To the Editor:
It has recently been shown in preclinical studies that local or systemic treatment with interleukin-12 (IL-12) produces profound antitumor effects causing regression of established tumors and tumor metastases.1,2 The antitumor activity of IL-12 is to a large extent mediated by interferon-γ (IFN-γ) secreted in increased amounts by T cells and natural killer (NK) cells.1
IL-12 had initially been viewed as a synergistic hematopoietic growth factor in vitro.3 However, when administered in vivo to mice it caused a dose-dependent suppression of bone marrow hematopoiesis.4 Similarly, in phase I trial of IL-12 in patients with advanced malignancies it caused anemia, thrombocytopenia, lymphopenia, and neutropenia.5
There is substantial evidence that cytokines (mainly IFN-γ) released during IL-12 treatment are implicated in the suppression of bone marrow hematopoiesis. However, both neutralization with monoclonal antibodies1 and reducing the release of IFN-γ (by means of predosing with IL-12)6,7 result in attenuation of the antitumor activity of IL-12, and thus cannot be considered as good methods to prevent the suppression of bone marrow hematopoiesis. In our previous experiments we showed that application of granulocyte colony-stimulating factor (G-CSF) results in correction of IL-12–induced granulocytopenia.8 However, coadministration of G-CSF with IL-12 led to even stronger suppression of thrombopoiesis. Moreover, as has been reported in a recent issue of BLOOD,pretreatment with IL-12 does not ameliorate the thrombocytopenia observed during subsequent administration of IL-12 to either humans or mice.7
Because erythropoietin (Epo) is used in the treatment of anemia of cancer,9 and has also been shown to occasionally correct thrombocytopenia,10 we decided to evaluate whether it could prevent the development of some of the side effects induced by IL-12 treatment.
Administration of IL-12 (kindly provided by Genetics Institute, Boston, MA) at a daily dose of 0.1 μg for 7 consecutive days resulted in anemia and thrombocytopenia (Table 1). Epo (rhuErythropoietin, Eprex, Cilag) administered at two daily doses of 20 U for 7 consecutive days nonsignificantly increased the level of hemoglobin as compared with controls. In accordance with our expectations, application of Epo prevented the development of IL-12–induced anemia. Additionally, unexpectedly Epo was also found to prevent IL-12–induced decrease in platelet count. However, taking into account the somewhat weaker thrombopoietic activity of Epo in humans11 as compared with mice, it remains to be seen whether Epo could be effective in correcting IL-12–induced thrombocytopenia in tumor patients.
The Influence of IL-12 and/or Epo on the Number of Platelets and the Hemoglobin Concentration in Peripheral Blood
| In Vivo Treatment . | Platelets (×109/mL) . | Hemoglobin (g/L) . |
|---|---|---|
| 0.1% BSA-PBS | 1004.5 ± 195.4 | 189.5 ± 25.0 |
| Epo | 853.6 ± 312.5 | 216.0 ± 21.9 |
| IL-12 | 612.9 ± 98.9-150 | 156.1 ± 8.5-150 |
| IL-12 + Epo | 887.6 ± 193.9-151 | 205.5 ± 29.3-151 |
| In Vivo Treatment . | Platelets (×109/mL) . | Hemoglobin (g/L) . |
|---|---|---|
| 0.1% BSA-PBS | 1004.5 ± 195.4 | 189.5 ± 25.0 |
| Epo | 853.6 ± 312.5 | 216.0 ± 21.9 |
| IL-12 | 612.9 ± 98.9-150 | 156.1 ± 8.5-150 |
| IL-12 + Epo | 887.6 ± 193.9-151 | 205.5 ± 29.3-151 |
Five to seven 9-week-old B6D2F1 melanoma-bearing male mice were used per group. Mice were treated intraperitoneally with IL-12 (0.1 μg/injection) and/or Epo (20 U/injection given twice daily) or with 0.1% BSA-PBS for 7 consecutive days. Blood was collected on day 8, 12 hours after the last injection, and analyzed using Sysmex-820 cell counter (Sysmex, Kyoto, Japan) adjusted to the analysis of rodent cells. Results represent the mean ± SD.
P < .01 versus controls.
P < .01 versus IL-12–treated mice (Student'st-test).
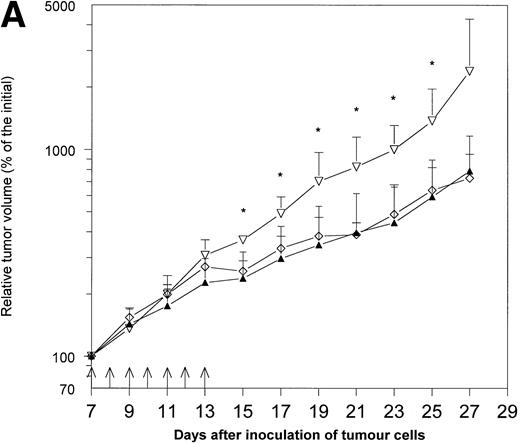
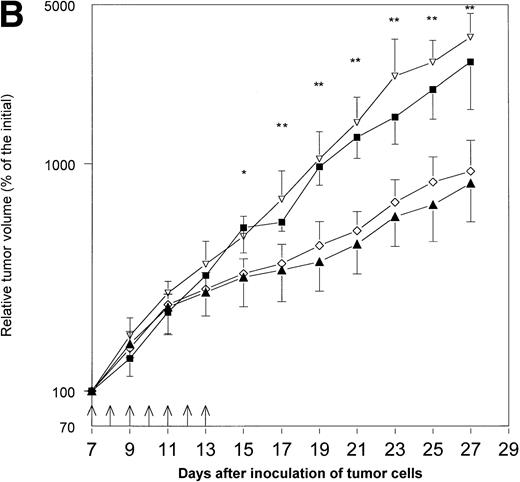
Because hematopoietic growth factors may occasionally stimulate the growth of neoplasms, including solid tumors,12 13 we decided to examine the influence of Epo application on the growth of MmB16 melanoma. Epo did not influence tumor growth when given alone and did not decrease the antitumor activity of IL-12 in this particular tumor model (Fig 1A and B).
The effects of treatment with IL-12 and/or Epo on MmB16 melanoma growth in B6D2F1 mice. Mice were inoculated with 1 × 106 melanoma cells into the footpad of the right hind limb and treated with the intratumoral injections (days 7 to 13, arrows) of IL-12 (0.1 μg/injection), Epo (20 U/injection; twice daily), or IL-12 in combination with Epo (the same doses). Tumor volume was determined as described previously.15 (A) A pilot study with four B6D2F1 mice per group. * P < .05: IL-12- and IL-12+ Epo–treated mice versus controls; ▴, IL-12 + Epo; ◊, IL-12; ▿, control. (B) Additional experiment with five to six B6D2F1 mice in each of the groups. * P < .05: IL-12- and IL-12+ Epo–treated mice versus controls and Epo-treated mice; ** P < .01: IL-12- and IL-12 + Epo–treated mice versus controls and Epo-treated mice (Student's t-test); ▴, IL-12 + Epo; ◊, IL-12; ▪, Epo; ▿, control.
The effects of treatment with IL-12 and/or Epo on MmB16 melanoma growth in B6D2F1 mice. Mice were inoculated with 1 × 106 melanoma cells into the footpad of the right hind limb and treated with the intratumoral injections (days 7 to 13, arrows) of IL-12 (0.1 μg/injection), Epo (20 U/injection; twice daily), or IL-12 in combination with Epo (the same doses). Tumor volume was determined as described previously.15 (A) A pilot study with four B6D2F1 mice per group. * P < .05: IL-12- and IL-12+ Epo–treated mice versus controls; ▴, IL-12 + Epo; ◊, IL-12; ▿, control. (B) Additional experiment with five to six B6D2F1 mice in each of the groups. * P < .05: IL-12- and IL-12+ Epo–treated mice versus controls and Epo-treated mice; ** P < .01: IL-12- and IL-12 + Epo–treated mice versus controls and Epo-treated mice (Student's t-test); ▴, IL-12 + Epo; ◊, IL-12; ▪, Epo; ▿, control.
As the antitumor activity of IL-12 may by potentiated by administration of some of the chemotherapeutics,14 it might be anticipated that IL-12 together with Epo could be successfully included into some of the combined chemoimmunotherapy schedules.
ACKNOWLEDGMENT
Supported by Grant No. 6 P207 058 07 from the State Committee for Scientific Research (K.B.N), Poland. Tomasz Stokłosa is a recipient of the Foundation for Polish Science Award.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal