Abstract
To determine the possible role of the BCR/ABL oncoprotein SH3 domain in BCR/ABL-dependent leukemogenesis, we studied the biologic properties of a BCR/ABL SH3 deletion mutant (▵SH3 BCR/ABL) constitutively expressed in murine hematopoietic cells. ▵SH3 BCR/ABL was able to activate known BCR/ABL-dependent downstream effector molecules such as RAS, PI-3kinase, MAPK, JNK, MYC, JUN, STATs, and BCL-2. Moreover, expression of ▵SH3 BCR/ABL protected 32Dcl3 murine myeloid precursor cells from apoptosis, induced their growth factor-independent proliferation, and resulted in transformation of primary bone marrow cells in vitro. Unexpectedly, leukemic growth from cells expressing ▵SH3 BCR/ABL was significantly retarded in SCID mice compared with that of cells expressing the wild-type protein. In vitro and in vivo studies to determine the adhesive and invasive properties of ▵SH3 BCR/ABL-expressing cells showed their decreased interaction to collagen IV- and laminin-coated plates and their reduced capacity to invade the stroma and to seed the bone marrow and spleen. The decreased interaction with collagen type IV and laminin was consistent with a reduced expression of α2 integrin by ▵SH3 BCR/ABL-transfected 32Dcl3 cells. Moreover, as compared with wild-type BCR/ABL, which localizes primarily in the cytoskeletal/ membrane fraction, ▵SH3 BCR/ABL was more evenly distributed between the cytoskeleton/membrane and the cytosol compartments. Together, the data indicate that the SH3 domain of BCR/ABL is dispensable for in vitro transformation of hematopoietic cells but is essential for full leukemogenic potential in vivo.
BCR/ABL ARE ONCOGENIC chimeric tyrosine kinases generated from the reciprocal t(9;22) (q34;q11) translocation in chronic myelogenous leukemia (CML) and in Philadelphia1acute lymphocytic leukemia (ALL). The translocation fuses a truncatedbcr gene with sequences upstream of the second exon of thec-abl gene.1,2 The BCR/ABL proteins p210 and p185 cause, CML- and ALL-like syndromes, respectively, in mice3,4 and are required for Philadelphia1 cell growth.5 Cytoplasmic BCR/ABL proteins6transduce oncogenic signals to the nucleus via several pathways.7-9 Signaling from BCR/ABL affects the function of intracellular regulators involved in apoptosis10 and growth factor-dependent proliferation11 and may intervene in cell-cell communication and cell-extracellular matrix interaction.12 13
BCR/ABL mutants have been used to define potential downstream effectors of wild-type BCR/ABL7-9 and to identify the BCR/ABL domains required for its transforming activity.14-19 Only a few of the signaling proteins, eg, Shc,7 RAS,20,21phosphatidylinositol 3-kinase (PI-3k),22 RAF,23BCL-2,10 c-MYC,24 and cyclin D1,25 appear essential for BCR/ABL-mediated leukemogenesis and/or colony formation by Philadelphia1 cells. These molecules, among others, are responsible for the BCR/ABL-dependent phenotype characterized by growth factor independence,11,26 protection from apoptosis,27abnormal adherence to the stroma,12,13 and leukemogenesis in vivo.3 Each of these functions may be regulated through different signaling pathways activated by distinct BCR/ABL domains.
Few domains or single amino acids are necessary for the full leukemogenic potential of BCR/ABL. The kinase domain,28 the 176-427 amino acids segment in the BCR portion of the protein,15 and the FLVRES motif in the SH2 domain7 are required for BCR/ABL oncogenic activity, because single amino acid substitutions or deletions in these regions result in impairment or abrogation of the transformation potential.
c-ABL SH3 domain mutations or deletions cause its oncogenic activation.29 However, the role of this domain in BCR/ABL-mediated leukemogenesis is unknown.
We report here that the SH3 domain is not required for the activation of BCR/ABL-dependent signaling pathways associated with the transformed phenotype in vitro. However, the same domain is important for BCR/ABL-mediated leukemogenesis in vivo because it regulates adhesion, invasion, and homing of leukemic cells.
MATERIALS AND METHODS
BCR/ABL Constructs
The p210 BCR/ABL(bcr exon 3/abl exon 2) wild-type (WT) cDNA was generated by replacing the EcoRI-BsrGI fragment of p185BCR/ABL in the pSRαMSVtkneo retroviral construct (gift of Dr C. Sawyers, UCLA, Los Angeles, CA) with that of the p210 BCR/ABL(bcr exon 3/abl exon 2) cloned in the sp65 plasmid (gift of Dr E. Canaani, Kimmel Cancer Institute, Philadelphia, PA). The p210BCR/ABL K1172R mutant was obtained from Dr C. Sawyers (UCLA). The p210 ΔSH3 BCR/ABL mutant was generated as follows: theHindII-BsrGI fragment obtained by digestion from SK-p210 BCR/ABL(bcr exon 3/abl exon 2) and the BsmI-NIaIV fragment obtained by digestion of the BCR/ABL PCR product from nucleotides 3201-3471 were ligated in-frame into theBsm I-BsrGI–less p210 BCR/ABL, generating the SK-ΔSH3 BCR/ABL plasmid, which lacks the SH3 domain from amino acids 959-1020 of p210 BCR/ABL(bcr exon3/abl exon2). TheEcoRI-BsrGI fragment, containing the deletion, was cloned into the EcoRI/BsrGI-digested pSRαp210BCR/ABL. The P1013L mutation of p210 BCR/ABL(b3a2) was generated by polymerase chain reaction (PCR)-based mutagenesis. The mutant 3′ primer oligonucleotide used for the proline to leucine substitution at the 1013 codon in the SH3 domain of p210 BCR/ABL was 5′-CAG ACT GTT GAC TGG CGT GAT GTA GTT GCT TAG GA-3′, where the underlined sequence replaced the wild-type codon CCA. The 5′ primer was 5′-TTC AGA AGC TTC TCC CTG ACA-3′. The wild-type p210 BCR/ABL cDNA was used as a template for PCR amplification of the mutant SH3 domain. The PCR product was subcloned into p210 BCR/ABL pBluescript as a Bsm I-HincII fragment and sequenced to verify the presence of the nucleotide substitutions. The P1013L mutation was then introduced into pSRαp210BCR/ABL by replacement of the wild-type with the mutated EcoRI-BsrGI fragment. The ΔSH2 mutant (from Dr R. Van Etten, Harvard Medical School, Boston, MA) in the pGD210 vector was subcloned into the pSRαMSVtkneo-p210 BCR/ABL(bcr exon 3/abl exon 2) by replacing the wild-type EcoRI-BsrGI fragment with that lacking the SH2 domain. The pSRαMSVtkneo Δ176-426 p185 BCR/ABL mutant was obtained from Dr A.M. Pendergast (Duke University Medical Center, Durham, NC). In the pSRαMSVtkneo construct, the BCR/ABL cDNAs are under the control of the long terminal repeat (LTR) of the murine sarcoma virus (MSV), whereas the neomycin resistance gene (neo) is driven by the herpes simplex thymidine kinase (tk) promoter.
32Dcl3 Cell Transfection
Constructs were electroporated into 32Dcl3 murine growth factor-dependent myeloid cells30 grown in Iscove's modified Dulbecco medium (IMDM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, penicillin/streptomycin (100 μg/mL each), and 15% WEHI-conditioned medium (WEHI-CM) as a source of interleukin-3 (IL-3). BCR/ABL-expressing clones were selected in G418 (1 mg/mL) and were maintained in IMDM-CM.
Infection of Bone Marrow Cells (BMC) With BCR/ABL Viruses
Helper-free retroviral stocks were prepared as described.13,30 BMC from C57BL/6TacfBR mice (The Jackson Laboratory, Bar Harbor, ME) treated with 5-fluorouracil (150 mg/kg body weight) 6 days before cell harvest were infected with BCR/ABL viruses or the insert-less virus in the presence of recombinant IL-3, Kit ligand, and IL-6 as described.31 Expression of BCR/ABL proteins was confirmed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting with anti-ABL monoclonal antibody (MoAb; Ab-3; Oncogene Science, Uniondale, NJ).
Northern Blot Analysis
Cells were starved of serum and growth factors during 5 hours of incubation in IMDM supplemented with 0.1% bovine serum albumin, 2 mmol/L L-glutamine, and penicillin/streptomycin (100 μg/mL). Total cellular RNA (10 μg/lane) was electrophoresed on agarose gels and blotted onto a nitrocellulose PROTRAN membrane (Schleicher & Schuell, Keene, NH). Full-length c-myc and c-jun cDNAs labeled with [α-32P]dCTP (Dupont NEN Research Products, Boston, MA) were used as probes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was used to control for equal RNA loading.
Western Blot Analysis
A rabbit anti-p85 PI-3k (UBI, Lake Placid, NY) was used for immunoprecipitation.20 Postnuclear cell lysate and immunoprecipitates were electrophoresed on SDS-polyacrylamide gels and Western immunoblotting was performed with anti-ABL MoAb (Oncogene Science), anti-p85 Ab (UBI), or anti-P.Tyr MoAb (UBI). Appropriate horseradish peroxidase-labeled antibodies were used for detection using ECL (Amersham Corp, Arlington Heights, IL).
Enzymatic Assays
Cells used in all the enzymatic assays were serum-starved and growth factor-deprived as described above.
RAS activation was determined by measuring GTP-bound RAS.20
PI-3k activity was measured in the anti-P.Tyr immunoprecipitates.22
MAPK activity was analyzed based on myelin basic protein (MBP; UBI) phosphorylation, measured as described.32 Eluted products were separated by 13% SDS-PAGE and transferred to nitrocellulose membranes. In vitro phosphorylation of MBP was visualized after exposure of the filters to X-AR films (Eastman Kodak, Rochester, NY). Western blot with anti-ERK2 antibody was performed to verify the precipitated amounts of MAPK.
JNK activity was analyzed as described,33 with some modifications. JNK was precipitated (3 hours at 40°C) with GST-c-JUN (amino acids 1-79) glutathione-Sepharose beads (Pharmacia Biotech, Piscataway, NJ) from 300 μg of whole cell extracts in 0.1 mL of cell lysis buffer (10 mmol/L Na2HPO4, 150 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.2% NaH3, 0.004% NaF, 1 mmol/L Na3VO4, 25 mmol/L β-glycerolphosphoric acid, 100 μg/mL phenylmethyl sulfonyl fluoride [PMSF], and 1 μg/mL each of aprotinin and leupeptin, pH 7.35). Immunoprecipitates were washed three times with phosphate-buffered saline containing 1% NP-40 and 2 mmol/L Na3VO4 and once with kinase reaction buffer (25 mmol/L HEPES, 25 mmol/L MgCl2, 2 mmol/L dithiothreitol [DTT], 0.1 mmol/L Na3VO4, and 25 mmol/L β-glycerolphosphoric acid). Kinase reaction buffer containing 2.0 μCi [γ32P]ATP (NEN) and 20 μmol/L of cold ATP (30 μL) was added and, after 20 minutes of incubation at 30°C, samples were electrophoresed on SDS-polyacrylamide gels, transferred to nitro-cellulose, and visualized after exposure to x-ray film.
STATs Assay
Serum and growth factor-starved cells were resuspended in lysis buffer containing 0.32 mol/L sucrose, 3 mmol/L CaCl2, 2 mmol/L Mg-acetate, 0.1 mmol/L EDTA, 10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L DTT, 0.5 mmol/L PMSF, and 0.1% (vol/vol) NP-40. After centrifugation (2,600 rpm for 5 minutes at 4°C), nuclei were washed with lysis buffer without NP-40, spun as described above, and resuspended in buffer containing 20 mmol/L HEPES, 60 mmol/L KCl, 0.5 mmol/L EDTA, 2.5 mmol/L DTT, 12% (vol/vol) glycerol. After 10 minutes on ice, nuclei were freeze-thawed four times and centrifuged (14,000 rpm for 15 minutes at 4°C). Electrophoretic mobility shift assays (EMSAs) were performed as follows. An oligonucleotide corresponding to the FcγRI probe (5′ AGCTTGTATTTCCCAGAAAAGGGA 3′; GAS motif is in bold) was used as wild-type probe. A mutant oligonucleotide (5′ AGCTTGTATGGATTGTCCAAGGGATC 3′; mutated GAS motif is in bold) was used as nonspecific (NS) competitor. The oligonucleotides were end-labeled with [γ-32P]ATP and T4 polynucleotide kinase. DNA probe (30,000 cpm) was incubated with 3 μg poly(dI-dC) and 10 μg of nuclear extract in incubation buffer (see above; 15 minutes at room temperature). Samples were electrophoresed in a 5% polyacrylamide gel in 0.25× Tris-borate-EDTA (TBE) buffer for 120 minutes at 4°C (10 V/cm). Radioactivity was visualized after exposure of the dried gels to x-ray film.
Clonogenic Assay
A total of 104 32Dcl3 or 105 BMC were plated in MethoCult H4230 semisolid methylcellulose medium (Stem Cell Technologies, Inc, Vancouver, British Columbia, Canada) as described.20 Colonies were scored on day 12.
Apoptosis Assay
Cells (2 × 105/mL) were incubated in IMDM supplemented with 10% FBS, 2 mmol/L L-glutamine, and penicillin/streptomycin (without WEHI-CM) for 24 and 48 hours. The percentage of apoptotic cells was determined using the TACS1 Klenow in situ apoptosis detection kit (Trevigen, Inc, Gaithersburg, MD) according to the manufacturer's protocol.
Detection of BCR/ABL-Positive Cells in SCID Mice
C.B-17/IcrTac-SCID (H-2Kd) male mice (Taconic Farms Inc, Germantown, NY) and C57BL/6-SCID-SzJ mice (The Jackson Laboratory) received 350 rads of total body irradiation and 1 day later were injected intravenously with 5 × 106 32Dcl3 cells (H-2Kk) or 106 BMC, respectively, expressing the indicated BCR/ABL proteins. The number of 32Dcl3 transfectants in bone marrow and spleen was analyzed 3 weeks later by flow cytometry measurement of mononuclear cells labeled with the fluorescein isothiocyanate (FITC)-conjugated anti–H-2Kk antibody (gift of Dr R. Korngold, Kimmel Cancer Institute). To assess the development of leukemia from BMC infected with the wild-type or the ΔSH3 BCR/ABL retrovirus, total RNA was isolated34 from 106bone marrow, spleen, and peripheral blood mononuclear cell suspensions 4 weeks after cell inoculation. BCR/ABL transcripts were detected by RT-PCR followed by Southern blotting as described,35 using the following primers: 5′ primer, AAGATGATGAGTCTCCGGGGC; 3′ primer, CGTCAGGCTGTATTTCTTCCA; and the probe spanning the b3/a2 junction-region, AGAGTTCAA AAGCCCTTC. To show that the reverse transcription-PCR (RT-PCR) reaction was equally efficient in each sample, 102 32Dcl3 transfectants carrying a ΔSH3+ΔSH2 BCR/ABL mutant were added to each cell sample before RNA extraction and the truncated ΔSH3+ΔSH2 BCR/ABL transcripts were coamplified with either the wild-type or the ΔSH3 BCR/ABL transcripts. Because RT-PCR was performed with reagents used in excess and only 102 cells were added to the samples, it is unlikely that ΔSH3+ΔSH2 BCR/ABL mRNA was a competitor for the BCR/ABL mRNA isolated from mouse tissues. The expected lengths of PCR products are as follows: wild-type BCR/ABL, 800 bp; ΔSH3 BCR/ABL, 605 bp; and ΔSH3+ΔSH2 BCR/ABL, 328 bp.
Homing Assay
Cells were labeled with [3H]-uridine (NEN Research Products, DuPont, Wilmington, DE; 0.5 μCi/mL IMDM-CM medium) for 24 hours at 37°C, washed, and injected intravenously into SCID mice (5 × 106 32Dcl3 cells/mouse and 106BMC/mouse). Bone marrow (from both femurs) and spleen cells were collected after 24 and 48 hours and deposited onto glass microfiber filters (Whatman International Ltd, Maidstone, UK). Cell-associated radioactivity was measured in a β-scintillation counter.
Invasion Chamber Assay
BCR/ABL-expressing 32Dcl3 cells (106/mL) or BMC (0.5 × 106/mL) suspended in 2 mL of IMDM without WEHI-CM were placed in the upper chamber of Biocoat Matrigel invasion chambers (Becton Dickinson, Bedford, MA); the lower chamber was filled with 2 mL of IMDM-CM as chemoattractant. Cells migrating into the lower chamber were counted 24 hours later. Cells remaining on the membrane were counted after washing and staining the membrane according to the manufacturer's protocol.
Invasion of Monolayer
This assay was performed as described36 with some modifications. Briefly, 104 32Dcl3 cell transfectants were suspended in 1 mL IMDM-CM and layered on stromal bone marrow fibroblast monolayers (third passage in 24-well plates for 24 hours at 37°C). Cells in suspension and those adhering to the monolayer were removed by washing and mechanical agitation monitored by phase-contrast microscopy. The remaining cells were collected after trypsinization and plated in MethoCult H4230 semisolid medium containing 15% WEHI-CM. Colonies were scored on day 12.
Adhesion to Stroma
Confluent stromal cell layers were prepared in 12-well plates from BMC cultured in Dulbecco medium supplemented with 10% FBS, 2 mmol/L L-glutamine, and penicillin/streptomycin, with or without 2 × 10−6 mol/L methyl-prednisolone (MP), as described.12 Before the experiments, adherent cells were treated with 10 μg/mL mitomycin C for 2 hours at 37°C. 32Dcl3 cell transfectants (104) were incubated on the layers for 1 hour at 37°C. Nonadherent cells were collected and adherent cells were harvested after trypsinization. Cells were plated in MethoCult H4230 semisolid medium and colonies were scored 12 days later.
Adherence to Substrates
32Dcl3 cells and BMC expressing wild-type or mutant BCR/ABL proteins were suspended (2 × 106/4 mL and 106/4 mL, respectively, IMDM supplemented with 0.1% bovine serum albumin) and incubated (4 hours at 37°C) in 6-well plates coated with the indicated substrate (Biocoat Cellware; Becton Dickinson, Bedford, MA). Nonadherent cells were removed and adherent cells were harvested after trypsinization and counted.
Flow Cytometry Analysis of Integrin Expression
Parental and BCR/ABL-expressing 32Dcl3 cells were washed and incubated with FITC antirat CD29 (integrin β1 chain), FITC antimouse CD49b (integrin α2 chain), or hamster antirat/mouse CD49a (integrin α1 chain) for 45 seconds at 4°C. For detection of the integrin α1 chain, cells were extensively washed and incubated with FITC antihamster IgG for 45 seconds at 4°C. Cells were washed and analyzed by flow cytometry. Cells stained with FITC antihamster IgG served as negative controls. All antibodies were from PharMingen (San Diego, CA).
Cellular Localization of Wild-Type and ΔSH3 BCR/ABL in 32Dcl3-Transfected Cells
Protein fractionation.
Clones of 32Dcl3 cell transfectants were lysed in hypotonic buffer (10 mmol/L Tris, pH 7.5, 5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L Na2VO4, 5 mmol/L leupeptin, 20 μg/mL aprotinin, 1 mmol/L benzamidin, and 1 mmol/L PMSF) at 107/mL as described.37 After 5 minutes on ice, lysates were homogenized in a Dounce homogenizer. Nuclei and debris were removed by 5 minutes of centrifugation at 1,000g and the supernatant was adjusted with NaCl until isotonic (100 mmol/L NaCl) and centrifuged at 100,000g for 40 minutes. The sedimented fraction was resuspended in RIPA buffer and designated the membrane fraction; the supernatant was designated the cytosol fraction. Protein concentration in the corresponding fractions of each cell lysate was adjusted after protein content measurements by the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories, Hercules, CA). As a control for similar levels of protein expression in cells used in the experiments, 5 × 105 cells of each clone were lysed in RIPA buffer and total cell extracts, along with the membrane and cytosolic fractions, were separated by SDS-PAGE. Proteins were detected by Western blotting with antibodies against c-ABL (Oncogene Science), anti-p120GAP (UBI), and anti-CD71 (transferrin receptor; PharMingen).
Confocal microscopy.
Cytospin preparations of 32Dcl3 cells from individual clones expressing wild-type or ΔSH3 BCR/ABL protein were fixed with methanol for 5 minutes and permeabilized in acetone for 2 minutes at −20°C.29 All subsequent steps were at room temperature. After 30 minutes of incubation in 1% goat IgG (Organon Teknika Corp, Cappel Research Products, Durham, NC), cells were incubated sequentially with anti-ABL MoAb (PharMingen; 10 μg/mL) for 45 minutes and FITC-(Fab′)2 goat antimouse Ig (Organon Teknika) at a 1:100 dilution with intermediate washings. Slides were mounted with Slowfade antiquenching agent (Molecular Probes, Eugene, OR) and examined for confocal microscopy using an Axiovert 100 Zeiss microscope and MRC-600 system (Bio-Rad Corp).
RESULTS
ΔSH3 BCR/ABL Signaling
BCR/ABL activates specific signaling pathways, inhibits apoptosis, stimulates growth factor-independent proliferation, and induces leukemic transformation when expressed in growth factor-dependent hematopoietic cell lines or in BMC. To determine whether the SH3 domain of BCR/ABL plays a role in these processes, the wild-type protein and an SH3 deletion mutant (ΔSH3) were expressed in myeloid precursor 32Dcl3 cells. The kinase-deficient K1172R BCR/ABL mutant, defective in many BCR/ABL functions,8 was used as negative control.
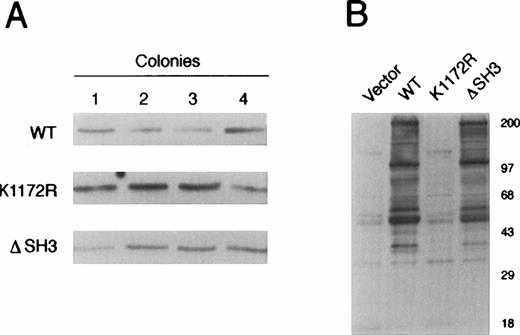
Several G418-resistant clones of 32Dcl3 cells transfected with a vector carrying wild-type or mutant BCR/ABL were analyzed for BCR/ABL protein expression. Four clones from each group were selected for further study (Fig 1A). Clones transfected with vector only or with the K1172R kinase-deficient BCR/ABL mutant served as negative controls. Experiments were performed with individual clones and pools of clones expressing the same BCR/ABL protein. As indicated by Western blotting with antiphosphotyrosine (P.Tyr) MoAb, tyrosine phosphorylated proteins of similar mass were detected in cell lysates from 32Dcl3 cells expressing wild-type or ΔSH3 BCR/ABL (Fig 1B).
BCR/ABL expression and protein tyrosine phosphorylation in individual 32D clone transfectants expressing wild-type or mutant BCR/ABL protein. (A) Expression of wild-type (WT), kinase-deficient mutant (K1172R), or ▵SH3 BCR/ABL proteins was examined in four 32Dcl3 transfectants by Western blotting with anti-ABL MoAb. (B) Phosphorylation of cellular proteins was analyzed after SDS-PAGE of cell lysates from the same clones as in (A) using anti-P.Tyr MoAb 4G10. Results are representative of three independent experiments.
BCR/ABL expression and protein tyrosine phosphorylation in individual 32D clone transfectants expressing wild-type or mutant BCR/ABL protein. (A) Expression of wild-type (WT), kinase-deficient mutant (K1172R), or ▵SH3 BCR/ABL proteins was examined in four 32Dcl3 transfectants by Western blotting with anti-ABL MoAb. (B) Phosphorylation of cellular proteins was analyzed after SDS-PAGE of cell lysates from the same clones as in (A) using anti-P.Tyr MoAb 4G10. Results are representative of three independent experiments.
32Dcl3 cell clones (4 for each transfectant), starved of serum and growth factors, were then assessed for the activation of several proteins involved in different signaling pathways. Wild-type and ΔSH3 BCR/ABL expression resulted in the activation of the same proximal (RAS, PI-3k, mitogen-activated protein kinase [MAPK], Jun kinase [JNK]) and distal (Myc, Jun, signal transducers and activators of transcription [STATs]) signaling molecules (Fig 2), whereas expression of the kinase-deficient K1172R BCR/ABL mutant did not activate any of the signal transduction proteins analyzed.
Activation of signal transduction pathways by BCR/ABL proteins. (A) Levels of GTP-bound RAS in BCR/ABL-expressing cells. Pools of clones expressing the indicated vectors were labeled with [γ32P] orthophosphate. RAS was immunoprecipitated and the proportion of GTP- and GDP-bound forms was analyzed by thin-layer chromatography. (B) PI-3k interaction with BCR/ABL proteins. (Left panel) PI-3k activity was detected in antiphosphotyrosine immunoprecipitates from a mixture of clones expressing the indicated vectors. (Right panel) p85 immunoprecipitates from the indicated mixtures of clones were analyzed after SDS-PAGE and Western blotting with anti-ABL antibody. p85 was detected with anti-p85 antibody after stripping the filter. (C) Activation of MAPK and JNK pathways by BCR/ABL proteins. (Left panel) MAPK and JNK activities were measured in the appropriate immunoprecipitates using, respectively, myelin basic protein (MBP) and GST-JUN as substrates. (Right panel) Expression of c-MYC and c-JUN mRNA was determined in total cellular RNA (10 μg) by Northern blotting. GAPDH was detected as a control for equal gel loading. (D) DNA binding activity of STAT proteins. EMSA was performed using nuclear extracts of the indicated clone mixtures and synthetic oligonucleotides containing the GAS motif as a probe. Results are representative of three to four independent experiments.
Activation of signal transduction pathways by BCR/ABL proteins. (A) Levels of GTP-bound RAS in BCR/ABL-expressing cells. Pools of clones expressing the indicated vectors were labeled with [γ32P] orthophosphate. RAS was immunoprecipitated and the proportion of GTP- and GDP-bound forms was analyzed by thin-layer chromatography. (B) PI-3k interaction with BCR/ABL proteins. (Left panel) PI-3k activity was detected in antiphosphotyrosine immunoprecipitates from a mixture of clones expressing the indicated vectors. (Right panel) p85 immunoprecipitates from the indicated mixtures of clones were analyzed after SDS-PAGE and Western blotting with anti-ABL antibody. p85 was detected with anti-p85 antibody after stripping the filter. (C) Activation of MAPK and JNK pathways by BCR/ABL proteins. (Left panel) MAPK and JNK activities were measured in the appropriate immunoprecipitates using, respectively, myelin basic protein (MBP) and GST-JUN as substrates. (Right panel) Expression of c-MYC and c-JUN mRNA was determined in total cellular RNA (10 μg) by Northern blotting. GAPDH was detected as a control for equal gel loading. (D) DNA binding activity of STAT proteins. EMSA was performed using nuclear extracts of the indicated clone mixtures and synthetic oligonucleotides containing the GAS motif as a probe. Results are representative of three to four independent experiments.
Functional Consequences In Vitro of ΔSH3 BCR/ABL Expression in Myeloid Precursor Cells
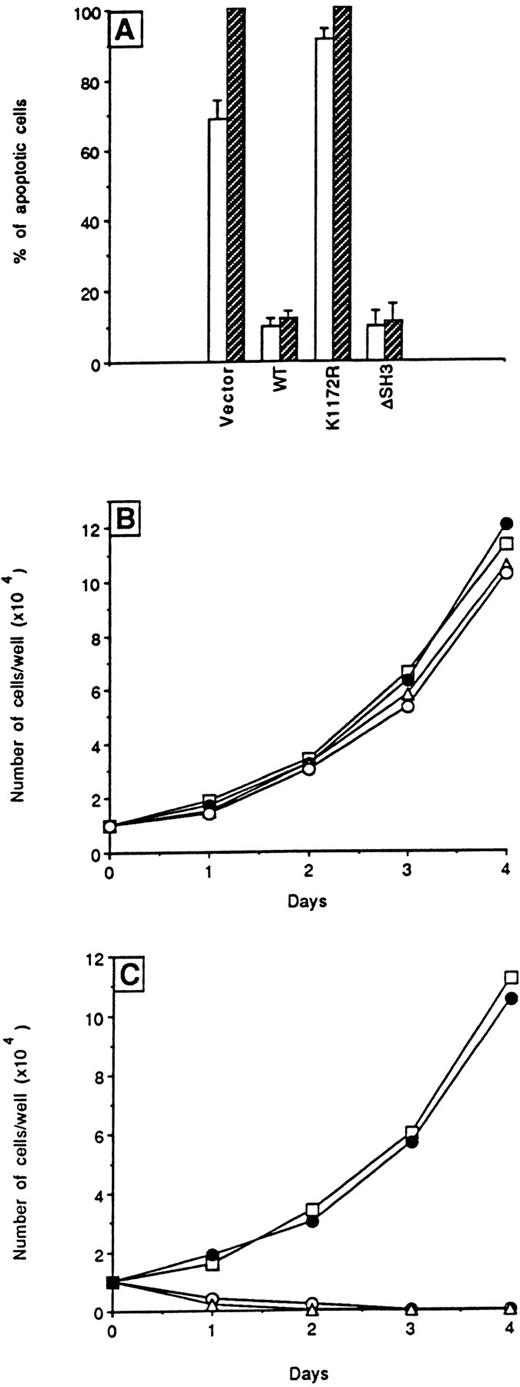
To assess the ability of ΔSH3 BCR/ABL to prevent apoptosis induced by cytokine deprivation, the percentage of apoptotic cells was determined in transfected 32Dcl3 cells starved of WEHI-CM. BCR/ABL wild-type or ΔSH3 mutant-expressing 32Dcl3 cells were resistant to apoptosis (Fig 3A), whereas cells carrying the K1172R kinase-deficient BCR/ABL mutant underwent apoptosis with kinetics (not shown) similar to that of control cells transfected with the vector. These results are consistent with the upregulation of BCL-2 levels only in wild-type and ΔSH3 transfectants (data not shown).
Effects of wild-type and ▵SH3 BCR/ABL on apoptosis and proliferation. (A) Cells were seeded without growth factors and apoptosis was quantitated at 24 (□) and 48 hours (▨) using an in situ apoptosis detection kit. Results represent the mean ± SD of four independent experiments using individual clones. In (B) and (C), cells from individual clones growing in IMDM-CM were plated with or without, respectively, added WEHI-CM in 96-well plates. The number of cells in each well was scored daily. Results are representative of four independent experiments using individual clones (○) vector, (□) WT, (▵) K1172R, and (•) ▵SH3.
Effects of wild-type and ▵SH3 BCR/ABL on apoptosis and proliferation. (A) Cells were seeded without growth factors and apoptosis was quantitated at 24 (□) and 48 hours (▨) using an in situ apoptosis detection kit. Results represent the mean ± SD of four independent experiments using individual clones. In (B) and (C), cells from individual clones growing in IMDM-CM were plated with or without, respectively, added WEHI-CM in 96-well plates. The number of cells in each well was scored daily. Results are representative of four independent experiments using individual clones (○) vector, (□) WT, (▵) K1172R, and (•) ▵SH3.
Individual clones transfected with any of the plasmids had similar proliferation rates in the presence of WEHI-CM as a source of growth factors (Fig 3B). However, only clones expressing the wild-type protein or the ΔSH3 BCR/ABL mutant were able to generate cell lines with similar proliferation rates in the absence of WEHI-CM (Fig 3C), and only these cells generated growth factor-independent colonies in semisolid methylcellulose medium (Table 1).
Effect of BCR/ABL Protein Expression on Colony Formation of 32Dcl3 and BMC
| Cells . | IL-3 . | Vector . | Wild-Type . | K1172R . | ΔSH3 . |
|---|---|---|---|---|---|
| 32Dcl3 | − | 0 | 994 ± 340 | 0 | 860 ± 192 |
| BMC | − | 0 | 7 ± 2 | 0 | 6 ± 3 |
| BMC | + | 31 ± 8 | 95 ± 17 | 35 ± 12 | 83 ± 13 |
| Cells . | IL-3 . | Vector . | Wild-Type . | K1172R . | ΔSH3 . |
|---|---|---|---|---|---|
| 32Dcl3 | − | 0 | 994 ± 340 | 0 | 860 ± 192 |
| BMC | − | 0 | 7 ± 2 | 0 | 6 ± 3 |
| BMC | + | 31 ± 8 | 95 ± 17 | 35 ± 12 | 83 ± 13 |
Colonies from 104 32Dcl3 cells (individual clones expressing the indicated proteins) or 105 BMC (infected with the viruses carrying the indicated constructs) were counted 12 days after plating in MethoCult H4230 semisolid medium with or without 0.1 U/mL rmuIL-3. Results are the mean ± SD from three independent experiments.
To examine the role of the SH3 domain in the ability of BCR/ABL to transform murine BMC in vitro, cells were infected with retroviruses carrying the different constructs. Expression of BCR/ABL was detected by SDS-PAGE and Western blotting (not shown), and clonogenic activity of the infected cells was measured in the presence of G418 (1 mg/mL) in semisolid methylcellulose medium with or without recombinant murine IL-3 (rmuIL-3). As compared with marrow cells infected with the K1172R kinase-deficient mutant or the insert-less virus, cells infected with wild-type BCR/ABL formed an increased number of colonies in the presence of threshold concentrations (0.1 U/mL) of IL-3 (Table 1). Colony formation was also observed in the absence of IL-3 (Table 1), consistent with a previous report.38 The extent of colony formation from marrow cells infected with the ΔSH3 BCR/ABL mutant was indistinguishable from that of wild-type BCR/ABL cells (Table 1). BCR/ABL transcripts were detected by RT-PCR in individual colonies recovered from methylcellulose (10 colonies tested from each group; data not shown) as described.39
Leukemogenic Potential of ΔSH3 BCR/ABL-Expressing Cells
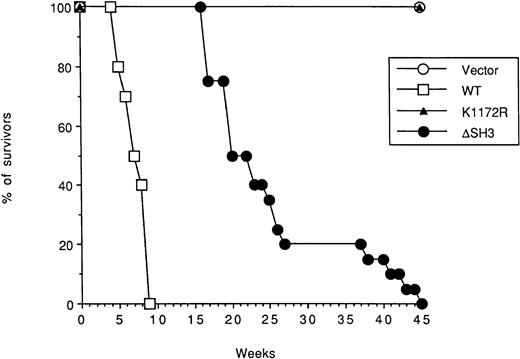
To assess the leukemogenic potential of the ΔSH3 BCR/ABL mutant, individual 32Dcl3 transfectant clones expressing BCR/ABL proteins or carrying the vector only were injected intravenously into SCID mice. Leukemia development was analyzed after 3 weeks. Spleen and bone marrow of mice (H-2Kd) injected with 32Dcl3 cells (H-2Kk) expressing wild-type BCR/ABL were massively infiltrated by these cells (38.7% and 44.9% of the cells, respectively; Table 2), as assessed by immunofluorescence with the anti–H-2Kk antibody. In contrast, spleen and bone marrow of mice implanted with 32Dcl3 cells expressing ΔSH3 BCR/ABL contained fewer leukemic cells (7.4% and 7.3%, respectively; Table 2). 32Dcl3 cells expressing the K1172R tyrosine kinase-deficient mutant or transfected with vector only were not detectable in either organ (not shown). Consistent with these results, mice injected with 32Dcl3 clones expressing wild-type BCR/ABL died within 6 to 9 weeks (6.2 ± 1.1 weeks) after injection, whereas mice injected with ΔSH3 BCR/ABL mutant died of leukemia after 17 to 45 weeks (25.4 ± 7.4 weeks, P < .001 as compared with the wild-type group; Fig 4).
Growth of 32Dcl3 Cells Expressing Wild-Type or ▵SH3 BCR/ABL in Hematopoietic Organs of SCID Mice
| Organs . | Wild-Type . | ΔSH3 . |
|---|---|---|
| Bone marrow | 44.9 ± 10.6 | 7.4 ± 4.5 |
| Spleen | 38.7 ± 3.8 | 7.3 ± 5.2 |
| Organs . | Wild-Type . | ΔSH3 . |
|---|---|---|
| Bone marrow | 44.9 ± 10.6 | 7.4 ± 4.5 |
| Spleen | 38.7 ± 3.8 | 7.3 ± 5.2 |
C.B-17/IcrTac-scid mice were injected intravenously with 5 × 106 32Dcl3 cells expressing the indicated BCR/ABL proteins. After 3 weeks, BMC and splenocytes were harvested and labeled with FITC-conjugated anti–H-2Kk antibody. Results are expressed as the percentage of positive cells (mean ± SD, 3 mice/group), as determined by flow cytometry.
Survival curves of mice injected with BCR/ABL-expressing 32D cells. SCID mice (5 mice/clone) were injected intravenously with 5 × 106 cells from each of the 4 clones analyzed expressing the indicated protein or carrying the empty vector. (○) Vector, (□) WT, (▵) K1172R, and (•) ▵SH3.
Survival curves of mice injected with BCR/ABL-expressing 32D cells. SCID mice (5 mice/clone) were injected intravenously with 5 × 106 cells from each of the 4 clones analyzed expressing the indicated protein or carrying the empty vector. (○) Vector, (□) WT, (▵) K1172R, and (•) ▵SH3.
At necropsy, histopathologic examination showed that leukemic cell infiltration of bone marrow, spleen, liver, lungs, and kidneys from mice of either group that succumbed to leukemia was similar (data not shown). Complete replacement of the red and white pulp by a diffuse cellular infiltrate was detected in spleens from mice injected with wild-type or ΔSH3 BCR/ABL mutant-expressing cells, and the infiltrates were composed primarily of blast cells with a low degree of myeloid and megakaryocytic differentiation (data not shown). Myeloid differentiation was confirmed by staining for chloroacetate esterase. Similar acute myeloid leukemic infiltrates were seen in liver, lungs, and kidneys; bone marrow was also involved and myeloid differentiation was more pronounced at this site (data not shown).
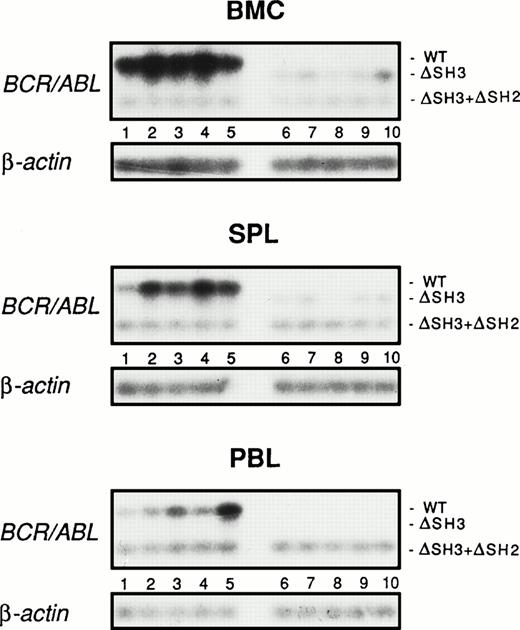
To further assess the leukemogenic potential of ΔSH3 BCR/ABL, leukemia development from mouse marrow cells infected with retroviruses carrying wild-type or ΔSH3 BCR/ABL and injected into preirradiated SCID mice was examined 4 weeks later by RT-PCR detection of BCR/ABL transcripts in peripheral blood, bone marrow, and spleen. To ensure that the results of RT-PCR detection of BCR/ABL transcripts quantitatively reflected the number of leukemic cells in the various tissues, the following steps were taken. (1) Marrow cells infected with a retrovirus carrying the wild-type or the ΔSH3 BCR/ABL mutant formed the same number of methylcellulose colonies in 0.1 U/mL of IL-3 and in the absence of the growth factor and expressed similar levels of BCR/ABL transcripts (not shown). (2) A total of 102 cells of a 32Dcl3 transfectant expressing a ΔSH3 + ΔSH2 BCR/ABL mutant were added to each cell suspension obtained from peripheral blood, bone marrow, or spleen, thus allowing the detection of a shorter BCR/ABL transcript used as an internal control of the RT-PCR procedure. (3) β-Actin transcripts were also amplified from an aliquot of each sample as an additional control. Levels of ΔSH3 BCR/ABL transcripts in bone marrow, spleen, and peripheral blood were much lower (7- to 10-fold less abundant in bone marrow and spleen, respectively) or even undetectable (in the peripheral blood) compared with the amount of wild-type BCR/ABL transcripts in the corresponding tissues (Fig 5).
Detection of BCR/ABL transcripts in hematopoietic organs of SCID mice inoculated with BMC infected with wild-type or ▵SH3 BCR/ABL. Preirradiated C57BL/6-SCID-SzJ mice were injected with 106 BMC infected with the wild-type (lanes 1 through 5) or ▵SH3 BCR/ABL (lanes 6 through 10) retrovirus. After 4 weeks, mononuclear cells from bone marrow (BMC), spleen (SPL), and peripheral blood (PBL) were harvested and BCR/ABL transcripts were detected by RT-PCR. To control the efficiency of RT-PCR in each sample, 102 32Dcl3 transfectants expressing the ▵SH3+▵SH2 BCR/ABL mutant were added to each sample before RNA extraction. As an additional control of the RNA preparation, β-actin transcripts were also amplified from each sample.
Detection of BCR/ABL transcripts in hematopoietic organs of SCID mice inoculated with BMC infected with wild-type or ▵SH3 BCR/ABL. Preirradiated C57BL/6-SCID-SzJ mice were injected with 106 BMC infected with the wild-type (lanes 1 through 5) or ▵SH3 BCR/ABL (lanes 6 through 10) retrovirus. After 4 weeks, mononuclear cells from bone marrow (BMC), spleen (SPL), and peripheral blood (PBL) were harvested and BCR/ABL transcripts were detected by RT-PCR. To control the efficiency of RT-PCR in each sample, 102 32Dcl3 transfectants expressing the ▵SH3+▵SH2 BCR/ABL mutant were added to each sample before RNA extraction. As an additional control of the RNA preparation, β-actin transcripts were also amplified from each sample.
In marked contrast, levels of ΔSH3+ΔSH2 BCR/ABL and β-actin transcripts were essentially identical in tissue samples from mice injected with cells carrying the wild-type or ΔSH3 BCR/ABL retrovirus (Fig 5).
Homing Ability of ΔSH3 BCR/ABL-Expressing Cells In Vivo
The homing ability of wild-type and ΔSH3 BCR/ABL-expressing 32Dcl3 transfectants was determined in SCID mice injected with [3H]uridine-labeled 32Dcl3 cells. Radioactivity in spleen and bone marrow from mice injected 24 or 48 hours earlier with cells expressing ΔSH3 BCR/ABL was twofold to threefold lower than that detected in the corresponding organs of mice injected with cells expressing wild-type BCR/ABL (Fig6A).
In vivo homing of BCR/ABL-expressing cells. Single-cell suspensions were prepared from bone marrow (BMC) and spleen (SPL) of mice 24 (▪) and 48 (▨) hours after injection with [3H]-uridine–labeled 32Dcl3 clones (A) or retrovirus-infected BMC (B) and deposited onto glass microfiber filters. Cell-associated radioactivity was determined as described in the Materials and Methods. Results are expressed as the percentage of injected radioactivity (mean ± SD from 3 mice in each group).
In vivo homing of BCR/ABL-expressing cells. Single-cell suspensions were prepared from bone marrow (BMC) and spleen (SPL) of mice 24 (▪) and 48 (▨) hours after injection with [3H]-uridine–labeled 32Dcl3 clones (A) or retrovirus-infected BMC (B) and deposited onto glass microfiber filters. Cell-associated radioactivity was determined as described in the Materials and Methods. Results are expressed as the percentage of injected radioactivity (mean ± SD from 3 mice in each group).
To assess whether the defect in homing was specifically due to the deletion of the SH3 domain, the homing ability of 32Dcl3 transfectants expressing either the ΔSH2 or the Δ176-426 BCR/ABL deletion mutants was examined and found to be essentially identical to that of wild-type BCR/ABL transfectants (Fig 6A). At 48 hours, radioactivity levels in spleen and bone marrow of mice injected with 32Dcl3 cells expressing K1172R BCR/ABL or carrying the empty vector were lower than those detected at 24 hours, probably reflecting the greater propensity of these cells, as compared with the wild-type or ΔSH3 BCR/ABL transfectants, to undergo apoptosis upon growth factor deprivation. Histopathologic examination showed no differences in organ distribution of the 32Dcl3 leukemic cells, and no radioactivity was detected in association with peripheral blood leukocytes at the time of the assay (data not shown). Thus, the ΔSH3 BCR/ABL-expressing cells that did not home to hematopoietic organs were likely eliminated from the mice. To exclude the possibility that the differences in the homing ability of 32Dcl3 transfectants expressing wild-type or ΔSH3 BCR/ABL were unique to these cells, murine BMC infected with a retrovirus carrying either construct were selected in G418-containing medium for 14 days, labeled with [3H]-uridine, and injected into mice. Radioactivity in spleen and bone marrow was assessed 24 and 48 hours later. The homing ability of ΔSH3 BCR/ABL-infected BMC was impaired, as compared with that of cells expressing wild-type BCR/ABL (Fig 6B).
Invasion and Adherence Abilities of ΔSH3 BCR/ABL-Expressing Cells In Vitro
Invasion and adherence ability of 32Dcl3 cell clones or murine BMC expressing wild-type or mutant BCR/ABL were analyzed in assays for invasion and interaction with specific substrates. In invasion chamber assays, a greater number of 32Dcl3 cells expressing the wild-type BCR/ABL passed through the membrane, as compared with cells expressing ΔSH3 BCR/ABL, K1172 BCR/ABL or carrying the vector only (Table 3). 32Dcl3 cells transfected with the P1013L BCR/ABL mutant, which is equivalent to the P131L c-abl mutant defective in binding to proline-rich target proteins,40 had also a reduced invasive potential (Table3). In contrast, 32Dcl3 cells transfected with ΔSH2 BCR/ABL or with the Δ176-426 BCR/ABL mutant behaved like the wild-type transfectants (data not shown).
Invasive and Adhesive Properties of 32Dcl3 and BMC Expressing BCR/ABL Proteins
| Function . | Vector . | Wild-Type . | K1172R . | ΔSH3 . | P1013L . |
|---|---|---|---|---|---|
| 32Dcl3 | |||||
| Invasion chamber* | |||||
| Membrane | 1.9 ± 0.4 | 7.0 ± 0.4 | 2.0 ± 0.2 | 2.9 ± 0.2 | 3.7 ± 0.8 |
| Well | 30.7 ± 4.8 | 83.2 ± 6.0 | 31.2 ± 2.7 | 39.7 ± 1.9 | 52 ± 8.0 |
| Invasion of monolayer† | 135 ± 32 | 621 ± 113 | 119 ± 27 | 237 ± 19 | |
| Adhesion to stroma‡ | |||||
| Stroma MP− | 0.06 ± 0.03 | 1.04 ± 0.16 | 0.08 ± 0.02 | 0.44 ± 0.06 | |
| Stroma MP+ | 3.18 ± 0.53 | 0.16 ± 0.03 | 2.68 ± 0.37 | 0.40 ± 0.21 | |
| Adhesion to substrate§ | |||||
| Collagen IV | 17.0 ± 4.0 | 156 ± 24 | 16 ± 8 | 21 ± 13 | 50 ± 20 |
| Laminin | 26 ± 15 | 80 ± 8 | 23 ± 7 | 26 ± 12 | 37 ± 8 |
| Fibronectin | 121 ± 22 | 21 ± 5 | 127 ± 21 | 25 ± 9 | 38 ± 5 |
| Poly-L-lysine | <1 | <1 | <1 | <1 | <1 |
| BMC | |||||
| Invasion chamber∥ | |||||
| Membrane | 0.6 ± 0.3 | 3.2 ± 0.3 | 0.7 ± 0.2 | 1.1 ± 0.3 | |
| Well | 5.0 ± 2.0 | 19.0 ± 3.6 | 4.3 ± 2.1 | 7.7 ± 1.5 | |
| Adhesion to substrate§ | |||||
| Collagen IV | 4.6 ± 0.6 | 16.2 ± 0.9 | 5.2 ± 0.2 | 6.8 ± 0.4 | |
| Laminin | 6.4 ± 0.9 | 16.5 ± 1.9 | 6.1 ± 0.9 | 7.3 ± 0.6 | |
| Fibronectin | 14.5 ± 1.5 | 6.5 ± 0.7 | 14.0 ± 1.9 | 6.8 ± 0.5 | |
| Poly-L-lysine | 58.7 ± 7.1 | 60.0 ± 8.5 | 59.3 ± 3.2 | 58.3 ± 4.2 |
| Function . | Vector . | Wild-Type . | K1172R . | ΔSH3 . | P1013L . |
|---|---|---|---|---|---|
| 32Dcl3 | |||||
| Invasion chamber* | |||||
| Membrane | 1.9 ± 0.4 | 7.0 ± 0.4 | 2.0 ± 0.2 | 2.9 ± 0.2 | 3.7 ± 0.8 |
| Well | 30.7 ± 4.8 | 83.2 ± 6.0 | 31.2 ± 2.7 | 39.7 ± 1.9 | 52 ± 8.0 |
| Invasion of monolayer† | 135 ± 32 | 621 ± 113 | 119 ± 27 | 237 ± 19 | |
| Adhesion to stroma‡ | |||||
| Stroma MP− | 0.06 ± 0.03 | 1.04 ± 0.16 | 0.08 ± 0.02 | 0.44 ± 0.06 | |
| Stroma MP+ | 3.18 ± 0.53 | 0.16 ± 0.03 | 2.68 ± 0.37 | 0.40 ± 0.21 | |
| Adhesion to substrate§ | |||||
| Collagen IV | 17.0 ± 4.0 | 156 ± 24 | 16 ± 8 | 21 ± 13 | 50 ± 20 |
| Laminin | 26 ± 15 | 80 ± 8 | 23 ± 7 | 26 ± 12 | 37 ± 8 |
| Fibronectin | 121 ± 22 | 21 ± 5 | 127 ± 21 | 25 ± 9 | 38 ± 5 |
| Poly-L-lysine | <1 | <1 | <1 | <1 | <1 |
| BMC | |||||
| Invasion chamber∥ | |||||
| Membrane | 0.6 ± 0.3 | 3.2 ± 0.3 | 0.7 ± 0.2 | 1.1 ± 0.3 | |
| Well | 5.0 ± 2.0 | 19.0 ± 3.6 | 4.3 ± 2.1 | 7.7 ± 1.5 | |
| Adhesion to substrate§ | |||||
| Collagen IV | 4.6 ± 0.6 | 16.2 ± 0.9 | 5.2 ± 0.2 | 6.8 ± 0.4 | |
| Laminin | 6.4 ± 0.9 | 16.5 ± 1.9 | 6.1 ± 0.9 | 7.3 ± 0.6 | |
| Fibronectin | 14.5 ± 1.5 | 6.5 ± 0.7 | 14.0 ± 1.9 | 6.8 ± 0.5 | |
| Poly-L-lysine | 58.7 ± 7.1 | 60.0 ± 8.5 | 59.3 ± 3.2 | 58.3 ± 4.2 |
Results represent the mean ± SD from three independent experiments using individual 32Dcl3 clones or mixtures of BMC expressing the indicated BCR/ABL proteins.
Values are the number of cells (×103) on the matrix membrane (membrane) or collected at the bottom of the chamber (well) (mean ± SD, 3 experiments).
Number of clonogenic cells infiltrating stromal bone marrow monolayers (mean ± SD, 3 experiments).
Nonadherent and adherent cells (after trypsinization) were plated in MethoCult H4230 semisolid medium. Results are given as the ratio between the number of colony-forming cells in the adhering versus the nonadhering fraction (mean ± SD, 3 independent experiments) in the presence of recombinant murine IL-3 (10 U/mL).
Number (×104) of cells adherent to the indicated substrates (mean ± SD, 3 experiments).
∥After infection with the retroviral constructs, BMC were grown for 14 days in G418-containing medium in the presence of Kit-ligand, IL-3, and IL-6.
A similar pattern was observed using BMC infected with wild-type, ΔSH3 BCR/ABL, or K1172R BCR/ABL mutant or carrying the empty vector and selected for 14 days in medium containing G418 (1 mg/mL) and Kit ligand, IL-3, and IL-6 (Table 3).
The reduced invasive potential of ΔSH3 BCR/ABL transfectants was confirmed on a fibroblast monolayer; as compared with cells transfected with wild-type BCR/ABL, fewer 32Dcl3 cells transfected with the ΔSH3 BCR/ABL mutant invaded the fibroblast monolayer (Table 3).
Adherence of 32Dcl3 transfectants expressing the various BCR/ABL proteins to bone marrow stroma cultured in the presence (+) or absence (−) of MP was also analyzed. Murine bone marrow progenitors, like human hematopoietic progenitor cells, adhere to MP+, but not to MP− stroma.12 As compared with 32Dcl3 cells expressing K1172R BCR/ABL mutant or transfected with vector only, a greater number of cells expressing wild-type BCR/ABL adhered to MP− than to MP+ stroma. Deletion of the SH3 domain from BCR/ABL reduced the adhesive ability of transfected cells to MP− stroma by approximately 2.5-fold, without a significant influence on the adhesion to MP+ stroma (Table 3).
To examine in more detail the defect in adhesive properties of 32Dcl3 and murine BMC expressing ΔSH3 BCR/ABL, we tested their ability to adhere to components of the basement membrane (ie, collagen IV and laminin) and to fibronectin, a component of the stroma. Cells expressing wild-type BCR/ABL showed increased adherence to collagen IV (3.5- to 9-fold) and to laminin (2.5- to 3.5-fold) and decreased adherence to fibronectin (2- to 6-fold) as compared with cells expressing the K1172R BCR/ABL mutant or carrying the vector only (Table3). Deletion of the SH3 domain from BCR/ABL or introduction of the proline to leucine mutation at codon 1013 of the BCR/ABL SH3 domain markedly reduced the ability of ΔSH3 BCR/ABL-expressing cells or 32Dcl3 cells transfected with the P1013L mutant to adhere to collagen IV- and laminin-coated, but not to fibronectin-coated plates, as compared with cells expressing the wild-type protein (Table 3). The number of ΔSH2- and Δ176-426- transfected 32Dcl3 cells adhering to collagen IV and laminin was similar to that of wild-type BCR/ABL transfectants (data not shown). None of the BCR/ABL proteins changed the adherence abilities of the cells to poly-L-lysine–coated plates (Table 3).
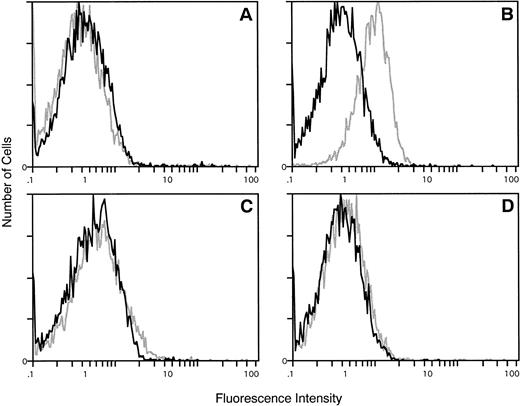
To determine whether the reduced adherence to laminin- and collagen IV-coated plates of ΔSH3 and P1013 BCR/ABL-expressing cells reflected changes in the expression of the corresponding integrin receptors, the levels of α1, α2, and β1 integrins were measured by flow cytometry in parental and BCR/ABL-transfected 32Dcl3 cells. β1 integrin was expressed at equivalent levels in parental and BCR/ABL-transfected 32Dcl3 cells, whereas α1 integrin levels were undetectable in these cells (data not shown). In contrast, α2 integrin was detectable in 32Dcl3 cells expressing wild-type BCR/ABL, but not in parental cells (Fig 7); interestingly, α2 integrin was not detectable in 32Dcl3 cells expressing ΔSH3 BCR/ABL or the P1013L mutant (Fig 7).
Cytofluorimetric detection of α2 integrin in parental and BCR/ABL-transfected 32Dcl3 cells. (A) Parental 32Dcl3 cells; (B) BCR/ABL-transfected 32Dcl3 cells; (C) ▵SH3 BCR/ABL-transfected 32Dcl3 cells; (D) P1013L BCR/ABL-transfected 32D cells. Representative of three separate experiments with similar results. Solid line, negative control; stippled line, α2 integrin.
Cytofluorimetric detection of α2 integrin in parental and BCR/ABL-transfected 32Dcl3 cells. (A) Parental 32Dcl3 cells; (B) BCR/ABL-transfected 32Dcl3 cells; (C) ▵SH3 BCR/ABL-transfected 32Dcl3 cells; (D) P1013L BCR/ABL-transfected 32D cells. Representative of three separate experiments with similar results. Solid line, negative control; stippled line, α2 integrin.
Intracellular Localization of Wild-Type and ΔSH3 BCR/ABL
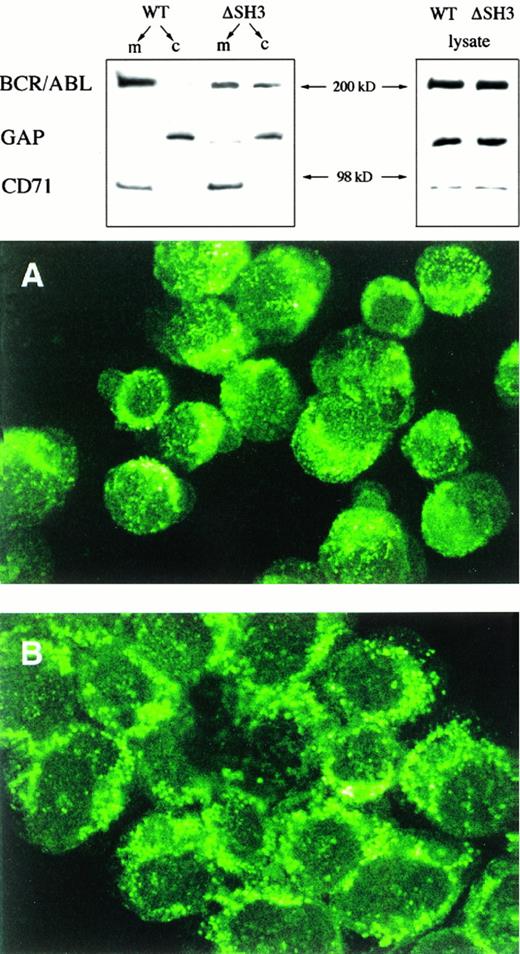
Because the SH3 domain has been reported to affect the intracellular localization of the Src tyrosine kinase,41 we analyzed localization of the BCR/ABL protein in the 32Dcl3 transfectants. Compared with wild-type BCR/ABL,which localizes mainly in the membrane fraction, ΔSH3 BCR/ABL was more evenly distributed between the membrane cytoskeleton and the cytosol compartments (Fig 8, top panel). Note that the transferrin receptor (CD71) was expressed only in the membrane fraction, whereas p120GAP was detected primarily in the cytosol (Fig8), showing that the preparation of membrane and cytosol fractions was sufficiently pure.
Intracellular localization of BCR/ABL proteins. (Top panel) Membrane (m) and cytosolic (c) BCR/ABL, p120 GAP, and transferrin receptor (CD71) were analyzed in BCR/ABL (wild-type and ▵SH3) transfectants by SDS-PAGE followed by Western blotting with anti-ABL, anti-GAP, and anti-CD71 MoAbs. As a control, the same proteins were also detected in total cell lysates. Results are representative of three different experiments. (Bottom panel) 32Dcl3 cells expressing wild-type (A) or ▵SH3 mutant (B) BCR/ABL were incubated with anti-ABL followed by FITC-conjugated goat antirabbit antibody and examined by confocal microscopy. Results are representative for three individual clones from each group (original magnification × 800).
Intracellular localization of BCR/ABL proteins. (Top panel) Membrane (m) and cytosolic (c) BCR/ABL, p120 GAP, and transferrin receptor (CD71) were analyzed in BCR/ABL (wild-type and ▵SH3) transfectants by SDS-PAGE followed by Western blotting with anti-ABL, anti-GAP, and anti-CD71 MoAbs. As a control, the same proteins were also detected in total cell lysates. Results are representative of three different experiments. (Bottom panel) 32Dcl3 cells expressing wild-type (A) or ▵SH3 mutant (B) BCR/ABL were incubated with anti-ABL followed by FITC-conjugated goat antirabbit antibody and examined by confocal microscopy. Results are representative for three individual clones from each group (original magnification × 800).
Confocal microscopy analysis showed a finely punctate localization for wild-type BCR/ABL, whereas ΔSH3 BCR/ABL exhibited a coarsely punctate pattern of cellular distribution (Fig 8, right panel). The use of an antibody directed to an ABL epitope did not affect the interpretation of these results, because the ratio of BCR/ABL to ABL proteins in transfected cells is approximately 3 to 5:1 and c-ABL localizes primarily to the nucleus.29
DISCUSSION
SH3 domains consist of a short conserved stretch of 50 amino acids that interact with proline-rich regions in other proteins,42thereby modulating function and/or cellular localization of the proteins involved in the interactions.43 For example, the SH3 domain-mediated interaction of oncogenic tyrosine kinases with the p85 subunit of PI-3k plays an important role in signal transduction.44,45 Deletion or mutation of the SH3 domain of the c-ABL nuclear tyrosine kinase is one mechanism whereby v-ABL is oncogenically activated.29 However, deletion of c-ABL carboxy-terminal sequences with retention of the SH3 domain and fusion with viral sequences also activates the oncogenic potential of v-ABL,46 47 suggesting that deletion/mutation of the SH3 domain is not strictly required for v-ABL activation.
The mechanism of ABL oncogenic activation after its joining with the truncated BCR gene and formation of the chimeric BCR/ABL oncogene48 differs from those involved in v-ABL activation. The SH3 domain and carboxy-terminal sequences of c-ABL are both intact in BCR/ABL, and BCR/ABL tyrosine kinase activity is autoregulated by BCR sequences.14 15 Thus, it is unclear whether the BCR/ABL SH3 domain plays a role in BCR/ABL-induced leukemogenesis. To test this, we generated a p210BCR/ABL SH3 deletion mutant (ΔSH3 BCR/ABL) and assessed the properties of hematopoietic cells constitutively expressing this mutant in vitro and in vivo.
The BCR/ABL SH3 Domain Is Not Required for Activation of Intracellular Signals Regulating Proliferation and Survival
Like wild-type BCR/ABL, ΔSH3, but not the K1172R mutant, induced growth factor-independent proliferation of transfected 32Dcl3 cells, inhibited their differentiation in the presence of granulocyte colony-stimulating factor (G-CSF; not shown), and protected the cells from apoptosis. Also, ΔSH3 BCR/ABL was capable of transforming murine BMC. The ΔSH3 BCR/ABL mutant induced signaling pathways leading to stimulation of RAS,7,20,49,50 PI-3k,22,51JNK,52 MAPK,53 and STATs54 and to increased c-MYC expression.8 This indicates that the SH3 domain is not required for the signaling to known BCR/ABL effectors. On the other hand, the SH3 domain might be involved in the activation of some of these BCR/ABL effectors, but its absence might be compensated by distinct pathways activated by other BCR/ABL domains, analogous to the involvement of different domains of BCR/ABL that interact with Grb-2 and/or Shc in RAS activation.7
The Leukemogenic Potential of BCR/ABL Is Impaired upon Deletion of the SH3 Domain
Unlike the functions discussed above, the in vivo leukemogenic potential of hematopoietic cells expressing the ΔSH3 BCR/ABL protein was significantly impaired. Hematopoietic tissues of SCID mice injected with cells expressing the mutant protein showed markedly reduced levels of BCR/ABL transcripts as compared with tissues from mice injected with cells expressing the wild-type protein. Moreover, infiltration of bone marrow and spleen by 32Dcl3 cells expressing the ΔSH3 BCR/ABL mutant was fivefold to sixfold lower than that in mice injected with wild-type BCR/ABL transfectants. Consistent with these data, survival of SCID mice injected with 32Dcl3 cells expressing ΔSH3 BCR/ABL was about fourfold longer than that of mice injected with cells expressing wild-type BCR/ABL. Thus, the presence of the SH3 domain is required for aggressive leukemia in vivo. The stage of differentiation of these leukemic cells in vivo was identical in all mice, excluding the possibility that delayed leukemogenesis from ΔSH3 BCR/ABL cells reflects an increased rate of cell differentiation. This conclusion is further supported by the observation that the transfected cells do not undergo granulocyte differentiation in vitro in the presence of G-CSF (data not shown). Moreover, in vitro proliferation rates of 32Dcl3 cells and BMC expressing the ΔSH3 mutant or wild-type BCR/ABL were similar, which argues against the possibility that differences in growth rate determine the in vivo leukemogenic potential of cells expressing the mutant oncogene.
Lack of the SH3 Domain Impairs Lodging of BCR/ABL-Expressing Cells in Bone Marrow and Spleen
Because proliferation and survival of ΔSH3 and wild-type BCR/ABL-expressing cells were comparable, we asked whether the impaired leukemogenic potential of ΔSH3 BCR/ABL-transfected cells might rest in defects in cell-cell and cell-extracellular matrix interactions that allow efficient lodging of leukemic cells in tissues. Indeed, such defective interactions were suggested in each of the assays performed to test the ability of ΔSH3 BCR/ABL-expressing cells to adhere to basement membrane and stroma substrates, to pass through artificial membranes, to infiltrate the stroma, and to lodge in vivo in bone marrow and spleen. Verfaillie et al13 suggested that abnormal trafficking of CML cells is dependent on the changes in their ability to adhere to stromal and basement membrane components (collagen IV, fibronectin, and laminin) due to elevated levels of α2 and α6 integrins. Analysis of integrin expression in 32Dcl3 cells transfected with wild-type and mutant BCR/ABL showed that, compared with parental cells, wild-type BCR/ABL induced expression of α2 integrin. Interestingly, the reduced ability of ΔSH3 BCR/ABL-expressing cells to adhere to collagen IV- and laminin-coated plates correlated with lack of α2 integrin expression in these cells. The α2β1 complex serves as ligand for laminin and collagens.55 Cells transfected with the P1013L mutant also lacked α2 integrin expression, raising the possibility that wild-type BCR/ABL activates the expression of α2 integrin via proline-rich proteins interacting with the SH3 domain. Several ABL SH3 binding proteins have been identified, such as 3BP-1,56 Abi-1,57Abi-2,58 AAP1,59 RIN1,60 and PAG.61 However, only two of them (RIN1 and PAG) were also described as BCR/ABL-binding proteins and, accordingly, as potential downstream molecules in the signaling from the SH3 domain of BCR/ABL.
The reduced ability of ΔSH3 BCR/ABL-expressing cells to home into bone marrow and spleen correlated with a reduced tissue infiltration by these cells at 3 or 4 weeks of leukemia growth (Table 2 and Fig 5) and with a marked survival prolongation of mice injected with ΔSH3 BCR/ABL 32Dcl3 cells as compared with those injected with wild-type BCR/ABL 32Dcl3 cells (Fig 4). Because the in vitro survival of ΔSH3 BCR/ABL-expressing cells under condition of growth factor deprivation was undistinguishable from that of wild-type BCR/ABL-expressing cells, it seems likely that the defect in homing of ΔSH3 BCR/ABL-expressing cells was the consequence of the impairment in their adhesion and infiltration abilities rather than of a reduced survival. However, it is also possible that a loss of survival and/or a reduced proliferation becomes only manifest when ΔSH3 BCR/ABL-expressing cells need to respond to cell-cell– or cell-extracellular matrix–mediated signals. By contrast, cells expressing the ΔSH2 or the Δ176-426 mutant were not defective in invasion, substrate interaction, or homing, even if defective in transformation and in the ability to activate various intracellular signaling pathways in hematopoietic cells.7,8,62 Thus, the leukemogenic potential of BCR/ABL involves distinct domains that affect, respectively, intracellular signaling or cell adhesion and motility. It has been postulated that SH3 domains are involved in intracellular localization of the interacting proteins because most proteins that contain SH3 domains associate with the cortical actin cytoskeleton and/or cellular membranes.63-65 In fibroblasts, activated Src associates with integrin-dependent cytoskeletal complexes,66 and this localization appears to depend, at least in part, on the Src SH3 domain.41
A recent study suggests that, in transfected 32Dcl3 cells, p210 BCR/ABL localizes to punctate structures similar to focal adhesions of epithelial cells.67 Such localization is believed to be important in regulating integrin function through phosphorylation and dephosphorylation of specific tyrosine residues.68 Integrin receptors play an important role not only in mediating cell-cell and cell-extracellular matrix interactions, but also in regulating cell proliferation, differentiation, and survival, possibly through the formation of multiprotein signaling complexes that trigger the activation of intracellular pathways in response to extracellular matrix-generated signals.68 69 Thus, loss of the BCR/ABL SH3 domain might not only impair the initial lodging of BCR/ABL-expressing cells into bone marrow and spleen, but also their subsequent proliferation.
The wild-type BCR/ABL protein is mainly found in the cytoskeletal/membrane fraction, whereas ΔSH3 BCR/ABL is more evenly distributed between the membrane and cytosolic compartments. The cellular redistribution of BCR/ABL protein upon removal of the SH3 domain is not unprecedented, because ΔSH3 Src mutant also did interact with focal adhesions and cytoskeletal matrix less than the wild-type protein, despite maintaining the myristylation site for membrane attachment.41 Interestingly, ΔSH3 Src mutant was reported to be unable to transform efficiently NIH-3T3 fibroblasts.70 Confocal microscopy after staining with anti-ABL antibody showed a coarsely punctate pattern for ΔSH3 BCR/ABL that contrasts with the finely punctate pattern of wild-type BCR/ABL (Fig 8). This might reflect protein aggregation or the association of ΔSH3 BCR/ABL with structures different from those to which wild-type BCR/ABL localizes.
In conclusion, the SH3 domain does not influence intracellular signaling that regulates proliferation and survival of BCR/ABL-transfected cells but is required for full leukemogenic potential in vivo. The molecular mechanism(s) underlying the impaired leukemogenic potential of ΔSH3 BCR/ABL-transfected cells appears to involve changes in the adhesion and motility of these cells. The correlation of this phenotype with lack of α2 integrin expression suggests a possible causative effect associated with disruption of signals generated by SH3-dependent protein-protein interactions.
Supported in part by a grant from the Elsa U. Pardee Foundation (T.S.) and a grant (DHP-3D) from the American Cancer Society (B.C.).
Address reprint requests to Tomasz Skorski, MD, PhD, or Bruno Calabretta, MD, Kimmel Cancer Institute, Jefferson Medical College, BLSB 630, 233 S 10th St, Philadelphia, PA 19107.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Activation of signal transduction pathways by BCR/ABL proteins. (A) Levels of GTP-bound RAS in BCR/ABL-expressing cells. Pools of clones expressing the indicated vectors were labeled with [γ32P] orthophosphate. RAS was immunoprecipitated and the proportion of GTP- and GDP-bound forms was analyzed by thin-layer chromatography. (B) PI-3k interaction with BCR/ABL proteins. (Left panel) PI-3k activity was detected in antiphosphotyrosine immunoprecipitates from a mixture of clones expressing the indicated vectors. (Right panel) p85 immunoprecipitates from the indicated mixtures of clones were analyzed after SDS-PAGE and Western blotting with anti-ABL antibody. p85 was detected with anti-p85 antibody after stripping the filter. (C) Activation of MAPK and JNK pathways by BCR/ABL proteins. (Left panel) MAPK and JNK activities were measured in the appropriate immunoprecipitates using, respectively, myelin basic protein (MBP) and GST-JUN as substrates. (Right panel) Expression of c-MYC and c-JUN mRNA was determined in total cellular RNA (10 μg) by Northern blotting. GAPDH was detected as a control for equal gel loading. (D) DNA binding activity of STAT proteins. EMSA was performed using nuclear extracts of the indicated clone mixtures and synthetic oligonucleotides containing the GAS motif as a probe. Results are representative of three to four independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.406/3/m_blod4022002.jpeg?Expires=1767766123&Signature=i8Qx1j6qvj9HKOIx9d6x7ab4BJxV798pvHXUKS1ZZpJw3THpHanBttYY51P7lChUUkw5CxXJaa3QwoMD5TnJRY5Ste214y7iznrLqK4ojnoq8ZE~r8tdl7cJsqDlZrMQK7~9KX-ifFtumed1AhJlwLc3cFFYN-d5MaWrjnIE0YPKq5Os~ce-qKudJPIDUgELJu1zNxFV~N2wf1eiylW6hswuF2-LDmDTiXJaW1hsL4d-1m5P3qALUACVE0sTeNQsh1hZM0B0R0rNdE0N9Bb-rwjNtbpeNWdnmu26cLXjJENQA3kNzrnyNJFnlZpptEpZbIaLhcIA~Zs7TxKI-oUMhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. In vivo homing of BCR/ABL-expressing cells. Single-cell suspensions were prepared from bone marrow (BMC) and spleen (SPL) of mice 24 (▪) and 48 (▨) hours after injection with [3H]-uridine–labeled 32Dcl3 clones (A) or retrovirus-infected BMC (B) and deposited onto glass microfiber filters. Cell-associated radioactivity was determined as described in the Materials and Methods. Results are expressed as the percentage of injected radioactivity (mean ± SD from 3 mice in each group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.406/3/m_blod4022006.jpeg?Expires=1767766123&Signature=caCwA5Zv7F6I9Kh46dlVUSmOVc3t2Mq5qjEWT2EziQifLKkdcun1JsyiPRC2ncgXTZv~OYoUadaOk2~UbBNdmHFszH4aB8irijUi6j5pqdwhls5i-jHYCc724hwo2UJcJtzq9MVBWTki9VKlCANn4Bku8UIoLsYkJyBzxilSkYfWHhhocU8Y1PsE2-lgO8HcZRbBeBn6FzRSY9n1RYK9J5XnO2TLfywUDqME2QqhYNvEBve-cZXQgCZQa5~QXyQJwVIIxFAgnfwy4XyD357~Va1cQIc3xPtbP5EkaBKO6xgaHbLiiqDCQMhnDER8NeYtfxFpJxrnY7cXO4mLCuQBxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal