Abstract
Cytokines produced by stromal cells induce the proliferation and differentiation of hematopoietic cells in the marrow microenvironment. We hypothesized that cross-talk between hematopoietic cells at different stages of differentiation and stromal cells influences stromal cytokine production and is responsible for maintaining steady-state hematopoiesis and responding to stress situations. We show that coculture of primitive CD34+ cells in contact with or separated by a transwell membrane from irradiated human bone marrow stromal layers induces a fourfold to fivefold increase in interleukin-6 (IL-6) and granulocyte colony-stimulating factor (G-CSF) levels in the stromal supernatant (SN) during the first week. Levels of both cytokines decreased to baseline after coculture of CD34+cells for 3 to 5 weeks. Coculture of more mature CD15+/CD14− myeloid precursors induced only a transient 1.5- to 2-fold increase in IL-6 and G-CSF at 48 hours. Neither CD34+ nor CD15+/CD14−cells produced IL-6, G-CSF, IL-1β, or tumor necrosis factor α. When CD34+ cells were cultured in methylcellulose medium supplemented with cytokines at concentrations found in stromal SN or supplemented with stromal SN, a fourfold to fivefold increase in colony formation was seen over cultures supplemented with erythropoietin (EPO) only. When cultures were supplemented with the increased concentrations of IL-6 and G-CSF detected in cocultures of stroma and CD34+ cells or when CD34+ cells were cocultured in methylcellulose medium in a transwell above a stromal layer, a further increase in the number and size of colonies was seen. The colony-forming unit–granulocyte-macrophage–stimulating activity of stromal SN was neutralized by antibodies against G-CSF or IL-6. These studies indicate that primitive CD34+ progenitors provide a soluble positive feedback signal to induce cytokine production by stromal cells and that the observed increase in cytokine levels is biologically relevant.
IN STEADY-STATE hematopoiesis, proliferation and differentiation of immature progenitors occurs in the bone marrow (BM) microenvironment. It is well known that cytokines produced locally by stromal cells are responsible for induction of cell proliferation and differentiation. However, it is less clear how steady-state levels of mature blood elements are maintained. Cytokines detectable in the blood of normal donors include interleukin-1β (IL-1β), IL-3, IL-6, and tumor necrosis factor α (TNF-α).1,2 In neutropenic patients, serum levels of IL-6, granulocyte-colony stimulating factor (G-CSF), and flt3-ligand, but not granulocyte macrophage-colony stimulating factor (GM-CSF) or IL-3, increase to the range of 500 to 1,000 pg/mL.2-11 It is unclear which signals are responsible for the increase in cytokine levels or if these increased levels of cytokines have functional importance. We hypothesized that cross-talk between cytokine-producing stromal cells and immature and mature hematopoietic cells is needed both to maintain steady-state hematopoiesis and to respond to stress situations.
Reverse transcription-polymerase chain reaction (RT-PCR) studies on BM biopsy specimens from healthy volunteers, reflective of in vivo steady-state hematopoiesis, have shown the presence of IL-6, stem cell factor (SCF), macrophage inflammatory protein-1α (MIP-1α), transforming growth factor β (TGF-β), TNF-α, and IL-1β but not G-CSF, GM-CSF, leukemia inhibitory factor (LIF), IL-3, or IL-1α mRNA.12 Stromal cells have also been shown to produce flt3-ligand.13 The in vivo microenvironment is mimicked at least in part by the in vitro stroma-dependent long-term bone marrow cultures (LTBMCs). As for steady state in vivo hematopoiesis, stromal supernatants contain picogram concentrations of a few cytokines including G-CSF, IL-6, and GM-CSF.14-16 Therefore, in the present studies we used the stromal LTBMC system to determine the effect of hematopoietic progenitors at different stages of differentiation on stromal secretion of cytokines and the potential of such cytokines to induce growth of committed clonogenic cells in vitro. We show that immature CD34+ cells but not maturing CD15+/CD14− myeloid cells induce an upregulation of cytokine production by stromal cells and that this is caused by a soluble factor derived from CD34+ cells. Thus, these studies show that hematopoietic cells can influence stromal production of cytokines.
MATERIALS AND METHODS
Cell separation.
Normal human BM was obtained from healthy volunteer donors after informed consent. Bone marrow mononuclear cells (BMMNCs) were separated by using Ficoll-Hypaque (Sigma, St Louis, MO) centrifugation. CD34+ cells were enriched either by sequential counterflow elutriation centrifugation and depletion of lineage committed progenitors using immunomagnetic beads17-19 or by selection with a Ceprate-LC CD34-avidin immunoadsorption column (CellPro Inc, Bothell, WA).20CD34+/HLA-DR− (DR−) and Lin−/CD34+/HLA-DR+(DR+) cells were purified by fluorescence-activated cell sorting (FACS), as previously described.18 Large cells in the “rotor off” fraction from counterflow elutriation centrifugation were separately subjected to FACS to obtain CD15+/CD14−(15+/14−) maturing myeloid precursors.
Stromal layers.
Human marrow stromal layers were established from BMMNCs as described.18 When confluent, the flasks were irradiated with 1,250 rads. Irradiated stromal feeders were subcultured in 24-well culture plates and maintained at 37°C by weekly replacement of half the medium with fresh LTBMC medium consisting of Iscove's modified Dulbecco's medium with 12.5% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), 12.5% horse serum (StemCell Technologies Inc, Vancouver, Canada), 2 mmol/L L-glutamine (GIBCO BRL, Gaithersburg, MD), 1,000 U/mL penicillin, 100 U/mL streptomycin, and 10−6 mol/L hydrocortisone.18
M2-10B4 cells (a kind gift from Dr C.J. Eaves, Terry Fox Laboratories, Vancouver, Canada) were cultured in RPMI-1640 plus 10% FCS. When confluent, cells were irradiated with 2,000 rads and subsequently maintained in LTBMC medium.
Liquid suspension cultures.
Ten thousand DR+ or DR− cells or 10,000 to 50,000 15+/14− cells were cocultured in contact with irradiated stromal layers or separated from stroma by a collagen-coated transwell insert (0.4-μm microporous filter; Costar Corp, Cambridge, MA) in 24-well culture plates in LTBMC medium. Cultures were incubated in a humidified 5% CO2 atmosphere at 37°C for up to 5 weeks and maintained by weekly replacement of half the medium with fresh LTBMC medium.
Supernatants (SNs).
SNs from irradiated cultures of human BM stroma or from cocultures of hematopoietic progenitors in contact with (stroma contact) or separated from (stroma noncontact) irradiated stromal layers were harvested 2, 4, or 7 days after a half-medium change during the first week, after initiation of the cultures, and on day 2 during the third, fourth, and fifth weeks. SNs were centrifuged at 400g for 10 minutes to remove cell debris and then frozen at −70°C until use.
Enzyme-linked immunosorbent assays (ELISA) for cytokines.
Cytokine concentrations were measured with commercially available ELISA kits for human IL-1β (sensitivity 0.3 pg/mL), IL-3 (sensitivity 7.4 pg/mL), IL-6 (sensitivity 0.35 pg/mL), IL-7 (sensitivity 6 pg/mL), G-CSF (sensitivity 10.9 pg/mL), SCF (sensitivity 3 pg/mL), LIF (sensitivity 4 pg/mL), basic fibroblast growth factor (bFGF; sensitivity 1 pg/mL), MIP-1α (sensitivity 2 pg/mL), human platelet-derived growth factor AB (PDGF-AB; sensitivity 8.4 pg/mL; all kits from R & D Systems, Minneapolis, MN), human GM-CSF (sensitivity 5 pg/mL; Endogen, Boston, MA), human TNF-α (sensitivity <10 pg/mL; Boehringer Mannheim, Indianapolis, IN), and human TGF-β (sensitivity 50 pg/mL; total [acid labile + acid stable] TGF-β measured; Genzyme, Cambridge, MA) according to the manufacturers' recommendations.
Expression of von Willebrand factor (vWf) by marrow cells.
To examine if marrow cells obtained after Ceprate-LC CD34-avidin immunoadsorption column contained endothelial cells, the cells eluting after adhesion to the column were stained with CD34-biotin followed by SA-670, HLA-DR phycoerythrin (PE) and anti-vWf antibody followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Control population of the same cells were stained with SA-670, IgG-PE, and the FITC-conjugated secondary antibody. Briefly, cells were first stained with the CD34 and HLA-DR antibodies followed by SA-670. Cells were washed, fixed with 4% paraformaldehyde at room temperature for 20 minutes, permeabilized by 0.1% Triton X-100 for 5 minutes on ice, and blocked with 3% bovine serum albumin. Cells were then sequentially stained with the vWf antibody and the secondary antibody. As a positive control, human microvascular endothelial cells (MECs) grown as described21 were stained for CD34 and vWf by the same technique. The expression of vWf by gated marrow cells in the DR−, DR+, and CD34−populations was analyzed by multiparameter flow cytometry.
Preparation of stromal cell RNA and Northern blot analysis.
Allogeneic irradiated human BM stroma was subcultured in 6-well tissue culture plates in LTBMC medium. Two days after the weekly replacement of half the medium with fresh LTBMC medium, 50,000 to 500,000 FACS-sorted CD34+ cells or 15+/14− cells were placed either alone or together into 0.4-μm transwell inserts above stromal feeders for 8 to 24 hours at 37°C. Control wells did not receive hematopoietic cells (stroma alone). After removal of the transwells containing hematopoietic cells, stromal layers were washed twice with phosphate-buffered saline (PBS) and stromal cells were detached by using trypsin plus EDTA for 5 minutes. After trypsin was neutralized with cold FCS, cells were pelleted by centrifugation at 4°C and immediately lysed with 0.35 mL lysis buffer and frozen. Total RNA was isolated by using the RNeasy method (Qiagen, Chatsworth, CA), estimated spectrophotometrically, electrophoresed on 1.2% agarose-formaldehyde gels (10 μg RNA per lane), and transferred to Nytran nylon membranes (Schleicher & Schuell, Keene, NH), as previously described.22,23 Blots were hybridized in the presence of 50% formamide, 2× Denhardt's solution, 5× sodium chloride/sodium phosphate/EDTA buffer (SSPE; Sigma), 0.1% sodium dodecyl sulfate (SDS), 0.1 mg/mL yeast RNA, and 0.1 mg/mL denatured salmon sperm DNA to 106 cpm/mL of a human G-CSF cDNA probe (R & D Systems) labeled with [α-32P]dCTP by random priming reaction.24 25 Membranes hybridized to cDNA probes were washed with high stringency buffer consisting of 0.1× sodium chloride/sodium citrate buffer (SSC; Boehringer Mannheim), 0.1% SDS at 65°C, and autoradiographed at −80°C. After removal of the first probe, membranes were rehybridized to a probe for β-actin as an internal standard of total RNA loaded per lane.
RT-PCR for G-CSF mRNA.
Total RNA was obtained as described previously from irradiated stromal feeders alone in 6-well tissue culture plates and from stromal feeders cocultured with FACS-purified CD34+ cells in transwell inserts (5 × 104 or 5 × 105 cells per transwell in 6-well plates; equivalent to 10,000 or 100,000 CD34+ cells, respectively, in 24-well plates).
A semiquantitative RT-PCR technique was used to determine the expression of G-CSF mRNA transcripts in stroma by using the one-step Titan RT-PCR system as per the manufacturer's instructions (Boehringer Mannheim). The following primer sets for human G-CSF were obtained from Clontech (Palo Alto, CA): upstream primer 5′ TTGGACACACTGCAGCTGGACGTCGCCGACTTT 3′ and downstream primer 5′ ATTGCAGAGCCAGGGCTGGGGAGCAGTCATAGT 3′. In initial experiments, we standardized RT-PCR quantitation of G-CSF mRNA transcripts in 0.1 to 4 μg total RNA from stromal layers cultured in the presence of 100 ng/mL TNF-α for 24 hours. We determined by serial cycle analysis between 25 and 40 cycles of PCR, that 35 cycles were optimal for semiquantitative detection of G-CSF mRNA transcripts. Conditions used were as follows: thermal cycling at 55°C for 30 seconds and 68°C for 1 minute for 35 cycles, followed by 7 minutes extension at 68°C. The amplified products from 1.5 μg total RNA per sample were resolved on 1.5% agarose gel in 1× Tris-borate-EDTA buffer, pH 8.0 and visualized with 0.5 μg/mL ethidium bromide. The negative controls were RT-PCR reactions performed in the absence of any template or in the absence of the enzyme. The RT-PCR–amplified products resolved on an agarose gel showed a single band of the expected size for the G-CSF product amplified by the Clontech primers, in comparison to a positive control, which was the 470-bp amplified product of the specific G-CSF cDNA fragment supplied by Clontech with the G-CSF Amplimer set. The mRNA for human β-actin was amplified for 35 cycles in the same PCR tube by using primers from GIBCO BRL as an internal control for RNA integrity and quantitation. The amplified product was detected as a single band of the expected size (353 bp). The intensity of the ethidium bromide–stained bands was quantitated by acquiring the image from a Gel Doc apparatus (Biorad, Hercules, CA) and Molecular Analyst (Molecular Bioscience Group, Hercules, CA) software.
Cytokines.
Recombinant human cytokines used included G-CSF (Neupogen; Amgen, Thousand Oaks, CA), GM-CSF (Immunex Corp, Seattle, WA), SCF (a kind gift from Amgen), LIF (R & D Systems), MIP-1α (R & D Systems), IL-3 and IL-6 (kind gifts from Dr G. Wong, Genetics Institute, Boston, MA), and EPO (Amgen).
Short-term methylcellulose cultures.
Cells were plated in methylcellulose (final concentration 1.12%) supplemented with 30% FCS, 3 IU recombinant EPO, and either 5% to 10% SN from the bladder carcinoma cell line 5637, 5 ng/mL IL-3, or combinations of recombinant human cytokines, as specified. For some experiments, cells were plated in methylcellulose medium in transwell inserts placed in 6-well plates containing similar methylcellulose medium supplemented with 5 ng/mL IL-3 alone or with 25% SN from irradiated stroma (M2-10B4 stromal cell line). Cells were also plated in transwell inserts placed in wells containing an intact irradiated stromal feeder layer (M2-10B4 stromal cell line) in the bottom chamber in methylcellulose medium that was not supplemented with exogenous cytokines except for EPO. Cultures were incubated in humidified 5% CO2 atmosphere at 37°C; burst-forming unit-erythroid (BFU-E), colony forming unit-mixed (CFU-MIX), and colony-forming unit–granulocyte-macrophage (CFU-GM) were enumerated at day 14 of culture, as previously described.26 High proliferative potential colony-forming cells (HPP-CFCs) were enumerated on day 28.
Inhibition of colony formation by anti–G-CSF and anti–IL-6 antibodies.
The effect of neutralizing antibodies against G-CSF and IL-6 on stroma-conditioned medium–induced colony formation was examined by adding the antibodies to methylcellulose cultures at final concentrations of 3 μg/mL anti–G-CSF, 5 μg/mL anti–IL-6, or 5 μg/mL normal goat IgG (all from R & D Systems) on day 0. Cultures were supplemented with additional antibodies at the same concentration on days 4, 7, and 11. Colonies were scored on day 14.
Statistics.
Results of data are reported as the mean ± standard error of the mean. Levels of significance were determined by two-sided Student'st-test.
RESULTS
Production of cytokines by irradiated human marrow stroma.
We determined by ELISA the concentrations of several cytokines known to influence the proliferation and differentiation of hematopoietic progenitors in SNs recovered from irradiated stromal feeders 2, 4, and 7 days after a weekly half medium replacement. Picogram amounts of IL-6, G-CSF, SCF, LIF, MIP-1α, TNF-α, and TGF-β were detected (Table 1). IL-1β, IL-3, IL-7, GM-CSF, bFGF, and PDGF-AB were not measurable. IL-6, SCF, and TGF-β increased between day 2 and 7 after medium replacement; the concentration of G-CSF remained constant, whereas TNF-α and MIP-1α levels decreased.
Cytokine Concentrations in Supernatants From Irradiated Stroma
| Cytokine . | Days After Half-Volume Medium Replacement . | ||
|---|---|---|---|
| Day 2 . | Day 4 . | Day 7 . | |
| IL-6 | 266 ± 40 (6) | 329 ± 94 (6) | 454 ± 47 (6)-150 |
| G-CSF | 48 ± 10 (6) | 50 ± 14 (6) | 44 ± 17 (6) |
| LIF | 14 ± 6 (4) | 19 ± 8 (4) | 20 ± 12 (4) |
| SCF | 36 ± 23 (6) | 78 ± 29 (6)-151 | 187 ± 74 (6)-152 |
| TNF-α | 36 ± 14 (4) | 24 ± 5 (4) | 13 ± 1 (4) |
| MIP-1α | 69 ± 17 (5) | 31 ± 9 (5) | 17 ± 7 (5)-150 |
| TGF-β | 1550 ± 430 (3) | 2537 ± 496 (3)-150 | 4097 ± 1426 (3) |
| Cytokine . | Days After Half-Volume Medium Replacement . | ||
|---|---|---|---|
| Day 2 . | Day 4 . | Day 7 . | |
| IL-6 | 266 ± 40 (6) | 329 ± 94 (6) | 454 ± 47 (6)-150 |
| G-CSF | 48 ± 10 (6) | 50 ± 14 (6) | 44 ± 17 (6) |
| LIF | 14 ± 6 (4) | 19 ± 8 (4) | 20 ± 12 (4) |
| SCF | 36 ± 23 (6) | 78 ± 29 (6)-151 | 187 ± 74 (6)-152 |
| TNF-α | 36 ± 14 (4) | 24 ± 5 (4) | 13 ± 1 (4) |
| MIP-1α | 69 ± 17 (5) | 31 ± 9 (5) | 17 ± 7 (5)-150 |
| TGF-β | 1550 ± 430 (3) | 2537 ± 496 (3)-150 | 4097 ± 1426 (3) |
All values shown as mean ± SEM in pg/mL. Numbers in parentheses represent number of experiments performed. TGF-β in fresh LTBMC medium is 2,100 pg/mL (cross-reactivity from fetal calf serum and horse serum in ELISA).
Comparison between day 2 and other days:
P < .02.
P < .002.
P < .05.
Effect of hematopoietic progenitors and precursors on cytokine production by stroma.
We next examined if immature hematopoietic progenitors affected stromal production of cytokines (Fig 1A and B). Previous studies from our group and others have shown that primitive progenitors are enriched in the DR− population and committed progenitors in the DR+ population. The average percentage of CFCs in DR− and DR+ cells is 2% and 8%, respectively; P < .001.18 The average percentage of long-term culture-initiating cells (LTC-ICs) in DR− cells is 1%.27 Coculture of DR− cells, enriched for LTC-ICs, with irradiated stromal layers induced a fourfold to fivefold increase in IL-6 and G-CSF concentrations in the medium on days 2, 4, and 7 during the first week of coculture. The more mature DR+ cell population, enriched for CFCs but not LTC-ICs, induced a similar increase in IL-6 and G-CSF levels. In the presence of either DR− or DR+ cells, IL-6 concentration was up to 2,643 pg/mL and G-CSF concentration was up to 637 pg/mL during the first week of culture. However, when the cultures were maintained for 3 to 5 weeks, the concentrations of both cytokines declined to levels comparable with control irradiated stroma (IL-6 range, 179 to 399 pg/mL; and G-CSF range, 0 to 51 pg/mL). Concentrations of LIF, MIP-1α, TNF-α, TGF-β, and IL-1β were not significantly altered by the presence of CD34+ progenitors (data not shown). SNs of DR− or DR+ cells incubated in LTBMC medium in the absence of stroma did not contain detectable levels of IL-6, G-CSF, IL-1β or TNF-α during the first week of culture.
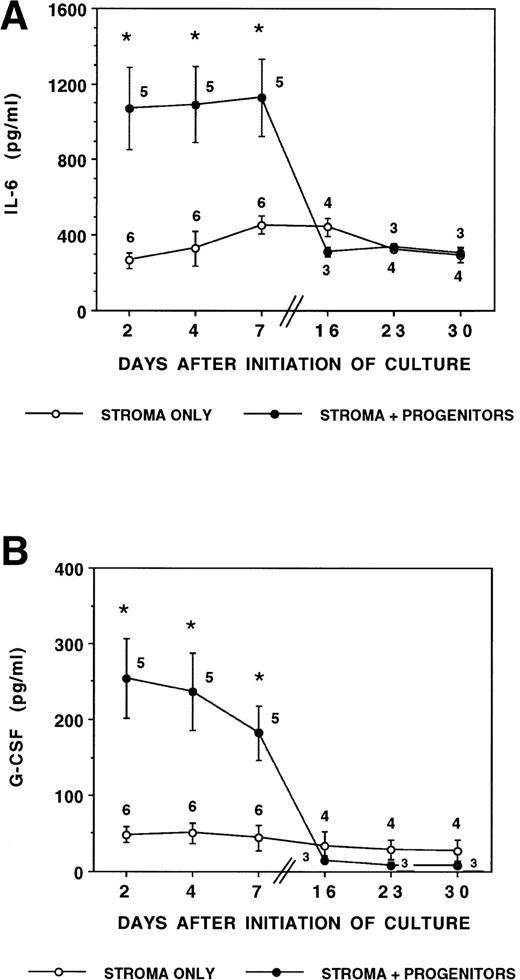
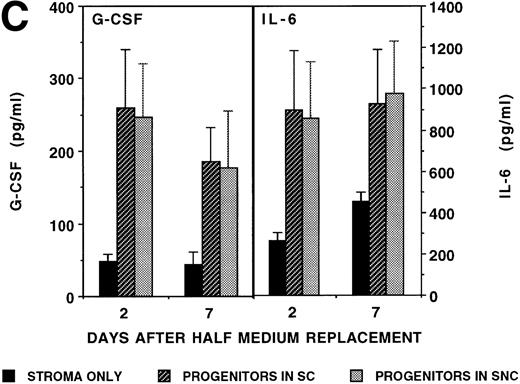
Stromal production of IL-6 and G-CSF is stimulated by CD34+ hematopoietic progenitors. Irradiated marrow stromal layers were subcultured with LTBMC medium in 24-well plates as described. A total of 10,000 CD34+/HLA-DR+or CD34+/HLA-DR− cells/well were seeded directly onto the stromal layers (stroma contact, SC) or in a transwell insert (stroma noncontact, SNC) on day 0. Cytokine levels were determined by ELISA in SNs obtained on days 2, 4, and 7 during the first week and on day 2 after a half medium replacement during weeks 3, 4, and 5. (A) Concentration of IL-6 in SN. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus progenitors (DR+ or DR− cells in either contact or noncontact): *P < .05. (B) Concentration of G-CSF in SN. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus progenitors (DR+ or DR− cells in either contact or noncontact): *P < .05. (C) Comparison between contact and noncontact cultures on G-CSF and IL-6 concentrations. N = 5.
Stromal production of IL-6 and G-CSF is stimulated by CD34+ hematopoietic progenitors. Irradiated marrow stromal layers were subcultured with LTBMC medium in 24-well plates as described. A total of 10,000 CD34+/HLA-DR+or CD34+/HLA-DR− cells/well were seeded directly onto the stromal layers (stroma contact, SC) or in a transwell insert (stroma noncontact, SNC) on day 0. Cytokine levels were determined by ELISA in SNs obtained on days 2, 4, and 7 during the first week and on day 2 after a half medium replacement during weeks 3, 4, and 5. (A) Concentration of IL-6 in SN. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus progenitors (DR+ or DR− cells in either contact or noncontact): *P < .05. (B) Concentration of G-CSF in SN. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus progenitors (DR+ or DR− cells in either contact or noncontact): *P < .05. (C) Comparison between contact and noncontact cultures on G-CSF and IL-6 concentrations. N = 5.
To evaluate if the changes in cytokine secretion were mediated by a cell-cell interaction (stroma-contact cultures) or by soluble progenitor-derived factors, we also measured cytokine levels in cultures in which DR+ or DR− cells were cultured separate from the stromal feeder by a transwell (stroma-noncontact cultures; Fig 1C). We have shown that human CD34+ cells cannot migrate through such 0.4-μm transwell membranes (unpublished observations). As was seen in stroma-contact cultures, increased levels of IL-6 and G-CSF were found in stroma-noncontact cultures.
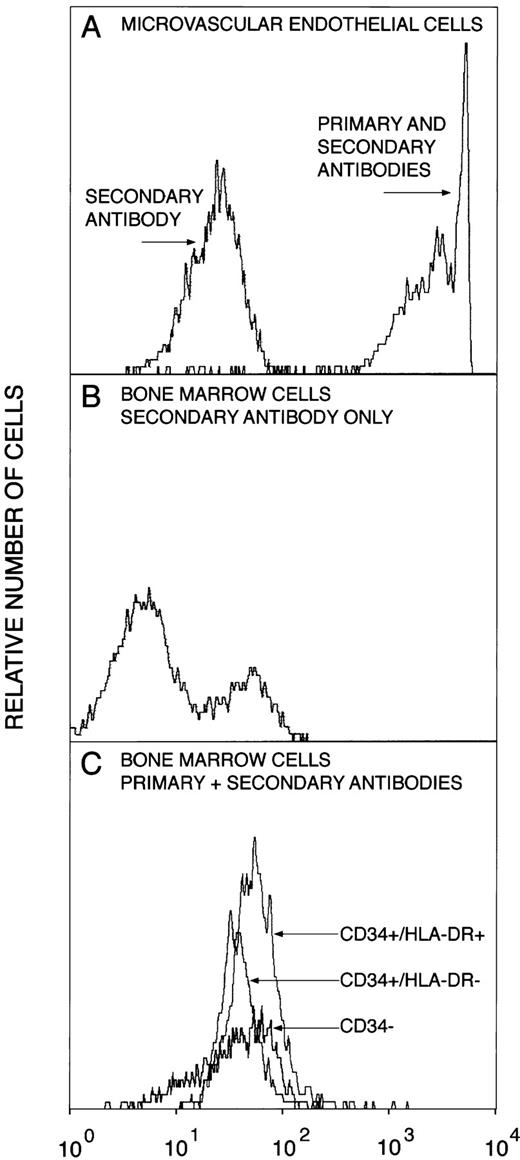
Because endothelial cells may express CD34, we examined if the CD34+ BM populations contained endothelial cells (Fig 2). Microvascular endothelial cells expressed extremely high levels of vWf and variable levels of CD34 (33% MECs were CD34+). In contrast, marrow cells that adhered to the CD34-immunoadsorption column did not express vWf. This was similar for the DR−, DR+, and for those cells that adhered to the column but after elution did not stain for CD34 (CD34−). These results indicate that the marrow cells obtained were not contaminated to any significant degree by endothelial cells.
Expression of vWf by CD34-immunoadsorption column purified marrow cells. Bone marrow cells adhering to the CD34-immunoadsorption column and control microvascular endothelial cells were stained for CD34, HLA-DR, and vWf and analyzed by flow cytometry, as described in the Materials and Methods. (A) Microvascular endothelial cells stained with either vWf antibody plus FITC-conjugated secondary antibody or with the FITC-conjugated secondary antibody; (B) bone marrow cells stained with the FITC-conjugated secondary antibody; (C) bone marrow cells stained with vWf antibody plus FITC-conjugated secondary antibody.
Expression of vWf by CD34-immunoadsorption column purified marrow cells. Bone marrow cells adhering to the CD34-immunoadsorption column and control microvascular endothelial cells were stained for CD34, HLA-DR, and vWf and analyzed by flow cytometry, as described in the Materials and Methods. (A) Microvascular endothelial cells stained with either vWf antibody plus FITC-conjugated secondary antibody or with the FITC-conjugated secondary antibody; (B) bone marrow cells stained with the FITC-conjugated secondary antibody; (C) bone marrow cells stained with vWf antibody plus FITC-conjugated secondary antibody.
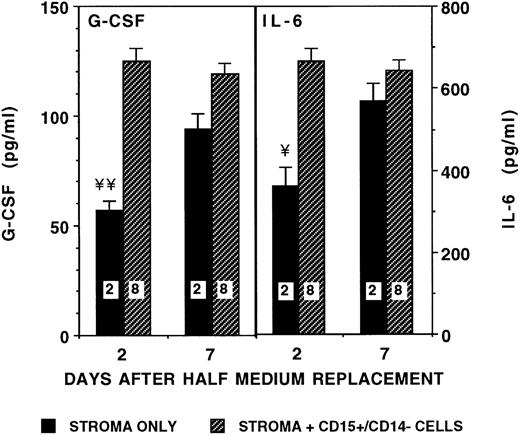
Because cytokine levels were no longer elevated in SNs of stroma cocultured for 3 to 5 weeks with CD34+ cells (Fig 1A and B), we hypothesized that, on differentiation, myeloid precursors may no longer affect stromal cytokine production. Therefore, we determined cytokine levels in cocultures of stroma by using FACS-purified 15+/14− maturing myeloid precursors. Although levels of G-CSF and IL-6 increased 1.5- to 2-fold at 48 hours after initiation of the cultures, concentrations of both cytokines became comparable with baseline stromal levels after 4 to 7 days of coculture (Fig 3). Again, no differences were seen on days 2, 4, or 7 between cultures in which 15+/14− cells were cultured in contact with or separated from the stromal layer (data not shown). As for CD34+ cells, 15+/14−precursors themselves did not produce detectable concentrations of IL-6, G-CSF, IL-1β, or TNF-α.
Effect of maturing myeloid precursors on stromal production of IL-6 and G-CSF. From 10,000 to 50,000 CD15+/CD14− myeloid precursors/well were seeded in contact (stroma contact, SC) or separated by a transwell insert (stroma noncontact, SNC) from irradiated stromal layers in 24-well plates. SNs were obtained 2 and 7 days after seeding, and cytokine levels were determined by ELISA. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus precursors (cells either in contact with or separated from stroma): ¥P < .005, ¥¥P < .001.
Effect of maturing myeloid precursors on stromal production of IL-6 and G-CSF. From 10,000 to 50,000 CD15+/CD14− myeloid precursors/well were seeded in contact (stroma contact, SC) or separated by a transwell insert (stroma noncontact, SNC) from irradiated stromal layers in 24-well plates. SNs were obtained 2 and 7 days after seeding, and cytokine levels were determined by ELISA. Numbers within the figure indicate number of experiments. Comparison between stroma only and stroma plus precursors (cells either in contact with or separated from stroma): ¥P < .005, ¥¥P < .001.
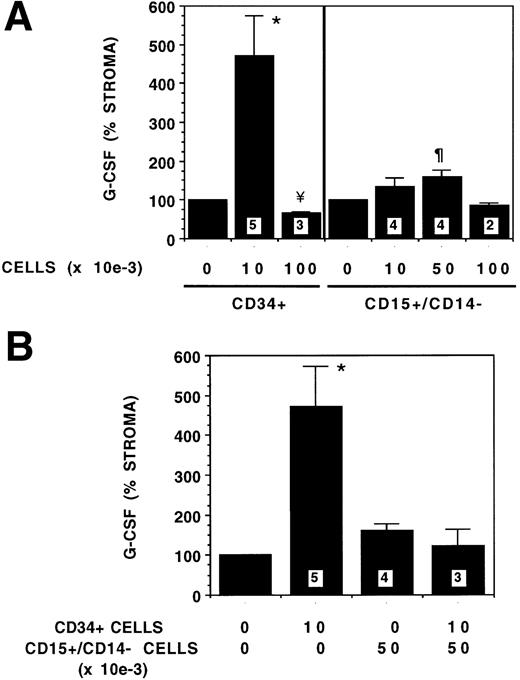
To examine the effect of cell concentration on stromal cytokine production, increasing numbers of CD34+ or 15+/14− cells were plated on stroma and G-CSF levels in SNs determined on day 4 (Fig 4A). However, the G-CSF level that was increased fivefold when 10,000 CD34+ cells were cocultured with stroma was decreased significantly (66% of SN of stromal feeders without progenitors) when the number of CD34+ cells was increased to 100,000. In contrast, different concentrations of 15+/14− cells altered stromal cytokine production only to a small extent, because coculture of 50,000 15+/14− cells increased G-CSF levels in SNs to 1.6-fold, whereas G-CSF levels were not significantly changed in the presence of 10,000 or 100,000 15+/14−cells.
Dose response effect of CD34+ and CD15+/CD14− cells on stromal G-CSF production. From 10,000 to 100,000 CD34+ or CD15+/CD14− cells were plated on stromal layers. SNs were obtained on day 4 and G-CSF levels were determined by ELISA. G-CSF levels shown as percent of stromal SN is 100%. Numbers within the figure indicate number of experiments. (A) The indicated numbers of CD34+ or CD15+/CD14− cells were plated on stromal layers. Comparison between stroma and other conditions: *P < .05, ¶P < .02, ¥P < .001. (B) Combinations of CD34+ and CD15+/CD14−cells were plated together on stromal layers. Comparison between stroma and other conditions: *P < .05.
Dose response effect of CD34+ and CD15+/CD14− cells on stromal G-CSF production. From 10,000 to 100,000 CD34+ or CD15+/CD14− cells were plated on stromal layers. SNs were obtained on day 4 and G-CSF levels were determined by ELISA. G-CSF levels shown as percent of stromal SN is 100%. Numbers within the figure indicate number of experiments. (A) The indicated numbers of CD34+ or CD15+/CD14− cells were plated on stromal layers. Comparison between stroma and other conditions: *P < .05, ¶P < .02, ¥P < .001. (B) Combinations of CD34+ and CD15+/CD14−cells were plated together on stromal layers. Comparison between stroma and other conditions: *P < .05.
To simulate the effect of prolonged coculture of CD34+cells with stroma, which results in a progressive decrease in CD34+ cells and increase in 15+/14− cells, we evaluated the effect of coculture of stroma with combinations of CD34+ cells and 15+/14− on G-CSF levels in the SNs (Fig4B). Coculture of increasing numbers of 15+/14− cells and decreasing numbers of CD34+ cells with stroma resulted in a progressive decrease in G-CSF levels. This effect was similar to the effect on G-CSF levels of prolonged coculture of CD34+ cells with stroma.
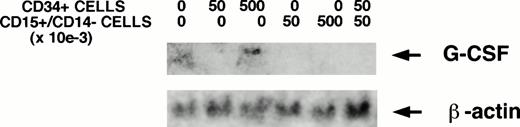
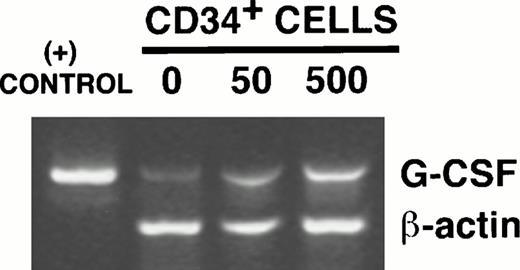
G-CSF mRNA transcripts were not detectable by Northern blotting in irradiated stromal layers in the absence of hematopoietic cells (Fig 5A). However, an increase in G-CSF mRNA was detected in stromal layers cocultured with CD34+cells in transwells. G-CSF mRNA remained undetectable by Northern analysis in stromal layers cocultured with low or high concentrations of 15+/14− cells alone or with 15+/14− cells and CD34+ cells together. The stimulation of G-CSF mRNA levels by CD34+cells was further confirmed by RT-PCR. G-CSF mRNA transcripts were detected in stroma cultured in the absence of CD34+ cells (Fig 5B). A dose-dependent increase in G-CSF mRNA was seen on coculture of stroma with 5 × 104 CD34+ cells/well in 6-well plates (twofold increase by densitometric analysis compared with stroma cultured in the absence of CD34+ cells) and further on coculture with 5 × 105 CD34+cells/well (fourfold increase by densitometric analysis compared with stroma cultured in the absence of CD34+ cells). Thus, although G-CSF protein levels are reduced in the SN in the presence of 100,000 CD34+ cells/well of 24-well plates, stromal G-CSF mRNA levels are increased. Because CD34+ cells were cultured in transwell inserts, which were removed before obtaining RNA from the stromal feeders in the lower chamber, these results further show that the G-CSF detected in the SN is produced by the stromal cells in response to stimulation by a soluble factor from CD34+cells.
CD34+ cells stimulate stromal G-CSF mRNA. The indicated numbers of FACS-sorted CD34+ cells, CD15+/CD14− cells, or both together were plated in transwell inserts above stromal feeders for 24 hours before obtaining stromal cell RNA. (A) Northern analysis, showing increased stromal G-CSF mRNA in the presence of CD34+ cells. (B) RT-PCR–amplified products resolved on 1.5% agarose gel, showing a dose-dependent increase in G-CSF mRNA transcripts with increasing numbers of CD34+ cells. The positive control was the specified 470 bp cDNA product of human G-CSF obtained from Clontech. The 353-bp amplified product of β-actin is shown as an internal control for RNA.
CD34+ cells stimulate stromal G-CSF mRNA. The indicated numbers of FACS-sorted CD34+ cells, CD15+/CD14− cells, or both together were plated in transwell inserts above stromal feeders for 24 hours before obtaining stromal cell RNA. (A) Northern analysis, showing increased stromal G-CSF mRNA in the presence of CD34+ cells. (B) RT-PCR–amplified products resolved on 1.5% agarose gel, showing a dose-dependent increase in G-CSF mRNA transcripts with increasing numbers of CD34+ cells. The positive control was the specified 470 bp cDNA product of human G-CSF obtained from Clontech. The 353-bp amplified product of β-actin is shown as an internal control for RNA.
The picogram concentrations of cytokines in stromal SNs stimulate progenitor growth.
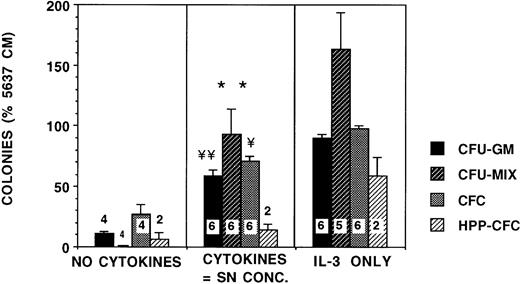
We then examined the biological relevance of these cytokine concentrations. Culture of CD34+ cells in methylcellulose medium in the presence of EPO and cytokines at the concentrations found in stromal SNs devoid of hematopoietic cells resulted in a fivefold increase in CFU-GM compared with cultures supplemented with EPO alone (Fig 6). This was 70% of CFU-GM growth seen in cultures supplemented with EPO and 5 ng/mL IL-3. Likewise, CFU-MIX growth in the presence of the cytokine combination was 50% to 60% of that seen with IL-3, whereas no CFU-MIX were seen in cultures supplemented with EPO alone. However, the growth of day-28 HPP-CFCs was not supported by these concentrations of cytokines. Addition of stromal SN itself to CD34+ clonogenic cultures increased growth of CFU-GM by 10-fold compared with cultures supplemented with EPO alone and resulted in a growth of 99% of CFU-GM and 70% CFU-MIX compared with IL-3 (Fig 7). The size of colonies in the presence of the nanogram concentration (5 ng/mL) of IL-3 was significantly larger than in cultures supplemented with the picogram concentrations of cytokines or with stromal SN.
Progenitor growth is stimulated by the concentrations of cytokines present in stromal SN. From 2,000 to 4,000 CD34+ cells/well were plated in methylcellulose cultures supplemented with 5 ng/mL IL-3 alone, 5% conditioned medium from 5,637 cells, or with a combination of recombinant human cytokines in concentrations comparable with stromal SN (50 pg/mL G-CSF, 5 pg/mL GM-CSF, 35 pg/mL SCF, 15 pg/mL LIF, 250 pg/mL IL-6, and 75 pg/mL MIP-1α). All cultures were supplemented with 3 IU/mL recombinant EPO. The no-cytokine cultures were not supplemented with any cytokines other than EPO. BFU-E, CFU-MIX, and CFU-GM colonies were enumerated on day 14 and HPP-CFC on day 28. Numbers within the figure indicate number of experiments. Comparison between no cytokines and SN concentrations are as follows: **P < .01, ¥P < .005, ¥¥P< .001.
Progenitor growth is stimulated by the concentrations of cytokines present in stromal SN. From 2,000 to 4,000 CD34+ cells/well were plated in methylcellulose cultures supplemented with 5 ng/mL IL-3 alone, 5% conditioned medium from 5,637 cells, or with a combination of recombinant human cytokines in concentrations comparable with stromal SN (50 pg/mL G-CSF, 5 pg/mL GM-CSF, 35 pg/mL SCF, 15 pg/mL LIF, 250 pg/mL IL-6, and 75 pg/mL MIP-1α). All cultures were supplemented with 3 IU/mL recombinant EPO. The no-cytokine cultures were not supplemented with any cytokines other than EPO. BFU-E, CFU-MIX, and CFU-GM colonies were enumerated on day 14 and HPP-CFC on day 28. Numbers within the figure indicate number of experiments. Comparison between no cytokines and SN concentrations are as follows: **P < .01, ¥P < .005, ¥¥P< .001.
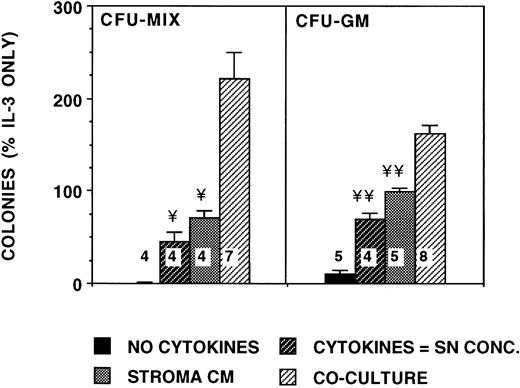
Stimulation of progenitor growth is increased in cocultures of CD34+ cells with stroma. A total of 2,000 or 5,600 DR+ cells/well were plated in methylcellulose medium in transwell inserts placed in 6-well plates containing similar methylcellulose medium. Cultures were supplemented with 5 ng/mL IL-3 alone or with 25% SN from irradiated stroma grown in a separate plate (stroma conditioned medium; M2-10B4 stromal cell line). Equal numbers of cells were also plated in transwell inserts directly above an intact irradiated stromal feeder layer (M2-10B4 stromal cell line) in the bottom chamber in methylcellulose medium not supplemented with exogenous cytokines (stroma coculture). Cells were also plated directly in 24-well plates in methylcellulose medium supplemented with cytokines comparable with stromal SN (SN concentration cytokines), as described for Fig 6. Numbers within the figure indicate number of experiments. Comparison between stroma coculture and other conditions are as follows: ¥P < .005, ¥¥P < .001.
Stimulation of progenitor growth is increased in cocultures of CD34+ cells with stroma. A total of 2,000 or 5,600 DR+ cells/well were plated in methylcellulose medium in transwell inserts placed in 6-well plates containing similar methylcellulose medium. Cultures were supplemented with 5 ng/mL IL-3 alone or with 25% SN from irradiated stroma grown in a separate plate (stroma conditioned medium; M2-10B4 stromal cell line). Equal numbers of cells were also plated in transwell inserts directly above an intact irradiated stromal feeder layer (M2-10B4 stromal cell line) in the bottom chamber in methylcellulose medium not supplemented with exogenous cytokines (stroma coculture). Cells were also plated directly in 24-well plates in methylcellulose medium supplemented with cytokines comparable with stromal SN (SN concentration cytokines), as described for Fig 6. Numbers within the figure indicate number of experiments. Comparison between stroma coculture and other conditions are as follows: ¥P < .005, ¥¥P < .001.
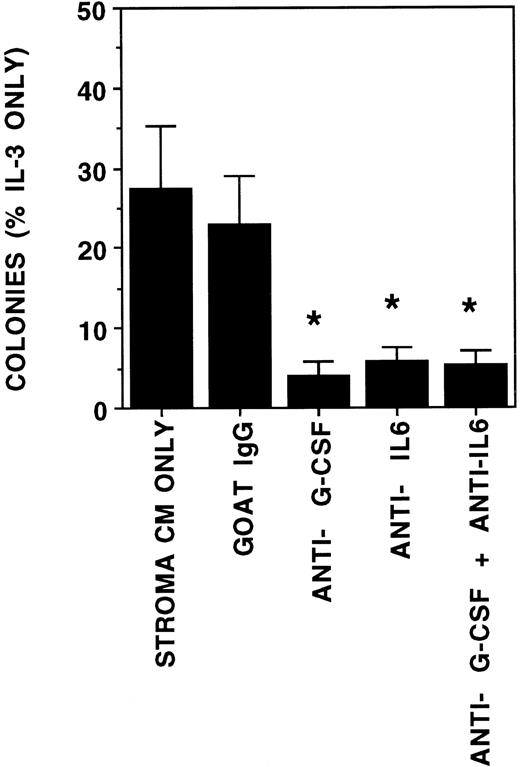
We further examined if the increase in cytokine concentrations was a result of coculture of progenitors with stroma increased colony growth. Coculture of progenitors in a transwell above stromal feeders increased CFU-GM growth 1.6-fold and CFU-MIX growth 3-fold when compared with cultures supplemented with SN from hematopoietic cell-free stromal feeders (Fig 7). Colony growth in coculture was also increased compared with cultures supplemented with cytokines in concentrations found in stromal cultures without CD34+cells. Colonies in the coculture conditions were larger than those in cultures supplemented with cytokines or stromal SN. The addition of neutralizing antibodies against G-CSF, IL-6, or both completely abrogated the stimulation of CFU-GM growth by stroma conditioned medium (Fig 8). The growth of BFU-E was not affected by the neutralizing antibodies (data not shown). Thus, stimulation of CFU-GM growth by stroma is largely attributable to the G-CSF and IL-6 present in the stromal SN.
Stimulation of CFU-GM growth is inhibited by antibodies against G-CSF and IL-6. A total of 2,000 DR+ cells/well were plated in methylcellulose cultures supplemented with either 5 ng/mL IL-3 alone or conditioned medium from irradiated human stroma (final concentration 50%). All cultures were supplemented with 3 IU/mL recombinant EPO. Neutralizing antibodies against human G-CSF, human IL-6, or normal goat IgG were added on days 0, 4, 7, and 11 to the indicated wells, which were supplemented with stroma conditioned medium. N = 4. Comparison between stroma CM or goat IgG and other conditions: *P < .05.
Stimulation of CFU-GM growth is inhibited by antibodies against G-CSF and IL-6. A total of 2,000 DR+ cells/well were plated in methylcellulose cultures supplemented with either 5 ng/mL IL-3 alone or conditioned medium from irradiated human stroma (final concentration 50%). All cultures were supplemented with 3 IU/mL recombinant EPO. Neutralizing antibodies against human G-CSF, human IL-6, or normal goat IgG were added on days 0, 4, 7, and 11 to the indicated wells, which were supplemented with stroma conditioned medium. N = 4. Comparison between stroma CM or goat IgG and other conditions: *P < .05.
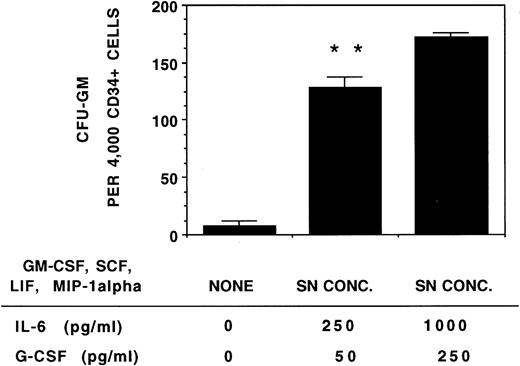
Supplementation of cultures with IL-6 and G-CSF at concentrations found in cocultures of stromal cells and CD34+ cells (1,000 pg/mL IL-6 and 250 pg/mL G-CSF) and low levels (equivalent to SN concentration) of other stromal cytokines also resulted in an increase in CFU-GM growth compared with cultures supplemented with cytokines in concentrations found in stromal cultures without CD34+cells (Fig 9).
Stimulation of progenitor growth is increased by the higher levels of IL-6 and G-CSF present in stromal cocultures. A total of 4,000 CD34+ cells/well were plated in methylcellulose medium supplemented with no cytokines or with a combination of recombinant human cytokines in concentrations comparable with stromal SN (5 pg/mL GM-CSF, 35 pg/mL SCF, 15 pg/mL LIF, and 75 pg/mL MIP-1α) and IL-6 and G-CSF either at the concentrations present in stromal SN (250 pg/mL IL-6 and 50 pg/mL G-CSF) or at the concentrations present in cocultures of stroma with CD34+ cells (1,000 pg/mL IL-6 and 250 pg/mL G-CSF). All cultures were supplemented with 3 IU/mL recombinant EPO. Colonies were enumerated on day 14. N = 4. Comparison between SN concentration cytokines and SN concentration cytokines with higher concentrations of IL-6 and G-CSF: **P < .01.
Stimulation of progenitor growth is increased by the higher levels of IL-6 and G-CSF present in stromal cocultures. A total of 4,000 CD34+ cells/well were plated in methylcellulose medium supplemented with no cytokines or with a combination of recombinant human cytokines in concentrations comparable with stromal SN (5 pg/mL GM-CSF, 35 pg/mL SCF, 15 pg/mL LIF, and 75 pg/mL MIP-1α) and IL-6 and G-CSF either at the concentrations present in stromal SN (250 pg/mL IL-6 and 50 pg/mL G-CSF) or at the concentrations present in cocultures of stroma with CD34+ cells (1,000 pg/mL IL-6 and 250 pg/mL G-CSF). All cultures were supplemented with 3 IU/mL recombinant EPO. Colonies were enumerated on day 14. N = 4. Comparison between SN concentration cytokines and SN concentration cytokines with higher concentrations of IL-6 and G-CSF: **P < .01.
DISCUSSION
This study confirms and extends previous reports showing that irradiated stromal feeders from long-term marrow cultures produce a number of growth-promoting and inhibitory cytokines in picogram concentrations.14-16 28 In addition, we show here that the concentration of at least two of these cytokines, G-CSF and IL-6, increases when DR− and DR+ populations, enriched in immature progenitors including CFCs and LTC-ICs, are cocultured in contact with or separated from stromal feeders. This suggests that these primitive progenitors produce a soluble factor that increases stromal cytokine production. Once these cells mature into terminally differentiated myeloid cells after 3 to 5 weeks of culture, cytokine concentrations return to basal levels. Finally, these changes in cytokine levels have potential biological relevance, because significantly more CFCs are induced to grow when cultured with cytokines at concentrations similar to those in stromal cultures seeded with CD34+ cells than in cytokines at concentrations similar to progenitor-free stromal cultures.
Cytokines produced locally in the marrow microenvironment are necessary for the induction of proliferation, differentiation, and maturation of hematopoietic stem cells into mature precursors and blood elements. Under steady-state conditions, production of normal precursors and blood cells remains fairly constant, whereas this process is accelerated under conditions of stress or after myelosuppression. However, the mechanisms that regulate steady-state and accelerated hematopoiesis are not well known. Our studies indicate that immature CD34+ progenitors may provide a positive feedback signal to stromal cells present in the marrow microenvironment to increase the availability of cytokines necessary for their proliferation and differentiation into more mature cells. The heterogeneity in the levels of IL-6 and G-CSF measured in the cocultures of CD34+ cells and stromal layers from various donors is in agreement with the range of concentrations of these cytokines reported from in vitro14,16 and in vivo2-7 studies and likely reflects inherent biological variability. Although the level of G-CSF in the SN was reduced when a large number of CD34+ cells were plated with stroma, the level of stromal G-CSF mRNA was increased, as detected by both Northern analysis and RT-PCR. This suggests that although both low and high numbers of CD34+ cells stimulate stromal G-CSF production, the reduced level of protein in the SN in the presence of higher numbers of progenitors may be a result of consumption of cytokines by the progenitors, which is in agreement with earlier reports.29-31 After prolonged coculture of CD34+ cells with stromal feeders, which results in the production of neutrophils and macrophages and the disappearance of the majority of the immature CD34+ cells, levels of IL-6 or G-CSF are not increased above those found in progenitor-free cultures. Of interest is the observation that coculture of CD34+cells enriched for CFCs and LTC-ICs increased IL-6 and G-CSF concentrations, whereas this was not seen for more mature precursors and neutrophils present in the CD15+ population. The addition of 10,000 to 100,000 15+/14−cells/well did not significantly reduce the level of cytokines in stromal SNs, nor was the level of stromal G-CSF mRNA detectably altered in the presence of these cells. Therefore, the return to baseline after prolonged coculture of CD34+ progenitors with stromal feeders is unlikely to be the result of consumption of the cytokines by maturing precursors. These data suggest that once sufficient mature precursors and blood cells are present or the number of CD34+ cells falls underneath a certain threshold, signals to increase cytokine levels may cease to be produced.
Recent studies indicate that leukemic blasts or myeloma cells stimulate stromal cytokine production.32,33 However, although coculture of malignant cells with marrow stromal feeders induces cytokine production, direct contact between these cells and stromal elements is required. In contrast, we show that coculture of normal human CD34+ cells either in direct contact with stromal cells or separated from the stromal cells by a 0.4-μm filter membrane results in equivalent increases in G-CSF and IL-6 levels. This indicates that the cytokine induction occurs through a soluble factor released by CD34+ cells and does not require cell-cell interaction. These results are in agreement with recent studies showing that CD34+ cells stimulate IL-6 production by osteoblasts via an unidentified soluble factor.31 However, CD34+ cells do not stimulate G-CSF production by osteoblasts, unlike the effect of CD34+ cells on irradiated stromal layers, which are composed of a variety of cell types. It is possible that differences in the culture media used for osteoblast culture31 and the LTBMC medium used in the present study, such as the concentration and source of serum and presence of hydrocortisone, may have contributed to the differences observed. IL-6 and G-CSF production in fibroblasts, monocytes, and endothelial cells can be upregulated by lipopolysaccharide, IL-1α, IL-1β, or TNF-α.34-41 However, SNs of CD34+ cells kept in culture do not contain IL-1β or TNF-α. Further, Taichman et al31 showed that neutralizing antibodies against IL-1 or TNF-α did not abrogate the stimulatory effect of CD34+cells on osteoblast cytokine production. Therefore, the nature of the soluble factor(s) responsible for this effect remains unidentified.
These in vitro results are reminiscent of what is observed clinically. Serum levels of IL-6 and G-CSF found in patients with neutropenia after chemotherapy or after transplant are elevated to a similar degree as what we observed in stromal cultures seeded with CD34+cells.2-10 Once the peripheral blood neutrophil count recovers to over 0.5 × 109/L, serum G-CSF levels decrease to baseline.3 Thus, the decrease in IL-6 and G-CSF levels seen in vitro in stroma-dependent cultures containing progressively increasing numbers of mature precursors and neutrophils mimics the cytokine/blood cell homeostasis observed clinically. Several clinical studies have indicated that these variations in cytokine levels have biological repercussions. The degree of G-CSF or IL-6 elevation after marrow aplasia correlates with the speed of hematopoietic recovery.3,10 Furthermore, Migliaccio et al15 have shown that conditioned medium from stromal cells obtained from patients with delayed engraftment after transplant contains significantly less G-CSF and supports growth of CFU-GM in vitro significantly less well than SNs from stromal cells obtained from normal individuals or patients who engrafted in a timely manner after transplant. Here we show that the concentrations of cytokines found in SNs of progenitor-free irradiated stromal cultures can support growth of CFU-GM and, to a lesser extent, more primitive CFU-MIX and HPP-CFCs. Routinely, in vitro clonogenic methylcellulose assays are supplemented with nanogram rather than picogram concentrations of one or more cytokines. Our studies show that a mixture of picogram concentrations of a number of cytokines produced in the marrow microenvironment is sufficient to induce growth of progenitors, although the colony size is smaller than when cultures are supplemented with 5 ng/mL IL-3. In addition, we have previously shown that the same cytokines in picogram concentrations (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 200 pg/mL MIP-1α, and 2 ng/mL IL-6) are capable of sustaining generation of CFU-GM for up to 5 weeks in long-term stroma-free cultures initiated with DR−cells.42 As is suggested by the in vivo effects of increased levels of G-CSF and IL-6, our in vitro studies show that a fivefold higher concentration of IL-6 and G-CSF significantly increases the growth of CFU-GM. Even greater increases in CFU-GM and CFU-MIX colony number and colony size were seen when CD34+ cells were cultured above the stromal feeders for 14 days. This suggests that additional stromal cytokines that are active on immature progenitors, such as IL-11 or flt-3 ligand, which were not measured and not added to the cultures, may also be important regulators of steady-state and stress hematopoiesis. Alternatively, the superior growth of CFCs in cocultures may be the result of the continuous supply of cytokines provided by the stromal layer in contrast to cytokine-or SN-supplemented cultures, in which picogram concentrations of cytokines are provided only at the initiation of the 2-week culture.
In conclusion, our studies show that cytokine production by the stromal cells of the marrow microenvironment is regulated by the presence or absence of hematopoietic progenitors at specific stages of differentiation. The nature of the positive feedback signal emanated by primitive CD34+ cells that induces cytokine production is currently under study.
ACKNOWLEDGMENT
The authors acknowledge the excellent technical assistance of Joseph Brazil, Dr Angela Panoskaltsis-Mortari and the University of Minnesota Cytokine Reference Laboratory, Todd Lenvik, Brad Anderson, and Nathalie Dellare.
Supported by National Institutes of Health Grant No. R01-HL-49930, the United States Department of Veterans Affairs, the Minnesota Medical Foundation, the University of Minnesota Bone Marrow Transplant Research Fund, and the University of Minnesota Hospital and Clinic. C.M.V. is a Scholar of the Leukemia Society of America.
Address reprint requests to Catherine M. Verfaillie, MD, Department of Medicine, Box 806 UMHC, 420 Delaware St SE, Minneapolis, MN 55455.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal