Abstract
The aim of this study was to define the need for specific collagen sequences and the role of their conformation in platelet adhesion to collagen under both static and flow conditions. We recently reported that simple triple-helical collagen-related peptides (CRPs), GCP*(GPP*)10GCP*G and GKP*(GPP*)10GKP*G (single-letter amino acid code, P* = hydroxyproline; Morton et al,Biochem J 306:337, 1995) were potent stimulators of platelet activation and were able to support the adhesion of gel-filtered platelets examined under static conditions. The present study investigated whether these same peptides were able to support platelet adhesion under more physiologic conditions by examining static adhesion with platelet-rich plasma (PRP) and adhesion under flow conditions. In the static adhesion assay, we observed 20% surface coverage with platelet aggregates. In marked contrast, there was a total lack of adhesion under flow conditions examined at shear rates of 50 and 300 s−1. Thus, the interaction of platelets with the CRPs is a low-affinity interaction unable on its own to withstand shear forces. However, the addition of CRPs to whole blood, in the presence of 200 μmol/L D-arginyl-glycyl-L-aspartyl-L-tryptophan (dRGDW) to prevent platelet aggregation, caused an inhibition of about 50% of platelet adhesion to collagens I and III under flow. These results suggest that the collagen triple helix per se, as defined by these simple collagen sequences, plays an important contributory role in the overall process of adhesion to collagen under flow. The monoclonal antibody (MoAb) 176D7, directed against the α2 subunit of the integrin α2β1, was found to inhibit static platelet adhesion to monomeric but not fibrillar collagens I and III. However, under flow conditions, anti-α2 MoAbs (176D7 anf 6F1) inhibited adhesion to both monomeric and fibrillar collagens, indicating that α2β1 is essential for adhesion to collagen under flow, independent of collagen conformation, whether monomeric or polymeric. To obtain further insight into the nature of the different adhesive properties of CRPs and native collagen, we investigated the relative importance of von Willebrand factor (vWF) and the integrin α2β1 in platelet adhesion to collagen types I and III, using the same shear rate (300 s−1) as used when testing CRPs under flow conditions. Our results, together with recent data of others, support a two-step mechanism of platelet interaction with collagen under flow conditions. The first step involves adhesion via both the indirect interaction of platelet glycoprotein (GP) Ib with collagen mediated by vWF binding to specific vWF-recognition sites in collagen and the direct interaction between platelet α2β1 and specific α2β1-recognition sites in collagen. This suffices to hold platelets at the collagen surface. The second step occurs via another collagen receptor (thought to be GPVI) that binds to simple collagen sequences, required essentially to delineate the collagen triple helix. Recognition of the triple helix leads to strengthening of attachment and platelet activation.
THE INTERACTION OF platelets with collagens in the vessel wall is one of the first and basic underlying mechanisms in a normal hemostatic response. Collagen is a unique ligand for platelets in that it both supports adhesion and causes platelet activation leading to platelet aggregate formation. Identification and characterization of adhesive domains in the collagen molecule and their interaction with specific receptors on blood platelets is of great importance for understanding the hemostatic process.
The mechanism of platelet-collagen interaction can be supported by a direct (primary) interaction mediated by collagen receptors with collagen and an indirect (secondary) interaction mediated by bridging molecules that bind both to platelet membrane receptors and to collagen. The α2β1 integrin (glycoprotein [GP] Ia/IIa, VLA 2, or CD49b/CD29), GPIV (GPIIIb or CD36), and GPVI (P62) have been proposed as direct collagen receptors.1 Among these direct receptors, the α2β1 integrin meets several of the criteria as a major collagen receptor for different collagen types (reviewed by Santoro et al,2 Sixma et al,3 and Sixma et al4). First, platelet adhesion under flow conditions to collagens type I, III, IV, and VI with blood from a patient whose platelets are deficient in the α2 subunit of the integrin α2β15 has been shown to be severely impaired.6,7 Second, using the monoclonal antibody (MoAb) 176D7 antibody against the α2 subunit, Saelman et al8showed that α2β1 is the main integrin involved in platelet adhesion under flow conditions to collagens types I through VIII. Two important studies investigating the role of GPIV indicate that this receptor is only of minor importance for platelet adhesion under flow conditions.9,10 It is now becoming clear that GPVI is of minor importance in the first phase of adhesion but plays a major role in the second phase, involving platelet activation leading to the formation of platelet aggregates.11 Both fibronectin and von Willebrand factor (vWF) are bridging molecules that are important for platelet adhesion to collagen.12-14 vWF is essential for conferring resistance of platelets to shear, with a more pronounced role under high shear forces.14 15
Fragmentation of collagen with cyanogen bromide suggested the existence of separate receptors for adhesion and platelet activation,16-21 a view supported by the effects of chemical modification of collagen on its platelet reactivity.22 Synthetic, triple-helical peptides have also been used to try to identify platelet-reactive sequences in collagen in view of the evidence that the collagen helical conformation is essential for its recognition of platelets and other cells.17,23-25 To examine the platelet reactivity of short, defined collagen sequences in triple-helical form, the sequence in question has been synthesized with additional Gly-Pro-Hyp triplets present to stabilize the helical structure.26 Helical peptides have also been synthesized covalently bonded at the C-terminus to facilitate correct alignment of the three constituent chains within the helix.27 Using this approach, platelet-reactivity has been found in a number of peptides based on the platelet-reactive collagen III fragmeny α1(III)CB426,28 and in a peptide containing residues 1263-1277 of the collagen IV α1(IV) chain.29 An α2β1-recognition sequence has been ascribed to residues 522-528 of the α1(III) collagen chain.28
These studies with synthetic peptides have been concerned with identifying highly specific platelet-reactive sequences in collagen. However, we have recently examined the platelet reactivity of very simple collagen-like peptides composed basically of only a repeat Gly-Pro-Hyp tripeptide structure and therefore ostensibly lacking highly specific collagen sequences. These peptides assumed spontaneously a very stable triple-helical conformation and, after cross-linking to yield a quaternary structure, were found, against expectation, to be extremely platelet-reactive.30 Their platelet reactivity was totally unaffected by anti-α2β1 MoAbs. These antibodies were without effect on adhesion measured under static conditions at room temperature, using gel-filtered platelets, and showed no inhibition of aggregation, measured at 37°C with platelet-rich plasma (PRP). These results suggested that the basic triple-helical structure of collagen may possess an intrinsic platelet reactivity independent of the presence of highly specific sequences.
The aim of the current study was to determine whether the structure of these simple collagen-related peptides (CRPs) was sufficient to support platelet adhesion under flow conditions. We first studied adhesion to the peptides under static conditions using more physiologic conditions than before (using PRP at 37°C) and then under physiologic flow conditions with whole blood. As an alternative approach to investigate whether recognition of the helix per se, independent of specific sequences, might contribute to adhesion to collagen under flow, we added the peptides to whole blood before perfusion over collagen. As a corollary to these studies, we also examined the relative vWF and α2β1 dependence of adhesion under low shear flow conditions to collagens I and III as monomers and fibers. In contrast to high shear conditions, we found that α2β1-dependent platelet adhesion to (monomeric) collagens I and III under low shear conditions can occur in the absence of vWF. Although adhesion to collagen as monomer is known to involve α2β1 under both static and flow conditions, adhesion to fibers has been reported under static conditions to be largely α2β1 independent.17 We consider it important, therefore, to establish the dependence on α2β1 of adhesion under flow to the collagens as fibers to confirm the essential role of this integrin as an adhesive receptor under flow conditions.
MATERIALS AND METHODS
Blood.
Whole blood, obtained from healthy volunteer donors who denied having taken aspirin or other platelet function inhibitors in the preceding week, was anticoagulated with 1/10 vol of 200 U/mL low molecular weight heparin in 0.15 mol/L NaCl (LMWH-blood; LMWH: Fragmin; Kabi Pharmacia, Stockholm, Sweden). In experiments in which the effect of the addition of D-arginyl-glycyl-L-aspartyl-L-tryptophan (dRGDW) and CRPs during perfusion was studied, whole blood was anticoagulated with 1/10 vol of 400 U/mL LMWH in 0.15 mol/L NaCl to prevent fibrin formation on the collagen surface.
Peptides/antibodies.
The peptides [Gly-Cys-Hyp-(Gly-Pro-Hyp)10-Gly-Cys-Hyp-Gly (CRP 1) and Gly-Lys-Hyp-(Gly-Pro-Hyp)10-Gly-Lys-Hyp-Gly (CRP 2); of which Hyp = hydroxyproline] were synthesized as described previously.30 Peptides were cross-linked [CRP 1 via the cysteinyl residues using N-succinimidyl 3-(2-pyridyldithio) propionate and CRP 2 via the lysyl residues using glutaraldehyde] as described.30
The dRGDW peptide was kindly provided by Dr J. Bouchaudon (Rhône-Poulenc-Rorer, Chemistry Department, Centre de Recherche de Vitry, Vitry sur Seine, France).31 Where indicated, 200 μmol/L dRGDW was added during perfusion experiments to prevent platelet aggregation in whole blood. dRGDW was added 15 minutes and CRPs (free peptide or cross-linked) 10 minutes before perfusion to the whole blood perfusate and incubated at 37°C. The CRPs were solubilized in 10 mmol/L acetic acid. Control perfusion experiments were performed in which the effect of the presence or absence of vehicle buffer on platelet adhesion to collagen type I and III was compared. No significant differences were observed. Furthermore, the addition of 100 μg/mL CRPs in this vehicle to whole blood showed no effect on the pH.
MoAb 176D7 and MoAb 6F1, directed against α2 subunit of the integrin α2β1, were kindly provided by Dr H.R. Gralnick (Department of Health and Human Services, National Institutes of Health, Bethseda, MD) and by Dr B. Coller (Mount Sinai Hospital, New York, NY), respectively. Both MoAbs inhibit adhesion under static conditions,32,33and the 176D7 MoAb has also been shown to inhibit adhesion under flow conditions.8 MoAb AK-2, directed against GPIb, was a generous gift of Dr M. Berndt (Baker Medical Research Institute, Prahan, Australia).34 In perfusion experiments, a 1:1,000 dilution was used.
Surfaces.
Glass coverslips (18 × 18 mm; Menzel Gläser, Braunschweig, Germany) and circular glass coverslips (Knittel Gläser, Braunschweig, Germany; surface area, 1.1 cm2) were soaked overnight in chromosulfuric acid, rinsed thoroughly with deionized water, and air-dried. Thermanox coverslips (Nunc, Inc, Napervillle, IL; surface area, 1.2 cm2) were soaked overnight in 80% ethanol, rinsed thoroughly with deionized water, and air-dried.
Human placental collagens type I and III (Sigma, St Louis, MO) were solubilized in 0.05 mol/L acetic acid (1 mg/mL) and sprayed on glass coverslips at a final density of 30 μg/cm2 with a retouching airbrush (Badger model 100; Badger Brush Co, Franklin Park, IL). Fibrillar collagens type I and III were prepared by dialysing the solubilized human placenta collagens type I and III twice for 24 hours at 4°C against a sodium phosphate buffer (20 mmol/L Na2HPO4), pH 7.4.14 35 Fibrillar collagens type I and III were also sprayed on glass coverslips at a density of 30 μg/cm2. CRP 1 or 2 (free peptide or cross-linked) were sprayed on Thermanox coverslips at a density of 10 μg/cm2. When the micro-spray method was used, a small surface area (0.13 cm2) in the center of a glass coverslip was sprayed with 300 μg/cm2 of CRP.
After coating or spraying, the coverslips were blocked for 90 minutes at room temperature with 1% human albumin solution (Behring, Marburg, Germany) in HEPES-buffered saline (HBS; 10 mmol/L HEPES, 150 mmol/L NaCl, pH 7.35) when used in a perfusion experiment or with 3% bovine serum albumin (BSA) in HBS when used in a static adhesion assay.
Electron microscopy.
Drops of collagen preparations before and after dialysis were applied to 400 mesh specimen grids covered with collodion films lightly coated with carbon. After 5 minutes, the drops were removed with filter paper; the preparations were either negatively stained with sodium phoshotungstate (pH 7.2) or positively stained with phosphotungstic acid (pH 3.4) and contrasted with uranyl acetate (1%).36The preparations were examined in a Philips EM 420 electron microscope (Philips, Eindhoven, The Netherlands) operated at 60 kV. Electron microscopy indicated the absence of micro fibrils or particulate structures in the acid-soluble monomeric collagen preparations (before dialysis).
Static adhesion assay.
Static adhesion experiments were performed in 24-well plates (Costar, Cambridge, MA) using circular coverslips (surface area, 1.1 cm2) or small Thermanox coverslips (surface area, 1.2 cm2). PRP was prepared by centrifugation of LMWH-blood at 120g for 10 minutes at 22°C. The platelet count was adjusted to 200,000 platelets/μL with platelet-poor plasma (PPP). Five hundred microliters of PRP were added per well and platelets were allowed to adhere for 1 hour at 37°C. Subsequently, the coverslips were removed, rinsed, and stained as described below.
When tested in the assay, the MoAb 176D7 was added to PRP 30 minutes before the assay was performed and incubated at 37°C.
Perfusions.
Perfusions were routinely performed in a single pass perfusion chamber of which the characteristics have been described by Henrita G. van Zanten et al.37 This single pass perfusion chamber has a modified parallel plate perfusion chamber with a slit height of 0.1 mm and a slit width of 2 mm,37,38corresponding with a flow rate of 60 μL/min (shear rate, 300 s−1). Blood was drawn through the perfusion chamber by a Harvard infusion pump (pump 22, model 2400-004; Harvard, Natick, MA). The perfusion time was 5 minutes. Some perfusions were repeated with the parallel plate plate perfusion chamber. The characteristics of this perfusion chamber have been described by Sakariassen et al.39 Duplicate coverslips were inserted in the chamber. Fifteen milliliters of whole blood was prewarmed at 37°C for 5 minutes and then recirculated through the chamber for 5 minutes at a wall shear rate of 300 s−1. Then, 15 mL of prewarmed HBS was drawn through the system to wash the coverslips. No significant differences in the extent of platelet adhesion were observed between the two perfusion chambers.
Evaluation.
After a static adhesion assay or after perfusion, the coverslips were removed, rinsed with HBS, fixed with 0.05% glutaraldehyde, dehydrated with methanol, and stained with May-Grünwald/Giemsa as previously described.40 Platelet adhesion was quantitated with a light microscope (1,000× magnification) connected to a computerized image analyser (AMS 40-10, Saffron Walden, UK). In experiments performed with the parallel plate perfusion chamber, three lines perpendicular to the flow direction were evaluated: one line in the center of the coverslip and two lines 3 mm upstream or downstream of this line, respectively. Platelet adhesion was expressed as the percentage of the surface covered with platelets.
Statistical analysis.
Results were expressed as the mean ± standard error of the mean (SEM) for data obtained from different experiments or as the mean ± standard deviation (SD) for data obtained from coverslips within one experiment. The Student's t-test was used to test for significance of differences between groups. P values less than .05 were considered significant.
RESULTS
Platelet adhesion to CRPs under static conditions.
Under static conditions, platelet adhesion, at least as good as that to collagen type I both monomeric and fibrillar, was observed to both CRPs as free peptide or cross-linked (Table 1). Platelet adhesion to the CRPs consisted solely of platelet aggregates ranging from intermediate to large in size. The morphology of the platelet aggregates was similar in all cases. Platelet aggregates adhering to cross-linked (-XL) CRP 1 are shown in Fig 1 by way of example. Adhesion to CRP 1-XL was significantly higher than to CRP 1. Adhesion to CRP 2-XL, which was more variable than to CRP 1-XL, was also higher than to the equivalent free peptide (CRP 2), but not significantly so. Addition of the platelet activation inhibitor indomethacin (30 μmol/L) had no effect on the extent of static platelet adhesion to CRP 1-XL or collagen type I (data not shown).
Platelet Adhesion to CRPs Under Static Conditions
| Surface . | Coverage . | +MoAb 176D7 . |
|---|---|---|
| Collagen I, M | 21.7 ± 0.5 (n = 8) | 2.4 ± 0.4 (n = 7) |
| Collagen I, F | 21.3 ± 1.2 (n = 7) | 20.0 ± 0.8 (n = 5) |
| CRP 1 | 20.2 ± 0.8 (n = 8) | 19.2 ± 0.3 (n = 6) |
| CRP 1-XL | 28.7 ± 1.1 (n = 6) | 28.2 ± 0.9 (n = 6) |
| CRP 2 | 19.4 ± 0.9 (n = 8) | 18.3 ± 0.4 (n = 6) |
| CRP 2-XL | 25.3 ± 6.0 (n = 6) | 27.6 ± 6.2 (n = 6) |
| 3% BSA | 0.9 ± 0.1 (n = 7) | — |
| Surface . | Coverage . | +MoAb 176D7 . |
|---|---|---|
| Collagen I, M | 21.7 ± 0.5 (n = 8) | 2.4 ± 0.4 (n = 7) |
| Collagen I, F | 21.3 ± 1.2 (n = 7) | 20.0 ± 0.8 (n = 5) |
| CRP 1 | 20.2 ± 0.8 (n = 8) | 19.2 ± 0.3 (n = 6) |
| CRP 1-XL | 28.7 ± 1.1 (n = 6) | 28.2 ± 0.9 (n = 6) |
| CRP 2 | 19.4 ± 0.9 (n = 8) | 18.3 ± 0.4 (n = 6) |
| CRP 2-XL | 25.3 ± 6.0 (n = 6) | 27.6 ± 6.2 (n = 6) |
| 3% BSA | 0.9 ± 0.1 (n = 7) | — |
Data are expressed as the percentage of surface covered by platelets. All peptides are sprayed at a concentration of 10 μg/cm2 on Thermanox coverslips. Collagen I was human placenta monomeric (M) or fibrillar (F) collagen type I sprayed on glass coverslips at 30 μg/cm2. MoAb 176D7, directed against the α2-subunit of the integrin α2β1, was added at a concentration of 20 μg/mL whole blood. Values are the mean ± SEM, obtained in at least two independent experiments.
Abbreviations: XL, cross-linked; n, number of evaluated coverslips.
Platelet adhesion to CRP 1-XL under static conditions (original magnification ×1,250).
Platelet adhesion to CRP 1-XL under static conditions (original magnification ×1,250).
In a previous study, we observed that static adhesion of gel-filtered platelets to the CRPs was not inhibited by a MoAb (6F1) against the α2 subunit of the integrin α2β1.30 We studied here the effect of another MoAb against α2β1 (176D7; 20 μg/mL) in the present static adhesion assay in which plasma proteins are present. Again, there was no effect of the antibody on static platelet adhesion to either CRPs or CRP-XLs (Table 1). Furthermore, the antibody clearly inhibited static platelet adhesion to monomeric but not to fibrillar collagen type I.
Platelet adhesion to CRPs under flow conditions.
In contrast to platelet deposition under static conditions, there was no adhesion to CRPs under flow conditions. No platelet deposition was observed on either peptide (free or cross-linked) at the two shear rates tested (50 and 300 s−1). Under the same conditions, adhesion to human collagen type I was 15.2% ± 2.3% at the higher and 4.1% ± 1.5% at the lower shear rate (Table 2). To ensure that the lack of adhesion to the peptides was not due to their removal during perfusion, a number of control experiments were performed. Denhardt-coating of glass coverslips, which has been described to retain collagen fragments on coverslips under flow conditions,19 was performed before spraying of the CRPs. Using this treatment, again no platelet deposition occurred during perfusion. Using a micro-spray method in which a very high concentration of peptide (300 μg/cm2) is sprayed onto a very small surface area, sufficient peptide was absorbed to allow its detection by May-G rünwald/Giemsa staining. However, platelet adhesion was again absent. When coverslips that had been used in a perfusion experiment were washed with HBS, and then were used to test static adhesion with blood (PRP) from the same donor, the same level and morphology of platelet adhesion was observed as on coverslips that had been used only to measure static adhesion (data not shown). The lack of adhesion under flow in this experiment was confirmed by staining parallel coverslips. In conclusion, these experiments confirm that CRPs and CRP-XLs do not support adhesion under flow conditions.
Platelet Adhesion Under Flow Conditions
| Surface . | Shear Rate (s−1) . | Coverage . |
|---|---|---|
| Collagen I | 300 | 15.2 ± 2.3 (n = 4) |
| CRP 1 | 300 | 0* (n = 12) |
| CRP 1-XL | 300 | 0* (n = 12) |
| CRP 2 | 300 | 0* (n = 12) |
| CRP 2-XL | 300 | 0* (n = 12) |
| Collagen I | 50 | 4.1 ± 1.5 (n = 3) |
| CRP 1 | 50 | 0 (n = 5) |
| CRP 2 | 50 | 0 (n = 5) |
| Surface . | Shear Rate (s−1) . | Coverage . |
|---|---|---|
| Collagen I | 300 | 15.2 ± 2.3 (n = 4) |
| CRP 1 | 300 | 0* (n = 12) |
| CRP 1-XL | 300 | 0* (n = 12) |
| CRP 2 | 300 | 0* (n = 12) |
| CRP 2-XL | 300 | 0* (n = 12) |
| Collagen I | 50 | 4.1 ± 1.5 (n = 3) |
| CRP 1 | 50 | 0 (n = 5) |
| CRP 2 | 50 | 0 (n = 5) |
Data are expressed as the percentage of surface covered by platelets. Peptides were sprayed at a concentration of 10 μg/cm2 on Thermanox coverslips. Collagen I was as in Table 1. Values are the mean ± SD (n = number of evaluated coverslips).
Peptides were also sprayed at 10 μg/cm2 on Denhardt-coated glass coverslips and micro-sprayed at a concentration of 300 μg/cm2 on glass coverslips as mentioned in the Materials and Methods.
Effect of CRPs and CRP-XLs on platelet deposition on human collagen type I and III under flow.
As an alternative approach, to investigate whether the adhesive properties of the CRPs contribute to adhesion to collagen under flow, we added these peptides to whole blood before perfusion over collagen. Because the cross-linked CRPs are highly platelet reactive30 and, when added to whole blood, will cause platelet aggregate formation that will affect platelet adhesion under flow conditions, we included the synthetic RGD-containing peptide, dRGDW (200 μmol/L), to prevent this. This adhesive recognition sequence for platelet receptor GPIIb/IIIa, the integrin αIIbβ3, has been shown to inhibit aggregate formation induced by collagen without inhibiting adhesion to collagen.41-43 The absence of aggregate formation after the addition of CRP 1 or 2 (free peptide or cross-linked, 100 μg/mL) was routinely measured by determining the platelet count before and after addition. Addition of CRP 1, CRP 1-XL, and CRP 2-XL resulted in a substantial decrease in platelet number that was prevented by preincubation with dRGDW.
Platelet adhesion under flow conditions (shear rate, 300 s−1) to human collagens type I and III in the presence of dRGDW resulted in a substantial increase in platelet deposition (from 18.4% ± 2.8% to 33.9% ± 7.2% [n = 4] in the case of collagen type I and from 20.3% ± 3.6% to 43.7% ± 0.5% [n = 3] for collagen type III). The same effect has been reported by Saelman et al31 and de Groot and Sixma.38 The addition of CRP 1, CRP 1-XL, or CRP 2-XL (100 μg/mL) together with dRGDW during perfusion resulted in an inhibition of platelet adhesion compared with a parallel perfusion to which only dRGDW was added (Table 3). Because the addition of 100 μg/mL of CRP is the highest possible experimental concentration achievable in the small perfusion system, this concentration was used in all experiments. The adhesion to collagen type I and III was diminished by 32.9% and 41.8%, respectively, after the addition of CRP 1 and by 53.2% and 51.7%, respectively, after the addition of CRP 1-XL. The latter is significantly more active than CRP 1 in inhibiting adhesion to collagen type III under flow conditions. Adhesion to collagen type I was only slightly inhibited and that to collagen type III only inhibited 33.6% after the addition of CRP 2, but the addition of CRP 2-XL caused inhibition of 56.9% and 68.4%, respectively.
Inhibition of Platelet Adhesion to Human Collagen Type I and III by CRPs in the Presence of dRGDW Under Flow Conditions
| . | Collagen I . | Collagen III . |
|---|---|---|
| Control | 31.6 ± 1.0 (n = 43) | 41.4 ± 0.8 (n = 37) |
| +CRP 1 | 21.2 ± 1.2 (n = 16) | 24.1 ± 1.0 (n = 17) |
| +CRP 1-XL | 14.8 ± 0.8 (n = 7) | 20.0 ± 0.8 (n = 8) |
| +CRP 2 | 28.9 ± 1.7 (n = 10) | 27.5 ± 1.3 (n = 3) |
| +CRP 2-XL | 13.6 ± 0.7 (n = 10) | 13.1 ± 1.0 (n = 3) |
| . | Collagen I . | Collagen III . |
|---|---|---|
| Control | 31.6 ± 1.0 (n = 43) | 41.4 ± 0.8 (n = 37) |
| +CRP 1 | 21.2 ± 1.2 (n = 16) | 24.1 ± 1.0 (n = 17) |
| +CRP 1-XL | 14.8 ± 0.8 (n = 7) | 20.0 ± 0.8 (n = 8) |
| +CRP 2 | 28.9 ± 1.7 (n = 10) | 27.5 ± 1.3 (n = 3) |
| +CRP 2-XL | 13.6 ± 0.7 (n = 10) | 13.1 ± 1.0 (n = 3) |
Data are expressed as the percentage of surface covered with platelets. The control perfusions were performed with dRGDW (200 μmol/L) alone present. The other perfusions were performed with both dRGDW and CRPs present. CRP 1 and 2 (free peptide or cross-linked) were added at a concentration of 100 μg/mL in whole blood. Collagens were human placental monomeric collagens. The perfusion experiments were performed at 300 s−1. Values are the mean ± SEM (n = number of evaluated coverslips) and were obtained in at least two independent experiments.
To exclude the possibility that the diminished level of adhesion in the presence of the CRPs is an indirect effect caused by the activating properties of the CRPs, platelets were stimulated with 15 μmol/L thrombin-receptor activating peptide (TRAP) in the presence of 200 μmol/L dRGDW. This had no effect on platelet adhesion to human collagen type III under flow conditions. Furthermore, FACS experiments, using a panel of MoAbs against various platelet membrane GPs (GPIb, CD 62, CD 63, GMP 33, CD31, and GP IIIA),44 showed that 100 μg/mL CRP-XL 1 and 15 μmol/L TRAP result in a similar level of membrane surface expression of these markers for platelet activation (data not shown).
Role for vWF and the integrin α2β1 in interactions with specific collagen sequences.
Our results as described here show that CRPs, simple collagen-like peptides, are not able to mediate platelet adhesion under flow. However, when added to whole blood, they cause a significant decrease in platelet adhesion to collagen types I and III under flow conditions. These data imply that the CRPs are involved in the adhesive process but are unable to mediate adhesion on their own. Apparently, they lack specific sequences that are present in native collagen and are necessary for primary adhesion under flow. The relative importance of the GPIb-bound vWF and integrin α2β1 in mediating platelet adhesion to native collagen under low shear conditions (300 s−1) has not been investigated in detail. To investigate the relative importance of vWF- and α2β1-recognition sequences in collagens I and III in mediating adhesion under these conditions, we undertook perfusion experiments using specific MoAbs directed against GPIb or α2β1.
The MoAb AK-2 (1:1,000) against the vWF binding site on GPIb reduced the adhesion to collagen type I by 52.0% and to collagen type III by 62.7% (Table 4). In both cases, a considerable extent of platelet deposition was still present. The addition together of both MoAb AK-2 and MoAb 176D7 (against the α2-subunit of α2β1; 20 μg/mL) resulted in a further inhibition of adhesion (80.7% and 88.6%, respectively; Table 4). The results suggest that the integrin α2β1 interaction with collagen is capable of partly mediating platelet adhesion to collagen in the absence of vWF under low shear conditions. This conclusion is supported by previous results obtained in perfusion experiments at a shear rate of 490 s−1 using von Willebrand disease (vWD) blood.14 We now repeated this latter experiment at a shear rate of 300 s−1 and furthermore tested the effect of addition of MoAb 176D7 to vWD blood during perfusion (n = 2 vWD type 3 patients). Again, substantial platelet adhesion was observed, of which the extent of adhesion of the vWD patient was almost as high as that to the control. The addition of MoAb 176D7 during perfusion with vWD blood resulted in 50% inhibtion of adhesion (data not shown).
Inhibitory Effect of Anti-GPIb MoAb Alone or in Combination With Anti-α2β1 MoAb on Platelet Adhesion to Monomeric Collagen Type I and III Under Flow Conditions
| Surface . | Control . | +MoAb AK-2 . | +MoAb AK-2 +MoAb 176D7 . |
|---|---|---|---|
| Collagen I | 25.4 ± 3.1 (n = 8) | 12.2 ± 0.9 (n = 9) | 4.9 ± 0.8 (n = 9) |
| Collagen III | 19.3 ± 3.1 (n = 9) | 7.2 ± 0.8 (n = 8) | 2.2 ± 0.2 (n = 8) |
| Surface . | Control . | +MoAb AK-2 . | +MoAb AK-2 +MoAb 176D7 . |
|---|---|---|---|
| Collagen I | 25.4 ± 3.1 (n = 8) | 12.2 ± 0.9 (n = 9) | 4.9 ± 0.8 (n = 9) |
| Collagen III | 19.3 ± 3.1 (n = 9) | 7.2 ± 0.8 (n = 8) | 2.2 ± 0.2 (n = 8) |
Data are expressed as the percentage of surface covered with platelets. MoAb AK-2 against GPIb was added in a 1:1,000 dilution in whole blood. MoAb 176D7, directed against the α2-subunit of the integrin α2β1, was added at a concentration of 20 μg/mL whole blood. The perfusion experiments were performed at 300 s−1. Values are the mean ± SEM, obtained in at least three independent experiments (n = number of evaluated coverslips).
vWF is an essential bridging molecule in mediating platelet adhesion to both monomeric and fibrillar collagen under high shear.14It is also known that adhesion to monomeric collagen under flow involves integrin α2β18 (compatible with our results described above under relatively low shear conditions). However, it is unclear to what extent adhesion under flow to collagen fibers is also α2β1-dependent. This is an important question, because, of course, collagen occurs in the vessel wall and elsewhere in vivo as fibers. We therefore analyzed the dependency on integrin α2β1 of platelet adhesion to both monomeric and fibrillar collagen type I and III in our static assay and under flow conditions.
Our results in the static adhesion assay confirmed the results of Morton et al17 that adhesion to fibrillar collagen type I (Table 1) and III (data not shown) is not inhibited with a MoAb (176D7) against the α2 subunit of the integrin α2β1. However, static platelet deposition to monomeric collagen type I and III is inhibited by 90.0% ± 2.0% (n = 7) and 63.9% ± 10.2% (n = 10), respectively. In contrast, under flow conditions, the MoAbs 176D7 and 6F1 (both against the α2 subunit of the integrin α2β1) cause a large decrease in platelet adhesion to both monomeric and fibrillar collagen types I and III (Table 5). These data confirm the important role of α2β1 under flow conditions irrespective of whether collagen is monomeric or fibrous.
Inhibitory Effect of Anti-α2β1 MoAbs on Platelet Adhesion to Monomeric and Fibrillar Collagen Type I and III Under Flow Conditions
| Surface . | Control . | +MoAb 176D7 . | +MoAb 6F1 . |
|---|---|---|---|
| Collagen I, M | 17.7 ± 1.3 (n = 20) | 1.5 ± 0.3 (n = 12) | 8.3 ± 0.7 (n = 8) |
| Collagen I, F | 20.7 ± 2.1 (n = 18) | 1.7 ± 0.4 (n = 12) | 6.4 ± 1.1 (n = 9) |
| Collagen III, M | 15.4 ± 0.9 (n = 19) | 4.6 ± 0.7 (n = 9) | 2.9 ± 0.3 (n = 9) |
| Collagen III, F | 17.9 ± 1.0 (n = 15) | 4.5 ± 1.2 (n = 6) | 6.0 ± 0.4 (n = 9) |
| Surface . | Control . | +MoAb 176D7 . | +MoAb 6F1 . |
|---|---|---|---|
| Collagen I, M | 17.7 ± 1.3 (n = 20) | 1.5 ± 0.3 (n = 12) | 8.3 ± 0.7 (n = 8) |
| Collagen I, F | 20.7 ± 2.1 (n = 18) | 1.7 ± 0.4 (n = 12) | 6.4 ± 1.1 (n = 9) |
| Collagen III, M | 15.4 ± 0.9 (n = 19) | 4.6 ± 0.7 (n = 9) | 2.9 ± 0.3 (n = 9) |
| Collagen III, F | 17.9 ± 1.0 (n = 15) | 4.5 ± 1.2 (n = 6) | 6.0 ± 0.4 (n = 9) |
Data are expressed as the percentage of surface covered with platelets. MoAb 176D7 and MoAb 6F1, directed against the α2-subunit of the integrin α2β1, were both added at a concentration of 20 μg/mL whole blood. The perfusion experiments were performed at 300 s−1. Values are the mean ± SEM, obtained in at least three independent experiments (n = number of evaluated coverslips).
Abbreviations: M, monomeric; F, fibrillar.
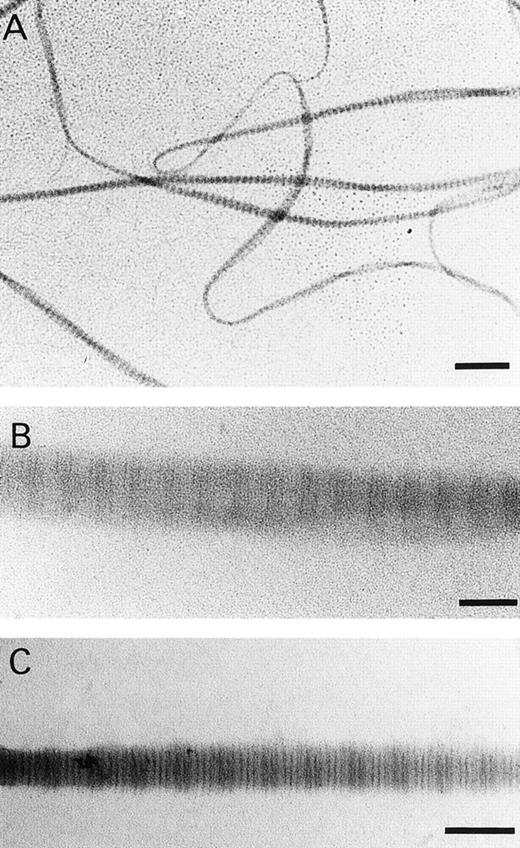
Because structural differences may exist between native collagen fibrils and those formed in vitro,45,46 we analyzed the fibrils formed in this study under the electron microscope. Fibrils were observed with a banding pattern, visualized either via positive or nagative staining, comparable to that observed by others using collagen obtained by a limited pepsin digestion,46 with a banding pattern similar but not identical to native collagen fibrils and indicating the presence of a 67-nm periodicity as in native collagen fibrils (Fig 2). The electron microscopical data and the similar behavior of our fibrils in static adhesion assay as that of intact native collagen type I fibrils (Morton et al17) strongly suggest that the fibrils used in this study closely resemble native collagen type I fibrils.
(A) Transmission electronic micrographs of fibrils formed in vitro of human collagen type I. (A) and (B) were stained with phosphotungstate and (C) was stained with phoshotungstic acid and uranyl acetate. Bar in (A) = 0.5 μm. Bar in (B) and (C) = 0.1 μm.
(A) Transmission electronic micrographs of fibrils formed in vitro of human collagen type I. (A) and (B) were stained with phosphotungstate and (C) was stained with phoshotungstic acid and uranyl acetate. Bar in (A) = 0.5 μm. Bar in (B) and (C) = 0.1 μm.
DISCUSSION
Earlier studies suggested that collagen-platelet interaction may be a two-step process involving the sequential recognition of separate platelet receptors, one mediating the initial adhesion and the other necessary for activation, each recognizing different structural elements in the collagen molecule.16,20-22 Several platelet surface proteins have been implicated in collagen-platelet interaction, including integrin α2β1, CD36, GPVI,1 and a 65-kD collagen species specific for collagen type.47
We demonstrated previously that simple CRPs, composed basically of a Gly-Pro-Hyp repeat sequence, were potent stimulators of platelet activation.30 The peptides were triple-helical and associated to form ordered aggregates that could be stabilized by cross-linking. As in the case for collagen, the triple-helical conformation and polymeric conformation structures were essential for CRP-induced platelet activation. The suggestion was made that the collagen triple helix per se may be platelet reactive and that collagen tertiary and quaternary structures may be sufficient alone to meet the structural requirements of collagen for platelet activation, without the need for highly specific collagen sequences. CRPs appear to activate platelets by the same mechanism as collagen, because both elicit the same specific signals.48-50 CRPs may be very useful therefore to better understand collagen-platelet interaction. Because activation by CRPs is not prevented by anti-integrin α2β1 MoAbs,30 it may be argued that this integrin is not involved directly in the activation mechanism. In accord with this, we have noted previously30 the relative ineffectiveness of the MoAb 6F1 in preventing collagen-induced platelet aggregation. In fact, there is now a considerable body of evidence that GPVI may be the activatory collagen-receptor,51-55 and this is probably the receptor recognized by the CRPs, because like collagen, CRPs do not activate GP VI-deficient platelets (Ichinohe et al55 and Kehrel et al56).57
CRPs immobilized on plastic support the static adhesion of gel-filtered platelets30 and, as shown here, the adhesion also of platelets in the presence of plasma proteins (PRP). Adhesion in both cases is α2β1-independent. We have found that adhesion to fibrillar, as opposed to monomeric, collagen under these conditions is also α2β1-independent. The receptor involved in this α2β1-independent static adhesion is not known for certain, although both CD369 and GPVI11,51 53 have been reported to play a role in platelet adhesion to collagen. This adhesion to CRPs might suggest that collagen tertiary and quaternary structures are sufficient alone to support the primary adhesion step as well as the subsequent activation. However, a crucial observation in the current work is that there is a complete absence of adhesion to the CRPs under flow. This suggests that CRP structure is not adequate to permit shear-resistant platelet adhesion and that the primary adhesion receptor responsible for the arrest of platelets on the collagen surface under flow must interact with specific, likely high-affinity, binding sites in collagen.
Interestingly, whereas CRPs did not themselves support adhesion under flow, they inhibited adhesion to collagen when added to whole blood before perfusion over collagen. In the latter case, both CRPs and platelets are in the fluid phase and the interaction between them is therefore not subjected to shear forces and the CRPs can presumably bind to a platelet receptor (eg, GPVI) under low-affinity conditions. Inhibition was 50% at 100 μg/mL, the highest concentration of peptide we could conveniently test (to conserve the use of peptide). Inhibition was less at lower CRP levels and it is possible that the inhibition may have been greater at peptide concentrations higher than 100 μg/mL. Surprisingly, the inhibitory activity appeared to require the cross-linked peptide. Thus, there was little if any inhibition by CRP 2 as free peptide. Whereas CRP 1 showed some inhibition, this was less than that by CRP 1-XL, and we believe the activity of CRP 1 may be due to some degree of cross-linking arising from spontaneous disulphide bond formation. This cannot occur with CRP 2, which has lysyl residues in place of cysteinyl residues in CRP 1. Presumably, CRP must bind to platelets by a multivalent mechanism requiring peptide in quaternary form. The exact mechanism of the inhibition is unclear but could indicate that the receptor binding CRP-XL is involved somehow in the overall adhesion process to collagen. It seems unlikely that the inhibition can be due to low-affinity binding of CRP to α2β1, because anti-α2β1 MoAbs do not prevent static adhesion to CRP. We believe it more likely that inhibition arises because of binding of CRP to GPVI, indicating that CRP sequences in collagen through their recognition of this receptor are directly or indirectly contributory to overall adhesion.
Our data show that CRPs support static adhesion but not adhesion under flow, indicating that this is a low-affinity interaction. This further implies that CRPs lack specific sequences that are present in native collagen and are necessary under flow conditions. Both integrin α2β1 and GPIb are known to be required for platelet adhesion to collagen under flow, the latter through its recognition of vWF bound to collagen.12-15 We have found no specific binding of vWF to CRPs (our unpublished data). Furthermore, using anti-GPIb and anti-α2β1 MoAbs and vWD blood used in perfusion studies we discovered the relative importance of vWF in supporting platelet adhesion to collagen under flow. However, at low shear conditions, some α2β1-dependent platelet adhesion to collagens I and III could occur in the absence of vWF. Concerning the relative importance of the integrin α2β1, there is no doubt of its requirement for platelet adhesion to monomeric collagen under flow.8 However, given that static adhesion to collagen fibers is α2β1-independent, a crucial question is the need for the integrin for adhesion to fibers under flow. Our finding that the integrin is essential for adhesion to both monomeric and fibrillar collagen confirms the view that this receptor is a critical adhesive receptor. Clearly, the failure of CRPs to support adhesion under flow can be partly attributed to the absence in the molecule of vWF binding sites and of specific α2β1-recognition sequences such as those tentatively identified in collagen.28
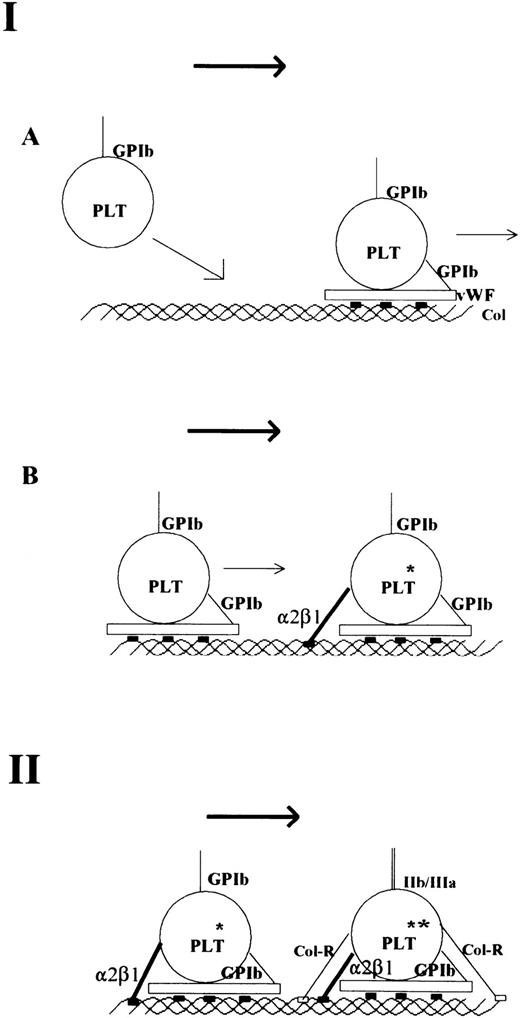
In conclusion, our results, together with recent results by others,11,56,58 support a two-step mechanism of collagen platelet interaction as speculated earlier.16,20-22 This mechanism is presented in diagrammatic form in Fig 3. The first step is the arrest of platelets. Under high shear conditions, the interaction of platelet GPIb with vWF12-15 bound to collagen through high-affinity vWF-binding sites in collagen is essential. This results in a marked decrease in velocity of platelets,58 which enables the direct interaction between collagen and the platelet integrin α2β1,5-8 again through specific recognition sites in collagen. The precise function of α2β1 in the process of activation is unclear, but an assisting role has been proposed.59 60 The second step, associated primarily with activation, involves low-affinity binding between platelets and collagen, which can occur because the platelets are held fast at the collagen surface. This low-affinity interaction involves a different receptor, likely GPVI, and simple (nonspecific) collagen sequences associated basically with generation of the collagen triple-helical structure. This second step leads to full activation and a concomitant increase in adhesion.
Model of platelet interaction with collagen under flow conditions. The first step is the arrest of blood platelets on collagen (A + B). The interaction between GPIb-vWF and specific and high-affinity vWF-binding sites in collagen results in a marked loss of velocity of the blood platelet (A). The actual arrest of platelets occurs after α2β1-collagen interaction (B). This may also result in some degree of activation (*). Both vWF and α2β1 bind to specific adhesive sites on collagen that are depicted as solid rectangles. The second step is the binding of other collagen receptors (Col-R; eg, GP VI) to simple collagen sequences (delineating the collagen triple helix) that are depicted as open rectangles. This results in full platelet activation (**), firm attachment, and GPIIb/IIIa activation.
Model of platelet interaction with collagen under flow conditions. The first step is the arrest of blood platelets on collagen (A + B). The interaction between GPIb-vWF and specific and high-affinity vWF-binding sites in collagen results in a marked loss of velocity of the blood platelet (A). The actual arrest of platelets occurs after α2β1-collagen interaction (B). This may also result in some degree of activation (*). Both vWF and α2β1 bind to specific adhesive sites on collagen that are depicted as solid rectangles. The second step is the binding of other collagen receptors (Col-R; eg, GP VI) to simple collagen sequences (delineating the collagen triple helix) that are depicted as open rectangles. This results in full platelet activation (**), firm attachment, and GPIIb/IIIa activation.
ACKNOWLEDGMENT
The authors thank Martin J.W. IJsseldijk and Glenda J. Heijnen-Snyder for their technical assistance. We further thank Eric G. Huizinga for critical advice on the manuscript and Gertrude Bunt for the electron micrographs.
Supported in part by the Thrombosis Foundation, The Netherlands (Grant No. 93.002). M.J.B., C.G.K., and L.F.M. are grateful to Medical Research Council for financial support.
Address reprint requests to Marilyn W. Verkleij, PhD, Department of Haematology, University Hospital Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal