF. BFU-E, CFU-GM, and CFU-GEMM, which respectively identify erythrocyte, granulocyte-macrophage, and multipotential progenitor cells, were scored in situ with an inverted microscope using standard criteria for their identification.29 30
Measurement of kinetics of diffusion, migration, and proliferation/survival effect of cytokines.
For diffusion experiments, SLF was added at final concentration of 10 ng/mL (600 μL in the chemotaxis buffer) to the lower chamber and washed 2.5 × 105 MO7e cells (100 μL volume in the chemotaxis buffer) were added to the upper chamber. At various time points, contents from each chamber were collected and centrifuged to separate cells from the buffer. Cells were fixed in 1% paraformaldehyde and viable cell numbers were counted within 24 hours by flow cytometry. Viable cells and dead cells were distinguished on side scatter and forward scatter channels. Supernatants were stored at −20°C until measurement of SLF concentration. SLF concentration was determined by the Quantikine (R&D Systems). For measurement of proliferation and survival effects of cytokines, 2.5 × 105 MO7e cells per well were added to 24-well plates containing chemotaxis buffer with SDF-1 (100 ng/mL), SLF (10 ng/mL), or control medium. At various time points, MO7e cells were harvested and fixed in 1% paraformaldehyde for viable cell counting by flow cytometry.
Calcium flux responses in M07e cells.
MO7e cells washed with PBS were loaded with 2.5 μmol/L FURA-2 AM in Hanks' balanced salt solution (HBSS) (Sigma Chemical Co), pH 7.4, supplemented with 0.05% BSA at 37°C for 45 minutes, and washed twice with PBS. FURA-2 AM-loaded cells were resuspended in HBSS supplemented with 0.05% BSA at 5 × 106 cells/mL, and placed in a continuously stirred cuvette at 37°C in a MSIII fluorimeter (Photon Technology Inc, South Brunswick, NJ). Fluorescence was monitored at 340 and 380 nm for excitation and 510 nm for emission. The data were recorded as the relative ratio of fluorescence excited at 340 and 380 nm. Data were collected every 1 second. SDF-1 was used at a final concentration of 50 nmol/L.
Statistics.
Results, shown as mean ± standard deviation (SD), are representative of at least three different experiments. Significant differences were determined by use of Student's t-test.
RESULTS
SDF-1 and SLF are efficient chemoattractants for MO7e cells and SLF, but not SDF-1, has chemokinetic activity.
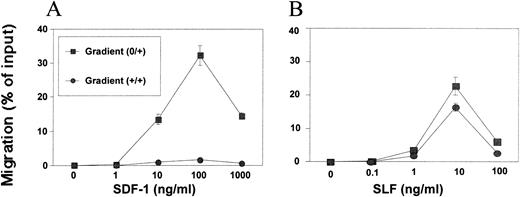
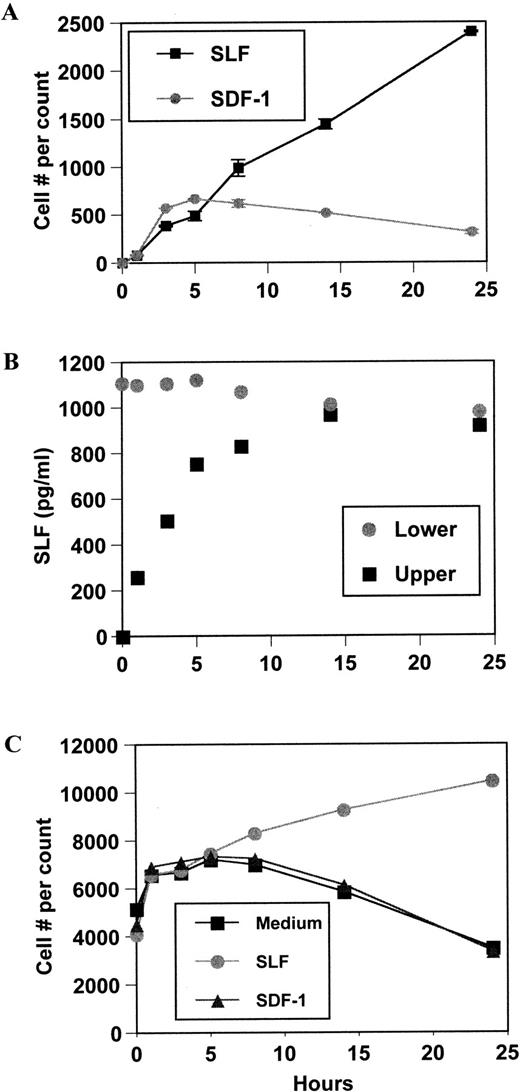
SDF-1 showed maximum cell attraction (over 30% MO7e cell migration) around 100 ng/mL in a positive gradient (0/+) during a 4-hour incubation period (Fig 1A). This optimum concentration range for MO7e cells was similar to the reported concentration for BM CD34+ cell chemotaxis.15At 1,000 ng/mL, cell migration was significantly decreased from maximal levels. SLF was also a good chemoattractant for MO7e cells. It usually attracted more than 20% of input cells at 10 ng/mL optimum concentration during a 4-hour incubation (Fig 1B). SLF-dependent MO7e migration was decreased significantly at 100 ng/mL. We examined MO7e cell migration over time up to 24 hours in the Transwell chemotaxis system. SDF-1–dependent migration occurred only within 5 hours and after 5 hours, no more migration was observed (Fig2A). However, SLF-dependent migration continuously increased during the 24-hour period (Fig 2A). We examined cytokine diffusion in the Transwell chemotaxis system using SLF as a model molecule. We added SLF at 10 ng/mL in the chemotaxis buffer to the lower chamber (volume 600 μL) and 100 μL of the chemotaxis buffer containing no SLF to the upper chamber, and measured SLF concentration by enzyme-linked immunosorbent assay (ELISA) in both chambers at different time points up to 24 hours. SLF concentrations in the upper and lower chambers reached complete equilibrium at the 14-hour time point (Fig 2B). After 3 and 5 hours, respectively, the SLF concentration of the upper chamber reached approximately half and 80% of SLF concentration of the lower chamber (Fig 2B). To exclude the possibility that the increased cell number in the lower chamber was due to indirect effects of SDF-1 and SLF on survival and proliferation of MO7e, we scored the viable cell number after incubation of MO7e cells in the chemotaxis buffer containing SDF-1, SLF, and medium at different time points. During the first 5-hour incubation, there is no difference in viable cell numbers among MO7e cells incubated in SDF-1, SLF, or medium (Fig 2C). After 5 hours, MO7e cells incubated in SLF slowly increased in cell number, while MO7e cells incubated in SDF-1 or medium began to decrease. There was 45% increase in MO7e cell number during a 19-hour incubation with SLF between the 5- and 24-hour time point (Fig2C). However, the cell number scored by chemotaxis experiment increased 500% in response to SLF in the lower chamber during the same time period (Fig 2A) suggesting most of the increased cell number in the chemotaxis system was due to SLF-dependent cell migration rather than proliferation. We noted that the 4- to 5-hour time point was the best time point to study chemotaxis and chemokinesis because the indirect effects of cytokines on a cell proliferation and survival were negligible and the chemotaxis assay system maintained an effective chemoattractant gradient during this period.
Differences in chemotactic and chemokinetic activities of SDF-1 and SLF on MO7e cells. Dose-dependent induction of MO7e cell migration into the lower chamber by (A) SDF-1 or (B) SLF in a positive gradient (0/+) or in a zero gradient (+/+) formed by equal concentration of SDF in both chambers.
Differences in chemotactic and chemokinetic activities of SDF-1 and SLF on MO7e cells. Dose-dependent induction of MO7e cell migration into the lower chamber by (A) SDF-1 or (B) SLF in a positive gradient (0/+) or in a zero gradient (+/+) formed by equal concentration of SDF in both chambers.
Kinetics of MO7e cell migration, diffusion of SLF, and survival/proliferation effects of SDF-1 and SLF. (A) MO7e cell migration was monitored during 24 hours at indicated time points after setting initial positive gradients with SDF-1 (100 ng/mL) and SLF (10 ng/mL). Viable cell numbers in the lower chambers were counted and the average and range of duplicate were shown. (B) Diffusion of SLF from the lower chamber to the upper chamber was monitored at indicated time points. SLF (10 ng/mL) was added to the lower chambers of the Transwell system to form a positive gradient. (C) MO7e cells were incubated in 24-well plates containing SLF (10 ng/mL), SDF-1 (100 ng/mL), or medium. At indicated time points, viable MO7e cells were counted (see Materials and Methods for details).
Kinetics of MO7e cell migration, diffusion of SLF, and survival/proliferation effects of SDF-1 and SLF. (A) MO7e cell migration was monitored during 24 hours at indicated time points after setting initial positive gradients with SDF-1 (100 ng/mL) and SLF (10 ng/mL). Viable cell numbers in the lower chambers were counted and the average and range of duplicate were shown. (B) Diffusion of SLF from the lower chamber to the upper chamber was monitored at indicated time points. SLF (10 ng/mL) was added to the lower chambers of the Transwell system to form a positive gradient. (C) MO7e cells were incubated in 24-well plates containing SLF (10 ng/mL), SDF-1 (100 ng/mL), or medium. At indicated time points, viable MO7e cells were counted (see Materials and Methods for details).
Chemokinetic activity can be defined as a chemoattractant's ability to induce random cell migration under a zero chemoattractant gradient (+/+). SLF, but not SDF-1, showed a strong chemokinetic effect on MO7e cells in a zero gradient (+/+) (Fig 1A and B). A clearer picture of the chemotactic activity of SDF-1 and the chemotactic and chemokinetic activity of SLF can be seen by use of a modified checkerboard assay shown in Table 1 and as described below.
Effects of SDF-1 and SLF on Migration of MO7e Cells Assessed by Checkerboard Assay
| SDF-1 Concentration (ng/mL) in Lower Chamber* . | SDF-1 Concentration (ng/mL) in Upper Chamber . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 10 . | 100 . | 1000 . | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0.3 | 0 | 0 | 0 | 0 |
| 10 | 13.4 | 6.6 | 0.9 | 0 | 0 |
| 100 | 32.2† | 31.2 | 1.7 | 1.6‡ | 0 |
| 1,000 | 14.4 | 14.3 | 11.8 | 4.2 | 0.6 |
| SLF Concentration (ng/mL) in Lower Chamber* | SLF Concentration (ng/mL) in Upper Chamber | ||||
| 0 | 0.1 | 1 | 10 | 100 | |
| 0 | 0 | 0 | 0 | 0.4 | 2.1 |
| 0.1 | 0.2 | 0 | 0.2 | 0.3 | 1.7 |
| 1 | 3.5 | 2.2 | 1.8 | 1.0 | 2.6 |
| 10 | 22.6§ | 23.5 | 20.4 | 16.3∥ | 1.7 |
| 100 | 6.0 | 7.5 | 5.9 | 5.1 | 2.6 |
| SDF-1 Concentration (ng/mL) in Lower Chamber* . | SDF-1 Concentration (ng/mL) in Upper Chamber . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 10 . | 100 . | 1000 . | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0.3 | 0 | 0 | 0 | 0 |
| 10 | 13.4 | 6.6 | 0.9 | 0 | 0 |
| 100 | 32.2† | 31.2 | 1.7 | 1.6‡ | 0 |
| 1,000 | 14.4 | 14.3 | 11.8 | 4.2 | 0.6 |
| SLF Concentration (ng/mL) in Lower Chamber* | SLF Concentration (ng/mL) in Upper Chamber | ||||
| 0 | 0.1 | 1 | 10 | 100 | |
| 0 | 0 | 0 | 0 | 0.4 | 2.1 |
| 0.1 | 0.2 | 0 | 0.2 | 0.3 | 1.7 |
| 1 | 3.5 | 2.2 | 1.8 | 1.0 | 2.6 |
| 10 | 22.6§ | 23.5 | 20.4 | 16.3∥ | 1.7 |
| 100 | 6.0 | 7.5 | 5.9 | 5.1 | 2.6 |
*Cells were added to the upper chamber and the indicated chemoattractants were added to either the lower or upper chamber. Results shown are the percentages of cells that migrated into the lower chamber and are representative of three independent experiments.
Figure used to determine MCTA of SDF-1 for Table 2.
Figure used to determine MCKA of SDF-1 for Table 2.
Figure used to determine MCTA of SLF for Table 2.
∥Figure used to determine MCKA of SLF for Table 2.
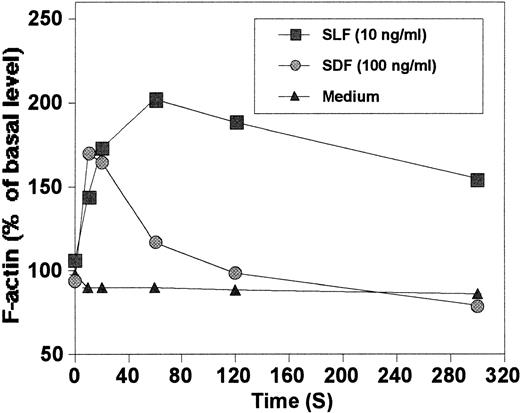
We evaluated the ability of SLF and SDF-1 to reorganize actin cytoskeleton in MO7e cells. SLF at 10 ng/mL, an optimum concentration for chemotaxis, was an efficient inducer of actin polymerization (Fig3). SDF-1 at 100 ng/mL, an optimum concentration for chemotaxis, induced actin polymerization in MO7e cells, similar to that reported in T cells by others16where they used a higher concentration of SDF-1 (1,000 ng/mL). F-actin polymerized by SLF depolymerized more slowly than that of SDF-1 demonstrating a difference in kinetics of actin reorganization.
Quantitation of chemotactic and chemokinetic activities.
To clarify the chemotactic and/or chemokinetic activities of chemoattractants, we have proposed a number of different formulae to quantitate and compare effects (Table 2) that are based on the data in Table 1. SDF-1 had a maximum chemotactic activity (MCTA) around 30% and the specific MCTA was about 0.3%/ng/mL. MCTA and specific MCTA of SLF were about 23% and 2.3%/ng/mL, respectively. Considering the molecular weights of the two chemoattractants tested (SDF-1, 8 kD; SLF, 31 kD), SLF was a far (25-fold) more efficient chemoattractant than SDF-1, although its MCTA was lower than that of SDF-1. Maximum chemokinetic activity (MCKA) of SLF was 16.3%, while that of SDF-1 was negligible. The chemotactic-chemokinetic index (CCI) shows the relative ratio of chemotactic activity to chemokinetic activity and can be used as quantitative criteria to determine whether a chemoattractant is a pure chemotactic factor or a chemotactic and chemokinetic factor. The CCI of SDF-1 was 20 meaning its chemotactic activity is 20 times stronger than its chemokinetic activity. CCI of SLF was 1.38 meaning its chemotactic activity is comparable to its chemokinetic activity. Relative chemokinetic activity (RCKA) is a figure to be used to assess the relative chemokinetic activity of a chemoattractant to the chemotactic activity. Lower RCKA, eg, less than 10%, suggests very low chemokinetic activity, while RCKA close to 100% suggests very high chemokinetic activity. Overall, these quantitations demonstrated that SDF-1 was a chemotactic, but not a chemokinetic factor, while SLF had both chemotactic and chemokinetic activities.
Quantitation and Comparison of Chemotactic and Chemokinetic Activities of SDF-1 and SLF on MO7e Cells
| Definitions* . | SDF-1 . | SLF . |
|---|---|---|
| Maximum chemotactic activity (MCTA) (%)† | 32.2 | 22.6 |
| Specific MCTA (%/ng/mL)‡ | 0.322 | 2.26 |
| Maximum chemokinetic activity (MCKA) (%)§ | 1.6 | 16.3 |
| Specific MCKA (%/ng/mL)∥ | 0.016 | 1.63 |
| Chemotactic-chemokinetic index (CCI)¶ | 20 | 1.38 |
| Relative chemokinetic activity (RCKA) (%)# | 5 | 72 |
| Definitions* . | SDF-1 . | SLF . |
|---|---|---|
| Maximum chemotactic activity (MCTA) (%)† | 32.2 | 22.6 |
| Specific MCTA (%/ng/mL)‡ | 0.322 | 2.26 |
| Maximum chemokinetic activity (MCKA) (%)§ | 1.6 | 16.3 |
| Specific MCKA (%/ng/mL)∥ | 0.016 | 1.63 |
| Chemotactic-chemokinetic index (CCI)¶ | 20 | 1.38 |
| Relative chemokinetic activity (RCKA) (%)# | 5 | 72 |
*All the calculations in this table were based on the data of checkerboard experiments as shown in Table 1. Net migration after subtraction of background migration should be used for calculation if the background level is high. Background migration is the cell migration occurring at zero concentration of a chemoattractant, and dependent on cell types and chemotaxis system used. In this experiment, the background is zero.
A chemoattractant's maximal ability to induce migration of a type of cells in a positive gradient. MCTA of SDF-1 (32.2%) was determined at 100 ng/mL of SDF-1 only in the lower chamber, and MCTA of SLF (22.6%) was determined at 10 ng/mL of SLF only in the lower chamber (see Table1 for these figures).
MCTA per ng/mL chemoattractant, eg, specific MCTA (0.322 %/ng/mL) of SDF-1 was calculated by dividing MCTA (32.2%) with the concentration of SDF-1 (100 ng/mL) used.
A chemoattractant's maximal ability to induce cell migration in a zero gradient formed by equal concentrations of the chemoattractant in both chambers. MCKAs of SDF-1 and SLF were determined at 100 ng/mL and 10 ng/mL, respectively in both the upper and lower chambers.
∥MCKA per ng/mL chemoattractant, eg, specific MCKA (1.63%/ng/mL) of SLF was calculated by dividing MCKA (16.3%) by the concentration of SLF (10 ng/mL) used.
¶The relative strength of the chemotactic activity compared with the chemokinetic activity of a chemoattractant for a type of cells. This equals MCTA divided by MCKA, eg, CCI (20) of SDF-1 was calculated by dividing MCTA (32.2%) by MCKA (1.6%).
#The relative chemokinetic activity of a chemoattractant for a type of cells. This equals the percentage of MCKA divided by MCTA, eg, RCKA (5%) of SDF-1 was calculated by dividing MCKA (1.6%) by MCTA (32.2%), and multiplying by 100.
Effects of SDF-1 and SLF on CD34+ cells.
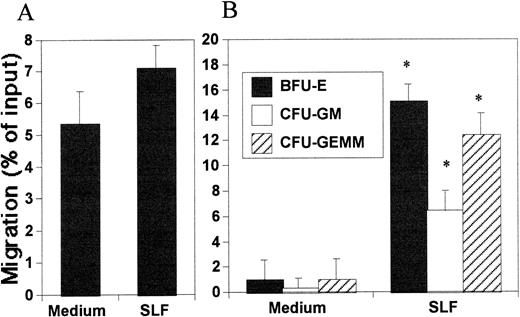
To better evaluate the relevance of the chemotactic effects of SDF-1 and SLF, we examined the effects of SLF and SDF-1 on CB and BM CD34+ cells. It has been reported that mouse SLF is both chemotactic and chemokinetic for mouse HPC.10 It was also reported that human SLF might not have any chemotactic activity toward human CD34+ cells.15 By counting the migrated cell number, the chemotactic effect of SLF was barely detectable on CD34+ cells (Fig 4A). However, the HPC colony-forming assay of migrated cells clearly showed the chemotactic effect of SLF on HPC on the total CD34+ cell population (Fig 4B). SLF attracted about 5% to 15% of HPC from input CD34+ cells within 5 hours. Because Okumura et al10 observed that murine SLF attracted murine HPC at 12- and 24-hour time points, we also examined the chemotactic effects of SLF on human HPC at a longer time point (14 hours). After 14 hours, SLF-dependent HPC migration reached up to 30%, which was twofold of that which occurred after 5 hours, and the optimal concentration of SLF for HPC attraction was about 50 ng/mL (data not shown). This appeared to be due to SLF-dependent migration not being sensitive to breakdown of the chemoattractant gradient, which can continue even after equilibration of SLF in both chambers (Fig 2A and B). We did all other HPC chemotaxis experiments using a 5-hour readout system. As shown in Fig 5A and B, SDF-1 is a strong chemotactic factor for human CD34+ cells, similar to that reported by others.15 In this context, when added together with SDF-1 to the in vitro migration system, SLF significantly increased SDF-1–dependent chemotaxis of CB and BM CD34+ cells during a 5-hour migration period (Fig 5A and B). The cooperativity between SDF-1 and SLF for HPC attraction was demonstrated by colony-forming cell assays (Fig 5C).
Chemotactic activity of SLF on HPC. Migration of cells into the lower chamber was assessed by either total CD34+cell counting (A) or HPC colony-forming cell assay (B) SLF (50 ng/mL) was added to the lower chamber to attract CB CD34+ cells for 5 hours. *Significant difference from control, P < .02.
Chemotactic activity of SLF on HPC. Migration of cells into the lower chamber was assessed by either total CD34+cell counting (A) or HPC colony-forming cell assay (B) SLF (50 ng/mL) was added to the lower chamber to attract CB CD34+ cells for 5 hours. *Significant difference from control, P < .02.
Combined effects of SDF-1 and SLF on CB CD34+ (A), BM CD34+ (B) and CB HPC cells. Cells migrated into the lower chamber were counted (A and B) or assayed for HPC (C) after the indicated chemoattractants (SDF-1 at 100 ng/mL for part A and B, 50 ng/mL for part C; SLF at 50 ng/mL for part A, B, and C) or control medium were added singly or in combination to either the upper or lower chambers. *Significant difference from controls (second bar to the left for part A and B, and medium for part C,P < .05). **Significant change from migration induced by either SLF or SDF-1 alone, P < .005.
Combined effects of SDF-1 and SLF on CB CD34+ (A), BM CD34+ (B) and CB HPC cells. Cells migrated into the lower chamber were counted (A and B) or assayed for HPC (C) after the indicated chemoattractants (SDF-1 at 100 ng/mL for part A and B, 50 ng/mL for part C; SLF at 50 ng/mL for part A, B, and C) or control medium were added singly or in combination to either the upper or lower chambers. *Significant difference from controls (second bar to the left for part A and B, and medium for part C,P < .05). **Significant change from migration induced by either SLF or SDF-1 alone, P < .005.
Combined chemotactic effects of SDF-1 and SLF on MO7e cells.
We evaluated the combined effects of SDF-1 and SLF in a positive gradient using MO7e cells as a model system because this cell line responded well to both SDF-1 and SLF (Tables 1 and 2, Fig 1). As shown in Fig 6A, the combination of SDF-1 and SLF induced additive effects. This suggested that SDF-1–dependent chemotaxis was not redundant or overlapping with SLF-dependent chemotaxis and that these two chemoattractants might cooperate in inducing MO7e cell migration. The additive effect was apparent within the range of the different concentrations of SDF-1 and SLF assessed (Fig 6B).
Combined effects of SDF-1 and SLF on MO7e cells. Migration of cells into the lower chamber after (A) SLF(10 ng/mL) and/or SDF-1 (100 ng/mL) were added to the lower chamber, or (B) SDF-1 and/or SLF were added to the lower chamber in combination at different concentrations (shown in ng/mL). *Significant difference from the second or third bar to the left, P < .05.
Combined effects of SDF-1 and SLF on MO7e cells. Migration of cells into the lower chamber after (A) SLF(10 ng/mL) and/or SDF-1 (100 ng/mL) were added to the lower chamber, or (B) SDF-1 and/or SLF were added to the lower chamber in combination at different concentrations (shown in ng/mL). *Significant difference from the second or third bar to the left, P < .05.
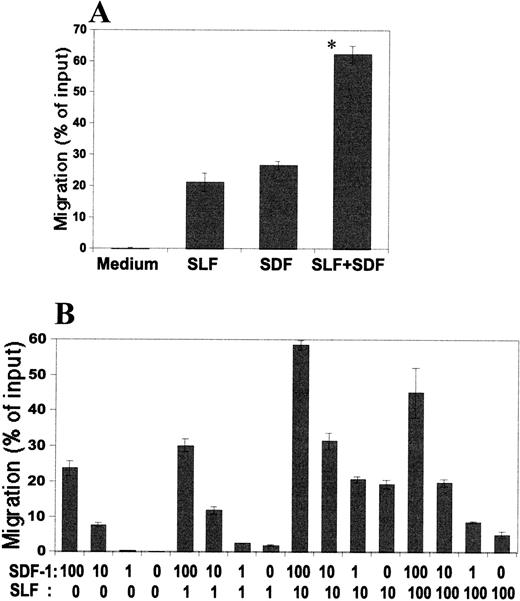
SDF-1 in a negative gradient inhibits SDF-1– and SLF-dependent chemotaxis and chemokinesis.
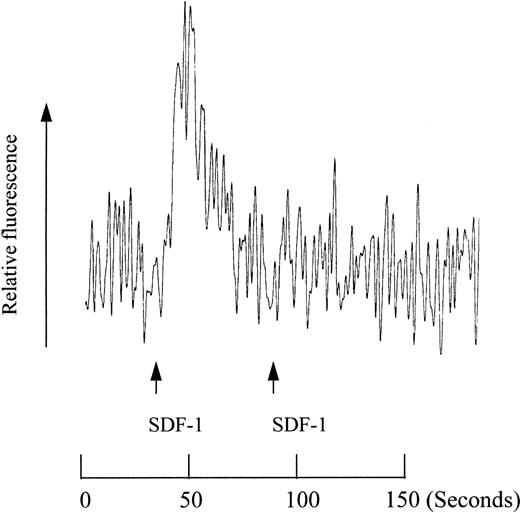
The presence of SDF-1 in the lower chamber (a positive gradient; 0/+) induced cell migration into the lower chamber, while negative (+/0) or zero (+/+) gradient did not attract cells (Table 1). These observations led us to hypothesize that SDF-1 in the upper chamber might inhibit the migration of cells into the lower chamber. We tested this inhibitory effect of SDF-1 on SLF-dependent migration of MO7e cells. SDF-1 (100 ng/mL) in the upper chamber decreased SLF-dependent migration occurring at its most effective concentration (10 ng/mL SLF for MO7e cells) by 40% to 50% (Fig7). However, SLF in the upper chamber had no inhibitory effect on SDF-1–dependent migration, even though it inhibited the migration of SLF-itself (Fig 7). Many chemokines are known to desensitize their receptors so that cells are unable to further react to the chemokines. A negative concentration gradient of SDF-1 is an effective condition for SDF-1 to bind cells and thus an efficient desensitization condition. In this context, we examined desensitization of calcium mobilization by SDF-1. As shown in Fig8, an initial SDF-1 treatment abolished MO7e cells' ability to induce calcium mobilization by a second treatment with SDF-1. So it is possible that SDF-1 in the upper chamber may desensitize M07e cells, and the desensitized cells cannot be attracted to SDF-1 in the lower chamber.
Inhibitory effects of SDF-1 or SLF in the upper chamber on MO7e cell migration with SDF-1 and/or SLF in the lower chamber. SDF-1 (100 ng/mL), SLF (10 ng/mL), and/or control medium were added to either the upper or lower chamber as indicated. Significant difference between bars, a and b (P < .01), a and c (P < .02), d and e (P < .002), and d and f (P < .0002).
Inhibitory effects of SDF-1 or SLF in the upper chamber on MO7e cell migration with SDF-1 and/or SLF in the lower chamber. SDF-1 (100 ng/mL), SLF (10 ng/mL), and/or control medium were added to either the upper or lower chamber as indicated. Significant difference between bars, a and b (P < .01), a and c (P < .02), d and e (P < .002), and d and f (P < .0002).
Desensitization of calcium mobilization in MO7e cells by SDF-1. SDF-1 (final concentration of 50 nmol/L) was used to activate MO7e cells at the indicated time points.
Desensitization of calcium mobilization in MO7e cells by SDF-1. SDF-1 (final concentration of 50 nmol/L) was used to activate MO7e cells at the indicated time points.
SDF-1, a poor mobilizer by itself, is an effective comobilizer for SLF.
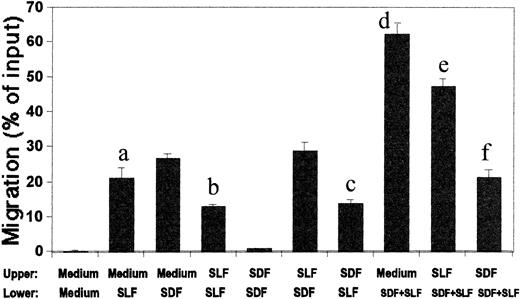
We used the in vitro migration system for MO7e cells to study the concept of mobilization. The two chemoattractants, SDF-1 and SLF, were added together to the upper chamber to form a negative inhibitory concentration gradient (Fig 9A and B). The rationale for this experiment was based on a hypothesis that the hematopoietic environment would produce and keep chemoattractants inside the BM microenvironment to inhibit unwanted mobilization of HPC into the PB system by continuously attracting them to the BM. SDF-1, added to lower chamber at 100 ng/mL in this in vitro mobilization system, mobilized less than 2% MO7e cells from the SDF-1 (100 ng/mL) and SLF (10 ng/mL)-containing upper chamber, showing that the environment formed by the two chemoattractants in the upper chamber greatly inhibited SDF-1-(in lower chamber)-dependent migration (Fig9A). In a positive gradient without this inhibitory negative gradient, SDF-1 (100 ng/mL) usually attracted more than 30% MO7e cells (Fig 1A). SLF, known to be an effective HPC mobilizer in mice and nonhuman primates, when added to lower chamber at 10 ng/mL, mobilized fourfold more MO7e cells than SDF-1 (Fig 9A). When added together to the lower chamber, SDF-1 increased the SLF-dependent mobilization significantly, in a far greater than additive fashion (Fig 9A). Because SDF-1 at 100 ng/mL is a relatively high concentration and a maximum dose for chemotaxis, we reduced the concentration of SDF-1 in the upper chamber to 20 ng/mL (Fig 9B), while maintaining the concentration of SLF in the upper chamber at 10 ng/mL, a maximally effective dose; SDF-1 still showed a strong antimobilization effect in a negative gradient (+/0) at this lower concentration. At this lower SDF-1 concentration in the upper chamber, we observed a similar enhancement of SLF-dependent MO7e mobilization by 20 ng/mL of SDF-1 in lower chamber (Fig 9B). As we increased the SDF-1 concentration in the lower chamber in this in vitro mobilization system, SLF-dependent mobilization was greatly enhanced by the added SDF-1 in the lower chamber (Fig 9B). Pertussis toxin, a G-protein coupled receptor inhibitor, did not block SLF-dependent migration, while it did block SDF-1–dependent migration in a preliminary experiment. Thus, we used pertussis toxin to determine if SDF-1 binding to its G-protein-coupled receptor was responsible for these effects. The comobilization activity of SDF-1 was inhibited by pretreatment of MO7e cells by pertussis toxin (Fig 9C), showing that this comobilization activity is mediated by specific signaling from a G-protein coupled receptor, most likely CXCR-4.19 Anti-SLF neutralizing antibody was used to show that the SLF-dependent migration was specific to added SLF (Fig 9C).
Model of in vitro mobilization by SDF-1 and SLF. (A) SDF-1 (100 ng/mL) and SLF (10 ng/mL) were added to the upper chamber to form a negative concentration gradient and SDF-1 (100 ng/mL) and/or SLF (10 ng/mL) were added to the lower chamber to mobilize MO7e cells from the upper chamber. (B) The concentration of SDF-1 at a lower concentration (20 ng/mL) with optimal concentration of SLF (10 ng/mL) was added to the upper chamber to form a less severe negative gradient of chemoattractants. SDF-1 at various concentrations (0, 20, 100, and 1,000 ng/mL) and SLF (10 ng/mL) were added to the lower chamber to mobilize the MO7e cells from the upper to the lower chamber. (C) Anti-SLF neutralizing antibody (10 μg/mL) and pertussis toxin (500 ng/mL) were used to respectively inhibit the effects of SLF and SDF-1 on migration of MO7e cells. SLF (10 ng/mL) and/or SDF-1 (100 ng/mL) was added to the lower chamber. SLF (10 ng/mL) and/or SDF-1 (20 ng/mL) was added to the upper chamber. * Designates significant changes from control (P < .02). ** 0% migration.
Model of in vitro mobilization by SDF-1 and SLF. (A) SDF-1 (100 ng/mL) and SLF (10 ng/mL) were added to the upper chamber to form a negative concentration gradient and SDF-1 (100 ng/mL) and/or SLF (10 ng/mL) were added to the lower chamber to mobilize MO7e cells from the upper chamber. (B) The concentration of SDF-1 at a lower concentration (20 ng/mL) with optimal concentration of SLF (10 ng/mL) was added to the upper chamber to form a less severe negative gradient of chemoattractants. SDF-1 at various concentrations (0, 20, 100, and 1,000 ng/mL) and SLF (10 ng/mL) were added to the lower chamber to mobilize the MO7e cells from the upper to the lower chamber. (C) Anti-SLF neutralizing antibody (10 μg/mL) and pertussis toxin (500 ng/mL) were used to respectively inhibit the effects of SLF and SDF-1 on migration of MO7e cells. SLF (10 ng/mL) and/or SDF-1 (100 ng/mL) was added to the lower chamber. SLF (10 ng/mL) and/or SDF-1 (20 ng/mL) was added to the upper chamber. * Designates significant changes from control (P < .02). ** 0% migration.
The BM environment has chemotactic activity toward human HPC cells.
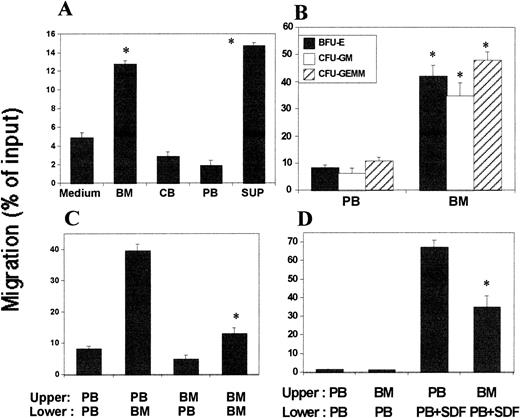
We evaluated the possibility that the BM microenvironment could have chemotactic activity on HPC against the PB system. We examined plasma from BM, CB, and PB for chemotactic activity. BM plasma, but not PB or CB plasma, showed significant chemotactic activity on human BM CD34+ cells (Fig 10A). It has been reported that mouse stromal cell lines express chemotactic factors for HPC.31 We included here the culture supernatants of mouse BM stromal cell line, M2-10B4, as a control and observed the culture supernatants had chemotactic effects on human CD34+cells. The sensitivities of CB and BM CD34+ cells to the BM plasma were not significantly different from each other (data not shown). BM plasma caused the migration of BFU-E, CFU-GM, and CFU-GEMM (Fig 10B). The lower chamber BM plasma attracted less CD34+cells when it was antagonized by the same BM plasma in the upper chamber showing a low chemokinetic activity of the BM plasma (Fig 10C).
Chemotactic and chemokinetic activities of the BM plasma on CD34+ cells. (A) Comparison of chemotactic activity of plasma from BM, CB, and PB on migration of BM CD34+ cells from the upper to lower chamber. Each plasma sample was diluted 1:2 and added to lower chamber. SUP is conditioned medium from the mouse stromal cell line, M2-10B4. (B) Chemotactic activity of BM plasma for human CB HPC present in a CD34+ population of cells. (C) Chemotactic and chemokinetic activity of BM plasma against PB plasma on CB CD34+ cells. PB and BM plasma used were diluted 1:2. (D) Inhibitory effect of the negative gradient of BM plasma on BM CD34+ cell migration induced by SDF-1. Diluted PB plasma (1:2) containing SDF-1 (50 ng/mL) was added to the lower chamber to attract human BM CD34+ cells from the upper chamber containing the indicated diluted plasma. *Significant changes from controls (medium for part A, PB for part B, PB/PB for part C, and third bar (PB/PB+SDF) to the left), P < .01.
Chemotactic and chemokinetic activities of the BM plasma on CD34+ cells. (A) Comparison of chemotactic activity of plasma from BM, CB, and PB on migration of BM CD34+ cells from the upper to lower chamber. Each plasma sample was diluted 1:2 and added to lower chamber. SUP is conditioned medium from the mouse stromal cell line, M2-10B4. (B) Chemotactic activity of BM plasma for human CB HPC present in a CD34+ population of cells. (C) Chemotactic and chemokinetic activity of BM plasma against PB plasma on CB CD34+ cells. PB and BM plasma used were diluted 1:2. (D) Inhibitory effect of the negative gradient of BM plasma on BM CD34+ cell migration induced by SDF-1. Diluted PB plasma (1:2) containing SDF-1 (50 ng/mL) was added to the lower chamber to attract human BM CD34+ cells from the upper chamber containing the indicated diluted plasma. *Significant changes from controls (medium for part A, PB for part B, PB/PB for part C, and third bar (PB/PB+SDF) to the left), P < .01.
Antimobilization effect of BM plasma in a negative gradient and mobilization of CD34+ cells from the effects of BM plasma by SDF and SLF.
We next tested a model that the BM environment may form a negative chemoattractant gradient for BM HPC to retain HPC within the BM. We set up an experiment such that BM plasma and CD34+ cells in the upper chamber and PB plasma in the lower chamber would mimic the in vivo BM and PB systems in terms of a chemotactic gradient. When we added SDF-1 in the lower chamber to induce migration, BM plasma in the upper chamber inhibited SDF-1–dependent cell migration into the lower chamber, suggesting an inhibitory negative chemoattractant gradient of the BM environment on HPC mobilization into the PB system (Fig 10D). We also examined the effect of SDF-1 and SLF on CD34+ cell mobilization into the lower chamber in this system. SDF-1 in the lower chamber (50 ng/mL) mobilized about 15% of input CD34+cells from this BM chemotactic environment, and SLF (50 ng/mL) increased the SDF-1–dependent mobilization by about 25% (data not shown).
DISCUSSION
The use of the formulae shown in Table 2 now allows a quantitative evaluation of the chemotactic versus chemokinetic activities of various effector molecules. This type of quantitative evaluation has not been previously applied to characterize chemoattractant molecules. This information should be of value in evaluating and comparing activities of multiple chemoattractants for a type of cells. Differences in the chemotactic and chemokinetic activities of SDF-1 and SLF suggest possible interacting roles of two chemoattractants in the migration of HPC. SDF-1 is a chemotactic factor that induces migration of cells and the direction of cell movement is determined by the concentration gradient of SDF-1. To our surprise, even low concentrations (eg, 1 ng/mL) of SDF-1 in a negative gradient could inhibit the effects of a positive gradient-dependent migration. This characteristic of SDF-1 suggests a possible important role for SDF-1 as a physiologic antimobilizing factor that under normal conditions may restrain the mobilization of HPC out of BM.
The in vitro chemotaxis system used in this study consists of two chambers, an upper (100 μL) and a lower (600 μL) chamber. Pores of the membrane (5 μm in diameter) separating the two chambers are smaller than most cells. However, it is big enough for small cytokines such as SDF-1 and SLF to diffuse from one chamber to the other. Because the upper chamber is relatively smaller than the lower chamber, the cytokine concentration in the lower chamber does not change much, while that of the upper chamber increases over time. It took 15 hours for a cytokine (SLF at 10 ng/mL was used in this study) to reach equalibrium by diffusion from the lower chamber to the upper chamber (Fig 2B). However, effective gradients of chemotactic factors for cell migration seem to be lost more quickly. SDF-1–dependent migration stopped after 5 hours when the two chambers were believed to lose much of their concentration difference. SLF induced consistent cell migration independently of diffusion and loss of a SLF concentration gradient. These results are in good agreement with the checkerboard assay experiments, where chemotactic and chemokinetic SLF induced cell migration in both positive (0/+) and zero (+/+) gradients, while chemotactic SDF-1 induced only in a positive gradient.
The mechanisms underlying cell movement in response to chemoattractants has not been clearly established. Small guanosine 5′-triphosphate (GTPase) molecules such as rho-like GTPases, Rac1 and 2, and CDC42Hs and actin regulation proteins, such as Wiskott-Aldrich syndrome protein and other actin binding proteins, have been suggested to be involved in cytoskeletal reorganization for formation of filopodia, lamellipodia, and stress fibers, important processes for cell movement.32,33 There is evidence that signals from tyrosine kinase via adapter protein NCK, and from G-protein–coupled receptors may regulate these actin regulation proteins.34 35 The receptor for SDF-1 is CXCR4, a G-protein-coupled receptor, and the receptor for SLF is c-kit, a receptor protein tyrosine kinase. It will be of interest to determine whether these small intracellular molecules are involved in receptor-mediated migration of stem and progenitor cells to the BM and mobilization out of the BM. In this context, it is of interest that actin polymerization induced by SDF-1 and SLF were different from each other in terms of kinetics (Fig 3). It remains to be determined whether the relatively slower kinetics of actin depolymerization is directly related to the chemokinetic nature of SLF-induced cell migration.
A possible problem in interpretation of experiments using primary CD34+ cells is the purity and possible roles of other cells in the cell population. BM and CB CD34+ cells used in this study were respectively 95% and 90% pure on average. We cannot rigorously rule out the possibility that SDF-1 and SLF might have indirect effects on cell migration by the induction and release of other cytokines from non-CD34+ cells or even CD34+ cells. However, these effects would have to be relatively rapid in terms of cytokine induction and diffusion and a response to the initial test cytokine. Chemoattractants are often believed to act in a microenvironment or on the surface of endothelial cell layers. SDF-1 has a high isoelectric point (10.9) and affinity for heparin.16 Although it has not been reported what concentrations of chemoattractants exist in these environments, BM stromal cells produce SDF-1 up to 800 ng/mL.16 It is reasoned that these surface or trapped chemoattractants in the microenvironment can form quite high chemoattractant concentrations. The optimal concentration for most chemokines as assessed in vitro are within a range from 10 to 1,000 ng/mL in the transwell chemotaxis system. We used 100 ng/mL SDF-1 for most experiments because this was an optimal amount of SDF-1.
SLF was reported to have both chemotactic and chemokinetic effects on murine HPC,10 but not on human PB and CB CD34+cells.15 However, the chemotactic activity of SLF on human CD34+ cells was assessed by counting total cell number, but not by functional assay. We observed that it was difficult to assess the chemoattractant effect of SLF by cell counting due to specificity of SLF for subtypes of CD34+ cells. We demonstrated by functional HPC colony assay that SLF has chemotactic activity on HPC and this effect is time-related and may be more specific for colony-forming HPC in contrast to a total population of CD34+ cells. We tried to examine whether c-kit–expressing CD34+ cells are better attracted to SLF than c-kit–negative cells. However, this was not possible because SLF did not attract enough CD34+ cells for immunostaining and flow cytometric analysis. Both SDF-1 and SLF showed no specificity for any particular colony-forming HPC and attracted all BFU-E, CFU-GM, and CFU-GEMM tested. However, it is possible that there may be uncharacterized chemoattractants specific for each type of colony-forming HPC. For T-cell subtypes, some chemokines showed specificity for certain subtypes of T cells, such as CD45RO+ memory cells or CD4+ helper T cells.36-38
It had not been previously reported that multiple chemoattractants could cooperatively affect cell migration. When added together to the lower chamber of the in vitro chemotaxis system to form a positive gradient (0/+), SDF-1 and SLF induced cell migration additively (Fig 6A and B). This result is of interest in that cells under the influence of “optimum” concentrations of one chemoattractant for cell migration still have the capacity to respond to the action of “optimum” concentrations of another chemoattractant. This additivity of two-chemoattractant–dependent migration was observed at different concentrations of these two chemoattractants.
Chemotactic effects of SDF-1 and SLF manifested an interesting phenomenon. When in a negative gradient, these chemotactic factors inhibited cell migration induced by a positive gradient of these chemoattractants (Table 1, and Fig 7). A possible explanation for this inhibition could be that the added chemoattractant in the upper chamber broke down the required stiffness of a positive chemoattractant gradient formed by the chemoattractant in the lower chamber. Another possibility is that the presence of SDF-1 in a negative gradient (+/0), thus binding to its receptors on cells, desensitizes the cell's ability to react to the chemoattractant in a positive gradient (0/+). We examined this desensitization effect of SDF-1 on MO7e cells by measuring calcium mobilization in response to SDF-1 treatment (Fig 8). An initial SDF-1 treatment abolished cells' ability to mobilize calcium when they were treated again by SDF-1. It is of interest that the inhibition of cell migration was observed between two different chemoattractants (Fig 7). SDF-1 in the upper chamber inhibited SLF-dependent migration, while SLF was inhibitory to only SLF itself, but had no effect on SDF-1–dependent migration. It appears that the effects of SDF-1 are dominant over SLF in the inhibition. This relationship between two chemoattractants suggests again the importance of SDF-1 as a possible antimobilizing factor for HPC mobilization. So it is conceivable that SDF-1 in the BM environment may attract HPC from the PB system and inhibit mobilization of HPC out of BM.
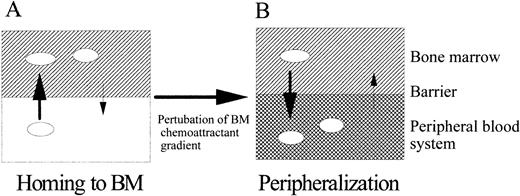
In the BM, many cytokines and chemoattractants, secreted from stromal cells or other accessory cells, are believed to form a suitable physiologic environment for HPC migration, proliferation, and differentiation.31 39 Chemoattractants may be especially important in guiding HPC to its suitable microenvironment as suggested in the diagram in Fig 11. Although it is not known how much SDF-1 and SLF exist in the BM environment, it is reasonable to think that these chemoattractants form various concentration gradients over the blood system and the BM microenvironment, attract HPC, and retain them in the BM microenvironment, unless, as shown in Fig 11, this attraction is broken by administered or induced effector molecules in the blood.
A model for homing and peripheralization of HPC by chemoattractants. This model considers: (A) homing of HPC to chemoattractants in the BM microenvironment and (B) mobilization of HPC out of the BM microenvironment when the negative gradient of the BM is broken by administering chemoattractants or known HPC mobilizers, which may act directly or induce expressions of chemoattractants outside of BM disturbing normal chemoattractant gradient around BM-PB system. The presence and concentration of chemoattractants are represented as intensity of gradient in the BM and PB compartment. Direction and size of arrows crossing two compartments respectively indicate the direction of cell migration and relative intensity of migration.
A model for homing and peripheralization of HPC by chemoattractants. This model considers: (A) homing of HPC to chemoattractants in the BM microenvironment and (B) mobilization of HPC out of the BM microenvironment when the negative gradient of the BM is broken by administering chemoattractants or known HPC mobilizers, which may act directly or induce expressions of chemoattractants outside of BM disturbing normal chemoattractant gradient around BM-PB system. The presence and concentration of chemoattractants are represented as intensity of gradient in the BM and PB compartment. Direction and size of arrows crossing two compartments respectively indicate the direction of cell migration and relative intensity of migration.
ACKNOWLEDGMENT
We thank Dr Ian Clark-Lewis for the generous gift of SDF-1.
Supported by public health service grants, R01 HL 56416, R01 HL 54037, and by a project in P01 HL 53586 from the National Institutes of Health, Bethesda, MD (to H.E.B.).
Address reprint requests to Hal E. Broxmeyer, PhD, Department of Microbiology/Immunology and the Walther Oncology Center, Indiana University School of Medicine, 975 W Walnut St, Indianapolis, IN 46202-5121.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal