Abstract
AL (amyloid light-chain) amyloidosis is an uncommon plasma cell disorder in which depositions of amyloid light-chain protein cause progressive organ failure and death in a median of 13 months. Autologous stem-cell transplantation is effective therapy for multiple myeloma and therefore, we evaluated its efficacy for AL amyloidosis. Patients with adequate cardiac, pulmonary, and renal function had stem cells mobilized with granulocyte-colony stimulating factor and were treated with dose-intensive intravenous melphalan (200 mg/m2). Response to therapy was determined by survival and improvement of performance status, complete response or persistence of the clonal plasma cell disorder, and change in the function of organs involved with amyloid at baseline. We enrolled 25 patients with a median age of 48 years (range, 29-60), all of whom had biopsy-proven amyloidosis with clonal plasma cell disorders. Twenty-two (88%) were Southwest Oncology Group performance status 1 or 2 within a year of diagnosis, and 16 (64%) had received no prior therapy. Predominant amyloid-related organ involvement was cardiac (n = 8), renal (n = 7), hepatic (n = 6), neuropathic (n = 3), and lymphatic (n = 1). Fifteen patients had one or two organ systems involved, whereas 10 had three or more involved. With a median follow-up of 24 months (12-38), 17 of 25 patients (68%) are alive, and the median survival has not been reached. Thirteen of 21 patients (62%) evaluated 3 months posttransplant had complete responses of their clonal plasma cell disorders. Currently, two thirds of the surviving patients (11 of 17) have experienced improvements of amyloid-related organ involvement in all systems, whereas 4 of 17 have stable disease. The improvement in the median performance status of the 17 survivors at follow-up (0 [range, 0-3]) is statistically significant versus baseline (2 [range, 1-3]; P < .01). Significant negative prognostic factors with respect to overall survival include amyloid involvement of more than two major organ systems and predominant cardiac involvement. Three patients have experienced relapses of the clonal plasma cell disorder at 12 and 24 months. Dose-intensive therapy should currently be considered as the preferred therapy for patients with AL amyloidosis who meet functional criteria for autologous transplantation.
THE MEDIAN SURVIVAL of patients with AL (amyloid light-chain) amyloidosis is only 13 months from diagnosis.1 Because this uncommon aggressive disease is characterized by the deposition of immunoglobulin light chains produced by clonal plasma cells, attempts at therapy have been based on the use of alkylating agents active against multiple myeloma. Several randomized prospective clinical trials have shown that treatment with oral melphalan and prednisone results in objective responses and prolonged survival (medians of 16 to 18 months).2,3 In the most recent of these trials, patients who experienced reductions in their serum or urine monoclonal proteins (M-proteins) had significantly prolonged survival versus nonresponders, although only 20% of patients treated with melphalan and prednisone on that trial were reported to respond in such a manner.4
Given the results with intermittent oral melphalan and prednisone, we considered it reasonable to investigate the use of dose-intensive intravenous melphalan to treat this disorder.5,6 Recently we described an initial experience of five patients with AL treated with dose-intensive melphalan and blood stem cells.7 One year post-therapy, these patients were found to have improvements in performance status and amyloid-related organ involvement, and three of them had complete hematologic responses with respect to their plasma cell disorders. We now report on our experience with autologous transplantation in those five and an additional 20 patients with AL.
MATERIALS AND METHODS
Patients
Between January 1, 1994 and September 31, 1996, 25 patients who had biopsy-proven amyloidosis in association with a clonal plasma cell proliferative disorder and were 18 to 61 years of age were enrolled in a phase-II clinical study whereby they were to receive dose-intensive intravenous melphalan (200 mg/m2) followed by blood stem cell autograft, provided they had adequate cardiac, pulmonary, hepatic, and renal function as previously described.7Patients with familial, secondary, senile cardiac, or other forms of amyloidosis were not included in this study.8,9 The first 15 patients enrolled to receive unselected blood stem cells, and the next 10 enrolled to receive CD34-selected blood stem cells (Isolex 300; Baxter Biotech, Deer Park, IL).10
Blood stem cells were mobilized with granulocyte-colony stimulating factor (G-CSF; 10 to 16 μg/kg; Filgrastim; Amgen, Thousand Oaks, CA), collected, and evaluated as previously described.11Dose-intensive melphalan was administered intravenously over 2 days (days −4 and −3), 100 mg/m2/d, and stem cells were infused 72 hours later (day 0). Patients received antibiotic prophylaxis with an oral quinoline and acyclovir beginning on day 3 after stem-cell infusion. Hematopoietic recovery was determined by daily blood counts and defined as days from stem-cell infusion to recovery of neutrophils >0.5 × 109/L for 2 consecutive days and of platelets >20 × 109/L for 2 days with independence from platelet transfusion. Toxicities were recorded and graded according to Southwest Oncology Group (SWOG) criteria. At the time of study entry, at follow-up 3 months after therapy, and annually thereafter, each patient was evaluated for performance status, for status of the clonal plasma cell proliferative disorder, and for extent, predominance, and response of amyloid-related organ involvement.
Plasma Cell Proliferative Disorder
Presence of a clonal plasma cell disorder was assessed by serum and urine immunofixation electrophoresis (SIFE and UIFE; Paragon, Baxter, Deerfield, IL) and by bone marrow biopsy with ≥2-cm core samples. The percentage of plasma cells in the bone marrow biopsy was estimated and clonality identified with standard immunohistochemical staining for light-chain isotypes (clonal dominance being a κ to λ ratio greater than 3 or less than 1). Amyloid depositions in marrow biopsy specimens stained with Congo red were graded as 0 (none detected), 1 (in blood vessels only), 2 (in blood vessels and in focal interstitial deposits in ≤2 high-power fields), and 3 (in prominent interstitial deposits in >2 high-power fields).
After therapy, a patient's plasma cell proliferative disorder was categorized as having completely responded or persisted. A complete hematologic response to therapy was defined both as the absence of detectable M-protein in SIFE and UIFE and by a bone marrow biopsy specimen containing less than 5% plasma cells with polyclonal staining (ie, without clonal dominance of κ or λ). Persistent disease was defined as the persistence of an M-protein by SIFE or UIFE or of clonal dominance. In a patient with a previously confirmed complete hematologic response, relapse was defined as the detection of the pretherapy M-protein by SIFE or UIFE or as the reversion to the prior Κ or λ clonal dominance on bone marrow biopsy.
Amyloid-Related Organ Involvement
At study entry the extent of amyloid-related organ involvement was evaluated in each patient. Organ system involvement was determined as previously described, and each patient's predominant organ involvement was determined based on clinical consensus.2 On follow-up evaluation, amyloid-related organ involvement was assessed as improved, stable, or worsened.
Cardiac involvement was defined as the presence of a mean left ventricular wall thickness on echocardiogram >11 mm in the absence of a history of hypertension or valvular heart disease, or as the presence of unexplained low voltage (<0.5 mV) on the electrocardiogram.12 Clinical status was based on history, physical examination, and New York Heart Association (NYHA) heart failure class. Patients who were NYHA class 1 with evidence of cardiac amyloid by echocardiogram or electrocardiogram were categorized as having asymptomatic cardiac involvement. Patients who were NYHA class 2 or higher with evidence of cardiac involvement were categorized as having predominant cardiac involvement (amyloid cardiomyopathy).
An improvement or response to therapy for patients with cardiac involvement was defined as a decrease of ≥2 mm in mean left ventricular wall thickness in patients with baseline wall thickness >11 mm or a decrease in two classes in NYHA class (ie, from 3 to 1). Stable disease was defined as no evidence of clinical response and no progressive disease by clinical, electrocardiographic, and echocardiographic evaluation. Progression of cardiac involvement post-therapy was defined by worsening clinical signs or symptoms, worsening NYHA class (ie, from 1 to 3), or by an increase of ≥2 mm in mean left ventricular wall thickness in a patient with baseline wall thickness ≤11 mm.
Renal involvement was defined as proteinuria >0.5 g/d. An improvement or response to therapy was defined as a 50% decrease in daily proteinuria without progressive renal insufficiency. Hepatic involvement was defined as hepatomegaly with an alkaline phosphatase >200 U/L. Improvement was defined as a decrease in liver span of ≥2 cm with a concomitant decrease of alkaline phosphatase by 50%. Neuropathic involvement was defined based on clinical history, autonomic dysfunction with orthostasis, gastric atony by gastric emptying scan, and abnormal sensory and/or motor findings on neurological examination. Improvement was defined as normalization of orthostatic vital signs and symptoms and resolution of gastric atony and of abnormal findings on neurological examination. Stabilization or worsening of renal, hepatic, and neuropathic involvement was defined by consensus based on clinical evaluation and appropriate noninvasive tests.
Statistics
Medians, ranges, means, and standard deviations (SD) were calculated, and regression analyses and other test statistics were performed with Minitab (Minitab, State College, PA) or with SAS (SAS Institute, Cary, NC).13
RESULTS
Patients
Between January 1, 1994 and October 1, 1996, 25 patients were enrolled and gave written informed consent as approved by our Institutional Review Board. The majority of patients were performance status 1 or 2, within a year of diagnosis, and minimally pretreated with oral melphalan and prednisone (Table 1). Fourteen had an M-protein in serum and urine, whereas 9 had an M-protein in urine only; 5 had κ and 18 λ M-proteins. With respect to marrow plasmacytosis, 7 had ≤5%, 14 had 5% to 10%, and 3 had >10% plasma cells on biopsy. The distribution of dominant amyloid-related organ involvement included cardiac (8), renal (7), hepatic (6), neuropathic (3), and lymphatic (1). With respect to multisystem involvement, 15 had one or two systems involved and 10 had three or more systems involved (Table 2).
Patient Characteristics at Enrollment (n = 25)
| Median age | 48 (range, 29-60) |
| Men | 13 |
| Women | 12 |
| SWOG performance status | |
| 1 | 10 |
| 2 | 12 |
| 3 | 3 |
| Months from diagnosis | |
| 0-3 | 4 |
| 3-12 | 18 |
| >12 | 3 |
| Prior oral melphalan | |
| 0 | 16 |
| <200 mg | 2 |
| >200 mg | 7 |
| Median number of biopsies positive for amyloid | 2 (range, 2-4) |
| M-protein in serum and urine | 14 |
| M-protein in urine only | 9 |
| M-protein light chain isotype: K/λ | 5/18 |
| Clonal bone marrow plasmacytosis only | 2 |
| Marrow amyloid depositions present | 18 |
| Grade 2 or 3 marrow amyloid depositions | 8 |
| Hypogammaglobulinemia | 17 |
| Howell-Jolly bodies | 7 |
| Median age | 48 (range, 29-60) |
| Men | 13 |
| Women | 12 |
| SWOG performance status | |
| 1 | 10 |
| 2 | 12 |
| 3 | 3 |
| Months from diagnosis | |
| 0-3 | 4 |
| 3-12 | 18 |
| >12 | 3 |
| Prior oral melphalan | |
| 0 | 16 |
| <200 mg | 2 |
| >200 mg | 7 |
| Median number of biopsies positive for amyloid | 2 (range, 2-4) |
| M-protein in serum and urine | 14 |
| M-protein in urine only | 9 |
| M-protein light chain isotype: K/λ | 5/18 |
| Clonal bone marrow plasmacytosis only | 2 |
| Marrow amyloid depositions present | 18 |
| Grade 2 or 3 marrow amyloid depositions | 8 |
| Hypogammaglobulinemia | 17 |
| Howell-Jolly bodies | 7 |
Baseline Patient Characteristics, Amyloid Organ Involvement, and Responses to Therapy
| Baseline Patient Data . | Amyloid Organ Involvement . | Survival and Responses . | |||||
|---|---|---|---|---|---|---|---|
| Patient . | Age/Sex . | Time (mo)* . | Predominant . | Other . | Status (mo) . | Plasma Cell Disease . | Amyloid . |
| 1 | 41M | 4 | Hepatic | Renal | Died, pretreatment | ||
| 2 | 42M | 3 | Neuropathic | Cardiac | Alive, +38 | CR | imp |
| 3 | 46M | 5 | Cardiac | Alive, +37 | CR | imp | |
| 4 | 39M | 1 | Neuropathic | Cardiac, lymphatic | Alive, +36 | CR | imp |
| 5 | 45F | 9 | Hepatic | Renal | Alive, +34 | CR | imp |
| 6 | 55F | 18 | Renal | Alive, +33 | PD | imp | |
| 7 | 53M | 33 | Renal | Alive, +31 | PD | W | |
| 8 | 42F | 6 | Hepatic | Cardiac, renal | Alive, +30 | CR | imp |
| 9 | 45M | 10 | Neuropathic | Cardiac | Alive, +29 | PD | S |
| 10 | 56M | 8 | Hepatic | Renal, neuropathic | Died at +22 | [CR]† | [imp]† |
| 11 | 60M | 8 | Renal | Alive, +25 | Relapse, +12 | S | |
| 12 | 55F | 4 | Hepatic | Renal | Alive, +23 | CR | imp |
| 13 | 29F | 4 | Renal | Cardiac | Alive, +24 | Relapse, +24 | S |
| 14 | 58F | 24 | Cardiac | Renal, hepatic | Died at +20 | [CR]† | [imp]† |
| 15 | 53M | 6 | Cardiac | Renal | Died at +5 | [PD]† | NA |
| 16 | 48M | 1 | Cardiac | Alive, +20 | PD | S | |
| 17 | 31F | 3 | Hepatic | Cardiac, renal, neuropathic | Alive +19 | CR | imp |
| 18 | 49F | 2 | Cardiac | Hepatic, renal, neuropathic | Alive +17 | PD | imp |
| 19 | 40M | 1 | Cardiac | Renal, hepatic | Died at +2 | NA | NA |
| 20 | 57F | 5 | Lymphatic | Cardiac, neuropathic | Died at +3 | [PD]† | [W]† |
| 21 | 39M | 3 | Cardiac | Neuropathic, hepatic | Died at +2 | NA | NA |
| 22 | 56F | 5 | Renal | Cardiac | Alive, +14 | CR | imp |
| 23 | 37F | 4 | Renal | Cardiac | Alive, +13 | PD | W |
| 24 | 56M | 5 | Renal | Alive +12 | Relapse, +12 | imp | |
| 25 | 48F | 6 | Cardiac | Renal, neuropathic | Died, pretreatment | ||
| Baseline Patient Data . | Amyloid Organ Involvement . | Survival and Responses . | |||||
|---|---|---|---|---|---|---|---|
| Patient . | Age/Sex . | Time (mo)* . | Predominant . | Other . | Status (mo) . | Plasma Cell Disease . | Amyloid . |
| 1 | 41M | 4 | Hepatic | Renal | Died, pretreatment | ||
| 2 | 42M | 3 | Neuropathic | Cardiac | Alive, +38 | CR | imp |
| 3 | 46M | 5 | Cardiac | Alive, +37 | CR | imp | |
| 4 | 39M | 1 | Neuropathic | Cardiac, lymphatic | Alive, +36 | CR | imp |
| 5 | 45F | 9 | Hepatic | Renal | Alive, +34 | CR | imp |
| 6 | 55F | 18 | Renal | Alive, +33 | PD | imp | |
| 7 | 53M | 33 | Renal | Alive, +31 | PD | W | |
| 8 | 42F | 6 | Hepatic | Cardiac, renal | Alive, +30 | CR | imp |
| 9 | 45M | 10 | Neuropathic | Cardiac | Alive, +29 | PD | S |
| 10 | 56M | 8 | Hepatic | Renal, neuropathic | Died at +22 | [CR]† | [imp]† |
| 11 | 60M | 8 | Renal | Alive, +25 | Relapse, +12 | S | |
| 12 | 55F | 4 | Hepatic | Renal | Alive, +23 | CR | imp |
| 13 | 29F | 4 | Renal | Cardiac | Alive, +24 | Relapse, +24 | S |
| 14 | 58F | 24 | Cardiac | Renal, hepatic | Died at +20 | [CR]† | [imp]† |
| 15 | 53M | 6 | Cardiac | Renal | Died at +5 | [PD]† | NA |
| 16 | 48M | 1 | Cardiac | Alive, +20 | PD | S | |
| 17 | 31F | 3 | Hepatic | Cardiac, renal, neuropathic | Alive +19 | CR | imp |
| 18 | 49F | 2 | Cardiac | Hepatic, renal, neuropathic | Alive +17 | PD | imp |
| 19 | 40M | 1 | Cardiac | Renal, hepatic | Died at +2 | NA | NA |
| 20 | 57F | 5 | Lymphatic | Cardiac, neuropathic | Died at +3 | [PD]† | [W]† |
| 21 | 39M | 3 | Cardiac | Neuropathic, hepatic | Died at +2 | NA | NA |
| 22 | 56F | 5 | Renal | Cardiac | Alive, +14 | CR | imp |
| 23 | 37F | 4 | Renal | Cardiac | Alive, +13 | PD | W |
| 24 | 56M | 5 | Renal | Alive +12 | Relapse, +12 | imp | |
| 25 | 48F | 6 | Cardiac | Renal, neuropathic | Died, pretreatment | ||
Abbreviations: CR, complete hematologic response; imp, improved; PD, persistent disease; W, worsened; S, stable; NA, not applicable.
Time from diagnosis to treatment.
Brackets indicate last recorded data.
Survival and Performance Status
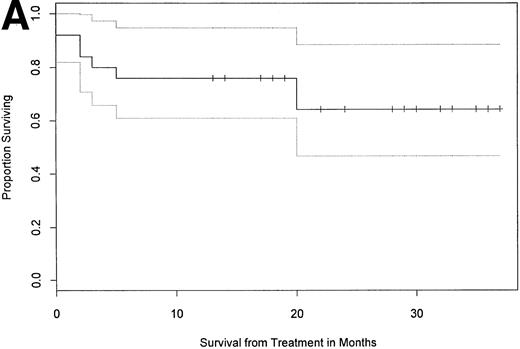
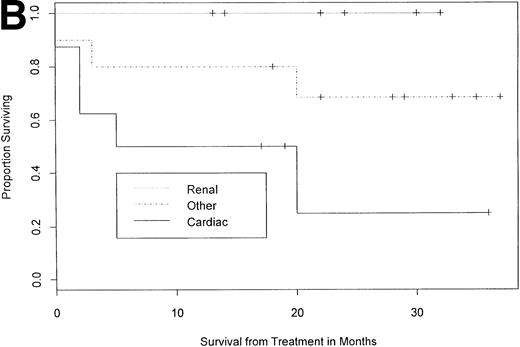
As of October 1, 1997, with a median follow-up of 24 months (12 to 38), 68% (17 of 25) of the patients were alive (Table 2 and Fig 1A). Eighty-seven percent of patients (13 of 15) with two or less major organ systems involved survived, as opposed to only 40% (4 of 10) surviving among those with more than two involved systems (P < .05, one-tailed Fisher's exact test). Eighty-two percent of patients without predominant cardiac involvement at baseline (14 of 17) were alive in follow-up compared with 38% of patients with predominant cardiac involvement (3 of 8; Table 2 and Fig1B; P < .05). Overall survival of patients with a baseline performance status of 1 was 80% (8 of 10), whereas that of patients with a baseline performance status of 2 or 3 was 60% (9 of 15;P = .402). With respect to the 17 patients surviving a year or more in follow-up, the difference between their median performance status before therapy (2 [range, 1 to 3]) and at follow-up (0 [range, 0 to 3]) was statistically significant (P < .05, Wilcoxon rank sum).
Overall survival and survival by category of organ involvement. With median follow-up of 24 months (12 to 38), 17 of 25 patients (68%) survived, and the median survival has not yet been reached as depicted in (A) a Kaplan-Meier plot showing the 95% confidence interval (CI; proportional survival 0.65, 95% CI 0.48 to 0.88). (B) Survival by predominant organ involvement is shown. Total and mean times of follow-up for the three cohorts are 149 and 21.3 months (renal, n = 7), 225 and 22.5 months (other, n = 10), and 101 and 12.6 months (cardiac, n = 8). Patients without predominant cardiac involvement had better overall survival than cardiac patients (one-tailed Fisher's exact test, P < .05).
Overall survival and survival by category of organ involvement. With median follow-up of 24 months (12 to 38), 17 of 25 patients (68%) survived, and the median survival has not yet been reached as depicted in (A) a Kaplan-Meier plot showing the 95% confidence interval (CI; proportional survival 0.65, 95% CI 0.48 to 0.88). (B) Survival by predominant organ involvement is shown. Total and mean times of follow-up for the three cohorts are 149 and 21.3 months (renal, n = 7), 225 and 22.5 months (other, n = 10), and 101 and 12.6 months (cardiac, n = 8). Patients without predominant cardiac involvement had better overall survival than cardiac patients (one-tailed Fisher's exact test, P < .05).
Two patients died before receiving chemotherapy (patients 1 and 25). Both had rapidly progressive amyloidosis and died after stem-cell collection but before being treated. One with predominant hepatic involvement died of massive gastrointestinal bleeding a month after collection, and the other with predominant cardiac involvement and a history of syncope died of hypoxia, hypotension, and cardiopulmonary failure within 24 hours of completing stem-cell collection.
Twenty-three patients received dose-intensive melphalan treatment. There were no deaths within 30 days of transplant. However, there were three deaths within 100 days of transplant, one in a patient with massive lymphatic involvement who died of cardiorespiratory failure, and two in patients with predominant cardiac involvement who experienced sudden cardiac events despite amiodarone therapy and an implanted cardiac defibrillator; hence, the 100-day posttransplant mortality was 13% (3 of 23), whereas, on an intention-to-treat basis, the overall early (<3 month) mortality associated with attempted therapy was 20% (5 of 25). Two more cardiac patients have died in follow-up, one at 5 months posttransplant of apparent sepsis and the other at 20 months posttransplant of secondary acute myelogenous leukemia. Additionally, at 20 months posttransplant, a patient with predominant hepatic involvement died in his sleep.
Responses of the Plasma Cell Disorders
At 3 months posttransplant, 62% of patients (13 of 21) experienced complete hematologic responses of their clonal plasma cell disorders. Eight patients without a serum M-protein (ie, with Bence-Jones protein and/or marrow isotype dominance) had complete hematologic responses, whereas 5 of 13 with serum M-proteins completely responded, resulting in a significant difference (P = .015, two-tailed Fisher's exact test). All patients with a κ isotype (4 of 4) responded completely; in contrast, only 9 of 17 with a λ isotype did so (P = .26). Three patients, all with predominant renal involvement and λ isotype disease, have relapsed: two (patients 11 and 24) at 12 and one (patient 13) at 24 months. Two had findings of recurrent serum M-proteins and the third of a recurrent urine M-protein. All three showed recurrent marrow isotype dominance with less than 5% total plasma cells staining for λ. Their amyloid-related organ involvement had not progressed at the time of relapse.
Responses of Amyloid Disease
Cardiac.
Eight patients had predominant cardiac involvement. Three had a history of syncope and all died: one postleukapheresis before receiving chemotherapy (see above), and two suddenly within 3 months after treatment. One year post-treatment, three of four surviving patients with predominant cardiac involvement experienced an improvement in their performance status to level 0, whereas the fourth experienced a deterioration in performance status caused by a leg ulcer and complications of hip replacement surgery, both sequelae of prolonged steroid use. One patient (patient 3), who had endomyocardial biopsy, echocardiographic, and clinical evidence of disease at baseline, experienced a >2-mm reduction of mean left ventricular wall thickness. Another patient (patient 18) improved two NYHA classes and is now class 1 without a diuretic requirement.
The follow-up data on eight patients with asymptomatic cardiac involvement and other predominant organ involvement are shown in Table 3. None of the six patients who experienced a complete hematologic response have progressed to symptomatic amyloid cardiomyopathy. Two patients with complete hematologic responses experienced reductions of their mean ventricular wall thickness of 2 and 4 mm, respectively, consistent with a cardiac response to therapy. One patient with persistent plasma cell disease and baseline low-voltage on the electrocardiogram experienced progression of cardiac amyloid with an increase of 3 mm of mean wall thickness to a mean thickness of 12 mm.
Status of Patients With Asymptomatic Cardiac Involvement at Baseline
| Patient . | Age*/Gender . | Months Since Therapy . | PS . | Response . | Baseline LVTM (cm) . | Follow-up LVTM (cm) . |
|---|---|---|---|---|---|---|
| 2 | 42M | 38 | 0 | CR | 1.2 | 1.2 |
| 4 | 39M | 36 | 0 | CR | 1.4 | 1.0 |
| 8 | 42W | 30 | 0 | CR | 0.9 | 0.7 |
| 9 | 45M | 29 | 1 | PD | 1.2 | 1.2 |
| 13 | 29W | 24 | 0 | CR | 0.7 | 1.0 |
| 17 | 31W | 19 | 0 | CR | 1.2 | 1.0 |
| 22 | 56W | 14 | 0 | CR | 1.5 | NA |
| 23 | 37W | 13 | 2 | PD | 0.9 | 1.2 |
| Patient . | Age*/Gender . | Months Since Therapy . | PS . | Response . | Baseline LVTM (cm) . | Follow-up LVTM (cm) . |
|---|---|---|---|---|---|---|
| 2 | 42M | 38 | 0 | CR | 1.2 | 1.2 |
| 4 | 39M | 36 | 0 | CR | 1.4 | 1.0 |
| 8 | 42W | 30 | 0 | CR | 0.9 | 0.7 |
| 9 | 45M | 29 | 1 | PD | 1.2 | 1.2 |
| 13 | 29W | 24 | 0 | CR | 0.7 | 1.0 |
| 17 | 31W | 19 | 0 | CR | 1.2 | 1.0 |
| 22 | 56W | 14 | 0 | CR | 1.5 | NA |
| 23 | 37W | 13 | 2 | PD | 0.9 | 1.2 |
These patients had asymptomatic amyloid cardiac involvement at baseline before dose-intensive melphalan therapy. Their predominant organs of involvement were not cardiac (see Table 2). Complete hematologic responses after therapy have been associated with improved functional status and, in two instances (patients 4 and 17), with reductions in the average thickness of the left ventricle by echocardiogram.
Abbreviations: PS, performance status; LVTM, mean left ventricular wall thickness (average of posterior and septal walls); CR, complete hematologic response; PD, persistent plasma cell disease.
Age at treatment.
Renal.
Fourteen patients with renal involvement, including 7 with predominant renal involvement, were treated and survived in follow-up as shown in Table 4. Three of 7 with predominant renal involvement and 6 of 7 with nonpredominant renal involvement improved in response to therapy. Three patients have stable disease and 2 have worsened disease, 1 caused in part by treatment-related toxicity (patient 23) with a creatinine increase from 0.9 to 4.9 mg/dL by day 15. The overall response rate for renal involvement then was 64% (9 of 14), and the median pretreatment and posttreatment levels of daily proteinuria in the 9 responders were significantly different (pretreatment, 6,030 mg [range, 645 to 23,595]; posttreatment, 1,573 [range, 102 to 7,560]; P < .01, Wilcoxon rank sum). Posttreatment albumin levels in the responders were also significantly improved over baseline (P < .01), whereas creatinine levels were not significantly altered (P = .183).
Renal and Hepatic Responses
| . | # . | Proteinuria (<150 mg/d) . | Alkaline Phosphatase (<120 U/L) . | Albumin (3.5-5.5 g/dL) . | Creatinine (<1.5 mg/dL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | Pre . | Post . | ||
| A | 6 | 11,370 | 1,573 | 124 | 108 | 2.3 | 4.0 | 1.0 | 0.9 |
| 7 | 12,800 | 11,400 | 104 | 77 | 2.2 | 2.5 | 1.0 | 4.1 | |
| 11 | 9,234 | 8,293 | 90 | 64 | 2.2 | 2.3 | 0.7 | 0.9 | |
| 13 | 9,653 | 7,320 | 135 | 111 | 2.1 | 3.1 | 0.8 | 0.9 | |
| 22 | 6,030 | 421 | 146 | 194 | 1.7 | 2.7 | 0.9 | 1.2 | |
| 23 | 16,390 | 21,625 | 59 | 61 | 1.2 | 1.7 | 0.9 | 7.1 | |
| 24 | 16,400 | 7,560 | 96 | 80 | 1.9 | 3.7 | 1.1 | 1.3 | |
| B | 5 | 645 | 102 | 184 | 113 | 5.1 | 4.9 | 1.3 | 1.4 |
| 8 | 16,300 | 4,218 | 490 | 520 | 3.4 | 4.8 | 1.6 | 1.3 | |
| 10 | 2,880 | 1,135 | 1,280 | 551 | 3.3 | 3.9 | 0.8 | 0.9 | |
| 12 | 3,548 | 1,595 | 413 | 199 | 3.3 | 5.7 | 0.8 | 0.7 | |
| 17 | 23,595 | 2,728 | 2,820 | 586 | 1.3 | 3.9 | 0.5 | 0.7 | |
| C | 14 | 3,542 | 992 | 362 | 278 | 4.0 | 5.1 | 1.5 | 1.7 |
| 18 | 2,420 | 4,189 | 1,488 | 160 | 2.7 | 3.3 | 0.9 | 1.3 | |
| . | # . | Proteinuria (<150 mg/d) . | Alkaline Phosphatase (<120 U/L) . | Albumin (3.5-5.5 g/dL) . | Creatinine (<1.5 mg/dL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | Pre . | Post . | ||
| A | 6 | 11,370 | 1,573 | 124 | 108 | 2.3 | 4.0 | 1.0 | 0.9 |
| 7 | 12,800 | 11,400 | 104 | 77 | 2.2 | 2.5 | 1.0 | 4.1 | |
| 11 | 9,234 | 8,293 | 90 | 64 | 2.2 | 2.3 | 0.7 | 0.9 | |
| 13 | 9,653 | 7,320 | 135 | 111 | 2.1 | 3.1 | 0.8 | 0.9 | |
| 22 | 6,030 | 421 | 146 | 194 | 1.7 | 2.7 | 0.9 | 1.2 | |
| 23 | 16,390 | 21,625 | 59 | 61 | 1.2 | 1.7 | 0.9 | 7.1 | |
| 24 | 16,400 | 7,560 | 96 | 80 | 1.9 | 3.7 | 1.1 | 1.3 | |
| B | 5 | 645 | 102 | 184 | 113 | 5.1 | 4.9 | 1.3 | 1.4 |
| 8 | 16,300 | 4,218 | 490 | 520 | 3.4 | 4.8 | 1.6 | 1.3 | |
| 10 | 2,880 | 1,135 | 1,280 | 551 | 3.3 | 3.9 | 0.8 | 0.9 | |
| 12 | 3,548 | 1,595 | 413 | 199 | 3.3 | 5.7 | 0.8 | 0.7 | |
| 17 | 23,595 | 2,728 | 2,820 | 586 | 1.3 | 3.9 | 0.5 | 0.7 | |
| C | 14 | 3,542 | 992 | 362 | 278 | 4.0 | 5.1 | 1.5 | 1.7 |
| 18 | 2,420 | 4,189 | 1,488 | 160 | 2.7 | 3.3 | 0.9 | 1.3 | |
All of these patients had amyloid-related renal involvement, and patients in groups B and C had both renal and hepatic involvement. The patients in group A had predominant renal involvement, those in B predominant hepatic, and in C predominant cardiac involvement. The columns give pre- and post-therapy values for laboratory tests obtained for each patient, the post-therapy value being from the most recent follow-up. (Normal values)
Hepatic.
Improvements have been observed in four of five patients with predominant and one of two with nonpredominant hepatic involvement, including elimination of symptoms and reduction in craniocaudal liver span from over 20 cm to normal size (<14 cm) usually accompanied by a >50% reduction in alkaline phosphatase levels (patient 5 is scored as improved; she had symptomatic hepatomegaly with a baseline alkaline phosphatase of 184 U/L and experienced a significant reduction in liver span, elimination of symptoms, and normalization of alkaline phosphatase; Table 4). The two remaining patients (8 and 14) were scored as having stable disease, although patient 8 had a reduction in liver span of >2 cm without a change in alkaline phosphatase level. The overall response rate for hepatic involvement then was 71% (5 of 7), and the median pretreatment and posttreatment levels of alkaline phosphatase in the five responders approached statistical significance (P = .059). In addition, correction of blood smear abnormalities at 1 year posttreatment was noted in four responding patients with the loss of Howell-Jolly bodies and target cells and with normalization of red blood cell morphology indicative of improved hepatosplenic function (the fifth responder, patient 10, had an emergent splenectomy 6 months posttransplant).
Neuropathic.
As reported previously, there were improvements in patients with predominant neuropathic involvement.7,14 Two of the three patients with predominant neuropathic involvement have had complete resolution of their autonomic and peripheral polyneuropathies, including resolution of orthostasis and gastric atony measured by a gastric emptying scan. In addition, patients 17 and 18 who had neuropathic involvement (orthostasis) experienced complete resolution of signs and symptoms. Therefore, the overall response rate for neurological involvement was 80% (4 of 5). Resolution of neuropathy has not been previously reported with oral chemotherapy and supports the concept that a reversible metabolic derangement may be the pathophysiological mechanism that underlies the polyneuropathy in this disease.15 16
Treatment and Toxicities
Twenty-two patients were treated with 200 mg/m2 melphalan; patient 15 with predominant cardiac involvement was treated with a reduced dose (100 mg/m2) because cardiac function worsened shortly before treatment. Data regarding toxicities of therapy and extent of transfusion requirements are summarized in Table 5. Patients were hospitalized for a median of 20 days (range, 15 to 42).
Data on Toxicities (SWOG ≥ Grade 2) of Dose-Intensive Melphalan and on Transfusion Support (n = 23)
| Toxicity | Frequency % (n) |
| Nausea/vomiting | 83 (19) |
| Diarrhea | 65 (15) |
| Mucositis | 91 (21) |
| Pulmonary edema | 35 (8) |
| Peripheral edema | 48 (11) |
| Non-GI bleeding | 17 (4) |
| GI bleeding | 22 (5) |
| Hepatic | 13 (3) |
| Renal | 35 (8) |
| Metabolic | 35 (8) |
| Sepsis | 26 (6) |
| Transfusion component | Median (range) |
| Units of red blood cells | 5 (0-14) |
| Platelet transfusions | 6 (0-28) |
| Toxicity | Frequency % (n) |
| Nausea/vomiting | 83 (19) |
| Diarrhea | 65 (15) |
| Mucositis | 91 (21) |
| Pulmonary edema | 35 (8) |
| Peripheral edema | 48 (11) |
| Non-GI bleeding | 17 (4) |
| GI bleeding | 22 (5) |
| Hepatic | 13 (3) |
| Renal | 35 (8) |
| Metabolic | 35 (8) |
| Sepsis | 26 (6) |
| Transfusion component | Median (range) |
| Units of red blood cells | 5 (0-14) |
| Platelet transfusions | 6 (0-28) |
Abbreviations: SWOG, Southwestern Oncology Group; GI, gastrointestinal.
Infectious Complications
There were no deaths caused by sepsis. With respect to infectious complications, six patients had fever with positive blood cultures:Streptococcus viridans (n = 3), coagulase-negativeStaphylococcus (n = 1), Candida kruseii (n = 1), and a species of Penicillium (n = 1). The latter two infections occurred in patients who received CD34-selected stem cells and were also diagnosed with Pneumocystis carinii pneumonia. Both patients were hyposplenic; one had replacement of her lymph nodes with amyloid and bilateral genitourinary fungal bezoars that were undiagnosed before therapy.10,17 The other was severely hypogammaglobulinemic at baseline (IgG <100 mg/dL). Both responded to antibiotics, although the patient with C kruseii died of cardiopulmonary failure on day 99 after completing antifungal therapy and had no evidence of infection postmortem. Whether T-cell–depleted CD34-selected autografts carry an increased risk of infection in certain categories of patients remains an open question.18
Renal and Hepatic Toxicities
One of seven patients (patient 23) with predominant renal involvement experienced the only episode of significant treatment-related renal toxicity. One of five patients with predominant hepatic involvement (patient 17) experienced jaundice and an increase in bilirubin to 18 mg/dL, which was attributed to drug toxicities (opiates, trimethoprim-sulfamethoxazole) and suspected veno-occlusive disease. The patient improved promptly and bilirubin returned to normal by the time of follow-up at 3 months.
Delayed Platelet Engraftment
Three patients had delayed platelet reconstitution. All other patients engrafted for platelets within 17 days. These three late engrafters had significant toxicities of therapy. Patient 8 who had received no prior oral melphalan engrafted on day 65 after experiencing grade-4 mucositis, grade-3 pulmonary edema, and grade-4 sepsis. Patient 11, who had received 320 mg of prior oral melphalan, engrafted on day 52 after experiencing grade-3 peripheral edema and grade-3 gastrointestinal bleeding. Both patients had evidence of splenic dysfunction (Howell-Jolly bodies).
Secondary Leukemia
The third patient who experienced delayed platelet engraftment (patient 14), in addition to receiving the largest amount of prior oral melphalan of the 23 patients (672 mg), had grade-3 mucositis and grade-3 pulmonary edema. Eighteen months posttransplant, this patient, a 61-year-old woman, developed acute myelogenous leukemia with a complex karyotype including deletion of chromosome 7. She died 2 months later, 20 months after treatment.
Stem-Cell Autografts and Transfusion Requirements
All patients were mobilized with G-CSF, usually 16 μg/kg subcutaneously daily for 4 to 5 days, with stem-cell collections beginning on day 4. A median of 8.9 × 106CD34+ cells/kg were collected (range 2.9 to 25.4) in two to three aphereses. The numbers of mobilized circulating baseline CD34+ cells in patients who had previously received more than 200 mg of oral melphalan pulse therapy were significantly less than in patients who had previously received less or no melphalan (mean ± SD; 48 ± 25 versus 78 ± 63 × 106/L;P = .028, two-tailed t-test). However, there were no significant differences between these groups with respect to CD34+ cells collected on individual days or in toto.
The numbers of CD34+ cells collected by leukapheresis on days 4 and 5 failed to correlate by regression analysis with patient age or gender, the baseline leukocyte count, the presence of Howell-Jolly bodies, or the number of blood volumes processed during leukapheresis. However, the number of peripheral CD34+cells circulating at baseline before leukapheresis was a weak but significant predictor of the number of CD34+ cells collected on day 4 (R2 = .316, P < .01), and the day-5 collection was correlated with the number of CD34+cells collected on day 4 (R2 = .435, P < .01).
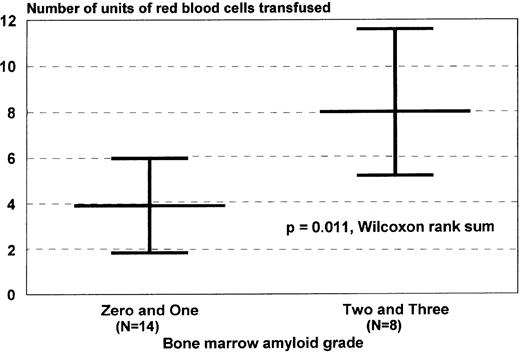
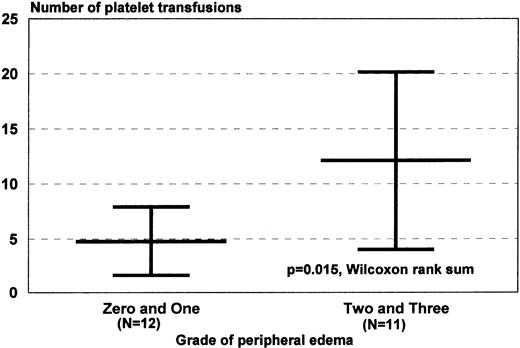
Neutrophil and platelet engraftment occurred in medians of 10 (8 to 17) and 14 days (8 to 65), respectively. There were no differences in neutrophil or platelet engraftment based on CD34+ cell dose or the use of unselected or CD34-selected cells, although differences in lymphocyte recovery were documented.10 With respect to transfusion of red blood cells and platelets in this transplant population, one that had a 39% incidence of bleeding complications, simple regression analysis was performed with baseline patient characteristics and graded toxicities of therapy to determine if there were significant correlations between any of those variables and transfusion requirements. There were significant correlations between marrow amyloid grade and units of red blood cells transfused and between the grade of peripheral edema and number of platelet transfusions (Fig 2).
Correlates of red blood cell and platelet transfusion requirements. Transfusion requirements were of interest in patients with AL undergoing dose-intensive therapy because of the association between AL, coagulation abnormalities, and vascular pathology. (A) Patients with higher marrow amyloid grades required more red blood cell transfusion support, a correlation identified initially by simple regression analysis (R2 = .46, P < .01). (B) Patients with higher grades of edema during the myelosuppressive period, caused by combinations of salt avidity, hypoalbuminemia, autonomic dysfunction, sepsis, or low cardiac output, required more platelet transfusions, perhaps because of increased vascular injury. This correlation was also identified initially by simple regression analysis (R2 = .31, P < .01). Peripheral edema was graded clinically by SWOG criteria. Values are medians and ranges.
Correlates of red blood cell and platelet transfusion requirements. Transfusion requirements were of interest in patients with AL undergoing dose-intensive therapy because of the association between AL, coagulation abnormalities, and vascular pathology. (A) Patients with higher marrow amyloid grades required more red blood cell transfusion support, a correlation identified initially by simple regression analysis (R2 = .46, P < .01). (B) Patients with higher grades of edema during the myelosuppressive period, caused by combinations of salt avidity, hypoalbuminemia, autonomic dysfunction, sepsis, or low cardiac output, required more platelet transfusions, perhaps because of increased vascular injury. This correlation was also identified initially by simple regression analysis (R2 = .31, P < .01). Peripheral edema was graded clinically by SWOG criteria. Values are medians and ranges.
DISCUSSION
Dose-intensive melphalan with blood stem-cell support for patients with AL amyloidosis represents an important therapeutic advance in the treatment of this rapidly fatal disease.19-22 Currently, with a median of 24 months follow-up (12 to 38), 68% (17 of 25) of patients are alive and the median survival has not been reached (Fig1A). Amyloid involvement of more than two major organ systems or presence of symptomatic cardiac involvement are significant negative prognostic factors with respect to overall survival. Eighty seven percent of patients (13 of 15) with up to two major organ systems involved have survived, as opposed to only 40% (4 of 10) surviving among those with more than two involved systems. Eighty-two percent of patients without symptomatic amyloid cardiomyopathy at baseline (14 of 17) were alive in follow-up compared with 38% of patients with symptomatic amyloid cardiomyopathy (3 of 8; Fig 1B). Conversely, involvement of no more than two major organ systems and absence of predominant cardiac involvement appear to be important positive prognostic factors. With respect to the 17 patients surviving a year or more in follow-up, the improvement in their performance status is statistically significant compared with baseline.
Complete hematologic responses were seen in 60% of surviving patients, most frequently in those without a serum M-protein or with a monoclonal protein of the κ isotype. The achievement of significant cytoreductive responses, as suggested by the frequency of complete responses, is likely to be responsible for the improvements observed in survival, performance status, and organ function. The degree to which such improvements are caused by cessation of amyloid deposition, resorption of existing amyloid depositions, or both, is unclear. Moreover, response of amyloid-related organ involvement and relapse of the plasma cell proliferative disorder can occur, suggesting a possible role for molecular methods that can identify the presence of minimal residual disease to ascertain prognosis and plan additional therapy if warranted.10 The rate of amyloid progression and clinical deterioration after hematologic relapse is also unclear at this time.
Amyloid cardiomyopathy, a fatal condition with a median untreated survival of less than 6 months from onset of symptoms, is rarely responsive to oral chemotherapy.23 It is encouraging that 38% (3 of 8) of patients with predominant cardiac involvement treated with dose-intensive therapy remain alive more than a year after treatment and that two have experienced significant improvements in performance status 1 year posttransplant. Of equal significance is the fact that no surviving patient with baseline asymptomatic cardiac involvement and a complete hematologic response has developed symptomatic amyloid cardiomyopathy. Indeed, two of these patients have shown a decrease in mean ventricular wall thickness of ≥2 mm. Nonetheless, patients with predominant cardiac involvement, particularly those with more than two organ systems involved, are clearly high-risk candidates for dose-intensive therapy and stem-cell transplant, with an early (<3 months) treatment-related mortality of 38% (3 of 8). However, for those with predominant cardiac and minimal other organ involvement who are NYHA class 3, consideration of eligibility for cardiac transplant (and subsequent stem-cell transplant) is clearly indicated.
Response rates in patients with predominant renal and hepatic involvement are also striking, particularly with respect to the reduction of proteinuria in the former and correction of red blood cell morphologic abnormalities in the latter. Loss of Howell-Jolly bodies on the peripheral blood smear is indicative of a return of splenic function and normalization of the lipid profile associated with hepatic and renal dysfunction. Moreover, the responses described in patients with predominant neuropathic involvement have not been described previously to occur after any other form of therapy.24
These data, with respect to survival, hematologic responses, and improvements in amyloid-related organ involvement, are dramatically different from those reported with oral chemotherapy. Because 18 of the patients (72%) studied were 3 to 12 months from diagnosis and a third of them had received some prior oral therapy, concerns regarding selection bias are reasonable. Patients with rapidly progressive AL often die within 3 months of diagnosis; therefore, the role of dose-intensive therapy in newly diagnosed untreated patients is a matter for further clinical study. Moreover, there may be a role for cytoreductive therapy with oral melphalan and prednisone, or other regimens, as a prelude to dose-intensive therapy. Both of these matters are currently being evaluated in a randomized clinical trial at our center.
With respect to stem-cell mobilization and hematologic recovery after autografting, it is clear that stem-cell mobilization with G-CSF is an effective method of stem-cell procurement. The overall quality of mobilization in AL patients, the majority of whom were not heavily pretreated, is similar to that observed in normal donors, and neutrophil and platelet recoveries did not appear to be significantly affected by amyloid marrow deposits.25 However, the increased red blood cell transfusion requirement in patients with higher amyloid marrow grades suggests that interstitial marrow depositions may slow erythropoietic recovery because of impaired production or increased destruction of red blood cells, or both. The apparent association between peripheral edema and the platelet transfusion requirement is equally intriguing (Fig 2). Possible explanations include either increased peripheral use or destruction (caused by vascular injury), decreased activity of thrombopoietin (caused by a leak into the interstitial space) causing decreased production, or both. These considerations are clinically relevant in view of the 39% incidence of bleeding, particularly gastrointestinal bleeding, that occurred in these patients during therapy. It was not unusual, for example, for patients to bleed from sites of previous tissue biopsies (eg, the rectum). Bleeding episodes may well be tied to the known abnormalities of coagulation and vascular integrity associated with AL.1,3 26
Our findings lead us to conclude that patients with performance status 0 to 2 who have predominant cardiac, renal, hepatic, or neuropathic involvement with one other organ system involved are highly likely to survive dose-intensive therapy and to benefit from it. Although the risks of renal and hepatic toxicity cannot be minimized, our data indicate that they are not contraindications to the use of this therapeutic approach. Patients with predominantly neuropathic involvement and performance status 3 caused by neuropathy are also highly likely to survive and to benefit from dose-intensive therapy despite their poor nutritional and functional status.
It would be reasonable to conclude that, because the results of this treatment are substantially better than those obtained with oral chemotherapy, patients are likely to experience prolonged disease-free survival. Because of the frequency with which patients improve and plasma cell proliferative disorders respond, we believe that dose-intensive therapy should be considered standard therapy for patients with AL who meet functional criteria for autologous stem-cell transplantation. Clearly, patients with predominant cardiac involvement and performance status 3 who are ineligible for cardiac transplant should not be treated with dose-intensive melphalan; however, effective diuretic therapy may result in sufficient improvement for some, allowing them to become candidates for less intensive regimens of intravenous melphalan (eg, 100 or 140 mg/m2).
Less than one third of the patients we treated had received more than 200 mg of prior oral melphalan, and no patient had evidence of myelodysplasia on bone marrow biopsy specimens or peripheral blood smears. However, patients who have received extended therapy with oral melphalan or have evidence of myelodysplasia subsequent to oral melphalan therapy may not be optimal candidates for stem-cell mobilization and transplantation, and in such cases appreciation and discussion of the risk of chemotherapy-induced leukemia are appropriate.
In summary, a new and frequently effective therapy, dose-intensive melphalan with blood stem-cell support, has been identified for a hitherto almost uniformly fatal systemic disease. The efficacy of this therapy is likely associated with obtaining a significant or complete response of the plasma cell proliferative disorder.27Furthermore, the existence of this therapy creates an impetus for physicians to diagnose AL early despite its multifaceted presentations and to consider treatment promptly in view of its inexorable progression. This would appear to make the critical issue not whether but to whom and when dose-intensive therapy should be offered.4,28,29 However, a caveat applies with respect to the security of the diagnosis of AL, because patients with secondary or familial variants of systemic amyloidosis are never candidates for dose-intensive melphalan. Therefore, the issue of diagnostic confidence in problematic cases becomes equally critical.8,30 31
ACKNOWLEDGMENT
We thank the staff of the Boston Medical Center blood bank and clinical laboratories; the Hematology-Oncology nurses in the Center for Cancer and Blood Disorders and on Atrium 7E, particularly Diane Sarnacki, RN; and members of the staff of the Clinical Trials Office, the Boston University School of Public Health, and the Amyloid Program, including Kathleen Finn, RN, Steven Evans, Salli Fennessey, and Ceit McCaleb.
Supported by grants from the Food and Drug Administration (FD-R-001346-01) (R.L.C.), the Arthritis Foundation (R.L.C.), the Amyloid Research Fund, the Sue Sellors Finley Cardiac Amyloid Research Fund, and the Stem-cell Laboratory Fund of BUSM and the Boston Medical Center.
Address reprint requests to Raymond L. Comenzo, MD, Director, Transfusion Medicine Program, H-303, Boston Medical Center, Boston, MA 02118.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal