Abstract
Donor lymphocyte infusions (DLI) can induce remissions in patients who have relapsed after allogeneic bone marrow transplantation (BMT). However, DLI frequently also result in significant acute and/or chronic graft-versus-host disease (GVHD). Several clinical and experimental lines of evidence have suggested that CD8+ T cells play a critical role in the pathogenesis of GVHD. To develop methods to reduce the incidence of GVHD associated with DLI, we administered defined numbers of CD4+ donor T cells after ex vivo depletion of CD8+ lymphocytes to 40 patients with relapsed hematologic malignancies after allogeneic BMT. Cohorts of patients received 0.3, 1.0, or 1.5 × 108CD4+ cells/kg. Overall, 12 of 38 patients (32%) evaluable for toxicity developed acute or chronic GVHD. However, 6 of 27 patients (22%) receiving 0.3 × 108 CD4 cells/kg developed GVHD compared with 6 of 11 patients (55%) who received ≥1.0 × 108 CD4 cells/kg (P = .07). Treatment-related mortality was low (3%), with 1 death related to infection in the setting of immunosuppression for GVHD. Disease responses after CD4+ DLI were documented in 15 of 19 patients (79%) with early-phase chronic myelogenous leukemia (CML) relapse, 5 of 6 patients (83%) with relapsed multiple myeloma, and 1 patient with myelodysplasia. For patients with early-phase CML relapse, the Kaplan-Meier probability of achieving complete cytogenetic remission was 87% and the probability of complete molecular response was 78% at 1 year after DLI. The median time to complete cytogenetic response and molecular response in patients with CML was 13 weeks (range, 9 to 30 weeks) and 34 weeks (range, 10 to 56 weeks), respectively. The median time to response in patients with multiple myeloma was 26 weeks (range, 15 to 62 weeks). All patients in this trial who developed GVHD demonstrated tumor regression, but the presence of GVHD was not required for patients to achieve a response, because 48% of responding patients never developed evidence of GVHD. Two patients with CML who did not respond at dose level 1 subsequently achieved complete cytogenetic remission after a second infusion of CD8-depleted cells at dose level 2. In patients with evidence of mixed hematopoietic chimerism who achieved a complete remission after DLI, cytogenetic analysis of marrow cells also demonstrated conversion to complete donor hematopoiesis in all evaluable patients. These studies suggest that relatively low numbers of CD8-depleted donor lymphocytes are effective in inducing complete remissions in patients with stable-phase CML and multiple myeloma who have relapsed after allogeneic BMT. Because of the relatively low risk of toxicity associated with the infusion of defined numbers of CD4+donor cells, further studies can be undertaken in the setting of persistent minimal residual disease to prevent relapse after allogeneic BMT.
DONOR LYMPHOCYTE infusions (DLI) have emerged as an effective strategy for the treatment of patients with chronic myelogenous leukemia (CML) who have relapsed after allogeneic bone marrow transplantation (BMT).1-6 This therapy appears to be most effective in patients with CML who have evidence of cytogenetic relapse or who have relapsed into stable phase.7-9 The use of DLI was initially envisioned as a means to induce graft-versus-host disease (GVHD), which, in turn, could eradicate recurrent leukemic cells by the induction of graft-versus-leukemia (GVL) activity. Nevertheless, the success of DLI has been limited to some extent by the morbidity and mortality associated with GVHD. Although some investigators have linked the induction of GVL activity following DLI to the induction of GVHD, there have been reports of patients treated with DLI who obtain a complete response in the absence of clinical GVHD. This finding suggests that the GVL response may be independent of the clinical development of GVHD.
T cells are presumed to be the principle mediators of GVHD in both animal models and humans.10-16 T cells are also implicated as the mediators of GVL.17-21 Specific T-cell subsets and T-cell number may both influence the incidence and severity of GVHD after DLI. The distinction between specific T-cell subsets inducing GVL and GVHD has been made in several preclinical models.18,20-23 The T-cell subset responsible for GVL or GVHD varies with the model.24 In humans, increased numbers of circulating CD8+ T cells have been observed in patients who develop GVHD after T-cell–depleted BMT compared with patients who do not develop GVHD.25 A lower incidence of GVHD after infusion of CD8-depleted bone marrow at the time of BMT compared with non–T-cell–depleted BMT has been reported.26,27 Depletion of CD8+ cells from the bone marrow may not be associated with an increased risk of relapse in these patients as it is in patients who receive pan-T-cell–depleted marrows. T-cell number is also important in the development of GVHD, and investigators have shown that increasing the number of T cells administered at the time of BMT is associated with an increased incidence of GVHD.28Infusion of increased numbers of T cells at the time of DLI may also be correlated with an increased incidence of GVHD.29
Because both T-cell number and CD8+ T cells have been implicated in the development of GVHD after BMT, we conducted a clinical trial to examine the toxicity and clinical efficacy of the infusion of defined numbers of selected T cells in patients with hematologic malignancies who have relapsed after allogeneic BMT. Three cohorts of patients received defined numbers of CD4+ T cells in a dose escalation trial. To assess the role of selective lymphocyte subsets on toxicity and efficacy, the infused cells were selectively depleted of CD8+ mononuclear cells ex vivo before infusion. Patients were observed after selective CD4+ DLI for the development of GVHD and for evidence of tumor response.
MATERIALS AND METHODS
Phase I/II clinical trial of DLI.
Forty patients with relapsed hematologic malignancies after allogeneic BMT were entered on phase I and phase II clinical trials to examine the toxicity and efficacy of different doses of CD4+ donor lymphocytes. Patients with CML had evidence of clinical relapse or greater than 10% Ph+ cells in the bone marrow when analyzed by standard techniques. Patients with hematologic malignancies other than CML had evidence of clinical relapse. Patients were required to have a performance status of 0-1 and adequate renal and hepatic function. All patients had HLA-identical sibling donors and were documented to be nonreactive in mixed lymphocyte culture before DLI. Patients were excluded if they had evidence of active grade II-IV acute GVHD or extensive chronic GVHD or were receiving immune suppressive medications to treat or prevent GVHD. In the initial phase I trial, 3 dose levels of cells were examined: (1) 0.3 × 108, (2) 1.0 × 108, and (3) 1.5 × 108 CD4+ cells per kilogram of patient weight. No interferon or other immunomodulatory agents were administered concurrently with or after DLI. After 5 to 7 patients were treated at each dose level, 23 additional patients were treated at dose level 1 to better define the toxicities and response at this dose level. These studies were approved by the Human Subjects Protection Committee of the Dana-Farber Cancer Institute and written informed consent was obtained from all patients and donors. Patient accrual was initiated in April 1994 and results were analyzed for all patients enrolled as of January 7, 1997.
Patient preparation.
All cytoreductive chemotherapy or immunotherapy was discontinued at least 1 week before DLI. No interferon was administered in preparation for DLI. No chemotherapy was administered after DLI; however, hydroxyurea could be used to control white blood cell counts, if needed, beginning 1 week after the infusion of donor cells. No immunosuppressive medications for GVHD prophylaxis were administered after DLI.
Collection and CD8 depletion of donor lymphocytes.
Using peripheral venous access, leukapheresis was performed for approximately 3 hours per session on a Cobe-Spectra collection device (Cobe BCT Inc, Lakewood, CO) on the original bone marrow donor. Mononuclear cells were isolated by density gradient centrifugation using Ficoll-Hypaque. CD8+ cells were depleted from the donor mononuclear cell fraction using anti-CD8 monoclonal antibody (MoAb; DFCI 2T8, IgG antibody) and rabbit complement and techniques that have been described previously for depletion of CD6+ T cells from bone marrow.30 31 Two cycles of depletion with MoAb and complement were performed on each pheresis product. Each cycle of ex vivo treatment consisted of incubation of donor mononuclear cells (2 × 107 cells/mL) with anti-CD8 MoAb at room temperature for 30 minutes followed by addition of baby rabbit complement (Pel Freeze, Rogers, AR) and further incubation for 30 minutes at 37°C. Cells were washed three times with tissue culture media (RPMI 1640; MediaTech, Herndon, VA) containing 2.5% human AB serum after completion of all treatments. The viability of leukocytes after depletion was confirmed by trypan blue dye exclusion. Aliquots of mononuclear cells obtained before and after CD8 depletion were analyzed for reactivity with directly fluorochrome-conjugated anti-CD4 and anti-CD8 MoAbs (Coulter Corp, Miami, FL) by flow cytometry (Coulter XL; Coulter Corp). The total number of CD4+ and CD8+ cells present after purging was calculated by multiplying the percentage of cells reactive with anti-CD4 and anti-CD8 MoAbs by the total number of nucleated cells remaining after in vitro treatment. CD8-depleted donor lymphocytes were infused over 10 to 15 minutes through an intravenous catheter. Pheresis and ex vivo treatment were repeated in a similar fashion at weekly intervals until the targeted cell number was reached.
Evaluation of toxicity and response.
Patients were evaluated at weekly intervals by physical exam, complete blood counts, and liver function tests for the first 12 weeks after infusion and then based on clinical conditions. Bone marrow and peripheral blood samples were obtained before the first infusion of donor lymphocytes, at 3 months after infusion, and subsequently when clinically indicated for analysis of morphologic, cytogenetic, and molecular response. The presence of acute and chronic GVHD was graded by standard criteria.32 Skin, liver, and bowel biopsies were performed when indicated to confirm the diagnosis of GVHD. Patients who developed GVHD were treated with prednisone. Cyclosporine was added if response to prednisone was inadequate. Patients must have been observed for greater than 8 weeks after DLI in the absence of additional chemotherapy to be considered evaluable for toxicity. Observation for at least 12 weeks without additional chemotherapy was required for evaluation of response. Patients who demonstrated no evidence of response or toxicity after 12 weeks of observation were eligible to receive additional infusion of donor lymphocytes at the next dose level. Results are analyzed as of October 1, 1997. Overall, 38 patients are evaluable for analysis of toxicity and 37 patients are evaluable for response. Two patients, 1 with multiple myeloma and 1 with CML in blast crisis, were not evaluable for toxicity or response due to rapid disease progression and were removed from study at 3 and 6 weeks, respectively, after infusion. At the time of removal from the study, neither patient had developed evidence of GVHD. One patient with relapsed AML was treated while in complete remission and is evaluable for toxicity but not response.
Cytogenetic and molecular studies.
Peripheral blood mononuclear cells (PBMC) and bone marrow cells were obtained before DLI and at regular intervals after DLI for at least 12 weeks. Cytogenetic analysis was performed on bone marrow samples using standard methods. In addition, mononuclear cells were obtained after Ficoll-Hypaque density gradient sedimentation and cryopreserved in media containing 10% dimethyl sulfoxide (DMSO) using standard techniques. For patients with CML who had no detectable metaphases with the Ph chromosome, these cryopreserved samples were used for detection of minimal residual disease. Cell samples were thawed and RNA extracted using standard techniques.33BCR-ABL transcripts were detected by a reverse transcriptase-polymerase chain reaction (RT-PCR) method using a double amplification method with nested primers as described previously.34 35 Analysis of PCR-negative samples was repeated three times to confirm a negative result.
Definitions of response.
Hematologic remission was defined as the return of normal blood counts and bone marrow cellularity. Cytogenetic response for patients with CML was defined as the absence of the Ph+ chromosome by routine cytogenetic analysis of bone marrow samples after DLI. Molecular remission for patients with CML was defined as the absence ofBCR-ABL transcripts by double amplification RT-PCR. In patients with multiple myeloma, a decrease in disease-related Ig by greater than 50% was defined as a partial response and complete elimination of monoclonal paraprotein with a normal bone marrow histology was defined as a complete response. In patients with acute leukemia or lymphoma, a complete response was defined as achievement of normal bone marrow histology and no evidence of disease by routine evaluation including appropriate x-rays or computer tomography (CT) scans.
Statistical methods.
Exact binomial confidence intervals are provided for the percentage of patients developing GVHD or responding to therapy. Associations between patient characteristics and outcome measures are assessed using the Fisher exact test.36 Predictive models were developed through univariate logistic regression. Odds ratios are calculated from the coefficients in logistic regression. Time to GVHD, cytogenetic response, and molecular response were calculated according to the method of Kaplan and Meier.37
RESULTS
Patient characteristics.
The clinical characteristics of the 40 patients who received CD4+ donor lymphocytes over a 38-month period are shown in Table 1. Twenty-five patients had CML; 7 had multiple myeloma (MM); 4 had acute myelogenous leukemia (AML); 1 had Ph+ acute lymphocytic leukemia (ALL); 1 had non-Hodgkin's lymphoma, diffuse large cell type (NHL); 1 had chronic lymphocytic leukemia (CLL); and 1 had myelodysplastic syndrome (MDS). CML was classified as being in stable phase, accelerated phase, or blast crisis according to the criteria used by the International Bone Marrow Transplant Registry.38 Thirty-nine patients had previously received T-cell–depleted allogeneic marrow as the only form of GVHD prophylaxis.30 39 One patient had received a non–T-cell–depleted transplant with cyclosporine/methotrexate for GVHD prophylaxis. The median length of time from transplantation to relapse was 21 months (range, 2 to 62 months) and the median time from relapse to cell infusion was 13 months (range, 1 to 75 months). Seven patients had a history of grade 1 GVHD after transplant and 33 patients had no prior history of GVHD. The median time on study of all evaluable patients is 50 weeks (range, 8 to 129 weeks). In the initial phase of the study, 17 patients were treated in a dose escalation schema to evaluate the toxicity of 3 doses of CD4+ donor lymphocytes obtained after ex vivo CD8 depletion. Cohorts of 5 patients received 0.3 × 108 and 1.0 × 108CD4+ cells/kg and 7 patients received 1.5 × 108 CD4+ cells/kg. To better define the toxicity and efficacy of dose level 1, 23 additional patients received infusions of 0.3 × 108 CD4+ cells/kg.
Patient Characteristics (N = 40)
| Age (median) | 45 yr | (19-59 yr) |
| Patient/donor | ||
| Sex | ||
| M/M | 11 | |
| F/F | 9 | |
| M/F | 9 | |
| F/M | 11 | |
| Disease | ||
| CML | 25 | |
| Cytogenetic relapse | 10 | |
| Stable phase | 9 | |
| Accelerated phase | 3 | |
| Blast crisis | 3 | |
| MM | 7 | |
| AML | 4 | |
| ALL | 1 | |
| NHL | 1 | |
| CLL | 1 | |
| MDS | 1 | |
| BMT/GVHD prophylaxis | ||
| CD6 T-cell depletion | 39 | |
| Cyclosporine/methotrexate | 1 | |
| Time from transplant to relapse (median) | 21 mo | (2-62 mo) |
| Time from relapse to DLI (median) | 13 mo | (1-75 mo) |
| Time from BMT to DLI (median) | 26 mo | (7-91 mo) |
| Interferon pre-DLI | 10 | |
| Follow-up (median) | 50 wk | (8-129 wk) |
| Age (median) | 45 yr | (19-59 yr) |
| Patient/donor | ||
| Sex | ||
| M/M | 11 | |
| F/F | 9 | |
| M/F | 9 | |
| F/M | 11 | |
| Disease | ||
| CML | 25 | |
| Cytogenetic relapse | 10 | |
| Stable phase | 9 | |
| Accelerated phase | 3 | |
| Blast crisis | 3 | |
| MM | 7 | |
| AML | 4 | |
| ALL | 1 | |
| NHL | 1 | |
| CLL | 1 | |
| MDS | 1 | |
| BMT/GVHD prophylaxis | ||
| CD6 T-cell depletion | 39 | |
| Cyclosporine/methotrexate | 1 | |
| Time from transplant to relapse (median) | 21 mo | (2-62 mo) |
| Time from relapse to DLI (median) | 13 mo | (1-75 mo) |
| Time from BMT to DLI (median) | 26 mo | (7-91 mo) |
| Interferon pre-DLI | 10 | |
| Follow-up (median) | 50 wk | (8-129 wk) |
Ranges are given in parentheses.
Composition of infused cells and efficacy of depletion of CD8+ cells.
CD8 depletion was performed on 67 pheresis products for the 40 patients on this study. A median of 1 (range, 1 to 5) leukopheresis session was required to achieve the targeted cell dose. An increasing number of leukopheresis sessions were required for increasing cell doses. At dose level 1, 24 patients required a single donor pheresis and 4 patients required 2 pheresis sessions to reach the targeted cell number. In 56 of the 67 depletions, no CD8+ cells were detected by direct immunofluorescence assay above background levels after ex vivo depletion. Eleven pheresis products contained small numbers of CD8+ cells detected by direct immunofluorescence after ex vivo depletion. These 11 products contained a median of 1 × 106 CD8+ cells/kg (range, 0.3 to 1.7 × 106 CD8+ cells/kg) after depletion. Eleven patients received single infusions with detectable CD8+cells (7 patients at dose level 1, 2 at dose level 2, and 2 at dose level 3). After CD8 depletion, the median composition of the infused cells in the 67 pheresis products included 51% CD3+ T cells (range, 31% to 80%), 23% CD14+ monocytes (range, 3% to 47%), 6% CD56+ natural killer cells (range, 0% to 21%), and 5% CD20+ B cells (range, 0% to 19%). The absolute median number of CD3+, CD4+, CD8+, CD56+, and CD20+ cells infused at each dose level is summarized in Table 2. Before depletion, CD8+ cells represented a median of 21% (range, 6% to 38%) of the mononuclear cells in the pheresis product.
Characteristics of Infused Cells After Ex Vivo CD8 Depletion
| Dose Level . | N . | Median No. of Pheresis Sessions/Patient . | CD3 . | CD4 . | CD8 . | CD56 . | CD20 . |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 1 | 3.5 (2.1-3.0) | 3.0 (2.1-3.7) | <0.01 (0-0.2) | 0.3 (0-0.9) | 0.4 (0.1-0.7) |
| 2 | 5 | 2 | 11.3 (8.1-13.1) | 9.4 (7.9-10.3) | <0.01 (0-0.1) | 1.0 (0.4-1.9) | 0.9 (0.2-2.4) |
| 3 | 7 | 2 | 13.2 (10.4-18.8) | 13.2 (10.9-16.8) | <0.01 (0-0.1) | 2.3 (1.4-6.4) | 1.6 (0.7-2.7) |
| Dose Level . | N . | Median No. of Pheresis Sessions/Patient . | CD3 . | CD4 . | CD8 . | CD56 . | CD20 . |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 1 | 3.5 (2.1-3.0) | 3.0 (2.1-3.7) | <0.01 (0-0.2) | 0.3 (0-0.9) | 0.4 (0.1-0.7) |
| 2 | 5 | 2 | 11.3 (8.1-13.1) | 9.4 (7.9-10.3) | <0.01 (0-0.1) | 1.0 (0.4-1.9) | 0.9 (0.2-2.4) |
| 3 | 7 | 2 | 13.2 (10.4-18.8) | 13.2 (10.9-16.8) | <0.01 (0-0.1) | 2.3 (1.4-6.4) | 1.6 (0.7-2.7) |
Values are the number of cells infused per patient × 107 cells/kg given as median (range in parentheses).
Incidence of GVDH after DLI.
Overall, 12 of 38 evaluable patients (32%; 90% confidence interval [CI], 19% to 46%) developed evidence of acute or chronic GVHD after CD8-depleted DLI. As shown in Table 3, 6 patients developed acute GVHD (4 had grade II and 2 had grade III). Three patients developed de novo extensive chronic GVHD, as manifested by mucocutaneous involvement and liver function test abnormalities. Three patients developed GVHD with simultaneous characteristics of both acute (liver and bowel) and chronic (mucocutaneous) GVHD. One patient treated at dose level 3 developed grade 3 acute GVHD and died of infectious complications related to immunosuppression. This was the only death in this patient population attributable to any cause other than disease relapse.
Toxicity Associated With CD4+ DLI
| Dose Level (CD4+ cells/kg) . | GVHD . | Neutropenia (ANC <500) . | Thrombocytopenia (Plt <20,000) . | ||
|---|---|---|---|---|---|
| Acute . | Chronic . | Acute + Chronic . | |||
| 1 (0.3 × 108) | 4/27 | 2/27 | 4/22 | 3/22 | |
| 2 (1.0 × 108) | 1/5 | 1/5 | 1/5 | 0/4 | |
| 3 (1.5 × 108) | 1/6 | 2/6 | 1/6 | 2/6 | 3/6 |
| Total | 6/38 | 3/38 | 3/38 | 7/33 | 6/32 |
| Dose Level (CD4+ cells/kg) . | GVHD . | Neutropenia (ANC <500) . | Thrombocytopenia (Plt <20,000) . | ||
|---|---|---|---|---|---|
| Acute . | Chronic . | Acute + Chronic . | |||
| 1 (0.3 × 108) | 4/27 | 2/27 | 4/22 | 3/22 | |
| 2 (1.0 × 108) | 1/5 | 1/5 | 1/5 | 0/4 | |
| 3 (1.5 × 108) | 1/6 | 2/6 | 1/6 | 2/6 | 3/6 |
| Total | 6/38 | 3/38 | 3/38 | 7/33 | 6/32 |
Although limited numbers of patients in the phase I study were treated at dose levels 2 and 3, the incidence of GVHD appeared to be higher in patients who received larger numbers of donor cells (P = .07). Six of 27 patients (22%) who received 0.3 × 108CD4+ cells/kg (dose level 1) developed acute or chronic GVHD, whereas 6 of 11 patients (55%) who received ≥1.0 × 108 CD4+ cells/kg (dose levels 2 and 3) developed GVHD. The median time to onset of GVHD in all patients who developed GVHD was 11 weeks (range, 7 to 42 weeks). There was no difference in the time to onset of GVHD in patients treated at different dose levels. The presence of detectable residual CD8+ cells after ex vivo treatment was not associated with subsequent GVHD.
Cytopenia after CD4+ DLI.
Peripheral cytopenias (absolute neutrophil count <500/μL or platelet count <20,000/μL) not related to disease progression were noted in 7 patients (Table 3). Six patients developed both neutropenia and thrombocytopenia related to DLI, and 1 patient developed thrombocytopenia alone. Only patients who demonstrated responses to DLI developed cytopenias related to lymphocyte infusion. Cytopenias related to DLI occurred in patients with and without evidence of GVHD.
The median time to the development of neutropenia and thrombocytopenia related to DLI was 12 weeks (neutropenia range, 8 to 62 weeks; thrombocytopenia range, 7 to 61 weeks). Of those patients evaluable for the development of cytopenia, 4 of 22 patients (18%) treated at dose level 1 and 3 of 11 patients (27%) treated at dose levels 2 and 3 developed neutropenia secondary to DLI. Three of 22 (14%) patients treated at dose level 1 and 3 of 10 (30%) patients treated at dose levels 2 and 3 developed thrombocytopenia after DLI. In all but 1 case, episodes of neutropenia and thrombocytopenia were transient after DLI. One patient with relapsed stable-phase CML treated at dose level 1 had prolonged pancytopenia. This patient received an infusion of CD6 T-cell–depleted marrow30 from the same donor without additional chemotherapy or radiotherapy conditioning and subsequently demonstrated engraftment with normal blood counts. No infectious or hemorrhagic complications related to neutropenia or thrombocytopenia were noted in any of these patients.
Response to CD4+ DLI.
Thirty-seven patients treated on this study are evaluable for response (Table 4). Follow-up for at least 12 weeks after DLI and measurable evidence of disease at the time of cell infusion were both required criteria for evaluable response. Patients evaluable for response after initial DLI included 24 with CML, 6 with multiple myeloma, 3 with AML, and 1 each with ALL, NHL, CLL, and MDS. Overall, 21 responses were noted (57%; 90% CI, 42% to 71%). Responses occurred frequently in patients with stable-phase or cytogenetic CML relapse (79%; 90% CI, 58% to 92%) and multiple myeloma (83%; 90% CI, 42% to 99%), but only 14% (90% CI, 1% to 52%) of patients with other hematologic malignancies responded to DLI. None of the 5 patients with accelerated phase or blastic phase CML responded to initial DLI.
Response to CD4+ DLI
| Dose Level (CD4+ cells/kg) . | CML Cytogenetic or Stable-Phase Relapse . | CML (accelerated or blast crisis) . | Multiple Myeloma (>50% decrease in paraprotein) . | ALL/AML NHL/CLL MDS . | |
|---|---|---|---|---|---|
| Cytogenetic Response . | Molecular Response . | ||||
| 1 (0.3 × 108) | 10/14 | 7/14 | 0/4 | 1/2 | 1/6 |
| 2 (1.0 × 108) | 1/1 | 1/1 | 0/1 | 2/2 | 0/1 |
| 3 (1.5 × 108) | 4/4 | 4/4 | 2/2 | ||
| Total | 15/19 | 12/19 | 0/5 | 5/6 | 1/7 |
| Dose Level (CD4+ cells/kg) . | CML Cytogenetic or Stable-Phase Relapse . | CML (accelerated or blast crisis) . | Multiple Myeloma (>50% decrease in paraprotein) . | ALL/AML NHL/CLL MDS . | |
|---|---|---|---|---|---|
| Cytogenetic Response . | Molecular Response . | ||||
| 1 (0.3 × 108) | 10/14 | 7/14 | 0/4 | 1/2 | 1/6 |
| 2 (1.0 × 108) | 1/1 | 1/1 | 0/1 | 2/2 | 0/1 |
| 3 (1.5 × 108) | 4/4 | 4/4 | 2/2 | ||
| Total | 15/19 | 12/19 | 0/5 | 5/6 | 1/7 |
Cytogenetic and molecular remissions after CD4+ DLI in patients with CML.
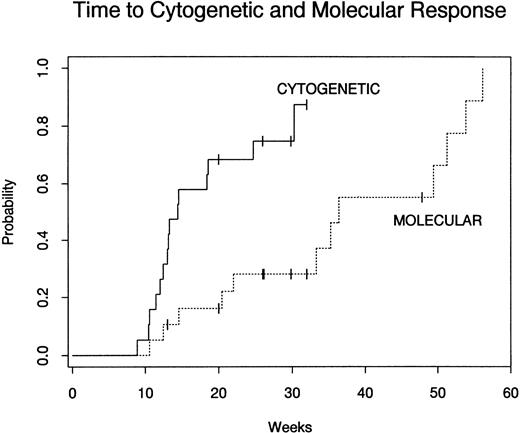
The probabilities of complete cytogenetic or molecular response for 19 patients with either cytogenetic relapse or stable phase CML relapse are shown in Fig 1. At 6 months after a single treatment course of donor lymphocytes, the Kaplan-Meier probability of complete cytogenetic response was 74% and complete molecular response was 28%. At 1 year after DLI, the probability of complete cytogenetic response was 87% and the probability of complete molecular response was 78%. Although the overall response rate was very high for patients with cytogenetic or stable-phase CML relapse, the median time to both cytogenetic and molecular response was prolonged. As shown in Fig 1, the median time to cytogenetic response from the time of first CD4+ DLI was 13 weeks (range, 9 to 30 weeks), whereas the median time to elimination of theBCR-ABL transcript detectable by PCR was 34 weeks (range, 11 to 56 weeks). No patient who has obtained a cytogenetic remission has relapsed with a median of 72 weeks (range, 25 to 127 weeks) of follow-up after obtaining cytogenetic remission. No patient who has obtained a molecular remission has had any subsequent blood or marrow samples positive for BCR-ABL transcript by PCR.
Probability of complete cytogenetic response and molecular remission after CD4+ DLI in patients with relapsed early-phase CML. Nineteen patients with cytogenetic or stable-phase CML relapse were evaluable for response. Three nonresponders received second infusions of CD4+ donor lymphocytes and were censored at the time of second infusion.
Probability of complete cytogenetic response and molecular remission after CD4+ DLI in patients with relapsed early-phase CML. Nineteen patients with cytogenetic or stable-phase CML relapse were evaluable for response. Three nonresponders received second infusions of CD4+ donor lymphocytes and were censored at the time of second infusion.
Response to CD4+ DLI in patients with MM.
Seven patients with MM received CD4+ DLI. Three patients had IgG, 2 patients had IgA, and 2 patients had light chain disease. One patient died 3 weeks after lymphocyte infusion related to rapidly progressive disease and was not evaluable for response. Six patients with MM are evaluable for response (Table 4). Five of 6 patients demonstrated decreasing numbers of plasma cells in the bone marrow associated with decreased numbers of monotypic Ig staining plasma cells. Three patients, 2 with light chain disease and 1 with IgG myeloma, achieved complete responses after DLI documented by complete elimination of detectable monoclonal plasma cells in marrow biopsies as well as complete elimination of detectable paraprotein in blood and urine. Two patients achieved a partial response with a reduction in myeloma-associated protein by greater than 50%. At the time of maximal response in these 2 patients, bone marrow biopsies continued to demonstrate persistent involvement with MM. Three of the 5 patients who responded had an initial increase in paraprotein level 4 weeks after DLI. Subsequent measurements demonstrated progressively decreasing levels of paraprotein. The median time to maximal response in patients with relapsed MM was 26 weeks (range, 18 to 62 weeks). In contrast to patients with CML, in whom responses have been durable in all patients thus far, 3 patients with MM who responded to DLI subsequently demonstrated progression. Two patients have remained in complete remission 12 and 28 months after CD4+ DLI.
Response to DLI in patients with acute leukemia and lymphoma.
Four patients with AML and 1 each with ALL, NHL, CLL, and MDS (RAEBT) received infusions of CD4+ donor lymphocytes. Seven of 8 patients with acute leukemia or lymphoma had evidence of active disease at the time of DLI. Six of 7 patients with active disease at the time of DLI had progression of their disease after DLI. The single patient with MDS was in hematologic relapse with 20% myeloblasts in the marrow at the time of DLI and achieved a complete remission 10 weeks after DLI. One patient with AML who had relapsed after allogeneic BMT had received one cycle of salvage chemotherapy and was in complete remission at the time of DLI. This patient remains in remission 48 weeks after DLI and has no evidence of GVHD.
CD4+ cell dose escalation for patients without evidence of response or toxicity.
Nine patients who were treated at the first dose level and did not develop toxicity or evidence of response received a second infusion of CD4+ donor cells at dose level 2. These included 3 patients with stable-phase CML, 3 patients with advanced CML, and 1 patient each with AML, CLL, and MM. The median time from the first infusion to the second infusion in these patients was 21 weeks (range, 12 to 28 weeks). Three patients with CML responded to the second infusion of CD4+ donor cells. None of these patients was classified as a responder for the previous analysis summarized in Table 4. One patient with stable-phase CML relapse demonstrated a complete cytogenetic response 26 weeks after the second infusion but has not achieved a molecular response. One patient with accelerated phase CML demonstrated a complete cytogenetic response after the addition of interferon 50 weeks after the second DLI. This patient achieved a complete molecular response with no detection of the BCR-ABLtranscript by PCR 68 weeks after the second infusion and remains in remission 30 months after the second infusion. This patient also developed extensive chronic GVHD 70 weeks after the second infusion. One patient with CML in accelerated phase developed a complete hematologic response after the addition of interferon 30 weeks after a second DLI. No other patients who received a second infusion of donor cells developed evidence of GVHD. Two patients with stable-phase CML have not shown evidence of response 7 and 9 months after a second DLI.
Relationship of GVHD to response after CD4+DLI.
All 12 patients who developed GVHD demonstrated a response to DLI. This included 8 patients with CML, 3 patients with MM, and 1 patient with MDS. Although the development of GVHD was strongly associated with response (P = .0004), responses were also noted in 9 patients without the development of either acute or chronic GVHD. Seven complete cytogenetic responses occurred in patients with early phase CML in the absence of GVHD. Two patients with MM also demonstrated responses, including 1 complete response, in the absence of GVHD.
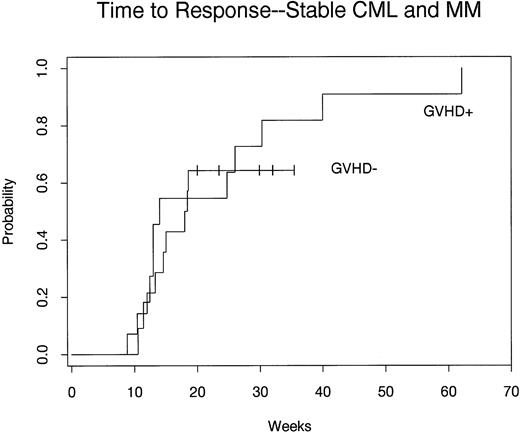
To better define the relationship between GVHD and response, we compared the time to response in patients who developed GVHD with patients that did not develop GVHD (Fig 2). This comparison included all 25 evaluable patients with cytogenetic or stable-phase CML relapse or MM. As shown in Fig 2, the probability of response was 100% in patients who developed GVHD compared with 72% in patients without GVHD. This difference was not significantly different (P = .75). The analysis shown in Fig 2 also demonstrates that the time required to achieve a response was very similar in patients who either developed or did not develop GVHD.
Probability of response after CD4+ DLI in 25 patients with relapsed early-phase CML or MM. Time to response is compared in patients who developed GVHD (n = 11) and patients who did not develop GVHD (n = 14).
Probability of response after CD4+ DLI in 25 patients with relapsed early-phase CML or MM. Time to response is compared in patients who developed GVHD (n = 11) and patients who did not develop GVHD (n = 14).
Factors associated with the development of GVHD and response after CD4 DLI.
A variety of clinical factors listed in Table 5 were examined for possible association with either GVHD or response after DLI. Each variable was analyzed by Fisher exact tests in the 38 patients evaluable for toxicity (GVHD) or in the 37 patients evaluable for response. Factors such as patient gender, age at DLI, time from transplant to DLI, prior interferon, and GVHD after BMT were not associated with the development of GVHD or response after DLI. Six patients (3 stable-phase CML and 3 advanced CML) received hydroxyurea to control increasing peripheral blood counts after DLI. Only 1 of these patients with stable-phase relapse responded to the initial course of DLI. Two additional patients who received hydroxyurea responded to a second infusion of CD4+ DLI. The number of cells infused and sex of the donor may influence the likelihood of developing GVHD. When analyzed together, infusion of cells at dose levels 2 or 3 from a female donor was significantly associated with the development of GVHD (P = .006). In addition to the development of GVHD, the only clinical variables associated with response were a diagnosis of CML or MM. There was no significant association of response with the dose of CD4+ cells infused.
Factors Associated With the Development of GVHD or Response After CD4+ DLI
| Clinical Variable . | GVHD . | Response . |
|---|---|---|
| Dose level 2 or 3 | .07 | .14 |
| Diagnosis of CML/MM | .39 | .04 |
| Female donor | .09 | .32 |
| Patient gender | .49 | 1.00 |
| Age >40 yr at DLI | .15 | .30 |
| Less than 1 yr post-BMT | 1.00 | .14 |
| Prior interferon | .12 | .26 |
| CD8+ cells infused | .71 | .73 |
| Development of GVHD | .0004 |
| Clinical Variable . | GVHD . | Response . |
|---|---|---|
| Dose level 2 or 3 | .07 | .14 |
| Diagnosis of CML/MM | .39 | .04 |
| Female donor | .09 | .32 |
| Patient gender | .49 | 1.00 |
| Age >40 yr at DLI | .15 | .30 |
| Less than 1 yr post-BMT | 1.00 | .14 |
| Prior interferon | .12 | .26 |
| CD8+ cells infused | .71 | .73 |
| Development of GVHD | .0004 |
Values are Fisher exact test P values.
Cytogenetic abnormalities in residual host nonleukemic cells.
In 9 patients with early-phase CML relapse, cells with cytogenetic abnormalities in the absence of the Ph chromosome were noted before the time of DLI. Presumably, these represented residual recipient cells with radiation- or chemotherapy-induced cytogenetic abnormalities that were unrelated to the malignant clone. Five of these patients had sex-mismatched transplants that definitively identified the abnormal metaphases as being derived from residual recipient cells. Two additional patients who received sex-mismatched transplants also had residual recipient metaphases with normal karyotypes detected by cytogenetic evaluation after BMT. Ten of these 11 patients achieved a complete cytogenetic remission after DLI with elimination of all metaphases containing the Ph chromosome. At the time of cytogenetic remission, both abnormal and normal cells unrelated to the Ph+ leukemic clone were also eliminated in every patient and were no longer detected on subsequent analysis.
DISCUSSION
Specific T-cell populations and the number of cells infused have both been implicated in the development of GVHD after BMT and after DLI. The primary objectives of the current phase 1 study were to identify the toxicities associated with infusion of CD4+ donor lymphocytes and to determine an appropriate dose of CD4+donor cells for use in future clinical trials of adoptive cellular therapy after allogeneic BMT. To accomplish these aims, cohorts of patients received defined numbers of CD4+ donor lymphocytes after ex vivo depletion of CD8+ cells. No patients received additional immune-stimulating or immune-suppressive agents, and no further cellular therapy was administered for at least 6 months after the initial course of therapy. As a result, we were able to evaluate both the role of selective lymphocyte populations and cell number and the risk of GVHD after DLI. The overall incidence of GVHD was relatively low, with only 12 of 38 (32%) patients developing acute or chronic GVHD. In a previous clinical trial of CD8-depleted DLI in 10 patients with relapsed CML, Giralt et al40 reported that only 3 patients developed acute or chronic GVHD after infusion of a mean of 0.9 × 108 mononuclear cells/kg. These results compare favorably with results of unfractionated DLI in 140 patients reported by Collins et al.9 In this large experience compiled from 25 transplant centers in North America, 76% of patients developed acute or chronic GVHD. However, the majority of patients in that report received unmanipulated infusions of more than 1 × 108 T cells/kg. By reducing the total number of T cells infused and removal of CD8+ cells, the overall incidence of GVHD appears to be reduced in the two trials of CD8-depleted DLI. The relative contributions of limiting the number of T cells infused or CD8+ depletion in the reduction in GVHD cannot be adequately assessed in the current phase I study. Further studies will be necessary to define the importance of these two factors in the development of GVHD after DLI.
Lowering the incidence of GVHD after DLI may have important clinical implications for the use of this treatment modality in larger numbers of patients after allogeneic BMT. With a relatively low incidence of GVHD in our study, we noted that treatment-related mortality associated with DLI was only 3%. In contrast, Collins et al9 reported that the probability of mortality not related to hematologic malignancy was 14% at 1 year and 18% at 2 years in recipients of unfractionated DLI. The lower incidence of treatment-related mortality in patients receiving CD8-depleted DLI is likely due to the finding that only 18% of patients developed severe acute GVHD (grade 3-4) or extensive chronic GVHD, and therefore very few patients required intensive immune-suppressive therapy for prolonged periods after CD8-depleted DLI. This relatively low incidence of severe toxicity associated with CD8-depleted DLI may be very helpful in planning future studies to evaluate the routine use of DLI in larger patient populations at high risk for relapse after allogeneic rather than in relatively small numbers of patients who have already demonstrated definitive evidence of relapse.41
In contrast to its effect on GVHD, CD8 depletion of donor lymphocytes does not appear to compromise the GVL effect in patients with early-stage CML relapse. In our study, the probabilities of achieving a complete cytogenetic and complete molecular response were 87% and 78%, respectively, at 1 year after DLI. These results are similar to the large multicenter experience reported by Collins et al9in which 74% of patients with early-stage CML relapse achieved complete cytogenetic remission after infusion of unfractionated donor lymphocytes. However, 90% of patients in the experience reported by Collins et al9 had morphologic evidence of relapse, compared with only 47% in the current study. Thus, despite the high response rate demonstrated after infusion of CD8-depleted cells, further studies may be necessary to directly compare the efficacy of CD8-depleted cells with unfractionated DLI. Of note, the cytogenetic and molecular remissions noted in our study also appear durable, with no recurrence of disease noted in any of these patients. As with unmanipulated infusions, patients with advanced-phase CML relapse did not respond well to CD8-depleted DLI. In our study, no responses were noted after a single course of therapy and only 2 patients with accelerated CML subsequently responded to a second infusion of CD8-depleted cells.
Two patients in this study had rapidly progressive disease and were not considered evaluable for toxicity or response. If these 2 patients are included in the analysis of toxicity, the overall incidence of GVHD in the study is 30%, compared with 32% if these patients are included. The incidence of GVHD at dose level 1 is also not significantly affected by the addition of these patients. With respect to response, 1 patient had CML in blast crisis and the other had MM. Inclusion of the patient with MM in our analysis of response would result in a response rate of 71% in this patient population. No significant changes are noted in the factors associated with either the development of GVHD or response by the addition of these 2 patients to the analysis.
Reports of previous clinical trials have noted that the time to complete cytogenetic response after DLI in patients with CML can be prolonged. However, the time required to achieve response has been difficult to define in previous studies, because many patients received additional infusions of donor cells weeks to months after the initial infusion. Moreover, many patients in other studies have also received additional immune stimulating therapy with interferon-α or other agents in conjunction with DLI. In our study, no patients with stable-phase CML relapse received a second infusion of donor cells less than 6 months after the initial infusion, and none received any other immune modulating therapy within the first year after DLI. As shown in Fig 1, the vast majority of cytogenetic responses occurred by 6 months after DLI in patients with early stage CML relapse. Occasional patients required longer than 6 months, with the probability of cytogenetic complete response increasing slightly from 74% at 6 months to 87% at 1 year after DLI. In contrast, molecular responses are achieved much more slowly, with only a 28% probability of patients being PCR negative by 6 months, compared with 78% by 12 months after DLI. These results suggest that patients with clinically stable CML relapse should be observed for evidence of a cytogenetic response without additional treatment for at least 6 months after DLI. Other treatment options, including retreatment with donor lymphocytes at a similar or increased dose of cells, addition of interferon-α or interleukin-2 (IL-2),42 or a second BMT may play an important role in patients who fail to respond to initial DLI. However, these additional treatments should not be considered until sufficient time has elapsed for adequate evaluation of their initial therapy.
Patients with relapsed MM were also noted to have a high response rate (83%) to DLI, comparable to patients with early-stage CML.43 However, unlike patients with CML, the response in patients with MM was not always complete or durable. Three patients developed complete responses to DLI and 2 others demonstrated partial responses. Although 2 patients remain in a complete remission, several patients who responded subsequently developed evidence of progressive disease. Interestingly, progressive disease in 2 patients occurred with the development of plasmacytomas that were noted while the bone marrow and serum paraprotein continued to show evidence of a response. This finding may suggest that the DLI reaction in patients with myeloma is primarily bone marrow directed and that extramedullary sites may remain sanctuary sites of disease. As with patients with CML, the time to response in patients with myeloma can be prolonged, and an initial increase in Ig after DLI should not be viewed as a failure to respond to DLI.
The results of this study demonstrate that the clinical development of GVHD is not required for GVL. Overall, 43% of patients in this study responded to CD8-depleted DLI without clinical evidence of GVHD. Of patients with early stage CML, 46% achieved a complete response without GVHD, whereas 40% of patients with myeloma had evidence of a response without GVHD. The time to response in patients who developed GVHD and those who did not was similar. Moreover, except for infusion of larger numbers of cells from female donors, there were no pre-DLI clinical characteristics identified that predicted for an increased risk of GVHD after DLI.
Although responses can occur in the absence of GVHD, the development of GVHD is nevertheless highly associated with response. This close relationship between GVHD and GVL has been noted in prior studies. Collins et al9 reported that 42 of 45 patients (93%) who developed a complete response also developed GVHD. In our study, GVHD was noted only in patients who developed a response after DLI. In fact, there were no patients in our study who developed GVHD and did not respond. This is in contrast to results of unmanipulated lymphocyte infusions, in which GVHD can occur in some patients in the absence of a response. This observation suggests that one possible effect of CD8 depletion may be the selective removal of those alloreactive T cells that may be responsible for GVHD in the absence of a GVL effect.
Although Fig 2 shows that the achievement of clinical response is not dependent on the development of GVHD, evidence of the GVL reaction is often noted in patients before the clinical development of GVHD. Although some patients developed evidence of GVHD simultaneously with response, there were no instances in our study in which development of GVHD preceeded a response. Evidence of the GVL reaction includes the decrease in white blood cell counts as well as decreasing numbers of Ph+ cells in the bone marrow that often occur before obtaining a complete cytogenetic response. In some cases, cytogenetic responses in patients with CML preceded the development of GVHD by weeks to months. A similar pattern has been noted in patients with MM, with a decrease in monoclonal protein often noted before development of GVHD. Taken together with the observation that GVHD was noted only in responding patients, these clinical findings suggest the possibility that GVL may induce GVHD, an association that has often been viewed in reverse.6,44 45 Although the potential mechanisms whereby this may occur in vivo are highly speculative, it is possible that GVL in vivo may generalize from a disease-specific or hematopoietic-restricted alloantigen-specific response to a more nonspecific reaction such as GVHD. As further experiments define the mechanism of GVL in vivo, it may be possible to begin to examine this hypothesis.
A previous report by Mackinnon et al29 suggested that infusion of increasing numbers of cells at the time of DLI was associated with an increased incidence of GVHD. However, the results of this study were somewhat difficult to interpret, because patients were eligible to receive additional infusions of donor cells 4 weeks after a previous infusion if there was no evidence of toxicity at that time. Although our study was not designed to have adequate power to detect differences in GVHD incidence between dose levels, a suggestion of an increased incidence of GVHD was noted in patients treated with ≥1.0 × 108 CD4+ cells/kg compared with patients treated with 0.3 × 108 CD4+cells/kg (55% v 22%). However, two large registry reports did not find a relationship between the number of cells infused and the risk of GVHD.8,9 These studies examined patients receiving greater than 3 or ≥4 × 108 mononuclear cells/kg compared with patients receiving fewer cells. The data presented in this study and others29,46 suggest that the cell number threshold for the development of significant GVHD may be well below the levels examined in these reports. With these conflicting results, the optimal dose of cells to be infused at the time of DLI has yet to be determined. The single infusion of 1 × 107CD3+ cells/kg reported by Mackinnon et al29 and 3 × 107 CD4+ cells/kg reported in our study appear to be associated with a relatively low incidence of GVHD. In future studies, the efficacy of lower doses of cells should be explored recognizing the often prolonged time to the development of GVHD as well as disease response after a single infusion of cells.
Whereas significant responses were noted in patients with early-stage CML and multiple myeloma, no significant responses were noted in patients with advanced CML or acute leukemia after a single course of cell infusion. The reason for this lack of response is not known. One possible explanation is the rapid progression of disease in patients with acute leukemia. As noted previously, the time to response after DLI is often prolonged and the GVL reaction may not have sufficient time to develop in patients with rapidly progressive disease. An alternative hypothesis is that acute leukemia cells do not contain suitable target antigens or may not be capable of proper antigen presentation at the cell surface. Interestingly, no evidence of GVHD was noted in these patients as well. Future studies to improve the efficacy of response in patients with advanced CML or acute leukemia may include the treatment of these patients while in a minimal disease state or the addition of cytokines, such as IL-2, which may lead to the activation of specific T-cell populations, promote proper presentation of target antigens, or amplify the GVL response in these patients.
The mechanism and specificity of the DLI reaction remains uncertain. T cells are believed to the primary mediators of GVL. Whereas almost all detectable CD8+ T cells were removed by the depletion, their participation in the GVL reaction after DLI cannot be excluded. Phenotypic analysis of circulating lymphocytes did not demonstrate the expansion of any specific effector population. In other studies, the analysis of T-cell repertoire in CML patients treated with CD8-depleted DLI demonstrates the clonal expansion of specific T-cell populations that coincide with the development of cytogenetic response.47 The identification of the expanded T cells, their CD4 or CD8 subtype, and the specificity of these cells has yet to be determined.
Whether DLI represents a disease-specific or allo-specific response is unclear. In sex-mismatched donor-recipient pairs, all patients who responded to DLI had cytogenetic conversion to donor hematopoiesis. This finding is consistent with others who have also demonstrated a conversion from a mixed chimeric state before infusion to complete donor hematopoiesis after infusion.6,29,48 In our study, we noted that radiation-induced cytogenetic abnormalities in residual host cells were also eliminated at the time of response. Taken together, these observations suggest that the DLI reaction is likely to be directed against allo-specific antigens rather than disease-specific targets.49 Minor HLA antigens represent possible examples of allo-antigens expressed on hematopoietic stem cells that may be targets of the GVL response,50 51 but other unrecognized targets may also exist. The restricted expression of minor histocompatibility antigens may also function to limit the toxicity of this treatment and limit the extent and severity of GVHD. The effectiveness of DLI in CML and MM may be due to the derivation of these tumors from hematopoietic stem cells or to their ability to express the GVL target antigens at relatively high levels compared with other cells. As further studies begin to identify the target antigens of the GVL response in patients with different hematologic malignancies, it may be possible to develop new clinical strategies to selectively amplify the antileukemia effects of treatment with donor cells.
Supported by National Institutes of Health Grants No. AI29530 and CA01730 and the Leonard Frankel Foundation for Leukemia.
Address reprint requests to Edwin P. Alyea, MD, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: edwin_alyea@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal