Abstract
Little is known concerning the interaction of thrombopoietin (TPO) with other megakaryocyte-active cytokines in directing the early events of megakaryocyte development. Culture of CD34+ cells in interleukins (IL) -1, -6, -11, plus stem cell factor (SCF; S) results in a 10- to 12-fold expansion in total cell numbers, whereas total CD41+ megakaryocytes are expanded ∼120-fold over input levels. Addition of TPO to IL-1, -6, -11, S generates a biphasic proliferation of CD41+ cells, accelerates their rate of production, and results in an ex vivo expansion of more than 200-fold. The addition of Flt-3 ligand (FL) increases CD41+ cell expansion to ∼380-fold over input levels. In the absence of TPO, ∼95% of the expanded cells show the phenotype of promegakaryoblasts; TPO and/or FL addition increases CD41 antigen density and ploidy in a subpopulation of promegakaryoblasts. A moderate (approximately sevenfold) expansion of megakaryocyte progenitor cells (colony-forming unit-megakaryocyte) occurs in the presence of IL-1, -6, -11, S, and the addition of TPO to this cocktail yields an ∼17-fold expansion. We conclude that early proliferative events in megakaryocyte development in vitro are regulated by multiple cytokines, and that TPO markedly affects these early developmental steps. However, by itself, TPO is neither necessary nor sufficient to generate a full proliferative/maturational in vitro response within the megakaryocyte compartment. TPO clearly affects terminal differentiation and the development of (some) high-ploidy human megakaryocytes. However, its limited in vitro actions on human cell polyploidization suggest that additional megakaryocyte-active cytokines or other signals are essential for the maximal development of human megakaryocytes.
MEGAKARYOCYTE development is a complex process in which a wide variety of regulatory signals work in cohort to direct a highly specific response to thrombopoietic demand.1,2 Like other lineages, megakaryocytes show a developmental hierarchy of cells ranging from the primitive, actively proliferating progenitor cells to the postmitotic, polyploid megakaryocytes undergoing maturational development.1 Within this lineage, promegakaryoblasts represent a transitional stage between the proliferating progenitor cells and the mature megakaryocytes.3,4 Promegakaryoblasts are restricted (or lacking) in proliferative potential, being the developmental stage at which megakaryocytes cease to proliferate and begin to acquire an increased DNA content. As such, they are endomitotic (a mechanism of acquiring a polyploid DNA content) and contain a lower DNA content than the earliest morphologically recognizable cell, the megakaryoblast.5 6
Megakaryocyte development is controlled by growth factors that function to regulate both the expansion of megakaryocyte numbers (proliferation) and terminal differentiation (maturation).7-9 Numerous pleiotropic cytokines are known to influence megakaryocytopoiesis, although their maturational and proliferative functions often overlap. Both interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are mitogenic cytokines that primarily stimulate the proliferative phases of megakaryocytopoiesis.10,11 In the absence of other cytokines, IL-3, a potent megakaryocyte CSF, induces early stages of megakaryocyte development in vitro. However, the resulting cells are underdeveloped, and additional differentiation-inducing factors, such as IL-6 or IL-11, are required for full megakaryocyte maturation.12,13 Both of these ILs primarily affect the later stages of megakaryocytopoiesis, and each synergizes with IL-3 or GM-CSF to increase the number, size, and DNA content of developing megakaryocytes.14 Interestingly, IL-l enhances megakaryocyte proliferation in the presence of IL-3 and IL-6,14 and also promotes long-term megakaryocytopoiesis, possibly by driving commitment to this lineage.15 Stem cell factor (SCF), the ligand for the c-kit proto-oncogene, is a multipotent hematopoietic cytokine that also stimulates megakaryocytopoiesis, and enhances IL-3- or GM-CSF-stimulated progenitor cell (colony-forming unit-megakaryocyte [CFU-Mk] or burst-forming unit-Mk [BFU-Mk]) proliferation, while having little effect on promoting megakaryocyte maturation (size or DNA content).16 A similar cytokine, the ligand for the Flt-3/Flk-2 tyrosine kinase receptor, is involved in regulating growth of primitive hematopoietic cells.17Initial reports indicated little or no effect of the Flt-3 ligand (FL) on megakaryocytopoiesis.18 However, a recent study suggests a role for FL in amplifying the megakaryocytic proliferative effects of GM-CSF, IL-3, and SCF.19
A number of the cytokines that influence megakaryocyte development also function in hematopoietic stem/progenitor cell proliferation. As mentioned, both IL-6 and IL-11 synergize with IL-3; they also support the proliferation of primitive hematopoietic cells, shortening the G0/G1 period, and triggering dormant progenitor cells into proliferation.20,21 Likewise, IL-1 supports the proliferation of primitive hematopoietic cells,22 but may do so indirectly.23 While having little colony stimulating activity alone, SCF synergizes with other cytokines, stimulating hematopoietic stem/progenitor cell growth and differentiation.24 The Flt-3 receptor is preferentially expressed on primitive hematopoietic cells and, in humans, is largely restricted to CD34+ cells; its ligand thus has an important role in regulating growth of hematopoietic stem cells.25
Although the existence of a specific humoral regulator of platelet production (thrombopoietin, TPO) was proposed as early as 1958,26 it was not until 1994 that several groups reported its isolation and/or cloning.27-32 TPO functions not only to induce megakaryocyte progenitor cell proliferation, but also to drive their terminal differentiation. Additionally, the receptor for TPO (c-mpl) is expressed on primitive hematopoietic progenitor cells, allowing TPO to regulate early hematopoietic progenitor cells as well.33-35 Gene inactivation studies have shown TPO to be the primary in vivo regulator of thrombocytopoiesis.36,37 Mice deficient in either TPO or its receptor (c-mpl) show a dramatic (∼85%) decrease in circulating platelets as well as bone marrow (BM) and splenic megakaryocytes.36,37 Also, the megakaryocytes of the TPO/c-mpl-deficient mice are smaller and exhibit a lower mean ploidy than those of control mice, but are structurally normal.38 The platelets in these animals also are reduced in number, but are both structurally and functionally normal, and are sufficient to prevent overt bleeding.36-38 The continued platelet production in these animals suggests that megakaryocyte-active cytokines other than TPO are capable of supporting, albeit at a reduced level, the in vivo processes of megakaryothrombocytopoiesis.
Although it is widely accepted that TPO is the primary regulator of megakaryocytopoiesis, the other megakaryocyte-active cytokines undoubtedly contribute to the in vivo regulation of this process. Proliferation of primitive hematopoietic progenitor cells, as well as early progenitor cells in the megakaryocyte lineage, are optimally stimulated by the synergistic interaction of multiple cytokines.11,15,39 40 To examine the interactions of TPO with other megakaryocyte-active cytokines on developing megakaryocytes, we examined its actions in combination with those cytokines that exhibit the dual capacity to affect both primitive hematopoietic progenitor cells as well as cells of the megakaryocyte lineage. We report that in serum-free, chemically defined media, the combination of IL-1, -6, -11, and SCF, in conjunction with TPO, is capable of significantly stimulating megakaryocytopoiesis using human BM CD34+ progenitor cells as a starting cell population. As well, Flt-3 ligand, when included in this cocktail of early acting cytokines, exerts an additional megakaryocytopoietic effect, further enhancing the total production of CD41+ cells. Specifically, the combination of these cytokines regulates the expansion of CD34+ cells into the megakaryocyte lineage, generating a 20-fold increase in megakaryocyte progenitor cell numbers, and a 200- to 300-fold increase in the number of CD41+promegakaryoblasts generated ex vivo.
MATERIALS AND METHODS
BM Samples
BM aspirates were obtained from healthy adults following informed consent, as approved by the University of Michigan Institutional Review Board. Low-density nonadherent cells were obtained as described elsewhere.9 Briefly, marrow cells were centrifuged over Ficoll (Histopaque-1077; Sigma Chemical Co, St Louis, MO), for 30 minutes at 400g. Low-density cells were collected, washed, and allowed to adhere to tissue-culture plastic in the presence of 5% fetal calf serum (FCS) overnight. Nonadherent (NALD) cells were collected, washed, and CD34+ cells isolated by immunomagnetic separation (Miltenyi Biotec, Sunnyvale, CA) according to the manufacturer's instructions. CD34+ cell populations averaged 89% ± 1% purity (mean ± SEM, n = 18).
Cell Cultures
Ex vivo cell expansion in suspension-phase cultures.
CD34+ cells were cultured in serum-free conditions at a concentration of 5 × 104/mL in liquid medium plus growth factors in 24-well plates (Costar, Cambridge, MA). The culture medium consisted of serum-free McCoy's 5A (GIBCO, Life Technologies, Grand Island, NY) nutrient-supplemented as previously described,9 and containing 1% Nutridoma NS (Boehringer Mannheim, Indianapolis, IN). Cytokines, used in various combinations, included recombinant human (rhu) IL-1 (10 ng/mL; R & D, Minneapolis, MN), rhuIL-6 (25 ng/mL; R & D), IL-11 (25 ng/mL; R & D), SCF (25 ng/mL; R & D), Flt-3 ligand (50 ng/mL; R & D), and TPO (50 ng/mL; a generous gift from Dr Dan Eaton, Genentech, San Francisco, CA). Cultures were incubated at 37°C in a humidified atmosphere containing 7% CO2, and samples removed for analysis at the indicated time points. For all cell culture assays, viable cell number was determined by trypan blue exclusion, and cell counts determined using a hemocytometer.
Hematopoietic progenitor cell assays.
Hematopoietic progenitor cells were cultured as described previously.9 41 Briefly, 1 to 2 × 103immunomagnetically separated CD34+ cells (as input cell population), or suspension-culture expanded cells were placed in 1 mL of semisolid media consisting of McCoy's 5A media (as described above), and containing 30% FCS (HyClone Laboratories, Logan, UT), 2-mercaptoethanol (5 × 10-5 mol/L), rhuIL3 (25 ng/mL; R & D), rhu erythropoietin (rhuEPO, 3 U/mL; Amgen Inc, Thousand Oaks, CA), rhuSCF (25 ng/mL; R & D), rhuG-CSF (100 ng/mL; Immunex, Seattle, WA), rhuGM-CSF (100 ng/mL; Immunex), penicillin/streptomycin (100 U/mL, 100 mg/mL, respectively; Life Technologies), and 0.9% methylcellulose (Fluka Chemical, Ronkonkoma, NY). Three replicate cultures were set up for each cytokine cocktail, for each marrow sample. Megakaryocyte colonies (CFU-Mk) were scored after 14 to 16 days in culture and at 21 to 24 days for BFU-Mk. Cultures were incubated at 37°C in a humidified atmosphere containing 7% CO2.
Flow Cytometry
Flow cytometry was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Phycoerythrin-labeled anti-CD34 or mouse IgG control (Becton Dickinson) was used to evaluate immunomagnetically separated starting cell populations. Anti-CD41 (anti-GPIIb/IIIa; a generous gift of Dr Kenneth Mann, University of Vermont, Burlington), and fluorescein isothiocyanate (FITC)-labeled anti-mouse IgG (Sigma Immunochemicals, St Louis, MO) were used to identify megakaryocytic cells. CD41+ megakaryocytic cells constituted an average of 1.8% ± 0.2% (mean ± SEM, n = 18) of the CD34+ cell population isolated (ie, input CD41+ cells) as determined by two-color flow cytometric analysis following staining with both anti-CD34-PE and anti-CD41-FITC. At least 10,000 to 20,000 cells were analyzed from each sample.
Antibody labeling.
A total of 105 cells were incubated with antibody (10 μg/mL) at room temperature for 30 minutes in Trisma-buffered phosphate-buffered saline (Tris-PBS), containing 1% bovine serum albumin, pH 7.6. Parallel samples were incubated with an isotype-specific, inappropriate antibody (MOPC; Becton Dickinson) to establish the background level of nonspecific staining. The incubations for CD41 antigen also included 5 mmol/L EDTA in the wash buffer to minimize selectin-mediated platelet binding that yields a falsely-elevated determination of CD41+cells.42 CD41+ cells are defined as those cells whose antigenic content exceeds that expressed by 97% of the cells that labeled with inappropriate antibody. The ex vivo expansion of CD41+ cells was determined by evaluating the total number of CD41+ cells (thus defined) per culture compared with the input numbers of CD41+ cells for each cytokine cocktail. Unless otherwise stated, total cellularity and CD41+ cell numbers were determined on days 10 to 12 of culture.
Megakaryocyte ploidy analysis.
To determine megakaryocyte DNA content, anti-CD41- or mouse MOPC-IgG-labeled cells were fixed/permeabilized by gradual addition of iced methanol during mixing. The cells were incubated for 30 minutes on ice, centrifuged, and stained with propidium iodide (PI; 50 μg/mL in PBS, with 100 U/mL RNAse; Sigma Chemical). For evaluation, control populations of CD34+ cells were labeled with MOPC-FITC and PI, and 2C/4C DNA content cells gated to determine the upper 97% limit of nonspecific FITC-fluorescence. Positive fluorescence (ie, CD41 antigen-positive cells) is thus defined as any fluorescence above this limit. DNA analysis for ploidy determination was done on these CD41+ gated cells, and at least 10,000 to 20,000 cells were analyzed per condition.
Cytocentrifugation, Cytological Staining, and Immunocytochemistry
For morphological analysis, cells were centrifuged onto the slide surface using a Cytospin centrifuge (Shandon, Pittsburgh, PA), air-dried for 2 to 3 minutes and fixed in absolute methanol. Cells were stained with May-Grünwald-Giemsa (Sigma). For immunocytochemistry, fixed cytospin preparations were blocked with species-specific blocking serum, and incubated with unconjugated anti-CD41 (45 minutes, 22°C). Antibody-labeled megakaryocytic cells were visualized using an avidin-biotin-alkaline phosphatase system (ImmunoPure ABC Phosphatase Staining Kit; Pierce, Rockford, IL), according to manufacturer's instructions.
Statistical Analysis
Statistical evaluations were performed using the Student'st-test.
RESULTS
Cytokine Stimulation of CD34+ Cells
Megakaryocytic precursor cells are known to respond to a variety of growth factor signals, showing a moderate expansion of megakaryocytes when stimulated with two to three growth factors.7,11 We reasoned that, as in other systems,39 43 multiple growth factor stimulation might better augment megakaryocyte development in vitro, and that specific combinations of megakaryocyte-active cytokines might yield a preferential ex vivo expansion of early cells of the megakaryocyte lineage. Our initial studies of two factor combinations yielded a modest proliferation of megakaryocytic precursor cells. For example, SCF plus TPO gave a 10- to 20-fold expansion of CD41+ cells (data not shown). Therefore, we decided to evaluate combinations of four to six growth factors, choosing those known to affect both hematopoietic stem/progenitor cells as well as megakaryocytes.
Preliminary investigations indicated that the omission of TPO from many cytokine combinations markedly reduced both CD41 antigen expression as well as the DNA content of megakaryocytes developing from CD34+ cells (see below). Therefore, we analyzed the actions of megakaryocyte-active cytokine combinations in the presence of optimal amounts of TPO. We observe that serum-free stimulation of CD34+ cells with combinations of at least four cytokines significantly increases megakaryocytic proliferation, resulting in a 40- to 150-fold expansion of CD41+ cells (Fig 1). The ex vivo expansion of CD41+ cells in a cytokine-cocktail containing TPO plus IL-1, IL-6, and IL-11 (herein after IL-1,6,11,T) yielded a 41-fold increase in megakaryocytic cells. Given the noted synergy of SCF and TPO,6 44 we next substituted SCF for each of the cytokines in IL-1,6,11,T. The addition of SCF to TPO-containing cytokine cocktails doubled the expansion of CD41+ cells. In the presence of SCF, we also observed that IL-1, IL-6, or IL-11 could be individually eliminated from the cocktail with little effect on cell numbers, CD41 antigen density, or DNA content (latter two not shown). Importantly, the combination of SCF plus IL-1, -6, and -11 provides an approximately twofold better proliferative stimulus than does the combination of IL-1 ,-6, -11 plus TPO, thus showing that TPO is not an obligate requirement for the ex vivo expansion of CD41+cells per se. An alternative, but less likely, explanation, is that the interaction of IL-1, -6, -11, and TPO might be inhibitory, allowing only a partial (but high) degree of expansion. This possibility was excluded by combining IL-1, -6, -11, SCF, and TPO as a cocktail. This cytokine mixture proved to be a powerful proliferative stimulus yielding a 219 ± 16.8-fold (mean ± SEM, n = 12) expansion of CD41+ cells.
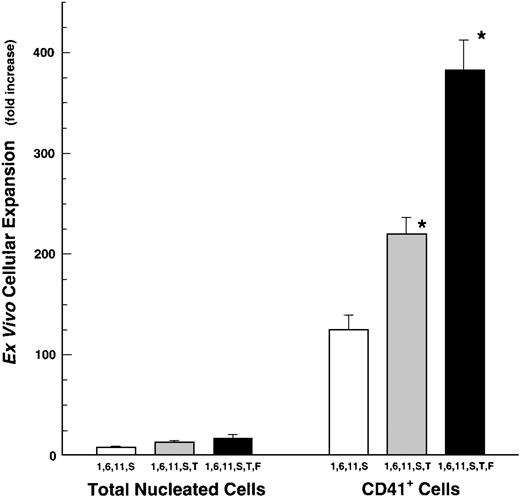
Cytokine stimulation of CD34+ cell expansion into CD41+ megakaryocytes. CD34+cells were cultured in various cytokine mixtures, composed of IL-1, -6, -11, SCF (S), and/or Thrombopoietin (T). Fold-increase values are mean ± SEM (n = 3). Input numbers of CD34+/CD41+ cells were 667 ± 289 per culture (n = 3). *P ≤ .05 compared with IL-1,6,11,S-driven CD41+ cell expansion in the absence of TPO.
Cytokine stimulation of CD34+ cell expansion into CD41+ megakaryocytes. CD34+cells were cultured in various cytokine mixtures, composed of IL-1, -6, -11, SCF (S), and/or Thrombopoietin (T). Fold-increase values are mean ± SEM (n = 3). Input numbers of CD34+/CD41+ cells were 667 ± 289 per culture (n = 3). *P ≤ .05 compared with IL-1,6,11,S-driven CD41+ cell expansion in the absence of TPO.
We next evaluated the action of the Flt-3/Flk-2 ligand (FL) on megakaryocyte production. Flt-3/Flk-2 ligand only partially substituted for SCF when added to a cocktail of IL-1, -6, -11, and TPO. However, when FL was included in the IL-1, -6, -11, SCF, TPO-cocktail, this combination of six cytokines (ie, 1, 6, 11, S, T, F) provided the most powerful stimulus to developing megakaryocytes generating a 381 ± 30-fold (mean ± SEM, n = 4) expansion of CD41+ cells (Fig 2). The cytokine-mediated expansion of CD41+ cells in these cocktails was preferential for this lineage, in that the expansion of CD41+ cells was 10 to 40 times greater than the expansion of total nucleated cells (Fig 2). This is true for cells grown in cocktails of either 4, 5, or 6 cytokines.
Ex vivo expansion of total nucleated cells (TNC) and CD41+ cells from human BM-derived CD34+cells. CD34+ cells were grown in IL-1, -6, -11, and SCF (S) with or without TPO (1,6,11,S or 1,6,11,S,T). Fold-increase values are mean ± SEM (n = 18, except for Flt3-ligand experiments where n = 4). Input numbers of CD34+/CD41+ cells were 857 ± 100 per culture. *P ≤ .05 comparisons are IL-1,6,11,S versus IL-1,6,11,S,T and IL-1,6,11,S,T versus the addition of FL.
Ex vivo expansion of total nucleated cells (TNC) and CD41+ cells from human BM-derived CD34+cells. CD34+ cells were grown in IL-1, -6, -11, and SCF (S) with or without TPO (1,6,11,S or 1,6,11,S,T). Fold-increase values are mean ± SEM (n = 18, except for Flt3-ligand experiments where n = 4). Input numbers of CD34+/CD41+ cells were 857 ± 100 per culture. *P ≤ .05 comparisons are IL-1,6,11,S versus IL-1,6,11,S,T and IL-1,6,11,S,T versus the addition of FL.
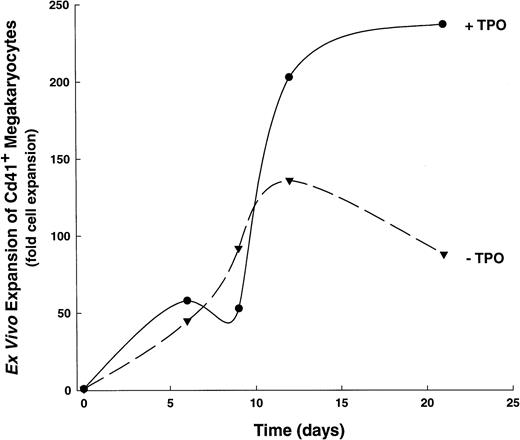
We also examined the actions of TPO on the kinetics of CD41+ cell development, determining its effects when added to the IL-1,6,11,S cytokine cocktail (Fig3). This cytokine combination was chosen because of its ability to stimulate significant megakaryocyte proliferation in the absence of TPO, thus allowing the segregation of TPO-mediated effects in a complex cytokine environment. When stimulated with IL-1, -6, -11, and SCF, human CD34+ cells produce optimal numbers of CD41+ cells over a 16- to 21-day period. In contrast, the addition of TPO (ie, IL-1,6,11,S,T) results in an increased rate of CD41+ cell development, such that significantly higher numbers (P ≤ .05, see Fig 3) of megakaryocytes are achieved by days 12-15 than when stimulated with IL-1,6,11,S alone. Moreover, CD34+ cells cultured in IL-1,6,11,S plus TPO show a biphasic production of CD41+ cells: a 25- to 50-fold increase occurs over the first 5 to 7 days, followed by a nadir on days 8-10, and a subsequent dramatic increase in cell production that reaches a 200-fold expansion of CD41+ cells by 16 to 21 days. The initial increase in total CD41+ cells at 5 to 7 days of culture seen in IL-1,6,11,S,T-containing cultures presumably represents a mature population of responding cells, because this increase correlates with the appearance of large cells with morphological characteristics of megakaryocytes, cytoplasmic processes, and the appearance of antigen-positive, platelet-sized particles (data not shown).
Developmental kinetics of CD41+ cell expansion. A single, representative kinetics experiment is shown out of six total experiments. (▾), CD41+ cell expansion in 1,6,11,S; (•), CD41+ cell expansion in IL-1,6,11,S,T.
Developmental kinetics of CD41+ cell expansion. A single, representative kinetics experiment is shown out of six total experiments. (▾), CD41+ cell expansion in 1,6,11,S; (•), CD41+ cell expansion in IL-1,6,11,S,T.
CD34+ Cells Stimulated by Multiple Cytokines Generate Large Numbers of CD41+Promegakaryoblasts
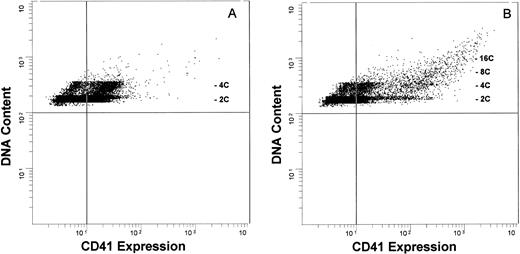
The marked ex vivo expansion of CD41+ cells suggested that a highly proliferative compartment was responding to these cytokines. To determine the level of cytokine action, we phenotypically characterized these cells with respect to the correlation between their DNA content and their expression of CD41. Developing megakaryocytes were analyzed by two-color flow cytometry, evaluating glycoprotein IIb/IIIa expression (CD41 antigen), cell size and complexity (forward-angle and side-angle light scatter, respectively), and DNA content. Megakaryocytes grown in the presence of IL-1,6,11,S express relatively low amounts of CD41 antigen (CD41Low cells, Fig 4A) and consist predominantly of 2C or 4C cells, with 7.4% ± 1.7% (mean ± SEM, n = 8) of these cells having a DNA content of ≥8C. May-Grünwald-Giemsa staining showed that these cells have the features of promegakaryoblasts, being relatively small, with a high nucleus:cytoplasm ratio, a (variably) lobulated nucleus, and basophilic cytoplasm. Immunocytochemical analysis confirmed the expression of low amounts of CD41 (data not shown). In contrast, the addition of TPO generated a population of cells expressing a markedly higher CD41 antigen level (CD41High cells) and increased the frequency of cells with higher DNA content (≥8C: 15.0% ± 7.8%, n = 8; Fig 4B). This subpopulation of CD41High cells is not seen in megakaryocyte populations grown in the absence of TPO, even after 28 days in culture. Immunocytochemical analysis again confirmed that the 2C/4C cells expressed a low to moderate amount of CD41 and, as expected, CD41 antigenic density correlated with ploidy, with the higher ploidy cells expressing the highest amount of antigen. Prolonging the culture period up to 28 days failed to increase the extent of polyploidization. To ensure that we were not biasing the culture system toward proliferation at the expense of differentiation, we expanded CD41+ cells in either an IL-1,6,11,S or an IL-1,6,11,S,T cytokine cocktail for 7 days, washed the cells, and recultured these cells in the presence of TPO only. This failed to enhance the degree of polyploidization over the ensuing 7 to 10 days of continued culture (data not shown). Thus, the presence of TPO is necessary for the expression of maximal amounts of CD41 antigen, but is insufficient to drive full endomitosis. We also investigated the possibility that endogenously produced transforming growth factor-β (TGF-β) might exert an inhibitory effect on megakaryocyte differentiation. Addition of saturating amounts of a neutralizing anti-TGF-β antibody to both IL-1,6,11,S- and IL-1,6,11,S,T-containing cultures yielded equivalent CD41+ cell expansion, CD41 antigen content, and ploidy distribution profiles to cultures in the absence of neutralizing antibody (data not shown). Thus, the lack of polyploidization was not influenced by inhibition of megakaryocytic development due to the presence of endogenously produced TGF-β.
Antigenic expression and DNA content of cytokine-expanded human megakaryocytic cells. Developing megakaryocytes analyzed by two-color flow cytometry, evaluating glycoprotein IIb/IIIa (CD41) antigen expression (FITC; abcissa) and DNA content (propidium iodide; ordinate). The vertical quadrant marker in the flow diagrams represents upper limit of nonspecific fluorescence, using an isotype-specific inappropriate antibody as a control.
Antigenic expression and DNA content of cytokine-expanded human megakaryocytic cells. Developing megakaryocytes analyzed by two-color flow cytometry, evaluating glycoprotein IIb/IIIa (CD41) antigen expression (FITC; abcissa) and DNA content (propidium iodide; ordinate). The vertical quadrant marker in the flow diagrams represents upper limit of nonspecific fluorescence, using an isotype-specific inappropriate antibody as a control.
Human Megakaryocyte Progenitor Cells Respond to Combinations of Megakaryocyte-Active Cytokines
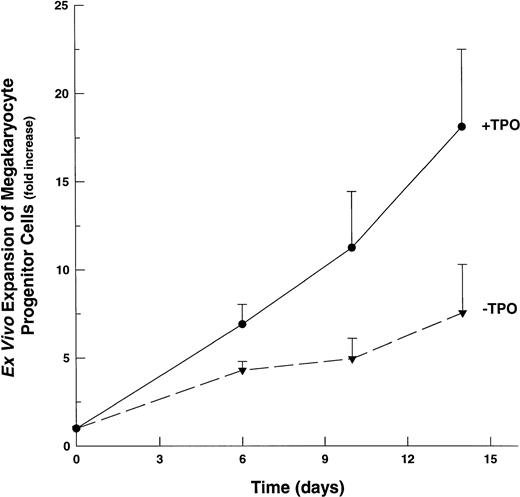
Although the majority of CD41+ cells grown in the presence of multiple-cytokine cocktails are promegakaryoblasts, the presence of 2C/4C, CD41+ cells suggested that some of these cells might be megakaryocytic progenitor cells, because a small percentage of these cells are known to express this lineage-specific antigenic marker.45 46 To assess the ability of multiple cytokine cocktails to affect megakaryocytic progenitor cell proliferation, we determined the number of CFU-Mk in suspension cultures at selected time points after their initiation. Unlike the marked (∼200-fold) expansion of promegakaryoblasts, we observed a modest (4.0-fold) expansion of CFU-Mk by day 6 in IL-1,6,11,S-based cultures (Fig 5). In the presence of TPO, the expansion of CFU-Mk continues over 14 days of culture, reaching a maximal increase of ∼17-fold. At all time points, the presence of TPO resulted in an approximate doubling in the ex vivo expansion of CFU-Mk.
Ex vivo expansion of megakaryocyte progenitor cells (CFU-Mk). CD34+ cells cultured in IL-1,6,11,S with or without TPO. Aliquots evaluated at indicated time points for the presence of CFU-Mk. The averages of three replicate cultures were determined for each cytokine combination, for each time point. Fold-increase values are mean ± SEM of the averaged (triplicate) values (n = 3). (▾), CFU-Mk expansion in 1,6,11,S; (•), CFU-Mk expansion in IL-1,6,11,S,T.
Ex vivo expansion of megakaryocyte progenitor cells (CFU-Mk). CD34+ cells cultured in IL-1,6,11,S with or without TPO. Aliquots evaluated at indicated time points for the presence of CFU-Mk. The averages of three replicate cultures were determined for each cytokine combination, for each time point. Fold-increase values are mean ± SEM of the averaged (triplicate) values (n = 3). (▾), CFU-Mk expansion in 1,6,11,S; (•), CFU-Mk expansion in IL-1,6,11,S,T.
DISCUSSION
Evaluation of the actions of multiple megakaryocyte-active cytokines on the ex vivo expansion of promegakaryoblasts from human CD34+ BM cells indicates that combinations of IL-1 ,-6, -11, SCF, FL, and/or TPO markedly affect this process. Each of these cytokines contributes to the ex vivo expansion of megakaryocytes, with TPO and SCF playing a predominant role. Although the addition of multiple cytokines increases the quantity of megakaryocytes, the overall quality of these cells remains the same. The use of multiple-cytokine combinations preferentially stimulates megakaryocyte expansion from CD34+ cells, as combinations of one to three cytokines gave only a modest (10- to 20-fold) increase in megakaryocyte numbers, whereas combinations of four to six megakaryocyte-active growth factors induced an 80- to 300-fold increase in CD41+cells. In sharp contrast, the respective increase in total nucleated cells generated using the same growth factor combinations ranged from 10- to 20-fold. The addition of TPO to any cytokine combination modestly increased the number of total nucleated cells while significantly increasing the total number of CD41+ cells, CD41 antigen-density, and the DNA content of a small subpopulation of CD41+ cells, as well as altering megakaryocyte developmental kinetics. These data are consistent with the concept that these cytokine cocktails act by influencing early (proliferative) events in megakaryocytic development, working predominately at the progenitor cell level. As a result, the predominant cell type produced in these cultures is the CFU-Mk progeny, ie, the promegakaryoblast, a transitional cell of relatively smaller size, lower antigen expression, and lower DNA content.
Our data show that a specific combination of five cytokines (ie, TPO plus the four megakaryocyte-active growth factors IL-1, -6, -11, and SCF) results in a marked expansion (>200-fold) of early megakaryocytes from CD34+ cells, whereas a six-cytokine combination (ie, the addition of FL to the above cocktail) yields a 50% increase in megakaryocyte numbers generating a 300-fold ex vivo expansion of CD41+ cells. Conversely, omission of TPO from any cytokine cocktail results in a reduction of the total number of CD41+ cells (with the resultant CD41+ cells being primarily CD41Low), and reduces the number of high ploidy (≥8C) cells. As a solitary cytokine, TPO generally supports a twofold to fourfold expansion of megakaryocytes6 (and our unpublished observations, September 1995). It also supports full differentiation, including proplatelet formation of megakaryocytes, increases the number of megakaryocyte colonies, and increases the number and size of megakaryocytes developed in vitro.27-30 Other investigations have shown that TPO, in conjunction with two to three other cytokines (IL-3, IL-6, or SCF), induces an 8- to 20-fold expansion of CD41+ cells from CD34+ cells.6 Bertolini et al47achieved a 58-fold increase in megakaryocytes using a seven-cytokine combination of IL-3, -6, -11, SCF, TPO, FL, and macrophage inflammatory protein-Iα (MIP-Iα). It is interesting to note that these cytokine combinations included IL-3. Although IL-3 is a megakaryocyte-active cytokine, we chose not to include it in our culture system because its presence obtunds megakaryocyte development (data not shown). Others have similarly reported a negative effect of IL-3 on megakaryocyte differentiation.6,44,48 49 It is unclear whether IL-3's pleiotropic actions favor commitment to other lineages or directly inhibit terminal megakaryocytopoiesis (see below). Nonetheless, our data indicate that IL-3 is not essential in the early phases of megakaryocytopoiesis, and that its presence is inhibitory to megakaryocyte differentiation.
In addition to TPO, both SCF and FL have a profound affect on megakaryocyte proliferation. Importantly, SCF, in conjunction with IL-1, -6, and -11, can substitute for TPO to yield a nearly equivalent proliferation of megakaryocytic precursors and, when added to TPO-containing cultures, SCF doubles the expansion of CD41+cells. Flt-2/Flk-3 ligand also augments megakaryocyte expansion. However, FL cannot fully replace SCF during the ex vivo expansion of promegakaryoblasts, demonstrating the presence of CD34+ or CD34+/CD41+ cells in our starting cell population that respond to SCF, but not FL. Other studies report similar effects for combinations of SCF, TPO, and/or FL. For example, Bertolini et al47 found FL to be significantly more effective than SCF for megakaryocyte progenitor cell proliferation, when using a cytokine combination that contained IL-3, IL-6, IL-11, and TPO. The mechanism of action for SCF and/or FL on megakaryocytopoiesis is not known, but may be due to their direct stimulation of CD34+ cells.50-52 Consistent with this, we observe an expansion of both promegakaryoblasts and megakaryocyte progenitor cells, suggesting that these factors either drive an influx of cells from a more primitive progenitor cell compartment (eg, BFU-Mk and/or earlier cells), or that they regulate lineage commitment. Under all multiple cytokine conditions tested in the absence of TPO, the majority of the megakaryocytic cells have a low CD41 antigen density (CD41Low) and a 2C/4C DNA content, and May-Grünwald-Giemsa staining and immunocytochemical localization indicate that the majority of these cells are promegakaryoblasts. In contrast, CD34+ cells grown in the presence of TPO show an increase in the proportion of cells with higher CD41 antigen density, and a proportion of these cells (≈15%) are morphologically mature and have a DNA content ≥8C. These data are similar to that of Debili et al,6 who observed a low mean ploidy and a variable CD41 antigen density in TPO-driven megakaryocytes (albeit with fewer cytokines), but also observed that ∼25% of ex vivo generated megakaryocytes were indistinguishable from marrow-derived cells.
It is interesting to note that neither the serum-free culture conditions reported here or elsewhere support the full polyploidization of human megakaryocytes, even when stimulated with optimal/maximal levels of TPO. TPO supports full megakaryocyte differentiation in vitro, including proplatelet formation by both human and murine megakaryocytes,53 and the generation of functional platelets.54 However, the majority of TPO-stimulated human cells are not mature megakaryocytes54 (and data herein), or show maturational defects6 or are of low ploidy.28,47,49 This limited ability to generate high-ploidy megakaryocytes from human CD34+ stem/progenitor cells is in sharp contrast to murine cells, where 35% to 55% of ex vivo-generated megakaryocytes have ploidy values of ≥64C.28 44 TPO's profound effect on megakaryocytopoiesis and thrombopoiesis in animals thus contrasts with its inability to support human megakaryocyte proliferation and differentiation in vitro. Even using complex mixtures of cytokines affecting both early and late megakaryocyte progenitor cells, the ability of TPO to affect terminal differentiation and develop higher ploidy of human megakaryocytes is limited, and the expanded CD41+ cells largely display the phenotype of promegakaryoblasts. This inability to fully support human megakaryocyte proliferation and differentiation in vitro suggests the absence of either extracellular matrix or other microenvironmental-derived signals, and/or that additional megakaryocyte-active cytokines are essential for maximal differentiation and polyploidization of human megakaryocytes.
Our data, using a culture system that does not include IL-3 (ie, IL-1,6,11,S), suggests that megakaryocyte differentiation is obtunded by the degree of proliferation. However, the addition of thrombopoietin to a cocktail of IL-1, -6, -11, and S results in both further proliferation and enhanced (but not full) maturation/polyploidization, showing that further maturation is cytokine-(ie, TPO) dependent. This contrasts with studies suggesting that (in IL-3-driven cultures) the proliferation of megakaryocytic progenitor cells somehow occurs at the expense of differentiation capacity, resulting in an inverse relationship between the degree of proliferation and ploidy.48,55,56 Mazur et al48,56 hypothesized an inverse relationship between mitosis and endomitosis during megakaryocyte differentiation, and that the extent of polyploidization that can be achieved by developing megakaryocytes is influenced by the prior mitotic history of their immediate precursor cells. Alternatively, Debili et al49 propose that the lack of higher ploidy (≥8C) megakaryocytes in IL-3-containing cultures is caused by a direct inhibitory effect, in which IL-3 favors proliferation of megakaryocyte progenitor cells by inhibiting the switch from mitosis to endomitosis, without necessarily inhibiting maturation-associated events. Taken together, our data and Debili's suggest that the presence of stimulatory or inhibitory extracellular signals mediates the onset of megakaryocyte polyploidization. Consistent with this, the in vivo response to acute thrombocytopenia includes both proliferation and differentiation as evidenced by an increase in number of megakaryocytes, as well as a shift to megakaryocytes of higher ploidy.8 57 Thus, the prior proliferative history in vivo does not seem to affect differentiation. Rather, the “cytokine profile” and/or extracellular matrix molecules of the megakaryocytic microenvironment undoubtedly regulates this process.
We conclude that early proliferative events in megakaryocyte development in vitro are regulated by multiple cytokines. Although TPO markedly affects these early developmental steps, it is neither necessary nor sufficient to generate a full proliferative response within the megakaryocyte compartment. Further, the simultaneous use of multiple megakaryocyte-active cytokines (eg, IL-1, -6, -11, SCF, FL, and TPO) allows a marked and preferential ex vivo expansion of early cells of the megakaryocytic lineage. The use of such cells as a starting population for purification studies should generate adequate numbers of early megakaryocytes for biochemical or molecular studies. Moreover, the expansion of these early megakaryocytes in the absence of TPO (ie, with IL-1, -6, -11, and SCF) will permit studies on the action of TPO in intracellular events such as signal transduction.
Supported in part by Grant No. HL514559 from the National Heart, Lung and Blood Institute.
Address reprint requests to Michael W. Long, PhD, Room 3570B, MSRB-II, University of Michigan, Ann Arbor, MI 48109-0688.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal