Abstract
An unusual group of human B-cell tumors with cellular features of chronic lymphocytic leukemia or lymphoplasmacytoid leukemia, together with high levels of a monoclonal IgG serum protein, has been investigated. Analysis of tumor-derived VH genes of neoplastic B lymphocytes was used to determine the clonal relationship between the IgM expressed or secreted by the tumor cells and the IgG serum paraprotein. In all five cases, VH gene sequences showed transcripts of IgM and IgG of common clonal origin. Sequences were derived from VH3 (4 of 5) and VH1 (1 of 5) families and were all highly somatically mutated with strong evidence for antigen selection. There was no intraclonal variation detectable in either IgM or IgG sequences. In 3 of 5 cases, in which monoclonal IgM and IgG were found in serum, the VH genes combined to Cμ or Cγ showed identical mutational patterns. However, in 2 of 5 cases, in which IgM was confined to cell expression with only monoclonal IgG in serum, sequences of the VH transcripts of IgM and IgG showed many shared mutations but also numerous differences. In these cases, the level of mutation was similar in IgM and IgG and both appeared to be antigen selected. In summary, the final neoplastic event in this group of tumors has apparently occurred at the point of isotype switch from IgM to IgG, leading to dual isotype synthesis. In the group that secreted both isotypes, the mutation pattern was identical, indicating either synthesis by a single cell, or silencing of mutational activity before switching. In the group that did not secrete IgM, cells of each isotype were distinct and reflected a divergent mutational history.
DURING NORMAL B-cell differentiation, a functional VH-DH-JH transcript is generated by a process of genetic recombination. A single VH gene is chosen from the available VHrepertoire consisting of approximately 51 potentially functional genes that can be grouped into structurally related families VH1 to VH7.1 Choice of D-segment gene and imprecision at the joints gives rise to heterogeneity in amino acid sequence at the third complementarity-determining region (CDR3). Therefore, the CDR3 sequence can be considered as a clonal signature for a B cell and, following neoplastic transformation, can act as a useful tumor marker.2
After a similar process of recombination in the light chain, a naive B cell expresses IgM and can bind antigen. The cell may then enter the lymphoid follicle and undergo a second process of sequence diversification by somatic hypermutation.3 Although the introduction of mutations has an element of randomness, the limited antigen in the germinal center will stimulate only those B cells that offer complementary sequences to antigen via the contact points in CDR1, CDR2, and CDR3.3,4 Therefore, the imprint of antigen selection will be a clustering of replaced amino acids in these regions. The next process in a maturing antibody response is a switch from IgM to another isotype, often IgG. The switching mechanism has been shown to involve deletional recombination with excision of the upstream constant regions.5 It appears that a considerable degree of somatic mutation can occur before switching,6,7leading to production of IgM memory cells.7 Further mutations may accumulate after switching,6 7 but the mutational mechanism appears silent in the fully differentiated plasma cell.
Neoplastic transformation can occur at several points in B-cell differentiation, from the pre-B cell to the plasma cell. Investigation of VH genes used by B-cell tumors is now providing information on the nature of the cell of origin and its clonal history. For example, it appears that the usage of VH genes by certain tumors is highly asymmetric, which may be important for pathogenesis.8-10 Analysis of VH gene sequences is also indicating whether the cell of origin has entered the germinal center, known to be the site of somatic mutation.3
In the case of chronic lymphocytic leukemia (CLL), a proportion of VH sequences are unmutated, consistent with a naive B cell.11 However, there are subsets of CLL, particularly those with abnormalities in chromosome 13q14, that have accumulated significant levels of mutation, indicative of a different maturation state.12 Follicular lymphoma and Burkitt's lymphoma have both undergone somatic mutation as expected, and mutations continue to accumulate in these tumors subsequent to the final transformation event leading to intraclonal heterogeneity.13-15 In contrast, splenic lymphoma with villous lymphocytes16 and myeloma17 have mutated VH sequences, but no intraclonal heterogeneity, suggesting that the final event in these tumors is at a postfollicular stage.18
Although it is possible to induce isotype switching in CLL cells in vitro,19,20 the ability of B-cell tumors to isotype switch in vivo was difficult to document before the development of VH gene probes. Coexistence of CLL and an isotype-switched plasma cell tumor such as myeloma is very rare, and commonly involves different light chain types, or different idiotypic antigens, indicative of biclonality.21 In an extensive literature survey of CLL and the more aggressive tumors that develop in approximately 10% of patients, genetic relatedness was a common finding only in cases of prolymphocytic transformation.22Transformation to myeloma showed clonal relatedness in only 2 of 8 cases, indicating that this type of disease progression arises relatively rarely via clonal evolution,22 a finding supported by more recent cases.23 However, transcripts of clonally related VH-Cμ have been found in patients with myeloma, which point to coexistence of a minor population of less mature B cells.24 25
We have had the opportunity to study an unusual subset of B-cell tumors with abnormal cells expressing or secreting both monoclonal IgM and IgG. Such cases are likely to be closer to lymphoplasmacytoid lymphoma (LPL) than to true CLL, although some share features with both entities. LPL is a distinct B-cell proliferative disorder with particular morphological and histopathological characteristics, and is frequently associated with a high secretion of monoclonal IgM.26 However, cases of CLL may also exhibit high levels of monoclonal Ig, usually IgM, and can show morphological or phenotypic lymphoplasmacytoid differentiation.27 On the other hand, cases of LPL may have blood and bone marrow involvement with cells difficult to distinguish from CLL.26 Our cases appear closer to LPL than CLL and have the additional rare feature of synthesizing both IgM and IgG. Because they may, therefore, reflect the point of isotype switch, we have used immunogenetic analysis to show the clonal history of these tumors.
MATERIALS AND METHODS
Patient selection.
The focus of this investigation was on patients with B-cell tumors who had evidence for synthesis of both IgM and IgG. From January 1989 to May 1996, we investigated in the Department of Hematology in Nantes, France, 611 cases of B-cell lymphoproliferative disorders (BLPD), with circulating abnormal lymphoid cells >5 × 109/L, and CD19 >30%. Among these, we observed 12 patients with a high serum monoclonal IgG. Four of these patients had the same light chain type in IgM and IgG and were selected for further study. An additional patient (BAR) presented in Southampton with renal stones, and was found to have IgM and IgG paraproteins of similar light chain type in serum. He had a normal blood count and has remained well for 6 years since diagnosis.
Morphological and histological classification of BLPD.
All May-Grünwald-Giemsa stained blood and bone marrow smears were reviewed and classified according to the French-American-British (FAB) group proposals.28 Similarly, all biopsy specimens were reviewed and classified according to the revised European-American classification of lymphoid neoplasms (REAL).26
Immunophenotypic analysis.
Peripheral blood mononuclear cells were prepared by separation on a Ficoll gradient and analyzed by indirect immunofluorescence, using a panel of monoclonal antibodies (MoAbs), including B3 and B6 (CD22 and CD23, respectively; Coulter, Miami, FL), leuM5 (CD11c) and B-B4 (CD138), a SYNDECAN-1 plasmacyte-specific MoAb29 (Diaclone Research, Besancon, France), using the FACSCAN (Becton Dickinson, San Jose, CA). Coexpression of CD5 and CD19 was assessed by double labeling using fluorescein isothiocyanate (FITC)-labeled IOT1a (CD5) and phycoerythrin (PE)-labeled IOB4 (CD19; Immunotech, Marseille, France). Surface Ig (sIg) expression was studied with FITC-labeled F(ab′)2 from goat polyclonal antibodies against human Igμ, Igδ, Igγ, Igκ, and Igλ chains. sIg fluorescence intensity was considered as strong when the difference between mean fluorescence of both anti-light chain types exceeded one log.
Analysis of VH genes.
Total RNA (5 to 50 μg) was isolated using RNAzol (Cinna Biotecx Labs Inc, Houston, TX) from mononuclear cell fractions prepared either from heparinized peripheral blood and stored as cryopreserved cells in dimethyl sulfoxide (0.9 to 1.5 × 107 cells; patients PAI, BLO, SAM, and LAR), or from a heparinized bone marrow aspirate (patient BAR). RNA (2 to 10 μg) was reverse transcribed to cDNA with Cμ and Cγ isotype-specific primers as described.30 A sample of cDNA was then amplified by the polymerase chain reaction (PCR) using a mixture of 5′-oligonucleotide primers that cover the VH1 to VH7 family potentially functional repertoire, together with nested downstream 3′-primers specific for the CH isotype. Amplification conditions and methods of cloning and sequencing of products have been described.30
RESULTS
Clinical and laboratory features of patients.
All patients had evidence of B-cell tumors that were synthesizing IgM and IgG, but the detailed nature of the tumors was variable. Four of five patients presented with a significant lymphocytosis with cell morphology typical of CLL. However, a few coexisting lymphoplasmacytoid cells were observed in two patients (BLO and SAM). One of five patients (BAR) had no lymphocytosis but had tumor cells in the bone marrow with morphology consistent with LPL. Histologic data were available in three of five cases (Table 1). According to the REAL classification, two patients showed a histopathologic picture of LPL, one in a bronchial biopsy specimen (BLO), the other in bone marrow and in spleen (red pulp infiltrate; SAM). A third patient (LAR) had an amyloid immunoglobulin light chain (AL)-type amyloid nephropathy on a kidney biopsy specimen. All patients had high levels of monoclonal IgG paraproteins in serum, and in one case (PAI) two IgG paraproteins were detected. Patients PAI, BLO, and BAR also had an additional IgM paraprotein of the same light chain type as the IgG (Table 2). In four of five cases, circulating tumor cells were present, and phenotypic analysis (Table 3) showed increased numbers of CD19+ lymphocytes. Only one case (PAI) showed an immunophenotypical profile characteristic of CLL (CD5+CD22+ CD23+), and these cells had an unusual strong sIg (++) expression (Table 3). Patients BLO, SAM, and LAR exhibited a CD5− CD22+CD23+/−, sIg++ surface phenotype more typical of LPL.26 31 The four cases with tumor cells in the blood were all negative for CD10, CD11c, and B-B4.
Clinical and Laboratory Features of B-Cell Tumors With High Serum IgG Paraprotein Levels
| Patient . | Age (yr) . | Splenomegaly . | Binet Stage . | Circulating Lymphocytes (×10−9/L) . | Classification . | |
|---|---|---|---|---|---|---|
| Morphology . | Histology . | |||||
| PAI | 68 | + | A | 15.7 | CLL | ND |
| BLO | 83 | + | B | 8.9 | CLL | LPL* |
| BAR | 69 | − | ND | Normal | LPL† | ND |
| SAM | 44 | + | A | 5.6 | LPL | LPL‡ |
| LAR | 70 | − | A | 9.1 | CLL | AL A§ |
| Patient . | Age (yr) . | Splenomegaly . | Binet Stage . | Circulating Lymphocytes (×10−9/L) . | Classification . | |
|---|---|---|---|---|---|---|
| Morphology . | Histology . | |||||
| PAI | 68 | + | A | 15.7 | CLL | ND |
| BLO | 83 | + | B | 8.9 | CLL | LPL* |
| BAR | 69 | − | ND | Normal | LPL† | ND |
| SAM | 44 | + | A | 5.6 | LPL | LPL‡ |
| LAR | 70 | − | A | 9.1 | CLL | AL A§ |
Abbreviations: CLL, chronic lymphocytic leukemia; ND, not determined; LPL, lymphoplasmacytoid lymphoma; AL A, AL amyloidosis.
Bronchial biopsy.
Bone marrow.
Bone marrow and lymph node.
Amyloidosis on kidney biopsy.
Serum Paraprotein Analysis
| Patient . | IgG . | IgM . | ||
|---|---|---|---|---|
| Type . | Concentration (g/L) . | Type . | Concentration (g/L) . | |
| PAI | 2 × IgGκ | 20 | IgMκ | 5 |
| BLO | IgGλ | 20 | IgMλ | 6 |
| BAR | IgGλ | 10 | IgMλ | 3.6 |
| SAM | IgGκ | 40 | — | — |
| LAR | IgGκ | 21 | — | — |
| Patient . | IgG . | IgM . | ||
|---|---|---|---|---|
| Type . | Concentration (g/L) . | Type . | Concentration (g/L) . | |
| PAI | 2 × IgGκ | 20 | IgMκ | 5 |
| BLO | IgGλ | 20 | IgMλ | 6 |
| BAR | IgGλ | 10 | IgMλ | 3.6 |
| SAM | IgGκ | 40 | — | — |
| LAR | IgGκ | 21 | — | — |
Immunophenotype of Tumor Cells
| Patient . | CD19 . | sIg . | CD5 . | CD22 . | CD23 . | CD38 . | |
|---|---|---|---|---|---|---|---|
| % . | Type . | ||||||
| PAI | 80 | 78 | MGκ++ | 86 | 47 | 37 | ND |
| BLO | 88 | 73 | Mλ++ | 1 | 82 | 46 | 0 |
| BAR | 16 | 19 | MGλ* | ND | ND | ND | ND |
| SAM | 48 | 48 | MGκ++ | 13 | 48 | 44 | 22 |
| LAR | 73 | 67 | MGκ++ | 20 | 34 | 0 | 6 |
| Patient . | CD19 . | sIg . | CD5 . | CD22 . | CD23 . | CD38 . | |
|---|---|---|---|---|---|---|---|
| % . | Type . | ||||||
| PAI | 80 | 78 | MGκ++ | 86 | 47 | 37 | ND |
| BLO | 88 | 73 | Mλ++ | 1 | 82 | 46 | 0 |
| BAR | 16 | 19 | MGλ* | ND | ND | ND | ND |
| SAM | 48 | 48 | MGκ++ | 13 | 48 | 44 | 22 |
| LAR | 73 | 67 | MGκ++ | 20 | 34 | 0 | 6 |
Tumor cells were obtained from blood, except for patient BAR in whom a bone marrow aspirate was analyzed.
Cytoplasmic Ig.
Circulating lymphoid cells expressed both sIgM and sIgG with similar light chain type in three patients (PAI, SAM, and LAR), but only IgM in patient BLO. In the case of BAR, only a bone marrow aspirate was available, and this had the cytological features of LPL. The tumor cells expressed CD19, and analysis of cytoplasmic Ig showed an IgMGκ+ population. However, in all cases, conclusions concerning dual expression of IgM and IgG were limited by the presence of high serum IgG paraprotein, which may be bound to Fc receptors.32
VH genes of tumor-derived VH-Cμ and VH-Cγ transcripts.
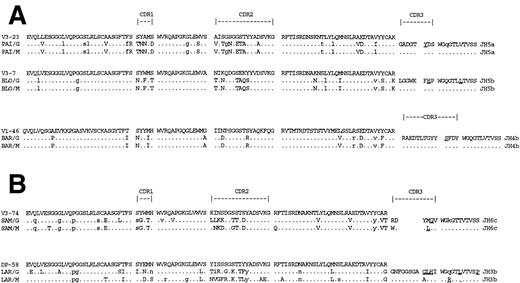
Identification of expanded tumor-derived VH sequences from repeated identical or closely similar CDR3 “clonal signature” sequences, obtained by PCR/cloning, has been validated previously.33 In all cases, amplification of VHin combination with Cγ yielded repeated sequences with clear CDR3 “clonal signatures” (Table 4). Tumor-derived deduced amino acid sequences are shown in Fig 1. Nucleotide sequences have been deposited in the European Molecular Biology Laboratory (EMBL) database (accession numbers: Z95481-95487). Other VH sequences obtained were individually different and were likely to be from normal B cells. For all patients, the same VH sequence was also found to predominate in sequences combined to Cμ (Fig 1), indicating derivation of both IgG and IgM from the same original B cell. Most of the tumors used VH genes from the VH3 family (4 of 5), with one out of five from VH1. The CDR3 sequences were short (6-14 amino acids), particularly when compared with typical CLL,11 and a range of JH genes was used, some of which were somatically mutated (Fig 1). There was no evidence for intraclonal variation in any of the tumor-derived sequences. All the IgG products were derived from the IgG1 or IgG2 subclass (data not shown); further assignment could not be made from the limited available length of constant region nucleotides.
Analysis of Tumor-Derived VH Genes
| Patient . | Ig Class . | VH Family . | GL Donor . | JH . | % Homology . | TumorDerived Clones . | Clones Sequenced . |
|---|---|---|---|---|---|---|---|
| PAI | IgG | VH3 | V3-23 | JH5a | 88.9 | 16/22 | 7 |
| IgM1 | VH3 | V3-23 | JH5a | 88.9 | 5/12 | 5 | |
| IgM2 | VH4 | V4-59 | (stop codon) | 5/12 | 4 | ||
| BLO | IgG | VH3 | V3-7 | JH5b | 93.2 | 7/12 | 7 |
| IgM | VH3 | V3-7 | JH5b | 93.2 | 9/12 | 6 | |
| BAR | IgG | VH1 | V1-46 | JH4b | 95.9 | 12/12 | 12 |
| IgM | VH1 | V1-46 | JH4b | 95.9 | 10/10 | 10 | |
| SAM | IgG | VH3 | V3-74 | JH6c | 90.1 | 9/9 | 6 |
| IgM | VH3 | V3-74 | JH6c | 90.9 | 6/8 | 4 | |
| LAR | IgG | VH3 | DP-58 | JH3b | 91.2 | 6/8 | 6 |
| IgM | VH3 | DP-58 | JH3b | 90.5 | 8/8 | 6 |
| Patient . | Ig Class . | VH Family . | GL Donor . | JH . | % Homology . | TumorDerived Clones . | Clones Sequenced . |
|---|---|---|---|---|---|---|---|
| PAI | IgG | VH3 | V3-23 | JH5a | 88.9 | 16/22 | 7 |
| IgM1 | VH3 | V3-23 | JH5a | 88.9 | 5/12 | 5 | |
| IgM2 | VH4 | V4-59 | (stop codon) | 5/12 | 4 | ||
| BLO | IgG | VH3 | V3-7 | JH5b | 93.2 | 7/12 | 7 |
| IgM | VH3 | V3-7 | JH5b | 93.2 | 9/12 | 6 | |
| BAR | IgG | VH1 | V1-46 | JH4b | 95.9 | 12/12 | 12 |
| IgM | VH1 | V1-46 | JH4b | 95.9 | 10/10 | 10 | |
| SAM | IgG | VH3 | V3-74 | JH6c | 90.1 | 9/9 | 6 |
| IgM | VH3 | V3-74 | JH6c | 90.9 | 6/8 | 4 | |
| LAR | IgG | VH3 | DP-58 | JH3b | 91.2 | 6/8 | 6 |
| IgM | VH3 | DP-58 | JH3b | 90.5 | 8/8 | 6 |
Abbreviation: GL, germline.
Deduced amino acid sequences of VH-Cγ and VH-Cμ transcripts from patients' tumor cells synthesizing both IgG and IgM isotypes. (A) Cases with both IgM and IgG serum parapoteins. (B) Cases with IgG serum paraproteins only. Comparisons are made with the closest germ line VH genes. Upper case, replacement mutations; lower case, silent mutations. Replacement mutations in JH are underlined.
Deduced amino acid sequences of VH-Cγ and VH-Cμ transcripts from patients' tumor cells synthesizing both IgG and IgM isotypes. (A) Cases with both IgM and IgG serum parapoteins. (B) Cases with IgG serum paraproteins only. Comparisons are made with the closest germ line VH genes. Upper case, replacement mutations; lower case, silent mutations. Replacement mutations in JH are underlined.
In the case of PAI, although two IgG paraproteins were observed in serum, only a single VH-Cγ transcript was identified, suggesting that either the two IgG serum paraproteins were clonally identical, or that the second paraprotein was being produced from a separate cell population. In contrast, two different repeated VH-Cμ transcripts were found for PAI, one of which was identical to the IgG-derived sequence (Table 4). The VH of the second IgM-derived transcript used the V4-59 gene segment but contained a stop codon at position 47 rendering it aberrant. This aberrant sequence was likely derived from the allelic chromosome of the tumor cells, and had apparently not undergone isotype switching.
Analysis of somatic mutations.
To assess the degree of somatic mutation, the VH sequences were compared with the closest germ line sequences in the databases. Although this approach does not allow for sequence polymorphism, it has become clear recently that the majority of polymorphic changes in human VH genes arise from deletions or insertions, and that sequence variation is at a low level.1 The percent homology of the VH sequences with the closest germ line genes was quite low (Table 4) indicative of extensive somatic mutation. Deduced amino acid sequences (Fig 1) show the positions of the mutations and indicate amino acid replacements. Comparison of the pattern of mutations in the sequences derived from IgG with those from IgM showed complete identity for patients PAI, BLO, and BAR (Fig 1A). In contrast, the pattern of mutation in IgG and IgM from patients SAM and LAR showed some common mutations but many differences, particularly in the CDRs (Fig 1B). However, the level of mutational activity was similar for both IgG and IgM, and there was no evidence for accumulation of mutations in switching from IgM to IgG. In fact, at several codons, there were mutations in the IgM-derived sequences, which were not found in the IgG sequences.
There was an apparent clustering of replaced amino acids in CDR1 and CDR2 in all sequences, and this was assessed for statistical significance, which points to antigen selection, using the method of Chang and Casali.34 The results (Table 5) show highly significant clustering of replacement amino acids in the CDRs for IgG- and IgM-derived sequences from all patients. Where mutations differed between IgG and IgM, both had strong evidence for antigen selection. There was also evidence for preservation of sequence in all the functional framework regions (FWRs; Table 5). A different pattern was observed in the nonfunctional IgM-derived V4-59 sequence from patient PAI (Table 5). In this aberrant gene, there was no clustering of somatic mutations in CDRs indicative of antigen selection, nor was there evidence for conservation of sequence in the FWR.
Distribution of Mutations in the Tumor-Derived VH Sequences
| Patient . | Ig Clone . | GL Donor . | R:Sobs (CDR)* R:Sobs (FWR) . | Rexp (CDR) . | P(CDR)† P (FWR) . |
|---|---|---|---|---|---|
| PAI | IgG/IgM1 | V3-23 | 2.8 (14:5) | 6.0 | <.001 |
| 1.0 (7:7) | <.001 | ||||
| IgM2 | V4-59 | 4.5 (9:2) | 6.0 | .100 | |
| (aberrant) | 10.0 (20:2) | .120 | |||
| BLO | IgG/IgM | V3-7 | 5.0 (10:2) | 4.0 | <.001 |
| 1.0 (4:4) | <.001 | ||||
| BAR | IgG/IgM | V1-46 | ∞ (4:0) | 2.0 | .001 |
| 3.0 (6:2) | .026 | ||||
| SAM | IgG | V3-74 | 4.3 (13:3) | 5.0 | <.001 |
| 1.6 (8:5) | <.001 | ||||
| IgM | V3-74 | 2.8 (11:4) | 5.0 | .002 | |
| 1.0 (6:6) | <.001 | ||||
| LAR | IgG | DP-58 | 12.0 (12:1) | 5.0 | <.001 |
| 1.6 (8:5) | <.001 | ||||
| IgM | DP-58 | 16.0 (16:1) | 5.0 | <.001 | |
| 0.6 (4:7) | <.001 |
| Patient . | Ig Clone . | GL Donor . | R:Sobs (CDR)* R:Sobs (FWR) . | Rexp (CDR) . | P(CDR)† P (FWR) . |
|---|---|---|---|---|---|
| PAI | IgG/IgM1 | V3-23 | 2.8 (14:5) | 6.0 | <.001 |
| 1.0 (7:7) | <.001 | ||||
| IgM2 | V4-59 | 4.5 (9:2) | 6.0 | .100 | |
| (aberrant) | 10.0 (20:2) | .120 | |||
| BLO | IgG/IgM | V3-7 | 5.0 (10:2) | 4.0 | <.001 |
| 1.0 (4:4) | <.001 | ||||
| BAR | IgG/IgM | V1-46 | ∞ (4:0) | 2.0 | .001 |
| 3.0 (6:2) | .026 | ||||
| SAM | IgG | V3-74 | 4.3 (13:3) | 5.0 | <.001 |
| 1.6 (8:5) | <.001 | ||||
| IgM | V3-74 | 2.8 (11:4) | 5.0 | .002 | |
| 1.0 (6:6) | <.001 | ||||
| LAR | IgG | DP-58 | 12.0 (12:1) | 5.0 | <.001 |
| 1.6 (8:5) | <.001 | ||||
| IgM | DP-58 | 16.0 (16:1) | 5.0 | <.001 | |
| 0.6 (4:7) | <.001 |
Abbreviations: GL, germline; CDR, complementary-determining region; FWR, framework region.
R:S is the ratio of replacement (R) to silent (S) mutations observed in CDRs or FWRs, as compared with those expected by chance alone.
Probability of obtaining the number of R mutations by chance alone.P < .05 is considered significant for either excess of R mutations (CDR) or lack of R mutations (FWR).
DISCUSSION
Assessment of the maturational status of B-cell tumors has, until recently, been based largely on morphological and phenotypical features. LPL is considered to arise from a mature B cell, often with cytoplasmic Ig, and commonly associated with a monoclonal serum paraprotein, usually of IgM type.26 Waldenstrom's macroglobulinemia is the most common lymphoma in this category and has been described as a distinct disorder of small lymphocytes showing maturation to plasma cells.26 However, many B-cell tumors, including CLL, also show some maturation to plasmacytoid cells. These observations suggest that B-cell tumors are not completely “frozen” at a point of differentiation.
Analysis of VH and VL genes is allowing further probing of the differentiation status of B-cell tumors. Because the V-gene germ line repertoire is largely mapped,1 it is possible to investigate the degree and pattern of somatic mutations that have occurred in the cell of origin. This process is activated in the germinal center; therefore, accumulation of mutations indicates that the cell has encountered this site.3 However, mutational activity can continue after neoplastic transformation, as seen clearly in follicular lymphoma in which clonally related cells have distinct intraclonal sequence variation.13,14 The distribution of mutations may also indicate a role for antigen selection, and, because antigen may persist,35 it could stimulate growth and differentiation of tumor cells.36 The cases we describe were characterized by circulating lymphoid cells with CLL morphology, but shared histopathologic and immunophenotypical features of LPL; thus, they may correspond rather to “lymphoplasmacytoid” leukemia than to true CLL. They have accumulated somatic mutations as expected from a mature cell, which has evidently passed through the germinal center. In fact, clustering of replacement amino acids in CDR1 and CDR2 is even more obvious than in the closely related splenic lymphoma with villous lymphocytes16 or in follicular lymphoma,13,14Burkitt's lymphoma,15 or myeloma.17,18,30,36 37 This significant clustering provides strong evidence for antigen selection up to and possibly beyond the final neoplastic event.
Isotype switch events in normal B cells appear to occur at about the same time as somatic mutation6 leading commonly, but not invariably, to mutated sequences in IgG+cells.38 A similar heterogeneity in mutational frequency is seen in the rare cases of IgG+ or IgA+CLL.39-41 Interestingly, in some cases of IgG+CLL, transcripts of Cμ and Cα combined to the tumor VH-DH-JH sequence have been observed.42 This suggests either that minor populations of cells with variant isotype switch products exist, or that a single population can generate alternative transcripts by trans-splicing of RNA.43 In one report, the fact that tumor cells expressed dual sIgG and sIgA would support the latter mechanism.40However, the argument for separate populations is supported by detection of a few mutational differences in transcripts in some cases42 and by the demonstration of deletional bi-allelic recombination in three cases of isotype-switched CLL.40
A further surprising finding is that blood lymphocytes from cases of conventional IgM+ CLL contain transcripts of tumor VDJ combined to a variety of alternative isotypes and that cells can be induced to synthesize the switched Ig.20,44,45 Thus, although isotype switching occurs rarely in CLL, transcripts are present. It is not known at present if these are produced by the bulk population or from minor populations that have undergone deletional switch recombination, although again there is some evidence for the latter.45
The cases we describe appeared to be arrested at a more mature point in differentiation. They all had high levels of monoclonal serum IgG, and, in three of five cases, an additional monoclonal IgM. Analysis of the VH genes of these three cases showed transcripts of identical VDJ sequences combined to both Cμ and Cγ. For PAI and BAR, there was no phenotypical evidence for two populations, but this cannot be ruled out. For BLO, only IgM expression was found, suggestive of a separate undetected minor population of isotype-switched cells. The only unequivocal way of confirming isotype heterogeneity among cell populations with identical VH sequences would be to separate out individual tumor cells and perform PCR at the single-cell level, and this is in progress. In the other two cases, transcripts of VDJ-Cμ and VDJ-Cγ were also obtained with CDR3 similarity indicating clear common clonal origin, but there were numerous distinct mutations. For these cases, the IgG+ and IgM+populations must be separate. This is in spite of the finding that the cells appeared to coexpress IgG and IgM, and confirms the dangers of phenotypic analysis of sIg32 particularly in the presence of high levels of serum IgG. All sequences had strong evidence for clustered replacement mutations characteristic of antigen selection, a feature lacking in the aberrant allelic VH gene identified in one of the patients. Therefore, it appears that the transformation event occurred at the level of an IgM+, somatically mutated, antigen-selected B cell, with characteristics of a memory B cell. The transformed cell proliferated giving rise to a neoplastic clone, which, in two cases, secreted IgM. This cell might be analogous to tumor cells of Waldenstrom's macroglobulinemia in which Vκ-genes have been found to be somatically mutated.46 However, in our cases, the cell was also able to undergo isotype switching to IgG, and a second tumor population was able to proliferate and differentiate to high level IgG secretion. In the IgM-secreting tumors, if the IgM and IgG are produced from different cells, the mutation mechanism must have been silenced before switching. In tumors not secreting IgM, mutation continued, possibly in both isotypes. This continuing mutational activity led to mutational divergence, with accumulation of some mutations in the IgM-derived sequences that were not found in the IgG-derived sequences. Curiously, no intraclonal variation was detectable, indicating that the neoplastic cells represent progeny of only single IgG and IgM variants. It also suggests that the current IgM+ population has lost the ability to switch. These tumors appear to have undergone neoplastic transformation at the pivotal point of isotype switch from IgM to IgG. They may in fact represent the bifurcation leading to memory cells or plasma cells,47 and the IgM+ cells could relate to the precursor cell identified in some cases of myeloma.24 25Therefore, immunogenetics has supported morphology in showing that B-cell tumors can respond to differentiation signals in the same way as normal B cells and has shown that the outcome can be two clonally related tumor populations.
Supported by the Leukaemia Research Fund, UK, and the European Myeloma Research Network (Biomed BMH1-CT93-1407).
Address reprint requests to Freda K. Stevenson, PhD, Molecular Immunology Group, Tenovus Laboratory, Southampton University Hospitals, Tremona Road, Southampton, SO16 6YD, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal