Abstract
Activation of Janus tyrosine kinases (Jak) and Signal transducers and activators of transcription (Stat) after ligation of major histocompatibility complex class I (MHC-I) was explored in Jurkat T cells. Cross-linking of MHC-I mediated tyrosine phosphorylation of Tyk2, but not Jak1, Jak2, and Jak3. In addition, the transcription factor Stat-3 was tyrosine phosphorylated in the cytoplasma and subsequently translocated to the cell nucleus. Data obtained by electrophoretic mobility shift assay suggested that the activated Stat-3 protein associates with the human serum-inducible element (hSIE) DNA-probe derived from the interferon-γ activated site (GAS) in the c-fos promoter, a common DNA sequence for Stat protein binding. An association between hSIE and Stat-3 after MHC-I ligation was directly demonstrated by precipitating Stat-3 from nuclear extracts with biotinylated hSIE probe and avidin-coupled agarose. To investigate the function of the activated Stat-3, Jurkat T cells were transiently transfected with a Stat-3 isoform lacking the transactivating domain. This dominant-negative acting Stat-3 isoform significantly inhibited apoptosis induced by ligation of MHC-I. In conclusion, our data suggest the involvement of the Jak/Stat signal pathway in MHC-I–induced signal transduction in T cells.
THE MAJOR histocompatibility complex class I (MHC-I) complex is known for its capacity to present peptide derived from intracellular proteins to cytotoxic T lymphocytes.1 Recently, there has been a growing interest in defining the intracellular signals and their consequences for cell activation after ligation of MHC-I molecules. Thus, it is now well established that ligation of MHC-I molecules expressed on T lymphocytes are critically involved in regulation of T-lymphocyte activation.2-6 In an effort to clarify the intracellular signal pathway(s) that operates after MHC-I ligation, we have previously shown that MHC-I cross-linking of human T lymphocytes induces tyrosine kinase and PLC-γ1 activity.7 It has been demonstrated by several groups that surface expression of the TCR/CD3 molecule is essential for MHC-I–induced signal transduction.7-9 In line with this, we have recently shown that the MHC-I molecule directly uses parts of the TCR/CD3 signal transduction machinery for signal transduction, but that MHC-I cross-linking leads to an alternative TCR/CD3 ζ-chain phosphorylation resulting in an altered activation of the ZAP70 tyrosine kinase and induction of apoptosis.10 Deletion of all but the four proximal amino acids from the intracellular domain of the MHC-I molecule does not alter its signal transduction capabilities,11,12 strongly suggesting that ligated MHC-I molecules, to transmit a signal, may associate with other signal transducing transmembrane molecules. Interestingly, it has been demonstrated that MHC-I molecules can associate with receptores for interleukin-2 (IL-2) and IL-4 as well as insulin and glucagon receptors.13-17
Ligand binding of many different cytokine receptors induces Jak tyrosine kinase activity and Jak's become noncovalently associated with the actual cytokine receptors. The Jak kinase family consists of Tyk2, Jak1, Jak2, and Jak3, which are characterized by the presence of two C-terminal kinase-related domains and the absence of SH2 domains.18,19 The Stat proteins are key substrates for the Jak kinases. Stat proteins are SH2-containing cytoplasmic proteins, which associate with the ligand activated cytokine-receptor/Jak kinase complex via the SH2 domain of the Stat proteins.19 Upon tyrosine phosphorylation, Stat proteins are released from the complex and form SH2 domain mediated homodimeric or heterodimeric complexes.19,20 These complexes are then translocated to the cell nucleus, where they regulate gene transcription through interaction with specific DNA sequences, most of which are related to the interferon-γ (IFN-γ) activated site (GAS), a regulatory element in the IFN-γ inducible genes.21 Presently, six members of the Stat family are identified and some of these exist in different isoforms.22 Each Stat protein functions in specific cytokine-receptor signal pathways. Stat-3, for example, is activated by various receptors such as the IL-2 receptor,23 IL-3 receptor,24 IL-5 receptor,25 IL-6 receptor,26 IL-9 receptor,27 IL-10 receptor,28 colony-stimulating factor-1,29epidermal growth factor (EGF) receptor,30,31and the thrombopoitin receptor.32 Evidence suggests that the specificity of Stat phosphorylation is not due to the specificity of Jak activation. Rather, it is hypothesized that the receptor complex determines which Stat proteins will be accessible to phosphorylation.19 The aim of the current study was to investigate the involvement of the Jak/Stat signal pathway after MHC-I ligation of human Jurkat T cells.
MATERIALS AND METHODS
Antibodies (Abs) and reagents.
Purified antihuman β2 microglobulin (anti-β2m) Ab from rabbit serum (DAKO A072; DAKO, Roskilde, Denmark). Purified control Ig from rabbit serum (DAKO X903). F101.01 monoclonal antibody (MoAb), which recognizes a conformational determinant on TCR33 was used as an ascites dilution. We used anti-CD3 (UCHT1) MoAb (DAKO M835); antiphosphotyrosine MoAb, IgG2b (UBI #05-321); purified antihuman Tyk2 from rabbit serum (UBI #06-275); purified antihuman Jak1 from rabbit serum (UBI #06-272); purified antihuman Jak2 from rabbit serum (UBI #06-255); purified antihuman Jak3 from rabbit serum (UBI #06-428); antihuman Stat1, MoAb, IgG2b (Affinity #S21120; Mamhead, Exeter, UK); antihuman Stat2, MoAb, IgG2a (Affinity #S21220); antihuman Stat-3, MoAb, IgG1 (Affinity #S21320); antihuman Stat4, MoAb, IgG1 (Affinity #S21420); antihuman Stat5, MoAb, IgG2b (Affinity #S21520); antihuman Stat6, MoAb, IgG2b (Affinity #S25420); antihuman ISGF3γ, MoAb, IgG1 (Affinity #129320); peroxidase-conjugated antimouse Ig from rabbit serum (DAKO P260); and purified peroxidase-conjugated antirabbit Ig from swine serum (DAKO Z196). Abs used for cell exposure were dialyzed against phosphate-buffered saline (PBS) before use and used at the indicated concentrations. Abs for immunoprecipitation were used according to the manufacturer's description. Biotin-conjugated Abs were prepared by reacting Ab with biotinsuccinimide (Sigma B-2643; Sigma, St Louis, MO), as described in Odum et al.34 Avidin (Sigma A9275) was used to cross-link biotin-conjugated Ab: natrium-orthovanadate (Na3VO4; Sigma S6508); protein A sepharose CL-4B (Pharmacia, Uppsala, Sweden); avidin-coupled agarose (KemEnTec, Copenhagen, Denmark); Ripa buffer (10 mmol/L Tris/HCl, pH 7.5, 1% NP-40, 0.25% deoxycholate wt/vol, 2 mmol/L EDTA, 10 mmol/L orthovanadate); lysis buffer for nuclear and cytoplasmic purification (10 mmol/L Tris/HCl, pH 7.5, 0.5% Triton X-100, 2 mmol/L EDTA, 10 mmol/L orthovanadate); buffer for nuclear purification (10 mmol/L KH2PO4, pH 6.8, 2.2 mol/L sucrose, 1 mmol/L MgCl2, 150 mmol/L NaCl, 10 mmol/L orthovanadate); buffer for cytoplasmic purification (10 mmol/L Tris/HCl, pH 7.5, 0.25 mol/L sucrose, 1 mmol/L MgCl2, 150 mmol/L NaCl, 10 mmol/L orthovanadate); electrophoretic mobility shift assay (EMSA) lysis buffer I (40 mmol/L Tris/HCl, pH 7.0, 2 mmol/L EGTA, 5 mmol/L MgCl2, 1 mmol/L phenylmethylsufonyl fluoride [PMSF], 0.1 mmol/L Na3VO4, 10 mmol/L NaF, 0.1 mmol/L NH4Molybdate, 10 μmol/L Pepstatin, 10 mmol/L β-glycerophosphate, 5 mmol/L dithiothreitol [DTT], 15 mmol/L p-nitrophenylphosphate, 10 μmol/L Leupeptin); and EMSA lysis buffer II (lysis buffer I supplemented with 50 mmol/L KCl, 300 mmol/L NaCl, 1.5% Ficoll). All buffers, except EMSA lysis buffers, were supplemented with a protease inhibitor cocktail according to the manufactures description (Boehringer Mannheim Gmbh #1697498; Boehringer Mannheim, Mannheim, Germany).
Cells.
Jurkat cell J76.25 was kindly provided by Dr C. Geisler (Institute for Medical Microbiology and Immunology, University of Copenhagen, Copenhagen, Denmark). Cells were grown in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS), fresh L-glutamine, and antibiotics. Cells were continuously tested to be mycoplasma free.
Cell stimulation.
Cells were preincubated with saturating amounts of biotinylated anti-β2m Ab or biotinylated control rabbit Ig (both 1:10 of batch concentration) using 10 μL antibody/106 cells in a final volume of 100 μL for 10 minutes at room temperature. Subsequently, cells were washed in PBS (37°C) and cross-linked with avidin (20 μg/106 cells) or UCHT-1 Ab (2.5 μL/106 cells) in a final volume of 100 μL at 37°C for various periods of time.
Immunoprecipitation.
Cells (3 × 107) were treated as described above, and the pellet was lysed in 1 mL ripa buffer and precleared several times with sepharose-coupled protein A (50% wt/vol slurry). Proteins were immunoprecipitated with saturating amounts of Ab and 30 μL sepharose-coupled protein A (50% wt/vol slurry). Immunoprecipitated proteins were washed, subjected to sodium dodecyl sulfate (SDS) polyacrylamide electrophoresis, and immunoblotted as described under Western blots.
Purification of nuclear and cytoplasmic cell fractions.
Cells (3 × 107) were treated as described above and the pellet was lysed in 2 mL lysis buffer for 30 minutes at 4°C. The nuclear and the cytoplasmic cell fraction were prepared exactly as described in Busch.35
Western blots.
Supernatants from the various cell fractions described above were electrophoresed on SDS-polyacrylamide gel and blotted on to a Hybond-ECL nitrocellulose membrane (Amersham #RPN 2020D; Amersham, Allerod, Denmark). The immunoblot was incubated with primary Ab according to the manufactures instruction for 2 hours, followed by incubation with 1/1,000 peroxidase-conjugated antimouse or antirabbit Ig for 1 hour, washed, and developed by ECL (Amersham #RPN 2106) using the manufacturer's instructions.
EMSA analysis.
Cells (107) were treated as described above and the cell membrane was lysed in EMSA lysis buffer I for 30 minutes at 4°C. The nuclear fraction was pelleted and lysed in EMSA lysis buffer II for 30 minutes at 4°C. The protein concentration in the lysate was determined using a bradford assay. The M67 hSIE oligo (5′-TGCAGTCGACATTTCCCGTAAATCGTCGA-3′, 3′-CAGCTGTAAAGGGCATTTAGCAGCTACGT-5′) was labeled with α32P-ATP and incubated with 10 μg of the lysate for 15 minutes at 4°C. Protein/DNA complexes were separated on a nondenaturing 6% polyacrylamide gel with 0.5 × TBE. Unlabeled competitor DNA (50-fold excess) or blocking Ab (1 μL) was incubated with the lysate 15 minutes before mixing with the labeled M67 probe.
Alternatively, proteins were precipitated from the nuclear extract (EMSA lysis buffer II extract) with biotinylated M67 probe and 50 μL avidin-coupled agarose. The precipitate was washed three times with PBS and subjected to anti–Stat-3 Western blotting as described above.
Cell transfection.
Cells (3 × 106) were transiently transfected with a Psg513 vector containing the sequence for Stat-3α or Stat-3β. The constructs were kindly provided by Dr E. Caldenhoven and are described in detail previously.25,36 Transfections were made using 10 μg of DNA and 10 μL LipofectAMINE (Life Technologia, Roskilde, Denmark), but otherwise in accordance with the manufacturer's instructions. Cells were grown for 40 hours and subsequently assayed for apoptosis and Stat-3 expression. Transfection efficiency was measured by transfecting cells with a CD20 construct. The construct was kindly provided by Dr K. Helin and Dr E. Harlow (The Cancer Center of Massachusetts General Hospital, Boston, MA). CD20 is a membrane protein that is exclusively expressed on B-cells and follicular dendritic cells.37 Cells were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD20 antibody (DAKO; F799) and the percentage of CD20+ cells was measured by flow cytometry using a FACScan (Becton Dickinson, Mountain View, CA).
Apoptosis analysis.
Apoptosis was in principle measured as described previously.6 10 Cells (106) were stimulated as described above. After 30 minutes of stimulation at 37°C, the cells were resuspended in RPMI 1640 supplemented with 10% heat-inactivated FCS (106 cells/mL) and cultured for 6 hours at 37°C. At the end of the culture period, the cells were pelleted, washed once in 2 mL 0.03% saponin (Sigma #S7900) in PBS, and reacted with 1 mL 0.4 μg/mL 7-aminoactinomycin D (7-AAD; Sigma #A9400) in 0.03% saponin for 25 minutes at room temperature in the dark. The samples were analyzed immediately by flow cytometry in a FACScan (Becton Dickinson) using a logarithmic fluorescence scale. The apoptosis data are presented as the percentage of sub-G1staining where the relevant background staining is subtracted.
RESULTS
MHC-I ligation induces tyrosine phosphorylation of Tyk2, but not Jak1-3.
In an attempt to define intracellular signal transduction pathways responsible for alterations in the proliferation kinetics after ligation of MHC-I, we focused on the involvement of the Jak/Stat pathway, which recently was documented to influence cell cycle kinetics.38
Lysates from MHC-I–ligated Jurkat T cells were immunoprecipitated with specific Abs against the tyrosine kinases Tyk2, Jak1, Jak2, and Jak3. The tyrosine phosphorylation of the different kinases was examined as a marker for their activation. Figure 1 shows that a small increment of Tyk2 phosphorylation was detected after 2 minutes of MHC-I cross-linking (Fig 1, lane 2), and maximal phosphorylation was seen after 5 minutes of stimulation (Fig 1, lane 3). In contrast, TCR/CD3 cross-linking did not induce significant tyrosine phosphorylation of the Tyk2 kinase (Fig 1, lane 5). The time-dependent phosphorylations of the Tyk2 kinase were not a result of sequestering or degradation of the Tyk2, because similar amounts of Tyk2 were immunoprecipitated (Fig 1, lower part). The inability of TCR/CD3 to induce Tyk2 phosphorylation was not due to suboptimal TCR/CD3 stimulation, because both MHC-I and TCR/CD3 antibodies were titrated and used at saturating conditions (data not shown). Furthermore, we have previously shown that the level of tyrosine phosphorylated proteins is higher in Jurkat T cells after TCR/CD3 ligation compared with MHC-I ligation7 using antibody concentrations similar to those in Fig 1. We were not able to observe any detectable tyrosine phosphorylation of Jak1, Jak2, or Jak3 after MHC-I ligation (data not shown).
Immunoprecipitates of Tyk2 obtained from lysates of Jurkat 76.25 cells after MHC-I or TCR/CD3 ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 through 4) before exposure to avidin for the indicated time (lanes 1 through 4) or exposed to anti-TCR/CD3 Abs (lane 5). Precipitates were immunoblotted with antiphosphotyrosine Ab (upper panel). Blots were stripped and reprobed with anti-Tyk2 Ab (lower panel).
Immunoprecipitates of Tyk2 obtained from lysates of Jurkat 76.25 cells after MHC-I or TCR/CD3 ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 through 4) before exposure to avidin for the indicated time (lanes 1 through 4) or exposed to anti-TCR/CD3 Abs (lane 5). Precipitates were immunoblotted with antiphosphotyrosine Ab (upper panel). Blots were stripped and reprobed with anti-Tyk2 Ab (lower panel).
MHC-I ligation induces tyrosine phosphorylation of Stat-3.
Because the activation of the Tyk2 kinase has been shown to be implicated in the tyrosine phosphorylation of Stat proteins, we investigated whether MHC-I cross-linking induced tyrosine phosphorylation of Stat1-6. Figure 2 shows immunoprecipitates of the 92-kD Stat-3 protein immunoblotted with antiphosphotyrosine antibody; a strong tyrosine phosphorylation of Stat-3 was observed after 5 minutes of MHC-I ligation (Fig 2, lane 2). Despite a pronounced effect of TCR/CD3 ligation on the overall level of tyrosine phosphorylation,7 no specific phosphorylation of immunopurified Stat-3 was observed after TCR/CD3 ligation (Fig 2, lane 3). The results were not due to sequestering or degradation of the Stat-3 protein, because similar amounts of Stat-3 were immunoprecipitated in all experiments (Fig 2, lower part). Ligation of MHC-I or TCR/CD3 did not induce detectable tyrosine phosphorylation of Stat1, Stat2, Stat4, Stat5, or Stat6 (data not shown).
Immunoprecipitates of Stat-3 obtained from lysates of Jurkat 76.25 cells after MHC-I or TCR/CD3 ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 4) before exposure to avidin for 5 minutes (lanes 1, 2, and 4) and/or exposed to anti-TCR/CD3 Abs for 5 minutes (lanes 3 and 4). Precipitates were immunoblotted with antiphosphotyrosine Ab (upper panel). Blots were stripped and reprobed with anti–Stat-3 Ab (lower panel).
Immunoprecipitates of Stat-3 obtained from lysates of Jurkat 76.25 cells after MHC-I or TCR/CD3 ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 4) before exposure to avidin for 5 minutes (lanes 1, 2, and 4) and/or exposed to anti-TCR/CD3 Abs for 5 minutes (lanes 3 and 4). Precipitates were immunoblotted with antiphosphotyrosine Ab (upper panel). Blots were stripped and reprobed with anti–Stat-3 Ab (lower panel).
MHC-I ligation induces nuclear translocation of tyrosine-phosphorylated Stat-3.
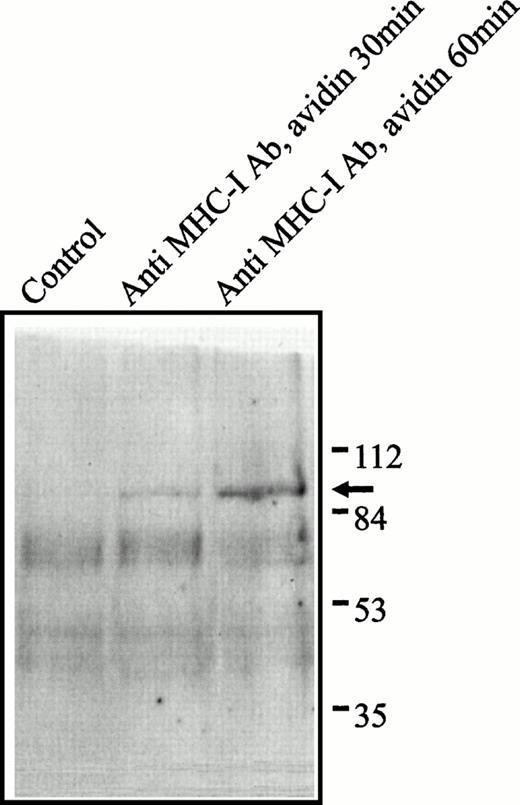
Tyrosine-phosphorylated Stat proteins are known to translocate to the nucleus, where they function as DNA-binding transcription factors.22 To determine whether tyrosine-phosphorylated proteins were translocated to the nucleus after MHC-I cross-linking, we prepared a highly purified nuclear fraction from Jurkat T cells. Figure3 shows an antiphosphotyrosine immunoblot of the nuclear fraction after MHC-I ligation. In contrast to the fast induction of several tyrosine-phosphorylated proteins in whole cell lysats after MHC-I ligation,7 only one single phosphotyrosine protein at 92 kD was present in the nucleus (weakly after 30 minutes of MHC-I ligation and more pronounced after 60 minutes stimulation; Fig 3). This suggests that the 92-kD protein is tyrosine phosphorylated in the cytoplasma and then subsequently translocated to the nucleus.
Phosphotyrosine blot of the nuclear fraction of Jurkat 76.25 cells after MHC-I ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 3) before exposure to avidin for the indicated time. The separated proteins were immunoblotted with antiphosphotyrosine Ab.
Phosphotyrosine blot of the nuclear fraction of Jurkat 76.25 cells after MHC-I ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 3) before exposure to avidin for the indicated time. The separated proteins were immunoblotted with antiphosphotyrosine Ab.
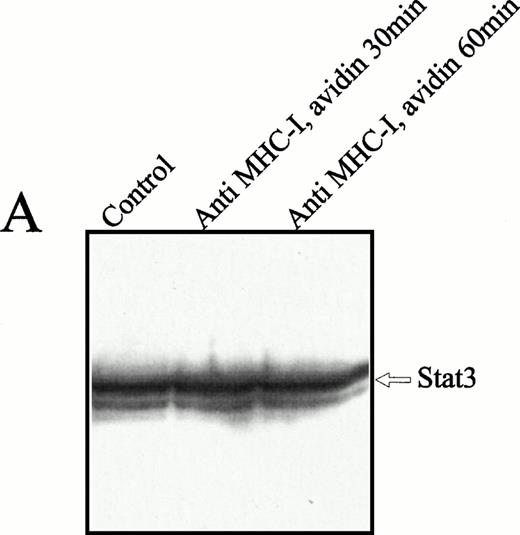
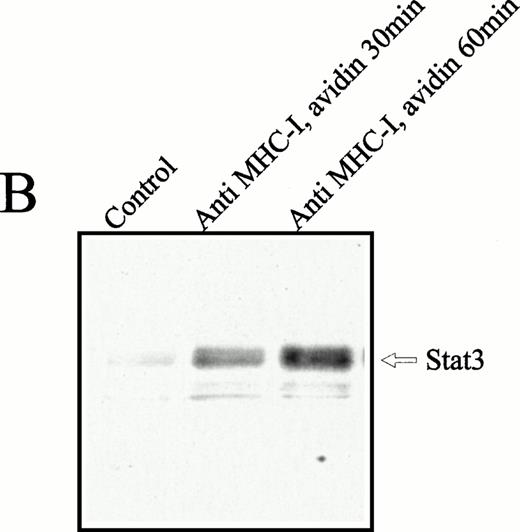
To examine whether the 92-kD nuclear translocated protein was identical to the Stat-3 protein, a study with comparable kinetics, as described above, was performed with anti–Stat-3 antibodies. The Stat-3 protein was present at high concentrations in the cytoplasma (Fig4A), regardless of cell activation. Ligation of MHC-I molecules induced a substantial increase in the nuclear Stat-3 concentration after 30 and 60 minutes of MHC-I ligation (Fig 4B). The weak bands under Stat-3 are impurities of the nuclear fraction that are not observed consistently. Similar studies were made with Stat1, 2, 4, 5, and 6, but none of these proteins was detected in the cell nucleus after MHC-I ligation (data not shown). From these results we conclude that MHC-I ligation induces tyrosine phosphorylation of cytoplasmic Stat-3 that subsequently translocated to the cell nucleus.
Anti–Stat-3 immunoblot of the cytoplasmic and nuclear fraction of Jurkat 76.25 cells after MHC-I ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 3) before exposure to avidin for the indicated time. Cells were separated in a cytoplasmic (A) or nuclear (B) fraction as described in the Materials and Methods. The separated fractions were immunoblotted with anti–Stat-3 Ab.
Anti–Stat-3 immunoblot of the cytoplasmic and nuclear fraction of Jurkat 76.25 cells after MHC-I ligation. Cells (3 × 107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 and 3) before exposure to avidin for the indicated time. Cells were separated in a cytoplasmic (A) or nuclear (B) fraction as described in the Materials and Methods. The separated fractions were immunoblotted with anti–Stat-3 Ab.
MHC-I–induced Stat-3 binds to specific DNA sequences.
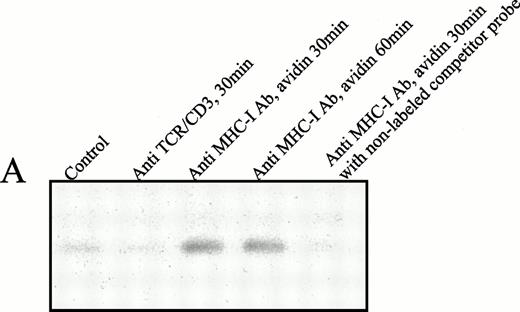
To examine whether the nuclear translocated tyrosine-phosphorylated Stat-3 protein was able directly to associate with DNA, an EMSA analysis was performed using the human serum-inducible element (hSIE) probe M67. The hSIE DNA-probe has been shown to associate with several Stat proteins, including Stat-3.30Figure 5A shows that nuclear proteins from Jurkat T cells 30 minutes after ligation of their MHC-I molecules contained proteins with affinity for the hSIE DNA probe (Fig 5A, lanes 3 and 4). The association was highly specific, because the association with the labeled probe was completely inhibited by a 50-fold excess of unlabeled probe (Fig 5A, lane 5). In contrast, ligation of the TCR/CD3 complex did not induce shifts in the mobility of the hSIE probe.
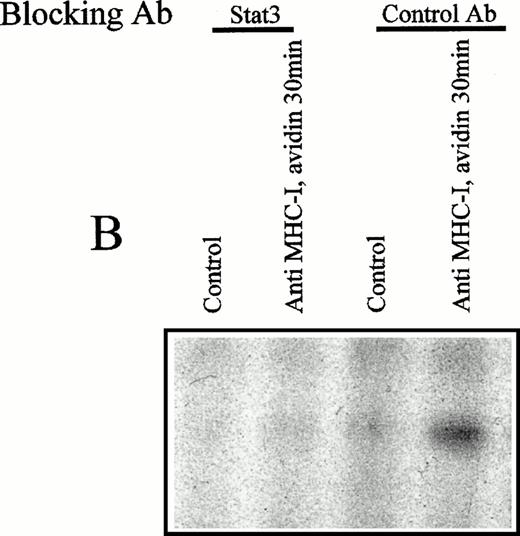
(A) EMSA analysis of nuclear proteins bound to the M67 hSIE DNA probe after MHC-I or TCR/CD3 ligation. Cells (107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 3 through 5) before exposure to avidin for the indicated time (lanes 1 and 3 through 5) or exposed to anti-TCR/CD3 Ab for 30 minutes (lane 2). Nuclear extracts were subjected to EMSA analysis as described in the Materials and Methods. The shifted DNA was blocked with 50-fold excess nonlabeled DNA (lane 5). (B) The EMSA analysis was performed as described under (A), but the nuclear extract was preincubated for 15 minutes with antibodies before the addition of the labeled M67 probe. Experiments shown in lanes 1 and 2 were preincubated with anti–Stat-3 antibody and those shown in lanes 3 and 4 with isotype control antibody (anti–ISGF-3γ). (C) Precipitation of Stat-3 from the nuclear extract. EMSA nuclear extract was made as described under (A) and precipitated with a biotinylated M67 probe and avidin-coupled agarose as described in the Materials and Methods. Cells (107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 through 4) before exposure to avidin for the indicated time (lanes 1 through 4). The precipitate were immunoblotted with anti–Stat-3 Ab.
(A) EMSA analysis of nuclear proteins bound to the M67 hSIE DNA probe after MHC-I or TCR/CD3 ligation. Cells (107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 3 through 5) before exposure to avidin for the indicated time (lanes 1 and 3 through 5) or exposed to anti-TCR/CD3 Ab for 30 minutes (lane 2). Nuclear extracts were subjected to EMSA analysis as described in the Materials and Methods. The shifted DNA was blocked with 50-fold excess nonlabeled DNA (lane 5). (B) The EMSA analysis was performed as described under (A), but the nuclear extract was preincubated for 15 minutes with antibodies before the addition of the labeled M67 probe. Experiments shown in lanes 1 and 2 were preincubated with anti–Stat-3 antibody and those shown in lanes 3 and 4 with isotype control antibody (anti–ISGF-3γ). (C) Precipitation of Stat-3 from the nuclear extract. EMSA nuclear extract was made as described under (A) and precipitated with a biotinylated M67 probe and avidin-coupled agarose as described in the Materials and Methods. Cells (107) were preincubated with PBS (lane 1) or saturating amounts of anti-β2M Ab (lanes 2 through 4) before exposure to avidin for the indicated time (lanes 1 through 4). The precipitate were immunoblotted with anti–Stat-3 Ab.
To verify that the shifted complex contained the Stat-3 molecule, an inhibition experiment was performed. Figure 5B shows an EMSA analysis in which the nuclear extract was treated with anti–Stat-3 or isotype control antibodies before incubation with the labeled M67 probe. No supershift was observed under these conditions, but the shifted band disappeared after treatment with the anti–Stat-3 antibody (Fig 5B, lane 2), implying that the anti–Stat-3 antibody and the M67 probe compete for binding to the same or nearby epitopes on the Stat-3 protein. To show that Stat-3 directly associates with hSIE in nuclear extracts from MHC-I ligated cells, Stat-3 was precipitated from the nuclear extract with biotinylated M67 probe and avidin coupled agarose and subsequently immunoblotted with anti–Stat-3 antibodies. Figure 5C shows that the Stat-3 protein in nuclear extracts from MHC-I–ligated cells interact with the M67 probe (Fig 5C, lanes 2 through 4). These results show that the MHC-I–induced nuclear Stat-3 protein can associate with the common Stat binding DNA sequence hSIE from the c-fos promoter.
Overexpression of Stat-3β inhibits MHC-I–induced apoptosis.
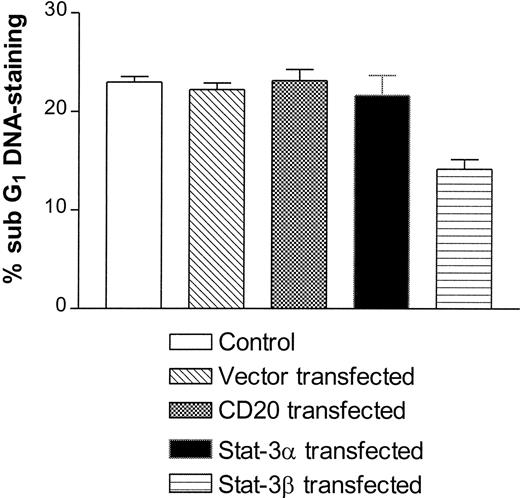
To assess the physiological function of Stat-3 activation after MHC-I ligation, Jurkat T cells were transfected with a normal Stat-3 (Stat-3α) or a Stat-3β construct. Stat-3β is an isoform that lacks the transactivating domain, and overexpression dominantly inhibits Stat-3–induced transcriptional activation.36 Apoptotic cells have condensed DNA, which leads to a lower stainability with 7-AAD.39 Apoptotic cells are therefore routinely measured by flow cytometry as the percentage staining below the G1DNA peak, where the relevant background staining is subtracted (percentage of sub-G1 staining). The results in Fig 6 show that transfection with Stat-3β significantly inhibits MHC-I–induced apoptosis of Jurkat T cells, as opposed to cells transfected with Stat-3α or a control CD20 construct. These results suggest that Stat-3 activation after MHC-I ligation is involved in the subsequent induction of apoptosis. Because Stat-3 is constitutively present in the cells, it is not possible to measure the transient transfection efficiency. To deduce the transfection efficiency, a CD20 construct was transiently transfected in parallel into Jurkat T cells using the same transfection procedures as with the Stat-3 constructs. The percentage of CD20+ cells was measured by flow cytometry. Transfection efficiency ranged from 35% to 45% (data not shown). MTT and proliferation analyses showed no difference in the viability or proliferation of Jurkat T cells transfected with either control or Stat-3α or Stat-3β constructs (data not shown), implying that Stat-3 transfection does not interfere with the endogenous proliferation or viability of Jurkat T cells.
Apoptosis measurement of MHC-I ligated Jurkat T cells. Cells were either untransfected or transiently transfected with the empty vector, CD20, Stat-3α, or Stat-3β. Cells were transfected as described in the Materials and Methods. Subsequently, apoptosis was measured as described in the Materials and Methods. The results are shown as the percentage of sub-G1 staining where the relevant background values are subtracted.
Apoptosis measurement of MHC-I ligated Jurkat T cells. Cells were either untransfected or transiently transfected with the empty vector, CD20, Stat-3α, or Stat-3β. Cells were transfected as described in the Materials and Methods. Subsequently, apoptosis was measured as described in the Materials and Methods. The results are shown as the percentage of sub-G1 staining where the relevant background values are subtracted.
DISCUSSION
MHC-I–induced signaling in T cells may lead to a number of functional alterations, including apoptosis, alteration of cell proliferation, cytokine secretion, and new surface phenotypes.2 5 To further clarify distinct signal pathways operating after MHC-I ligation, the engagement of the Jak/Stat signal pathway in MHC-I–induced signaling in human T cells was investigated in the present work.
We provide here evidence that MHC-I ligation of human T cells induces tyrosine phosphorylation of the Tyk2 tyrosine kinase. In contrast, none of the other Jak family tyrosine kinases (Jak1, Jak2, or Jak3) was activated after MHC-I ligation. Immunoprecipitation of the Stat-family proteins Stat-1 through Stat-6 showed that the 92-kD Stat-3 protein was tyrosine phosphorylated in response to MHC-I ligation, whereas no detectable tyrosine phosphorylation of Stat1, Stat2, Stat4, Stat5, and Stat6 was observed. In accordance with a previous study by Beadling et al,40 we did not observe activation of proteins from the Jak and Stat families after TCR/CD3 ligation.
Tyrosine-phosphorylated Stat proteins make homodimers and/or heterodimers in the cytoplasm and are subsequently translocated to the cell nucleus by a currently unknown mechanism.20,22 To elucidate whether tyrosine-phosphorylated proteins were translocated to the nucleus upon MHC-I ligation, a highly purified nuclear fraction was obtained from Jurkat T-cell lysates. In the nuclear fraction, a 92-kD tyrosine-phosphorylated protein was observed after 30 minutes of MHC-I ligation that became more intensively phosphorylated after 60 minutes of stimulation. Because the induction of tyrosine-phosphorylated proteins in the cytolasma is observed within 5 seconds,7this suggests that the protein was tyrosine phosphorylated in the cytoplasma and subsequently translocated to the cell nucleus.
Studies were performed to elucidate whether the 92-kD protein was identical to the Stat-3 protein. Immunoblotting of a purified nuclear lysats with anti–Stat-3 antibodies showed that the Stat-3 protein was indeed observed in the nucleus after 60 minutes of MHC-I ligation. Together, these results strongly suggest that MHC-I ligation of T cells induces tyrosine phosphorylation and subsequent nuclear translocation of the Stat-3 transcription factor.
We tested whether nuclear supernatant from MHC-I–stimulated cells could associate with the hSIE DNA probe derived from the c-fos promoter, a DNA sequence known to associate with various activated Stat proteins, including Stat-3.30 In a gel-shift assay (EMSA), nuclear supernatant from MHC-I–ligated Jurkat T cells induced a clear shift in the mobility of the hSIE probe, indicating an association between the hSIE DNA probe and one or more of the proteins in the nuclear supernatant. Furthermore, the shifted complex was absent in nuclear extracts preincubated with anti–Stat-3 antibodies, and the Stat-3 protein could be coprecipitated from nuclear cell extracts of Jurkat T cells with a biotinylated hSIE probe and avidin-coupled agarose. Together, these results demonstrate that the Stat-3 protein from MHC-I–ligated cells specifically interacts with DNA representing a common Stat binding sequence.
To investigate the functional consequences of MHC-I–induced Stat-3 activation, Jurkat T cells were transfected with constructs encoding Stat-3α or Stat-3β. When cells were transiently transfected with the nontransactivating isoform Stat-3β, a significant reduction in the MHC-I–induced apoptosis of Jurkat T cells was observed. Because of technical limitations, we were only able to transiently transfect about 40% of the cells; therefore, it is hard to judge whether the dominant negatively acting Stat-3β totally abolish apoptosis after MHC-I ligation or whether other signals participate. Nonetheless, the data suggest that activated Stat-3 is involved at least in a significant part of the intracellular signals leading to apoptosis after MHC-I ligation. We tried to produce Jurkat T cells stabily transfected with the Stat-3β construct, but, unfortunately, the attempts were not successful.
Of particular interest, we have recently found that JNK activity is critically involved in MHC-I–induced apoptosis.6 Thus, in future studies, it will be of interest to elucidate the cooperation between JNK and Stat-3 in MHC-I–induced apoptosis.
It is well established that the cytoplasmic domain of the MHC-I molecule is not essential for MHC-I–induced signal transduction11,12; therefore, it is highly likely that the MHC-I molecule associates with and uses the signal transduction devices of other transmembrane molecules. The MHC-I molecules have been shown to be either physically or functionally coupled to other signal transducing proteins.2 This report presents evidence that MHC-I ligation mediates signals through the Jak/Stat signal pathway. It is therefore tempting to suggest that MHC-I molecules either directly or indirectly associate with receptor(s) for growth regulatory cytokine(s). An alternative explanation is that the MHC-I signal induces secretion of intracellular stores of cytokines to the media, which then mediate the activation of the TYK2 kinase and the Stat-3 protein through their respective receptors. However, this scenario is highly unlikely, because fresh Jurkat T cells exposed to antibody-depleted culture supernatants from 2 to 10 minutes with MHC-I–ligated cells did not show any tyrosine phosphorylation of Stat-3 (data not included).
The MHC molecules associate both directly and functionally with the IL-2 and IL-4 receptors16,17,41; therefore, these receptors could be likely candidates through which the MHC-I molecule activates TYK2 and Stat-3. To our knowledge, no single receptor has been shown to exclusively phosphorylate TYK2 and Stat-3. This suggests that the MHC-I molecules do not uncritically use cytokine receptors and mediate the same signal as these; teleologically, this would also be very inappropriate in a complex biological system. Rather, it is likely that the MHC-I molecule by ligation induces specific changes in the receptor to which it associates and thereby induces a specific signal.
The precise physiological function of Stat proteins is currently not well understood. One or more Stat proteins are activated in response to almost all known cytokines. This suggests that Stat proteins are involved in various physiological signals, as diverse as signals leading to growth progression through cell cycle from G1 to G2 to signals leading to growth inhibition and activation-induced cell death. Of particular interest, Toshio Hirano's group has recently demonstrated that mutation of the Stat-3 binding domain in the cytoplasmic part of the gp130 chain of the IL-6 receptor abolished IL-6–induced growth arrest.42 Subsequently, the same group showed that dominant-negative forms of Stat-3 inhibited IL-6–induced growth arrest at G0/G1.43 These observations are in accordance with the observations on Jurkat T cells described in this report and our recent data showing that MHC-I ligation induces a particular anergic-like phenotype of the ζ-chain and ZAP70 tyrosine kinase,10 reduces colony formation in semisolid culture,44 and is a key inducer of growth arrest and apoptosis.4,6,10,45-47 We have previously shown that staphylococcal enterotoxins exert a profound inhibitory effect on IL-2–induced Jak3 and Stat protein activation,38 a process dependent on MHC class II binding by the staphylococcal enterotoxin (N.Ø., unpublished observation).
Even though there has been an enormous interest in the Jak/Stat signal pathway, relatively little is known about its biological function. Our hypothesis is that the MHC-I molecule uses other transmembrane molecules for signal transduction, which may induce both MHC-I–specific signals and modulation of cytokine receptor signaling. The nature of this signal molecule(s) and the extent of its modulation remain to be clarified.
ACKNOWLEDGMENT
The authors thank Dr Erik Caldenhoven (University Hospital Utrecht, Utrecht, The Netherlands) for providing the Stat-3 constructs and Tania Palm and Mette Jeppesen for excellent technical assistance.
Supported by the Danish Medical Research Council, the Novo Nordic Foundation, the Beckett Foundation, the Lundbeck Foundation, the Leo Research Foundation, and the Alfred Benzon Foundation. N.Ø. is supported by the Danish Allergy Research Center.
Address reprint requests to Søren Skov, MSc, Cell Cybernetics Laboratory, Institute of Medical Microbiology and Immunology, The Panum Institute, University of Copenhagen, Blegdamsvej 3, Bldg 22-5, 2200 Copenhagen N, Denmark; e-mail:s.skov@sb.immi.ku.dk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal