Abstract
The Fas/Fas ligand system is involved in uncontrolled apoptosis, which ultimately leads to the loss of T lymphocytes in human immunodeficiency virus (HIV)-infected individuals. The signal transduced by Fas receptor involves the activation of an acidic sphingomyelinase, sphingomyelin breakdown, and ceramide production. Our recent reports have shown that L-carnitine inhibits Fas-induced apoptosis and ceramide production both in vitro and in vivo. The aim of this study was to study, in a preliminary fashion, the impact of long-term L-carnitine administration on CD4 and CD8 absolute counts, rate, and apoptosis in HIV-1–infected subjects. The generation of cell-associated ceramide and HIV-1 viremia was also investigated. Eleven, asymptomatic, HIV-1–infected subjects, who refused any antiretroviral treatment despite experiencing a progressive decline of CD4 counts, were treated with daily infusions of L-carnitine (6 g) for 4 months. Immunologic and virologic measures and safety were monitored at the start of the treatment and then on days 15, 30, 90, and 150. L-carnitine therapy resulted in an increase of absolute CD4 counts, which was statistically significant on day 90 and 150 (P = .010 and P = .019, respectively). A positive, not significant trend was also observed even in the change in absolute counts of CD8 lymphocytes. L-carnitine therapy also led to a drop in the frequency of apoptotic CD4 and CD8 lymphocytes. This reduction occurred gradually, but changes in actual values between each time point and baseline were strongly significant (P = .001 at the end of the study compared with the baseline). A strong reduction (P = .001) in cell-associated ceramide levels was found at the end of the study. In general, HIV-1 viremia increased slightly. No toxicity related to L-carnitine therapy was observed and dose reductions were not necessary. In HIV-1–infected subjects, long-term infusions of L-carnitine produced substantial increases in the rate and absolute counts of CD4 and, to a lesser degree, of CD8 lymphocytes. This was paralleled by a reduced frequency of apoptotic cells of both subgroups and a decline in the levels of ceramide. No clinically relevant change of HIV-1 viremia was observed.
LYMPHOCYTES FROM subjects infected with the human immunodeficiency virus type 1 (HIV-1) undergo an inappropriate programmed cell death (apoptosis), a major mechanism for the decline of CD4 and CD8 cells that is crucial to the progression towards the overt acquired immunodeficiency syndrome (AIDS).1-8 Indeed, lymphocyte apoptosis correlates with disease progression and lower CD4 counts: a high degree of apoptosis has been detected in patients with AIDS in comparison with long-term nonprogressors.1-8
Recent studies have provided evidence that the Fas-Fas ligand (FasL) system is involved in the molecular mechanism of T-cell depletion in HIV-1 infection and AIDS.9-12 Thus, viral gene products, such as gp120 and HIV-1 Tat, have been shown to greatly accelerate apoptotic death in T lymphocytes after the T-cell receptor triggering that is mediated via the Fas-FasL interaction. Furthermore, an increased expression of Fas on peripheral T cells from patients with HIV-1 infection has been found that correlates with disease progression, CD4 cell depletion, and a strong increase in sensitivity of patient T cells for Fas-mediated apoptosis ex vivo.9-12The apoptotic signal transduced by Fas receptor involves the activation of an acidic sphingomyelinase, sphingomyelin breakdown, and ceramide production.13,14 Ceramide acts as an endogenous mediator of apoptosis in several cell lines13-17 and also enhances HIV-1 replication.18,19 Patients with AIDS have significantly higher lymphocyte-associated ceramide levels than healthy individuals and HIV-1–infected long-term nonprogressors have less elevated lymphocyte-associated ceramide levels than subjects with evolving disease.8,20,21 Remarkably, this is paralleled by a lower frequency of apoptotic CD4 and CD8 cells in long-term nonprogressors than in patients with AIDS.8 21
In a preliminary, clinical study, short-term treatment of AIDS patients with L-carnitine resulted in a substantial reduction in the frequency of apoptotic CD4 and CD8 cells, paralleled by reduced levels of cell-associated ceramide.22 Of note, L-carnitine did not seem to influence viral replication directly (H. Mitsuya, NCI, personal communication, May 1995). These in vivo results were supported by in vitro studies. In fact, experiments conducted using the Fas-sensitive HuT78 and U937 cell lines have shown that L-carnitine inhibits both apoptosis and ceramide production triggered by Fas cross-linking, and this appears to involve the inhibition of an acidic sphingomyelinase.23 By contrast, the activity of a Fas-activated neutral sphingomyelinase, which does not seem to be implicated in the generation of ceramide, relevant to Fas-induced apoptosis, is not significantly influenced by L-carnitine.23
Taking everything into consideration, it is reasonable to deduce that therapies directed at downmodulating the generation of ceramide may slow the progression of HIV-1 infection by reducing apoptotic lymphocyte death, even if unable to affect viral replication directly.
This background prompted us to investigate the impact of long-term treatment, with a highly purified L-carnitine preparation, on absolute counts, rate, and apoptosis in both CD4 and CD8 lymphocytes, ceramide generation, and HIV-1 viremia. To perform this pilot trial, we enrolled HIV-infected individuals who fulfilled the following eligibility criteria: (1) each subject had to be living in the community of San Patrignano,24 which is devoted to the rescue of drug addicts and, (2) each individual had rejected the opportunity of antiretroviral treatment despite experiencing a progressive decline in CD4 cell counts.
MATERIALS AND METHODS
Study site.
San Patrignano is a private residential community for the rehabilitation of drug users, located in northern Italy (Corione, Rimini, Italy24). The San Patrignano Community was established in 1979 for individuals wishing to undergo rehabilitation. The physical property, extending for 1,000 acres, consists of several residential buildings and extensive pastural and agricultural lands. Treatment, which is free of charge, lasts for a mean time of 3 years. The community rules prohibit the use of illicit or psychotropic drugs; most all of the guests (>95%) are smokers, with a maximum of 10 cigarettes a day allowed. A maximum amount of one glass of wine a meal was also allowed. To ensure abstinence from drug use, guests are not allowed to leave the community during the first year of residency. Since 1980 more than 10,000 drug addicts have been treated, 2,200 with HIV infection.
Study population.
In accordance with the San Patrignano Medical Center protocol, all newly entering drug users undergo the following examinations: physical examination, chest x-ray, electrocardiogram, intradermal purified proteic derivative from M. tuberculosis (PPD; 5 IU), delayed skin tests (multitest Merieux), and routine blood analyses, including serology for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis. HIV-infected individuals are further investigated for lymphocyte subsets, serology for human T-cell lymphotropic virus type I (HTLV-I), Toxoplasma gondii, and cytomegalovirus; abdominal echography is also performed. At least once a year, a serum and plasma sample from each guest is obtained and stored, respectively at −20°C and −80°C.
For any type of medical problem, guests were sent to the Community Medical Center, which includes an out-patient facility, a day-hospital unit (16 beds), and a 36-bed ward. Chemoprophylaxis for HIV-1–related opportunistic infections is administered after standard criteria. In autumn, influenza vaccination is offered to all guests, with a mean adhesion rate of 50%. Antiretroviral therapy is offered according to the National Institutes of Health guidelines.
Patients and L-carnitine treatment.
Eleven, asymptomatic, HIV-1–infected, male subjects living in the Community of San Patrignano (mean age, 33 ± 6.1 years; range, 27 to 43; mean CD4 cell counts, 305 ± 133 cells/mL; range, 61 to 558) gave written informed consent to participate in this pilot study. The study design was approved by the Internal Ethical Committee of S. Patrignano taking into account that the compound is freely available in Italy and had no untoward effects. The patients were chosen among those with CD4 lymphocyte counts between 200 to 500/μL; those with CD4 >500 were not considered because they were stable; those with CD4 <200 were not considered because they were rapid progressors. The patients had been staying a mean of 51 ± 4 months (range, 19 to 96) at the Community of San Patrignano24 and had stable CD4 counts until when, over the last 12 months of follow-up, progressively declining counts were shown. Despite this, all patients freely refused to undergo antiretroviral therapy with zidovudine and/or didanosine and were selected on their willingness to be treated with L-carnitine. Obviously, it was made clear to the patients that L-carnitine treatment did not take the place of antiretroviral therapy. All subjects had stable body weight, or maintained their weight within a 10% variation range during the last 3 months and had albumin levels above 4 g/dL and total carnitine levels comparable to those in healthy individuals. None had persistent or severe diarrhea in the previous 3 months and nutritional support was not requested. None of these subjects had clinical or laboratory evidence of kidney dysfunction and the Karnofsky score was >90 in all subjects. Notably, all of the individuals were HCV positive, with the exception of patients no. 6684 (HBV positive) and 6457 (negative). Patient no. 6438 was both HBV and HCV positive and patients no. 6466 and 2000 had a past episode of Herpes zoster infection.
The patients were administered 6 g/day highly purified L-carnitine (Carnitor, Sigma Tau, Pomezia, Italy), intravenously, in normal saline over a 2-hour period each day for 4 months. During the trial of L-carnitine therapy, a complete physical examination was performed and both blood counts and a biochemical profile were obtained at baseline and then regularly each week throughout the entire study period. No opportunistic infection intercurred during the present trial. Patients were ranked on the Karnofsky performance score by the same investigator on each visit over the course of the trial. To evaluate the frequency of apoptotic CD4 and CD8 cells and the levels of peripheral blood mononuclear cell (PBMC)-associated ceramide, blood samples were taken at baseline (T0), on day 15 (T1), on day 30 (T2), on day 90 (T3), and on day 150 (T4). HIV-1 viremia was measured at T0 and T4. Blood samples were taken at least 18 hours after the previous infusion of L-carnitine taking into account that the half-life of L-carnitine after intravenous infusion is less than 1 hour and that plasma concentrations return to base level within 10 hours.25
PBMC isolation.
PBMCs were separated from heparinized peripheral blood by Lymphoprep gradient centrifugation (Nicodem, Oslo, Norway), washed twice with phosphate-buffered saline (PBS), incubated for 8 hours in RPMI 1640 supplemented with 5% fetal calf serum (FCS), L-glutamine, HEPES, and antibiotics at 37°C, and then used for cytofluorometric analysis of phenotype and apoptosis and extracted for ceramide level determination.
Phenotypic analysis of apoptotic cells.
The absolute counts of cells bearing the CD4 or CD8 phenotype were determined by flow cytometry. In brief, PBMCs were stained with an anti-CD4 and an anti-CD8 monoclonal antibody labeled with fluorescein, according to the manufacturer's instructions (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA).
Quantification and phenotypic analysis of apoptotic cells in lymphocytes was performed by staining apoptotic cells with 7-amino-actinomycin D (7-AAD) (Sigma Chemical Co, St Louis, MO) as prevously described.26 This method discriminated between early and late apoptotic cells, evaluating the increase of 7-AAD fluorescence of apoptotic cells due to the alteration of their membrane permeability. Briefly, for the staining of apoptotic and dead cells, PBMCs were incubated with 20 μg/mL of 7-AAD in 100 μL of PBS (without Ca2+ and Mg2+, Irvine, Santa Ana, CA) containing 2% newborn calf serum (Irvine) and 0.1% sodium azide (Sigma) (PBSAz) for 20 minutes at 4°C protected from light; the cells were then analyzed in their staining solution by flow cytometry analysis. For staining of surface antigens, 10 μL of each anti-CD4 and anti-CD8 fluorescein isothiocyanate (FITC) monoclonal antibodies (Becton Dickinson) in 100 μL of PBSAz were added to 1 × 106 cells followed by incubation for 15 minutes at 4°C. For determination of background staining, cells were incubated with 10 μL each of mouse IgG1 FITC (Becton Dickinson). After one wash with 2 mL of PBSAz, the supernatant was removed and the cell pellet was resuspended in 1 mL of PBSAz. The spectral properties of 7-AAD allow the staining of apoptotic cells by fluorescence emission in the red channel FL-3 (650 nm < wavelenght < 850 nm) and the easily and simultaneous labeling of cell surface antigens (FL1 and FL2).
Identification of apoptotic cells was assessed also by considering scatter characteristics, based on the evidence that apoptotic cells can easily be distinguished from viable cells via measurement of forward scatter (FSC) and side scatter (SSC) light parameters, which are proportional, respectively, to cell diameter and internal granularity.27 Indeed, while living cells display relatively high FSC/low SSC properties, cells undergoing programmed cell death shift to a lower FSC/higher SSC compartment, consistent with the cellular changes occurring during apoptosis (reduction of cell size and cytoplasmic volume and chromatin condensation).27,28
Ceramide measurement (diacylglycerol kinase assay).
PBMCs (2 × 106 cells), isolated as above, were pelleted and resuspended in ice cold methanol:chloroform:water (2.5:1.25:1). Incubation was stopped by immersion of samples in methanol/dry ice (−70°C) for 10 seconds followed by centrifugation at 4°C in a microfuge. Lipids were extracted and then incubated with Escherichia coli diacylglycerol (DAG) kinase (DAG kinase assay kit and 32P-adenosine triphosphate [ATP] γ [specific activity 3 Ci/mmol/L], from Amersham, Buckinghamshire, UK). Ceramide phosphate was then isolated by thin layer chromatography using CHCl3/CH3OH/CH3COOH (65/15/5, vol/vol/vol) as solvent. Authentic ceramide (type III; from bovine brain; Sigma) was identified by autoradiography at retention factor (Rf) 0.25. Specific radioactivity of ceramide-1–phosphate was determined by scintillation counting of corresponding spots scraped off the gel. Quantitative results for ceramide production were obtained from comparing the experimental values with a linear curve of the ceramide standards and are expressed as pmoles of ceramide-1–phosphate/106 cells.
Plasma polymerase chain reaction (PCR) for HIV-1 RNA determination.
Peripheral blood was collected from each subject in tubes containing acid-citrate dextrose and processed within 6 hours. Plasma was separated from whole blood by centrifugation and stored in liquid nitrogen. We used 200 μL of plasma to estimate the HIV-1 RNA copies per milliliter. The application of quantitative PCR was performed using the Amplicor detection system (Roche, Branchburg, NJ) and the gene AMP 9600 thermal cycler (Perkin Elmer, Norwalk, CT). Shortly, the HIV-1 RNA was extracted from each sample using guanidinium, reverse transcribed, and amplified by recombinant thermus thermophilus (rTth) DNA polymerase and detected using a method based on changes in optical density produced by reactions mediated by horseradish peroxidase. A noncompetitive, internal control was introduced to monitor sample and reaction interference.
Statistical analysis.
For each of the several parameters of interest, differences were calculated from each later time point and T0, and these differences tested against a null hypothesis of no change by using the Wilcoxon signed rank test; individual two-tailed P values are reported and the Hochberg adjustment for multiple comparisons was also used to correct for analysis of four changes from baseline for each parameter.29 For HIV-1 viremia, the logs of the ratios of values at T0 and T4 were tested using the Wilcoxon signed rank test. Spearman rank correlation was performed between ceramide levels and apoptotic CD4 and CD8 cells on each of the time points throughout the study period.
RESULTS
CD4 counts.
The absolute CD4 cell counts increased during the period when L-carnitine was administered (Table 1). There was a significant increase in CD4 counts at T3 and T4 compared with T0 (P = .010 and P = .019), but a positive trend was already evident at T1 and T2, even though the differences were not statistically significant in comparison with the baseline values (P = .56 for T1 v T0 and P = .52 for T2v T0) (Table 2). The mean net change from baseline at the end of the study was an increase of 101.09 ± 114.71 (range, −60 to +337 cells/μL). Two patients had an increase of more than 100%, two patients more than 50%, and three patients approximately 30% over the baseline CD4 cell counts. In three patients, the treatment with L-carnitine did not result in a significant gain of CD4 cells and a slight decrease was observed at the end of the study compared with the baseline. However, in one of these subjects, slightly increased counts were observed at T2 and in another of these subjects on T3.
CD4, CD8, and HIV RNA Values at Different Time Points
| Variable . | Time Points . | No. . | Mean . | St. Dev. . | Min . | Median . | Max . |
|---|---|---|---|---|---|---|---|
| CD4 | T0 | 11 | 305.2 | 139.4 | 61 | 331 | 558 |
| T1 | 11 | 317.7 | 136.3 | 62 | 311 | 504 | |
| T2 | 11 | 322.0 | 142.3 | 100 | 337 | 522 | |
| T3 | 11 | 377.5 | 154.5 | 100 | 390 | 580 | |
| T4 | 11 | 406.3 | 167.9 | 101 | 476 | 670 | |
| CD8 | T0 | 11 | 860.7 | 353.7 | 352 | 844 | 1,460 |
| T1 | 11 | 926.3 | 392.9 | 419 | 918 | 1,745 | |
| T2 | 11 | 920.3 | 425.7 | 525 | 737 | 1,865 | |
| T3 | 11 | 947.5 | 405.4 | 401 | 922 | 1,577 | |
| T4 | 11 | 1,025.1 | 700.0 | 395 | 947 | 2,887 | |
| HIV (log10) | T0 | 11 | 3.67 | 0.64 | 2.60 | 3.84 | 4.52 |
| T1 | NA | ||||||
| T2 | 10 | 3.82 | 0.72 | 2.65 | 4.13 | 4.52 | |
| T3 | NA | ||||||
| T4 | 11 | 3.89 | 0.54 | 2.9 | 4.07 | 4.51 |
| Variable . | Time Points . | No. . | Mean . | St. Dev. . | Min . | Median . | Max . |
|---|---|---|---|---|---|---|---|
| CD4 | T0 | 11 | 305.2 | 139.4 | 61 | 331 | 558 |
| T1 | 11 | 317.7 | 136.3 | 62 | 311 | 504 | |
| T2 | 11 | 322.0 | 142.3 | 100 | 337 | 522 | |
| T3 | 11 | 377.5 | 154.5 | 100 | 390 | 580 | |
| T4 | 11 | 406.3 | 167.9 | 101 | 476 | 670 | |
| CD8 | T0 | 11 | 860.7 | 353.7 | 352 | 844 | 1,460 |
| T1 | 11 | 926.3 | 392.9 | 419 | 918 | 1,745 | |
| T2 | 11 | 920.3 | 425.7 | 525 | 737 | 1,865 | |
| T3 | 11 | 947.5 | 405.4 | 401 | 922 | 1,577 | |
| T4 | 11 | 1,025.1 | 700.0 | 395 | 947 | 2,887 | |
| HIV (log10) | T0 | 11 | 3.67 | 0.64 | 2.60 | 3.84 | 4.52 |
| T1 | NA | ||||||
| T2 | 10 | 3.82 | 0.72 | 2.65 | 4.13 | 4.52 | |
| T3 | NA | ||||||
| T4 | 11 | 3.89 | 0.54 | 2.9 | 4.07 | 4.51 |
T0, baseline; T1, day 15; T2, day 30; T3, day 90; T4, day 150.
Abbreviations: NA, not available; St. Dev., standard deviation.
Changes in Actual Values of Immunologic Parameters Between Time Points Indicated
| Variable . | Time Points . | Mean . | No. . | St. Dev. . | Min . | Median . | Max . | P . |
|---|---|---|---|---|---|---|---|---|
| CD4 | T1-T0 | 12.55 | 11 | 61.03 | −82 | 1 | 118 | .56 |
| T2-T0 | 16.82 | 11 | 78.70 | −103 | 34 | 177 | .52 | |
| T3-T0 | 72.27 | 11 | 79.52 | −39 | 59 | 221 | .01* | |
| T4-T0 | 101.09 | 11 | 114.71 | −60 | 106 | 337 | .019 | |
| CD8 | T1-T0 | 65.55 | 11 | 225.73 | −347 | 96 | 443 | .33 |
| T2-T0 | 59.55 | 11 | 225.17 | −238 | 78 | 563 | .64 | |
| T3-T0 | 86.82 | 11 | 244.49 | −272 | 78 | 570 | .28 | |
| T4-T0 | 164.36 | 11 | 569.18 | −372 | 117 | 1585 | .76 | |
| Apoptotic CD4 | T1-T0 | −14.04 | 11 | 11.81 | −33.4 | −12.0 | −0.2 | .0010* |
| T2-T0 | −31.24 | 11 | 16.10 | −56.9 | −35.5 | −6.0 | .0010* | |
| T3-T0 | −30.73 | 11 | 18.02 | −55.4 | −33.6 | −1.3 | .0010* | |
| T4-T0 | −40.45 | 11 | 16.35 | −64.8 | −42.4 | −20.1 | .0010* | |
| Apoptotic CD8 | T1-T0 | −5.51 | 11 | 5.35 | −16.5 | −6.5 | 1.2 | .0137* |
| T2-T0 | −12.25 | 11 | 7.19 | −25.2 | −13.9 | −0.9 | .0010* | |
| T3-T0 | −10.58 | 11 | 9.64 | −34.1 | −11.7 | 1.5 | .0029* | |
| T4-T0 | −22.78 | 11 | 9.11 | −44.7 | −21.2 | −9.1 | .0010* | |
| Ceramide | T1-T0 | −6.18 | 11 | 19.32 | −56.0 | 0 | 17.0 | .55 |
| T2-T0 | −1.55 | 11 | 19.82 | −51.0 | −1.0 | 29.0 | .85 | |
| T3-T0 | −19.55 | 10 | 20.63 | −64.0 | −12.0 | 0.0 | .002* | |
| T4-T0 | −35.8 | 11 | 35.27 | −109.0 | −33.0 | 10.0 | .0029* |
| Variable . | Time Points . | Mean . | No. . | St. Dev. . | Min . | Median . | Max . | P . |
|---|---|---|---|---|---|---|---|---|
| CD4 | T1-T0 | 12.55 | 11 | 61.03 | −82 | 1 | 118 | .56 |
| T2-T0 | 16.82 | 11 | 78.70 | −103 | 34 | 177 | .52 | |
| T3-T0 | 72.27 | 11 | 79.52 | −39 | 59 | 221 | .01* | |
| T4-T0 | 101.09 | 11 | 114.71 | −60 | 106 | 337 | .019 | |
| CD8 | T1-T0 | 65.55 | 11 | 225.73 | −347 | 96 | 443 | .33 |
| T2-T0 | 59.55 | 11 | 225.17 | −238 | 78 | 563 | .64 | |
| T3-T0 | 86.82 | 11 | 244.49 | −272 | 78 | 570 | .28 | |
| T4-T0 | 164.36 | 11 | 569.18 | −372 | 117 | 1585 | .76 | |
| Apoptotic CD4 | T1-T0 | −14.04 | 11 | 11.81 | −33.4 | −12.0 | −0.2 | .0010* |
| T2-T0 | −31.24 | 11 | 16.10 | −56.9 | −35.5 | −6.0 | .0010* | |
| T3-T0 | −30.73 | 11 | 18.02 | −55.4 | −33.6 | −1.3 | .0010* | |
| T4-T0 | −40.45 | 11 | 16.35 | −64.8 | −42.4 | −20.1 | .0010* | |
| Apoptotic CD8 | T1-T0 | −5.51 | 11 | 5.35 | −16.5 | −6.5 | 1.2 | .0137* |
| T2-T0 | −12.25 | 11 | 7.19 | −25.2 | −13.9 | −0.9 | .0010* | |
| T3-T0 | −10.58 | 11 | 9.64 | −34.1 | −11.7 | 1.5 | .0029* | |
| T4-T0 | −22.78 | 11 | 9.11 | −44.7 | −21.2 | −9.1 | .0010* | |
| Ceramide | T1-T0 | −6.18 | 11 | 19.32 | −56.0 | 0 | 17.0 | .55 |
| T2-T0 | −1.55 | 11 | 19.82 | −51.0 | −1.0 | 29.0 | .85 | |
| T3-T0 | −19.55 | 10 | 20.63 | −64.0 | −12.0 | 0.0 | .002* | |
| T4-T0 | −35.8 | 11 | 35.27 | −109.0 | −33.0 | 10.0 | .0029* |
T0, baseline; T1, day 15; T2, day 30; T3, day 90; T4, day 150.
Abbreviation: St. Dev., standard deviation.
Significant at .05 level after Hochberg adjustment for multiple comparison.
CD8 counts.
At each time point over the period when L-carnitine was administered, we observed a trend towards increased absolute CD8 cell counts, but the impact of L-carnitine therapy did not result in statistically significant gain (Table 1). The net change from baseline to the end of the study was an increase of 164.36 ± 569.18 (range, −372 to +1,585 cells/μL). At the end of the study, six patients had increased counts (range, 154 to 1585) (Table 2). In particular, one patient had an increase of more than 100%, three patients more than 50%, and two patients 30%. The remaining five patients had a slight decline (range, 123 to 372) compared with the baseline.
Apoptotic CD4 and CD8 cells.
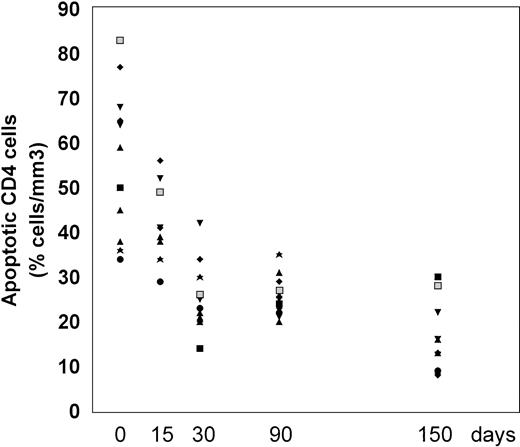
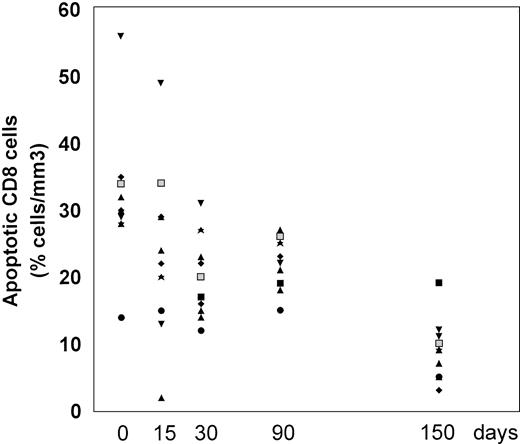
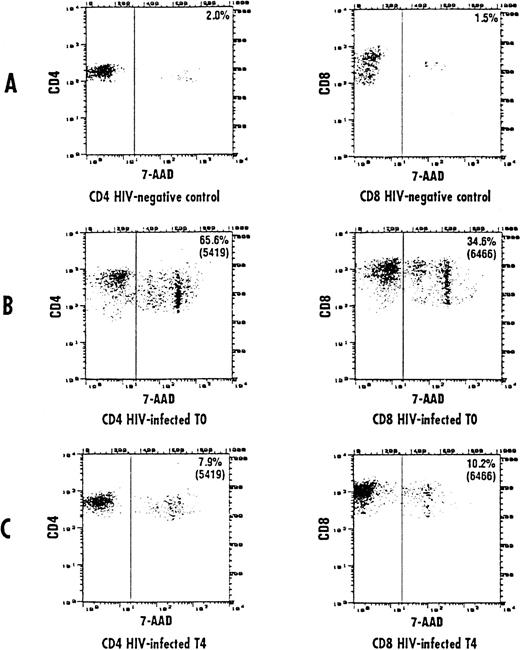
The frequency of apoptosis was investigated using the 7-AAD staining method that allows the detection of apoptotic cells, which are stained by 7-AAD because of their altered membrane integrity in contrast to living cells. Using this method, L-carnitine therapy led to a significant drop in the frequency of apoptotic CD4 and CD8 lymphocytes compared with the baseline (Fig 1 and 2, respectively). This reduction occurred gradually, but changes in actual values between each time point and T0 were always strongly significant (P values were between .001 and .014) (Table 2). The mean net change from baseline at the end of the study was a reduction of 40.4 ± 16.35 (range, 20.1 to 64.8) and 22.78 ± 9.11 (range, 9.1 to 44.7) in the percentage of apoptotic CD4 and CD8 cells, respectively (P < .001 for both subgroups). Representative cytofluorimetric profiles of these experiments are shown in Fig 3. As expected in control individuals, the majority of CD4 and CD8 cells were not stained by 7-AAD (Fig 3A). In contrast, HIV-positive individuals at baseline (T0) had a very high frequency of CD4 and CD8 cells stained with 7-AAD (ie, apoptotic cells) (Fig 3B); the L-carnitine treatment (T4) strongly reduced the frequency of cells stained with 7-AAD to a level comparable to that found in HIV-negative controls (Fig 3C).
Percentage of CD4 cells undergoing apoptosis before and after 15, 30, 90, and 150 days of L-carnitine treatment (each patient is indicated by a code). The apoptotic CD4 cells were analyzed by flow cytometry after 7-AAD as described in Materials and Methods. (▪) 2000; (⧫) 5419; (▴) 5716; (▴) 5762; (▾) 6438; (⧫) 6457; (★) 6464; (▪) 6466; (•) 6655; (▴) 6684; (▾) 7173.
Percentage of CD4 cells undergoing apoptosis before and after 15, 30, 90, and 150 days of L-carnitine treatment (each patient is indicated by a code). The apoptotic CD4 cells were analyzed by flow cytometry after 7-AAD as described in Materials and Methods. (▪) 2000; (⧫) 5419; (▴) 5716; (▴) 5762; (▾) 6438; (⧫) 6457; (★) 6464; (▪) 6466; (•) 6655; (▴) 6684; (▾) 7173.
Percentage of CD8 cells undergoing apoptosis before and after L-carnitine treatment (each patient is indicated by a code). The apoptotic CD8 cells were analyzed by flow cytometry after 7-AAD as described in Materials and Methods. The symbols are the same as those in Fig 1.
Percentage of CD8 cells undergoing apoptosis before and after L-carnitine treatment (each patient is indicated by a code). The apoptotic CD8 cells were analyzed by flow cytometry after 7-AAD as described in Materials and Methods. The symbols are the same as those in Fig 1.
Representative cytofluorographic assessment of CD4 and CD8 cells stained with 7-AAD (apoptotic cells). Two-color immunofluorescence staining was performed on CD4 and CD8 lymphocytes to analyze the staining by 7-AAD. Representative results are shown for CD4 and CD8 T cells from a control individual (A) and an HIV-infected person at baseline (B) and after 150 days of L-carnitine treatment (C). The vertical bar represents the threshold above which 7-AAD staining was considered positive.
Representative cytofluorographic assessment of CD4 and CD8 cells stained with 7-AAD (apoptotic cells). Two-color immunofluorescence staining was performed on CD4 and CD8 lymphocytes to analyze the staining by 7-AAD. Representative results are shown for CD4 and CD8 T cells from a control individual (A) and an HIV-infected person at baseline (B) and after 150 days of L-carnitine treatment (C). The vertical bar represents the threshold above which 7-AAD staining was considered positive.
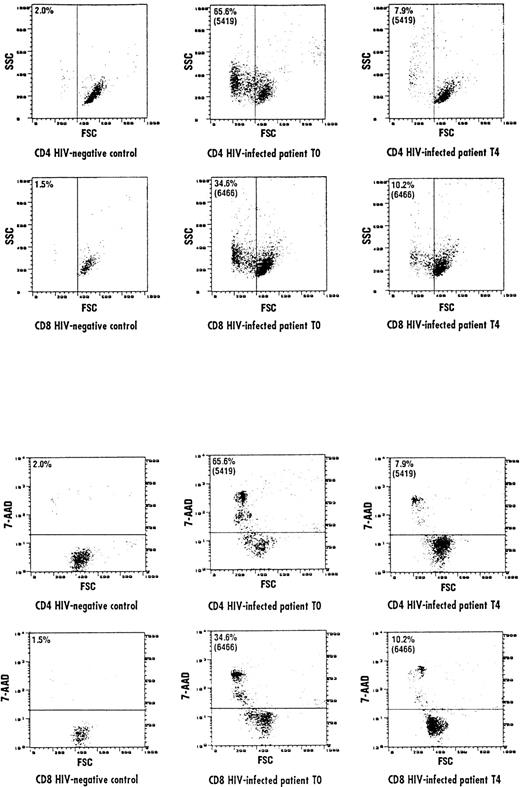
The results of experiments performed by investigating the FSC/SSC characteristics of CD4 and CD8 cells were comparable to the results obtained by the 7-AAD staining method. In the representative experiments shown in Fig 4A, both CD4 and CD8 cells in the HIV-positive donor (T0) shifted to a lower FSC/higher SSC compartment, indicating that these lymphocytes were engaged into the apoptotic pathway, a process that was not observed in lymphocytes from the control individuals. The L-carnitine treatment (T4) of HIV-positive patients resulted to shift both CD4 and CD8 cells to higher FSC/lower SSC compartment, comparable to that observed in normal individuals. Remarkably, CD4 and CD8 lymphocytes from the HIV-positive donor (T0), which had decreased cell size (FSC), were precisely those that incorporated 7-AAD (Fig 4B). L-carnitine therapy (T4) led to an increase of CD4 and CD8 cells with higher FSC, which did not incorporate 7-AAD.
Representative cytofluorographic experiment of apoptotic CD4 and CD8 cells assessed by evaluating FSC and SSC parameters. Apoptotic cells are represented as a function of both parameters. (A) Represents FSC/SSC lymphocyte compartments in an HIV-negative control and an HIV-positive individual at baseline (T0) and after 150 days of L-carnitine treatment (T4). A shift to a lower FSC/higher SSC was observed in the HIV-positive individual compared with the normal control, but L-carnitine treatment shifted FSC/SSC parameters to the levels observed in the HIV-negative control. Accordingly, CD4 and CD8 cells in the HIV-positive individual at baseline (T0) with decreased FSC were precisely those that incorporated 7-AAD (B). After 150 days of L-carnitine treatment (T4), CD4 and CD8 cells with higher FSC did not incorporate 7-AAD, as seen in the normal individual. The horizontal bar represents the threshold above which 7-AAD staining was considered positive.
Representative cytofluorographic experiment of apoptotic CD4 and CD8 cells assessed by evaluating FSC and SSC parameters. Apoptotic cells are represented as a function of both parameters. (A) Represents FSC/SSC lymphocyte compartments in an HIV-negative control and an HIV-positive individual at baseline (T0) and after 150 days of L-carnitine treatment (T4). A shift to a lower FSC/higher SSC was observed in the HIV-positive individual compared with the normal control, but L-carnitine treatment shifted FSC/SSC parameters to the levels observed in the HIV-negative control. Accordingly, CD4 and CD8 cells in the HIV-positive individual at baseline (T0) with decreased FSC were precisely those that incorporated 7-AAD (B). After 150 days of L-carnitine treatment (T4), CD4 and CD8 cells with higher FSC did not incorporate 7-AAD, as seen in the normal individual. The horizontal bar represents the threshold above which 7-AAD staining was considered positive.
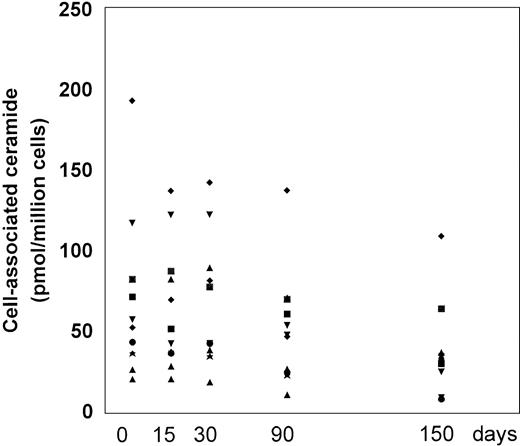
Ceramide levels.
The PBMC-associated ceramide levels were significantly higher at baseline in the patient group in comparison to healthy individuals (10.2 ± 2.3 pmol/106 cells), according to our previous work.8 20 L-carnitine therapy led to a progressive decline in the ceramide levels with respect to the baseline (Fig 5and Table 2). We measured decreased levels of PBMC-associated ceramide on each time point over the course of the study and at the end of the study, the changes were statistically significant compared with the baseline (P = .003). The mean net change from baseline at the end of the study was a reduction of 35.8 ± 35.3 (range, decrease of 109 to a gain of 10) pmol/106 cells. At each time point, the levels of ceramide correlated only weakly to moderately with the frequency of apoptotic CD4 and CD8 cells; a statistically significant correlation was found between ceramide levels and apoptotic CD4 cells on baseline and T1 (r = 0.71 and r = 0.73, P = .015 and .011, respectively) (Table 3).
Cell-associated ceramide levels expressed as picomoles/106 cells before and after L-carnitine treatment (each patient is indicated by a code). Fresh PBMC were isolated and the lipid extracts were assayed for endogenous ceramide with the DAG-kinase assay as described in Materials and Methods. The symbols are the same as those in Fig 1.
Cell-associated ceramide levels expressed as picomoles/106 cells before and after L-carnitine treatment (each patient is indicated by a code). Fresh PBMC were isolated and the lipid extracts were assayed for endogenous ceramide with the DAG-kinase assay as described in Materials and Methods. The symbols are the same as those in Fig 1.
Spearman Rank Correlations Between Ceramide Levels and Apoptotic CD4 and CD8 Cells
| Variable . | Time Points . | r . | P . | No. . |
|---|---|---|---|---|
| Apoptotic CD4, Ceramide | T0 | 0.71 | .015 | 11 |
| T1 | 0.73 | .011 | 11 | |
| T2 | 0.26 | .43 | 11 | |
| T3 | −0.24 | .48 | 11 | |
| T4 | −0.15 | .66 | 11 | |
| Apoptotic CD8, Ceramide | T0 | 0.37 | .26 | 11 |
| T1 | −0.063 | .85 | 11 | |
| T2 | −0.15 | .66 | 11 | |
| T3 | −0.37 | .26 | 11 | |
| T4 | −0.13 | .70 | 11 |
| Variable . | Time Points . | r . | P . | No. . |
|---|---|---|---|---|
| Apoptotic CD4, Ceramide | T0 | 0.71 | .015 | 11 |
| T1 | 0.73 | .011 | 11 | |
| T2 | 0.26 | .43 | 11 | |
| T3 | −0.24 | .48 | 11 | |
| T4 | −0.15 | .66 | 11 | |
| Apoptotic CD8, Ceramide | T0 | 0.37 | .26 | 11 |
| T1 | −0.063 | .85 | 11 | |
| T2 | −0.15 | .66 | 11 | |
| T3 | −0.37 | .26 | 11 | |
| T4 | −0.13 | .70 | 11 |
T0, baseline; T1, day 15; T2, day 30; T3, day 90; T4, day 150.
Abbreviation: r, Spearman rank correlation coefficient.
HIV-1 viremia.
HIV-1 viremia was measured at baseline, after 30 days, and at the end of the study. The large variability in the baseline levels of plasma HIV-1 RNA (log10) (range, 2.6 to 4.52 copies/μL) reflects the differing viral burdens between patients (Table 1). Mean HIV viremia levels increased slightly between baseline and T4 (mean log10 gain = 0.22; median gain, 0.14; range, −0.45 to +1.09).
Safety of treatment.
No toxicity related to L-carnitine therapy was observed and none of the subjects investigated required reductions in the dose of L-carnitine. In addition, even though we did not observe any significant improvements in the Karnofsky scores compared with the baseline (all of the patients had a Karnofsky score of >90 at enrollment), all of the subjects reported, with no exception, a sense of improved well-being by the second week of L-carnitine treatment.
DISCUSSION
Recent work from our laboratory has shown that L-carnitine downmodulates ceramide generation by interfering with the Fas-induced apoptotic signal,23 a major mechanism for the loss of CD4 and CD8 cells during the progression of the infection towards AIDS. The finding that short-term treatment of AIDS patients with L-carnitine significantly reduced both the levels of lymphocyte-associated ceramide and the frequency of apoptotic CD4 and CD8 cells22 added weight to the in vitro results. Ceramide has been shown to initiate signalling leading to lymphocyte apoptosis in response to several mechanisms, which are implicated in the inappropriate apoptosis seen in HIV-1–infected individuals, such as the chronic expression of Fas and Fas ligand and the unregulated activation of the receptor for tumor necrosis factor.13-17 Furthermore, ceramide enhances HIV-1 replication,18,19 and its cellular levels strongly increase after experimental HIV-1 infection30 and in peripheral blood lymphocytes from HIV-infected patients.8 The role of ceramide in the progression of HIV-1 infection is also supported by the finding that long-term nonprogressors, who remain clinically healthy and immunocompetent over an extended period of time, have lower lymphocyte-associated ceramide levels than subjects with AIDS.8 20
In this pilot study, we show evidence that L-carnitine therapy of HIV-1–infected subjects may have a significant impact on CD4 absolute counts, one independent predictor of the risk of developing AIDS-related complications. We were unable to set up an appropriate control group because we consider the placebo administration in AIDS patients unethical. Moreover, in the same group of patients, multiple CD4 counts were done before the initiation of the study, therefore, excluding artifactual increases of the data generated during the trial period. The absolute CD4 cell counts increased over the period when L-carnitine was administered and there was a significant increase compared with the baseline after 90 and 150 days of treatment, but a positive trend was already recognized after 15 and 30 days, even though the differences were not statistically significant.
The analyzed individuals represent a unique population of infected subjects, all of whom were living in the Community of San Patrignano, being exposed to the same environmental influences and with comparable nutritional regimens. Remarkably, in the majority of individuals, there was a past history of HBV or HCV infection and no opportunistic infection was detected during the trial period.
They had stable CD4 counts over an extended period of time, but progressively declining CD4 counts were recognized over the last 12 months of their stay in the Community before the enrollment in the trial. Because they refused to undergo any treatment with antiretroviral drugs, an option for enrollment in the study was offered, even though none of them had carnitine deficiency as shown by comparable plasma levels in healthy individuals.
The impact of L-carnitine therapy was not limited to CD4 cells, as even CD8 cell absolute counts and rate of change exhibited a trend towards improvement. Indeed, L-carnitine therapy resulted in an overall gain of CD8 cells.
These results were paralleled by a strong reduction in the frequency of apoptotic CD4 and CD8 cells and, even though this reduction occurred gradually, changes in actual values between each time point and baseline were always strongly significant.
The improvement in CD4 and CD8 absolute counts and the reduction in apoptosis appear to have progressive decline in cell-associated ceramide levels as the common denominator. Furthermore, at each time point, we found that ceramide levels correlated weakly to moderately with the frequency of apoptotic CD4 and CD8 cells and the correlation was statistically significant at baseline and T1 (r = 0.71 and r = 0.73, P = .015 and P = .011, respectively).
Remarkably, most of the above modifications occurred with only moderate (± 0.5 log maximum) changes in the plasma viral load, with the exception of one patient, in whom HIV-viremia increased by 1.1 log, but the same individuals showed a significant increase in CD4 cell counts and subjectively reported an increased sense of well-being and resistance to fatigue. Taken together, our data suggest that long-term L-carnitine administration may have a substantial impact on the chief immunologic abnormality associated with HIV-1 infection, the loss of CD4 T cells, through downmodulating the generation of ceramide and reducing the rate of apoptotic lymphocyte death, without affecting the HIV-1 viremia levels, thus suggesting that a dissociation exists between changes in viremia and CD4 depletion. Indeed, L-carnitine administration, although not affecting the viral life cycle, interrupts the HIV-infection–associated ceramide generation and consequently the lymphocyte apoptosis, which is considered a crucial factor in the pathogenesis of AIDS.
Additional mechanisms contributing to the antiapoptotic effects of L-carnitine, beside the inhibition of ceramide generation, cannot be ruled out, as shown by recent studies reporting its ability to prevent the disruption of mitochondrial transmembrane potential,31,32 an early and irreversible step in the effector phase of apoptotic cell death.33 Moreover, an abnormal redox cellular state is associated with HIV infection and could be at least partially involved in the T-cell depletion in AIDS.34,35 Indeed, oxidative stress has been implicated in apoptosis, and it may provide effector mechanisms for the final common pathway of programmed cell death. L-carnitine, when administered in vivo, is very effective in inhibiting oxygen radical production.36 Thus, the antiapoptotic effect of L-carnitine could also be due to its antidepolarizing action at the mitochondrial level and antioxidant activity.
The safety of treatment and the occurrence of toxicity related to the treatment was closely monitored in our study. None of the subjects investigated reported any toxicity related to L-carnitine therapy and dose reductions were not necessary. As L-carnitine therapy was able to increase CD4 counts in this small group of patients who had been experiencing decline in CD4 counts, this suggests that such therapeutic benefits may be real. The use of L-carnitine as an adjuvant to current combination antiretroviral therapy in a randomized comparison against the same therapies excluding L-carnitine is actually under investigation.
In conclusion, our results indicate that L-carnitine targets the immune system and suggest that complementing antiretroviral therapy with L-carnitine may be an attractive approach to the management of HIV-1–infected patients and a comparative evaluation seems justified. The potential of L-carnitine to protect the host against the toxicity of nucleoside analogs adds further weight to this idea.37-40
Address reprint requests to Claudio De Simone, MD, PhD, Department of Experimental Medicine, Via Vetoio 10, Coppito 2, 67100 L'Aquila, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal