Abstract
Inherited deficiency of factor XIIIA subunit (FXIIIA) is an autosomal recessive disorder that is characterized by a life-long bleeding tendency and complications in wound healing. Molecular genetic studies have shown the deficiency can be due to small sequence changes within the FXIIIA gene, such as point mutations or microdeletions. On molecular analysis of the FXIIIA gene in an FXIII-deficient patient, of United Kingdom origin, we identified a putative homozygous missense mutation, Arg408Gln. However, the father of this patient is homozygous normal for arginine at codon 408. Having proved paternity in this pedigree by microsatellite analysis, we examined the FXIIIA RNA of the patient by reverse transcriptase-polymerase chain reaction and found the paternal allele to lack exons 4 through 11 inclusive. Hence, a huge deletion extending from intron 3 to intron 11 and the Arg408Gln mutation are jointly responsible for FXIIIA deficiency in this family. This is the first finding of such a large deletion in the FXIIIA gene.
THE TRANSGLUTAMINASE factor XIII (FXIII) is essential for normal hemostasis. FXIII is the last enzyme to be activated in the blood coagulation pathway. Activated FXIIIA′ catalyzes ε(γ-glutamyl) lysine cross-links between fibrin molecules,1 which increases the mechanical strength of blood clots and makes them less susceptible to proteolytic cleavage. FXIIIA′ cross-links fibronectin and thrombospondin to anchor the blood clots to the site of injury2,3 and fibrin to α2-antiplasmin to increase clot resistance to plasmin.4In addition, FXIIIA′ can also cross-link fibronectin, vitronectin, collagen, and lipoprotein(a) in the extracellular matrix. Furthermore, it is important in stimulation of connective tissue cells and the processes involved in inflammation and wound healing.5-8
Intracellular FXIII exists as a dimer of two FXIIIA molecules, whereas the circulating plasma FXIII is composed of two FXIIIA and two FXIIIB subunits.9 This tetramer is activated in the presence of thrombin and Ca2+ by separation of the two subunits and cleavage of the 37 amino acid activation peptide from the N-terminal of the FXIIIA molecule.10 In the majority of cases, inherited FXIII deficiency is due to mutations in the gene for the FXIIIA subunit.
The FXIIIA gene extends over greater than 160 kb, contains 15 exons,11 and produces a transcript of 3.9 kb, the coding region of which is translated into a polypeptide of 731 amino acids.12,13 A number of mutations in the FXIIIA gene have been described in recent years.14-17 Three microdeletions have also been reported.18-20 We describe the first example of a very large deletion in the FXIIIA gene. This deletion extends from intron 3 to intron 11.
MATERIALS AND METHODS
Subjects.
A male patient suffering from a bleeding diathesis characteristic of inherited FXIII deficiency was studied. This patient and his family originate from the Yorkshire geographical region in the United Kingdom. The patient bled from the umbilical cord a few days after birth and has had severe bleeding into muscles, into subcutaneous tissues, around joints, and into gums and the mouth during teething. Bleeding manifestations have been controlled very successfully with long-term regular prophylaxis with a factor XIII concentrate. The patient lacks platelet and plasma FXIIIA subunit.
Isolation of genomic DNA and total RNA.
Genomic DNA was extracted from peripheral blood using standard procedures. The peripheral blood mononuclear cells (PBMCs) were separated from whole blood using lymphoprep (Nycorma, Birmingham, UK), according to the manufacturer's instructions. The acid guanidinium thiocyanate-phenol method described by Chomczynski and Sacchi was used to isolate total RNA from the PBMCs. 21
Short tandem repeat (STR) analysis.
The STR, (AAAG)n, present in the 5′ upstream region of the FXIIIA gene was amplified by the polymerase chain reaction (PCR) in a total volume of 100 μL containing 50 ng genomic DNA, 1 μmol/L each of the forward (dCAACAGAGCAAGACTTCA) and reverse (dCATTTTAGTGCATGTTCAAA) primers, 0.2 μmol/L dNTPs, 1× PCR buffer (Promega, Birmingham, UK), and 2 U of Taq DNA polymerase (Promega). The PCR was processed through 35 cycles of 92°C for 30 seconds, 54°C for 30 seconds, and 72°C for 60 seconds, followed by an extension step at 72°C for 5 minutes. Five microliters of the reaction was analyzed using 12% nondenaturing polyacrylamide gels as described in Sambrook et al.22 The PCR product expected was about 130 bp, with the exact size being dependent on the precise number of AAAG repeats present.
PCR of genomic DNA, reverse transcriptase-PCR (RT-PCR) of total RNA, and sequence analysis of PCR products.
These methods were performed as described previously.16
RESULTS
FXIII levels and activity.
The methods used for measurement of plasma FXIIIA and FXIIIB subunit levels and FXIIIA activity were as detailed in Fear et al.23 As can be seen in Table1, the proband is deficient in FXIII activity and FXIIIA subunit levels but has a low level of plasma FXIIIB subunit present. The parents have a lower than average FXIII activity and FXIIIA levels but normal FXIIIB subunit levels. This finding suggests that the parents are probably heterozygous for FXIIIA.
Plasma FXIII Concentration and Activity
| Relation . | FXIII Subunit A . | FXIII Subunit B . | FXIII Activity . |
|---|---|---|---|
| Father | 40 | 108 | 68 |
| Mother | 66 | 110 | 82 |
| Patient | 0 | 39 | 0 |
| Relation . | FXIII Subunit A . | FXIII Subunit B . | FXIII Activity . |
|---|---|---|---|
| Father | 40 | 108 | 68 |
| Mother | 66 | 110 | 82 |
| Patient | 0 | 39 | 0 |
Plasma FXIII subunits A and B and FXIII activity were measured as described previously.23 All results are expressed as the percentage of normal pooled plasma.
Mutation analysis.
Exons 2 to 15 of the FXIIIA gene (coding region) were amplified individually by PCR as described previously and PCR products were sequenced directly with the amplimers.16 Nucleotide sequence analysis showed that there was a sequence change within codon 408, from CGG → CAG (the mutation occurring in a CpG dinucleotide), correlating to an amino acid change of arginine to glutamine (Fig 1; as reported by us previously16). The patient was found to be homozygous for this mutation. Molecular analysis of the remaining members of this pedigree showed that the mother, brother, and daughter of the patient were heterozygous for this same Arg408Gln mutation. However, the father of the patient was found to be homozygous for the CGG normal allele (Fig 2), despite showing lower than normal FXIIIA levels and activity (Table 1). The pedigree was examined further to resolve this discrepancy in the allele inheritance pattern.
DNA sequence analysis of exon 10 of the FXIIIA gene. The G → A mutation, Arg408Gln, identified is indicated. The patient appears to be homozygous for this sequence change.
DNA sequence analysis of exon 10 of the FXIIIA gene. The G → A mutation, Arg408Gln, identified is indicated. The patient appears to be homozygous for this sequence change.
Pedigree of the FXIII-deficient family studied. The inheritance pattern of the Arg408Gln, CGG → CAG mutation identified is presented. The father is homozygous normal at this sequence.
Pedigree of the FXIII-deficient family studied. The inheritance pattern of the Arg408Gln, CGG → CAG mutation identified is presented. The father is homozygous normal at this sequence.
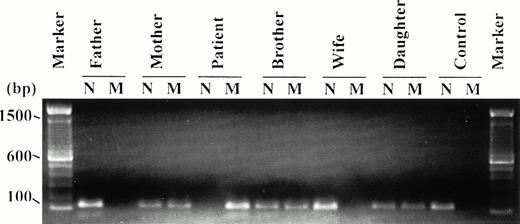
To confirm that the missense mutation Arg408Gln is causative, the frequency of this mutation was examined in 124 unrelated normal individuals by the technique of amplification refractory mutation system-PCR (ARMS-PCR).24 Exon 10 of the FXIIIA gene was amplified from each individual using the normal forward primer, N (dTGCAATGCAGGCATGTATCG), or the mutant forward primer, M (dTGCAATGCAGGCATGTATCA), and normal reverse primer (dAAACAGCACTTTCCTCCAGC). PCR conditions were 35 cycles of 92°C for 30 seconds, 60°C for 30 seconds, and 72°C for 60 seconds. The ARMS-PCR products obtained are displayed in Fig3 and authenticate the results of sequence analysis in our pedigree. From the patient, a PCR product is only obtained using the mutant forward primer confirming the patient is mutant homozygous. A PCR product is only obtained using the normal forward primer in both the father and wife, indicating that they are both normal homozygous. The mother, brother, and daughter all give PCR products using both the normal and mutant forward primers, thus confirming that they are heterozygous for this mutation. As expected, the normal control only gives a product using the normal forward primer, and this was the pattern obtained from all the 124 unrelated normals analyzed. Hence, the Arg408Gln mutation was not detected in 248 alleles, confirming that it is not a common population polymorphism and that, therefore, this mutation is likely to be responsible for FXIII deficiency in this family.
ARMS-PCR products of 172 bp obtained using the normal and mutant forward primers. All relations are defined with respect to the patient. N, normal forward primer; M, mutant forward primer. The molecular weight marker used is the 100-bp ladder (BRL). Only the mutant primer gives a PCR product from the patient, indicating that the patient is mutant homozygous. Both the normal and mutant primers give PCR products from the mother, brother, and daughter, suggesting that they are all heterozygous for this mutation. The father, wife, and unrelated normal control give a PCR product with the normal primer but not with the mutant primer, confirming that they are normal homozygous. This latter pattern was obtained from all the 124 normal controls analyzed.
ARMS-PCR products of 172 bp obtained using the normal and mutant forward primers. All relations are defined with respect to the patient. N, normal forward primer; M, mutant forward primer. The molecular weight marker used is the 100-bp ladder (BRL). Only the mutant primer gives a PCR product from the patient, indicating that the patient is mutant homozygous. Both the normal and mutant primers give PCR products from the mother, brother, and daughter, suggesting that they are all heterozygous for this mutation. The father, wife, and unrelated normal control give a PCR product with the normal primer but not with the mutant primer, confirming that they are normal homozygous. This latter pattern was obtained from all the 124 normal controls analyzed.
Paternity test.
Six microsatellite markers, roughly equally spaced on human chromosome 6, were selected and used to investigate paternity in this pedigree. As can be seen in Table 2, the patient appears to have inherited allele B from the father, thus proving paternity, and allele C from the mother. The patient's heterozygous brother has also inherited allele C from the mother but allele A from the father. This suggests that the paternal allele A is probably the normal allele, whereas paternal allele B may be the one causing FXIIIA deficiency.
Paternity Analysis in the FXIII-Deficient Family Studied
| Microsatellite Marker . | Father . | Mother . | Patient . | Brother . | ||||
|---|---|---|---|---|---|---|---|---|
| A . | B . | C . | D . | B . | C . | A . | C . | |
| D6S271 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
| D6S264 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| D6S257 | 2 | 1 | 3 | 4 | 1 | 3 | 2 | 3 |
| D6S285 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
| D6S305 | 1 | 1 | 2 | 3 | 1 | 2 | 1 | 2 |
| D6S259 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
| Microsatellite Marker . | Father . | Mother . | Patient . | Brother . | ||||
|---|---|---|---|---|---|---|---|---|
| A . | B . | C . | D . | B . | C . | A . | C . | |
| D6S271 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
| D6S264 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| D6S257 | 2 | 1 | 3 | 4 | 1 | 3 | 2 | 3 |
| D6S285 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
| D6S305 | 1 | 1 | 2 | 3 | 1 | 2 | 1 | 2 |
| D6S259 | 1 | 2 | 3 | 4 | 2 | 3 | 1 | 3 |
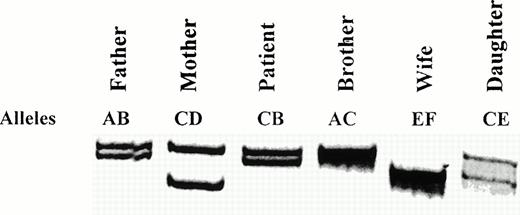
Verification of the presence of two alleles for the FXIIIA gene.
The STR present 5′-upstream of the FXIIIA gene was amplified by PCR from all members of the pedigree. The PCR products obtained are presented in Fig 4. Both the patient and his father clearly show two bands of differing molecular weights, resulting from a varying number of AAAG repeats. This indicates the presence of two FXIIIA alleles, at least in this region of the gene. The inheritance pattern obtained for this STR again confirms paternity in this pedigree.
Segregation of the FXIIIA (AAAG)n STR alleles. All relations are defined with respect to the patient. Alleles are arbitrarily labeled AB for the father, CD for the mother, and EF for the wife. The alleles inherited by each family member are indicated.
Segregation of the FXIIIA (AAAG)n STR alleles. All relations are defined with respect to the patient. Alleles are arbitrarily labeled AB for the father, CD for the mother, and EF for the wife. The alleles inherited by each family member are indicated.
Analysis of FXIIIA gene transcripts.
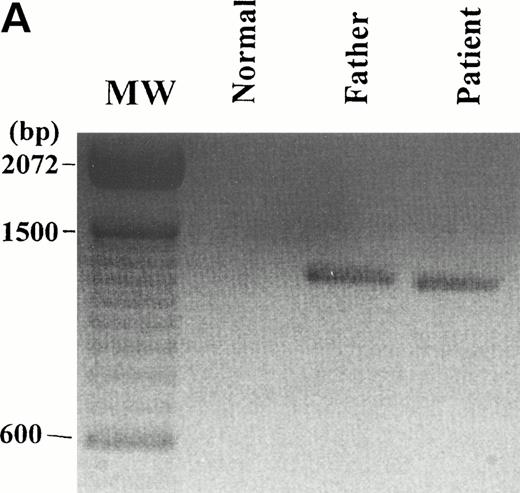
The FXIIIA gene transcript was amplified by RT-PCR as four overlapping fragments A, B, C, and D, as described previously.18 The expected size PCR products were obtained for all fragments A to D (data not presented). When the whole coding region of the FXIIIA mRNA was amplified as one fragment by RT-PCR, an amplification product much shorter than the expected size of 2.28 kb was obtained from total RNA from both the patient and his father (Fig5
A). The 2.28-kb PCR product comprising the full coding region could only be amplified by performing a second nested PCR (data not shown) and, hence, is not visible in Fig 5A, in which the products of only a single round of primary PCR are shown. The shorter, approximately 1.2-kb, RT-PCR product (Fig 5A) was not observed in any of 9 further unrelated individuals analyzed.
RT-PCR and sequence analysis of the FXIIIA mRNA. (A) The full coding region of the FXIIIA mRNA was amplified by RT-PCR as described previously.16 The molecular weight marker (MW) used is the 100-bp ladder (BRL). A shorter product of approximately 1.2 kb is clearly evident in both the patient and his father. The 2.28-kb RT-PCR product from the normal length transcript is not visible because that could only be produced by performing a second nested PCR (data not shown). (B) DNA sequence analysis of the approximately 1.2-kb RT-PCR product from the patient shows that it lacks exons 4 through 11 inclusive. Sequence analysis of this shorter product from the father produced an identical result (data not presented). This shorter RT-PCR product is likely to be from a transcript transcribed from allele B of the father, which gives rise to FXIII deficiency in this family.
RT-PCR and sequence analysis of the FXIIIA mRNA. (A) The full coding region of the FXIIIA mRNA was amplified by RT-PCR as described previously.16 The molecular weight marker (MW) used is the 100-bp ladder (BRL). A shorter product of approximately 1.2 kb is clearly evident in both the patient and his father. The 2.28-kb RT-PCR product from the normal length transcript is not visible because that could only be produced by performing a second nested PCR (data not shown). (B) DNA sequence analysis of the approximately 1.2-kb RT-PCR product from the patient shows that it lacks exons 4 through 11 inclusive. Sequence analysis of this shorter product from the father produced an identical result (data not presented). This shorter RT-PCR product is likely to be from a transcript transcribed from allele B of the father, which gives rise to FXIII deficiency in this family.
Nucleotide sequence analysis of the shorter, approximately 1.2-kb FXIIIA transcript from both the patient and his father showed the loss of exons 4 through 11 inclusive (Fig 5B). This indicates a deletion in the FXIIIA gene, beginning at intron 3/exon 4 and ending at exon 11/intron 11, in the paternal allele A in this family.
DISCUSSION
The present study describes the molecular genetic analysis of inherited FXIIIA deficiency in a family originating from the Yorkshire region in the United Kingdom. The patient studied was eventually found to be a compound heterozygote.
A missense mutation, Arg408Gln, inherited through the maternal line was identified.16 Arg-408 in the factor XIIIA subunit is located within the catalytic core domain that is made up of about 330 amino acid residues between Asn-185 and Arg-515.25 In fact, it is in the immediate vicinity of the catalytic triad (Cys-314, His-373, and Asp-396), specifically Asp-396.26 Arginine is a highly charged basic amino acid and Arg-408 may well be involved in hydrogen bonding or salt bridge formation with key residues around the active site. Glutamine is a neutral polar amino acid, and it is plausible that the Arg408Gln mutation results in a conformational change around the catalytic triad. This would grossly affect the activity of the mutant protein. Such a mutant FXIIIA protein would be difficult to study functionally by expression in recombinant cells as similar structural instability problems would be encountered. However, because the Arg408Gln mutation was not detected in 248 normal alleles, it is likely to be the causative mutation for FXIII deficiency in our pedigree.
The second sequence change, inherited through the paternal line, was a deletion of introns 3 through 11 inclusive. This deletion results in an FXIIIA transcript in which exon 3 is joined to exon 12 during RNA processing, and exons 4 to 11 inclusive are absent. The reading frame is maintained and a polypeptide of 351 amino acid residues would be predicted to be synthesized. This shorter product would lack 380 amino acid residues from Ileu-106 to Glu-485, corresponding to exons 4 through 11. Amino acid residues 106 to 485 of the FXIIIA subunit represent part of the β-sandwich and almost all of the catalytic core domain.25 Thus, it is probable that the shorter polypeptide is unable to adopt an appropriate conformation. Because the core domain and the catalytic triad are absent from the mutant protein, it is likely to be inactive and may in fact form an unstable structure.
A number of sequence changes in the highly polymorphic FXIIIA gene have been described in recent years.14-17 However, only three deletions have been reported previously. All of these are microdeletions: a dinucleotide AG deletion at the exon 3 acceptor site18; a 13-bp deletion within exon 3, extending from codons 82 to 8619; and a 4-bp deletion in exon 11.20 These three microdeletions each cause a frameshift and result in premature termination of translation. This is the first finding of a major deletion in the FXIIIA gene, and it extends from intron 3 through intron 11.
Although the size of each of the 15 exons present in the FXIIIA gene is known, the sizes of all of the 14 introns separating these exons have not been determined. During the cloning of the FXIIIA gene by Ichinose and Davie,11 a total of 121 clones was isolated. These clones contained all the exons that encode the FXIIIA cDNA sequence. Restriction digestion and Southern blotting analysis showed 25 of these clones to be unique and the remainder to be overlapping. The overlapping clones spanned greater than 160 kb, but contained gaps within introns then designated B, D, F, H, J, and M.11Thus, sizes for only 8 of the 14 introns are known. This suggests that the size of the gene for the FXIIIA subunit may be substantially greater than 160 kb. The deletion we have identified in our study extends from somewhere within intron 3/exon 4 to exon 11/intron 11. Introns D, F, H, and J, which have not yet been cloned or sequenced, lie within the deletion we have identified. Because their exact sizes are unknown, it is very difficult to estimate the actual size of the DNA sequence deleted. However, it is probable that the deletion is larger than 100 kb, because 9 of the 14 introns, representing more than half of the FXIIIA gene, are lost from the deleted allele.
Repetitive DNA sequences can give rise to gene deletions by promoting recombinational instability. Alu sequences flanking deletion breakpoints have been noted in a number of human genetic diseases involving defects in the genes for α-globin, β-globin, apolipoprotein B, adenosine deaminase, etc.27 Deletion is thought to occur by meiotic (or mitotic) recombination between chromosomes misaligned at these partially homologous sequences. It is worth noting that the 4 deletions identified so far in the FXIIIA gene are around exons 3 and 11. It may be that there are particular sequences present within or around introns 3 and 11 that influence the efficacy of DNA replication in these regions or indeed influence events such as DNA recombination resulting in large deletions. Sequence analysis of these regions around exons 3 and 11 may provide some insight into how the deletion we report may have occurred.
To conclude, we have identified a missense Arg408Gln mutation and a very large deletion, extending from introns 3 through 11, in the FXIIIA subunit gene in a factor XIII-deficient family. The deletion maintains the translation reading frame but is predicted to produce a polypeptide which is shorter by 380 amino acid residues and lacks most of the β-sandwich and the catalytic core domain of the protein.
ACKNOWLEDGMENT
The authors thank their patient and his family for their cooperation and support.
Supported by the Wellcome Trust, Northern and Yorkshire Regional Health Authority, and the West Riding Medical Research Trust.
Address reprint requests to Rashida Anwar, PhD, Molecular Medicine Unit, Department of Medicine, University of Leeds, Clinical Sciences Building, St James's University Hospital, Leeds LS9 7TF, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal