Abstract
The thrombopoietic efficacy of recombinant forms of c-mplligand is being actively investigated in preclinical studies using daily dosing schedules. However, a comprehensive kinetic study of the thrombopoietic response to a single injection of recombinant c-mpl ligand has not been performed. Here, we present the results of a detailed kinetic analysis of the platelet response to a single intravenous administration of pegylated recombinant murine megakaryocyte growth and development factor (PEG-rmMGDF) in mice. In addition, we compare the efficacy of single versus daily dosing in stimulating platelet production. A single intravenous injection of PEG-rmMGDF produced a marked and dose-dependent elevation in platelet number and a moderate increase in mean platelet volume (MPV). After administration of 25 or 250 μg/kg of PEG-rmMGDF, platelet number was first increased on day 3 and peaked at 2.7-fold (25 μg/kg) and 5.7-fold of normal (250 μg/kg) on day 5. Thereafter, platelet number declined and returned to baseline by days 9 and 14, with the 25 and 250 μg/kg doses, respectively. MPV began to increase on day 2 after PEG-rmMGDF, reaching maximum values of 1.2-fold (25 μg/kg) and 1.5-fold of normal (250 μg/kg) on day 4. Subsequently, MPV declined and was downregulated on days 6 to 7 (25 μg/kg) and day 8 (250 μg/kg). Based on these results, we evaluated the platelet response to PEG-rmMGDF administered intravenously as a single dose versus daily for 5 days. A single administration of 100 μg/kg produced a higher platelet number on day 5 than daily administration of 100 or 20 μg/kg for 5 days. However, the thrombocytosis was less sustained after single versus daily dosing. The smaller platelet number increase on day 5 after daily dosing reflected the production of larger platelets, rather than suppression of thrombopoiesis. Our results indicate that PEG-rmMGDF administered as a single intravenous dose potently stimulates platelet production in mice, challenging the need for its daily administration. Adoption of an intermittent administration schedule of this cytokine could be more efficacious and is merited in future clinical trials.
THROMBOPOIESIS IS A complex, multistep process that begins with the commitment of a pluripotent hematopoietic stem cell to a differentiation pathway.1 The committed megakaryocytic progenitor cell then undergoes proliferation and subsequently differentiates into a mature megakaryocyte, which ultimately releases platelets into the circulation.2Experimental induction of immune thrombocytopenia in animals has served as a model system to study the mechanisms that regulate megakaryocytopoiesis and platelet production.3-5 An increase in platelet size was detected as early as 8 to 12 hours after induction of acute severe or moderate immune thrombocytopenia in rodents and preceded the increase in platelet number.3-5Furthermore, injection of plasma from thrombocytopenic animals into mice resulted in increases of platelet number and size.6-8The thrombopoietic activity present in thrombocytopenic plasma was attributed to thrombopoietin, the humoral regulator of thrombopoiesis that long has been believed to exist and sought after.9Thrombopoietin was thought to be responsible for the alterations in the platelet indices observed after induction of immune thrombocytopenia,10 but whether this was indeed the case remained to be determined.
The recent discovery and cloning of the c-mplligand11-15 have provided an essential tool to characterize the role of thrombopoietin in thrombopoiesis. Several in vitro and in vivo studies, along with the c-mpl receptor and ligand knock-out mouse models, have identified c-mpl ligand as thrombopoietin, the primary regulator of thrombopoiesis.16-19 Further data have indicated the potential usefulness of this novel cytokine in the clinical setting. For instance, megakaryocyte growth and development factor (MGDF), a truncated recombinant form of c-mpl ligand, has been shown to be effective in alleviating and even abrogating thrombocytopenia following myelosuppressive therapy in mice.20,21 In the bone marrow transplant setting, Fibbe et al22 showed that although treatment of lethally irradiated mice with recombinantc-mpl ligand did not stimulate platelet recovery, pretreatment of donor mice with this cytokine did accelerate the reconstitution of platelets and erythrocytes in recipient mice. On the other hand, Molineux et al23 demonstrated an enhanced stimulation of platelet recovery in lethally irradiated mice treated with a pegylated form of MGDF after marrow transplantation.
Most preclinical studies performed to evaluate the thrombopoietic effects of this clinically promising agent have used a daily subcutaneous or intraperitoneal injection schedule. However, no detailed kinetic studies of the thrombopoietic response to a single administration of this cytokine had been reported. Therefore, we conducted a detailed kinetic analysis of the thrombopoietic response to a single intravenous administration of pegylated recombinant murine MGDF (PEG-rmMGDF), in mice. In an earlier report, we described the kinetics of the megakaryocytic changes produced by the single injection.24 Here, we present the detailed analysis of the consequent platelet response. In addition, we compare the platelet responses to single versus daily dosing of PEG-rmMGDF.
MATERIALS AND METHODS
Experimental Design
First, we defined the serial effects of PEG-rmMGDF administered intravenously as a single dose (25 or 250 μg/kg) on platelet number and size in mice. These parameters were measured at frequent intervals (12 hours, 24 hours, 36 hours, daily at days 2 to 10, and day 14) after PEG-rmMGDF administration. Independent groups of mice were used for the different doses at each time point, ie, mice were bled only once. The 25 μg/kg dose was chosen because of its repeated use in daily administration schedules in other studies of this cytokine. A 10-fold larger dose (250 μg/kg) was used based on our hypothesis that administration of a single large dose of this cytokine may be more advantageous than daily administration of smaller doses. Next, we compared in two separate experiments the platelet responses to PEG-rmMGDF given intravenously as a single dose of 100 μg/kg, or daily for 5 days at 100 μg/kg or 20 μg/kg. Specifically, we determined platelet indices on days 5, 7, 10, and 12 after initiation of PEG-rmMGDF treatment. Independent groups of mice were used in these experiments except in experiment 1, where mice bled on days 5 and 7 after PEG-rmMGDF administration, were bled again on days 10 and 12, respectively.
MGDF Reagent
PEG-rmMGDF is a recombinant truncated murine c-mpl ligand encompassing the erythropoietin-like domain of the native c-mplligand, thrombopoietin.25 Derivitization with polyethyleneglycol (pegylation) increases the in vivo potency of this product by roughly 10-fold to 20-fold, largely by delaying its clearance and thus prolonging its plasma half-life.21 26PEG-rmMGDF was kindly provided by AMGEN Inc (Thousand Oaks, CA) as a clear, aqueous solution. An initial lot of PEG-rmMGDF used in our kinetic studies demonstrated higher thrombopoietic potency than a second lot that was used in subsequent experiments. No data on the relative biologic activity of the different PEG-rmMGDF lots determined by bioassays, were provided by the supplier.
On the day of administration, the stock solution was diluted in phosphate-buffered saline (PBS) containing 1% isologous-mouse serum.
Animals Studied
Young adult C57BL/6J male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and quarantined for 4 weeks before use. At the time of the experiments, mice were 10 to 12 weeks old and weighed 25 to 35 g. They were anesthetized with methoxyflurane before blood collection via cardiac puncture or from the orbital sinus or abdominal aorta.
MGDF Administration
In all experiments, PEG-rmMGDF was injected intravenously via a lateral tail vein after induction of vasodilation by warming under an examination lamp. The intravenous route of injection was chosen in an attempt to achieve the highest possible plasma levels of PEG-rmMGDF. In the single dose schedule, PEG-rmMGDF was administered as a single injection on day 0, while it was given on days 0 through 4 in the daily administration schedule. An equivalent volume of PBS with 1% isologous-mouse serum (carrier) was injected intravenously into control mice. Blood samples were obtained for platelet counting and sizing at frequent intervals (12 hours to 14 days) after PEG-rmMGDF/carrier administration.
Platelet Number Determination
Blood was collected by orbital sinus bleeding into Unopette pipettes and immediately diluted in Unopette reservoirs containing 1% ammonium oxalate. Platelets were counted on a hemacytometer by phase-contrast microscopy (magnification ×500).
Mean Platelet Volume (MPV) Measurement
Blood was collected by cardiac puncture into K2 EDTA at a final concentration of 12.5 mmol/L. Platelets were separated on self-generated Percoll density gradients. Percoll density medium (Pharmacia, Inc, Piscataway, NJ) was made isotonic for mouse blood with 10× Dulbecco's PBS and then diluted with an isotonic solution of the same buffer to a 40% concentration. A total of 5 mmol/L K2 EDTA was added to the medium to prevent platelet aggregation in the gradients. Continuous density gradients were generated by centrifugation of 9 mL volumes of the diluted Percoll medium at 20,000g for 20 minutes at 22°C in a fixed-angle rotor. A total of 200 μL of each blood sample was diluted 1:1 with Hanks' balanced salt solution, layered on top of the density gradient, and centrifuged at 1,000g for 10 minutes at 22°C. The portion of the gradient containing platelets was collected for MPV measurement. MPV was determined with a Coulter particle analyzer (Model ZM with Channelyzer 256; Coulter Scientific Instruments, Hialeah, FL). The instrument's calibration was verified with 2.02 μm diameter latex beads before each use. Data were accumulated on each platelet sample until a count of 2,000 was achieved in the peak channel. The volume distributions were analyzed as log-normal distributions. The data were expressed as MPVs.
Analysis of Platelet Size by Flow Cytometry
Blood was collected by orbital sinus bleeding into Unopette pipettes and immediately diluted 1:40 with normal saline solution. Blood samples were then incubated for 30 minutes at room temperature with a saturating concentration (10 μL of 1:1000 dilution) of a rat monoclonal antibody to mouse platelets (4A5),27 shown to be directed to a 74-kD glycoprotein present on murine platelets28 and to be highly platelet specific27 (kindly provided by Dr S. Burstein, University of Oklahoma, Oklahoma City, OK). Platelets were then fluorescently labeled with 5 μL of fluorescein isothiocyanate (FITC)-goat antirat IgG F(ab')2 (Biosource, Camarillo, CA), by incubating for 30 minutes at room temperature. Before flow cytometric analysis, samples were diluted 1:20 with normal saline solution. Platelets incubated with FITC-goat antirat IgG F(ab')2 only, served as the negative control. Flow cytometric analysis was performed on a FACScan (Becton-Dickinson, San Jose, CA). Cells positive for 4A5, identified as platelets, were selected for analysis by electronic gating. Forward angle light scatter was used as an index of platelet size. The proportion of large platelets was defined as the percentage of platelets larger in size than 99% of platelets of control mice (mice injected with carrier only). Ten thousand platelets were examined for each sample.
Electron Microscopy
Blood was collected from the abdominal aorta of methoxyflurane-anesthetized mice into ACD/PGE1 containing syringes and immediately dripped into 9 vol of 1.5% gluteraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4, with 1% sucrose, and fixed for 1 hour at room temperature. The red blood cells were pelleted by centrifugation for 20 minutes at 400g. The supernatant containing the platelets was collected, and platelets were pelleted by centrifugation for 10 minutes at 3,200g. The fixed platelets were washed three times with 0.1 mol/L cacodylate buffer (pH 7.4) by centrifugation for 10 minutes at 2,300g, then were postfixed in osmium tetroxide.29 The platelet samples were dehydrated through a graded series of ethanol, infiltrated with toluene and subsequently with a resin composed of dodecenyl succinic anhydride (DDSA), nadic methyl anhydride (NMA), and tri(dimethyl amino methyl) phenol (DMP-30; Ted Pella, Redding, CA) and LX-112 (Ladd Research Industries, Burlington, VT), and finally embeded in 100% of the same resin. Thin sections were cut and stained with uranyl acetate and lead citrate and viewed with a Philips 301 transmission electron microscope (Philips Electronic Instruments, Inc, Mahwah, NJ).
Statistical Analyses
The Kruskal-Wallis test,30 a nonparametric analog of the analysis of variance (ANOVA) F-test, was used to detect overall differences in median platelet number and size when comparisons involved more than two groups. Because of the small sample sizes, a permutation test31 (STATXACT software package; CYTEL Software Corp, Cambridge, MA)32 was used to determine theP values. When the number of permutations was less than 10,000, an exact P value was calculated, whereas, when the number of permutations exceeded 10,000, an estimate of the P value was obtained using the Monte Carlo method, based on 10,000 permuted samples. With 10,000 permutations, the estimated P value is within 1.3% of the true P value with 99% confidence. When a significant difference was detected by the Kruskal-Wallis test or when only two groups were being compared, the Wilcoxon-Mann-Whitney rank sum test30 was used. In the case of multiple comparisons, a Bonferroni correction33 was made to maintain the overall type 1 error level of α = .05. The corrected α was obtained by dividing 0.05 by the number of comparisons. P values were calculated using the permutation test as described above.
Statistical analyses of nonparametric nature were performed comparing medians, whereas, figures shown depict arithmetic means ± standard error of means (SEM).
RESULTS
Kinetics of the Platelet Response to PEG-rmMGDF
Platelet number.
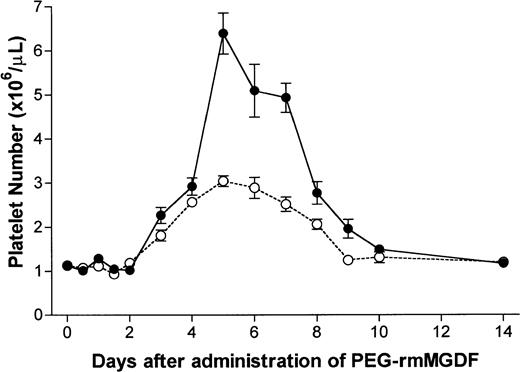
PEG-rmMGDF administered as a single intravenous dose of 25 or 250 μg/kg resulted in a profound thrombopoietic stimulation. Platelet number peaked with both doses on day 5, at 2.7-fold and 5.7-fold of normal with the 25 and 250 μg/kg doses, respectively (Fig 1). The corrected α for analysis of data shown in Fig 1 was .004. After a 2-day delay, with both doses, platelet number increased on day 3 (P = .0006 and P < .0001, with the 25 and 250 μg/kg doses, respectively), and reached approximately 2.5-fold of normal by day 4. With the 25 μg/kg dose, platelet number remained elevated for the next 4 days and returned to baseline on day 9. In contrast, with the 250 μg/kg dose, platelet number continued to increase beyond day 4, reaching a peak of 5.7-fold of normal on day 5. The platelet number remained elevated through day 10 (P = .002). With both doses, the rate of decline in platelet number from peak values was consistent with the 4-day life span of normal murine platelets.34
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on platelet number in mice. PEG-rmMGDF produced a profound and dose-dependent increase in platelet number. With both PEG-rmMGDF doses, a 2-day delay was observed before platelet number began to increase; and peak values were reached on day 5. Each data point represents the mean ± SEM of platelet number; for the 25 μg/kg dose, n = 4 to 10 mice per time point for the MGDF group and n = 13 for controls, and for the 250 μg/kg dose, n = 5 to 11 mice per time point for the MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on platelet number in mice. PEG-rmMGDF produced a profound and dose-dependent increase in platelet number. With both PEG-rmMGDF doses, a 2-day delay was observed before platelet number began to increase; and peak values were reached on day 5. Each data point represents the mean ± SEM of platelet number; for the 25 μg/kg dose, n = 4 to 10 mice per time point for the MGDF group and n = 13 for controls, and for the 250 μg/kg dose, n = 5 to 11 mice per time point for the MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
MPV.
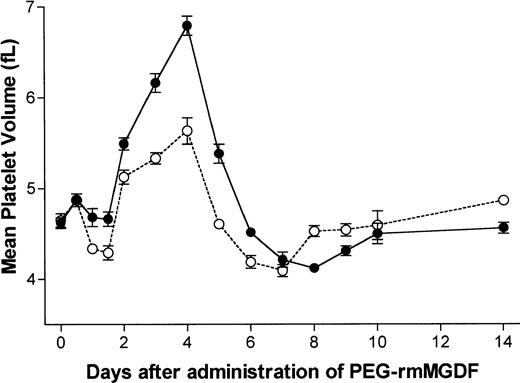
An increase in MPV was first detected on day 2 after PEG-rmMGDF (P= .0007 and P = .0003 for the 25 and 250 μg/kg doses, respectively), preceding by 1 day the increase in platelet number (Fig 2). The corrected α for analysis of data shown in Fig 2 was .004. Changes in MPV before day 2 were not significantly different from control. MPV reached maximum values of 1.2-fold and 1.5-fold of normal with the 25 and 250 μg/kg doses on day 4, respectively. Noteworthy is the sharp decrease in MPV on day 5, time of the peak in platelet number. Thereafter, MPV declined to below baseline on days 6 (P = .003) and 7 (P < .0001) with the 25 μg/kg dose, and on day 8 (P < .0001) with the 250 μg/kg dose. MPV returned to baseline values on days 8 and 9 after the 25 and 250 μg/kg doses, respectively.
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on MPV in mice. PEG-rmMGDF caused a dose-dependent increase in MPV, which began on day 2 and peaked on day 4. Each data point represents the mean ± SEM of MPV; for the 25 μg/kg dose, n =4 to 10 mice per time point for the MGDF group and n = 13 for controls, and for the 250 μg/kg dose, n = 5 to 7 mice per time point for the MGDF group and n = 18 for controls. Data from control mice were pooled to represent the day 0 data point.
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on MPV in mice. PEG-rmMGDF caused a dose-dependent increase in MPV, which began on day 2 and peaked on day 4. Each data point represents the mean ± SEM of MPV; for the 25 μg/kg dose, n =4 to 10 mice per time point for the MGDF group and n = 13 for controls, and for the 250 μg/kg dose, n = 5 to 7 mice per time point for the MGDF group and n = 18 for controls. Data from control mice were pooled to represent the day 0 data point.
To test whether c-mpl ligand is responsible for the early increase in platelet size noted after experimental induction of immune thrombocytopenia, a single large dose (250 μg/kg) of PEG-rmMGDF was injected intravenously into mice, and MPV was determined at 12 hours and 24 hours after administration. No change in MPV was detected 12 hours after this dose in the MGDF-treated mice compared with control mice at α = .05 (Table 1). Similarly, no change in MPV was noted 24 hours after 250 μg/kg of PEG-rmMGDF. Doubling the dose to 500 μg/kg (250 μg/kg given twice, 12 hours apart) also did not produce a change in MPV at 24 hours. These results suggested that c-mpl ligand is not responsible for the early increase in platelet size observed after experimental immune thrombocytopenia.
MPV Early After PEG-rmMGDF
| Group . | MPV at 12 hr (fL) . | MPV at 24 hr (fL) . |
|---|---|---|
| Control | 4.91 ± 0.06 | 4.64 ± 0.14 |
| (2) | (4) | |
| MGDF 250 μg/kg | 4.88 ± 0.05* | 4.68 ± 0.10† |
| (5) | (7) | |
| MGDF 500 μg/kg | 4.77 ± 0.13† | |
| (4) |
| Group . | MPV at 12 hr (fL) . | MPV at 24 hr (fL) . |
|---|---|---|
| Control | 4.91 ± 0.06 | 4.64 ± 0.14 |
| (2) | (4) | |
| MGDF 250 μg/kg | 4.88 ± 0.05* | 4.68 ± 0.10† |
| (5) | (7) | |
| MGDF 500 μg/kg | 4.77 ± 0.13† | |
| (4) |
Values shown represent mean ± SEM. Numbers in parentheses indicate number of mice per group.
Not significantly different from MPV of control mice P = 0.857, Wilcoxon-Mann-Whitney.
Not significantly different from MPV of control mice P = 0.708, Kruskal-Wallis.
Platelet size by flow cytometry.
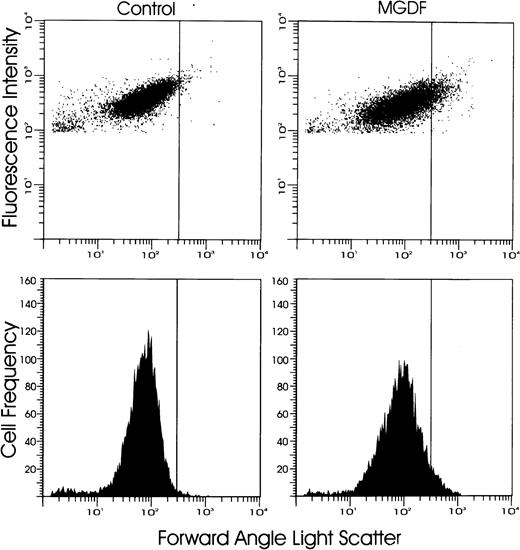
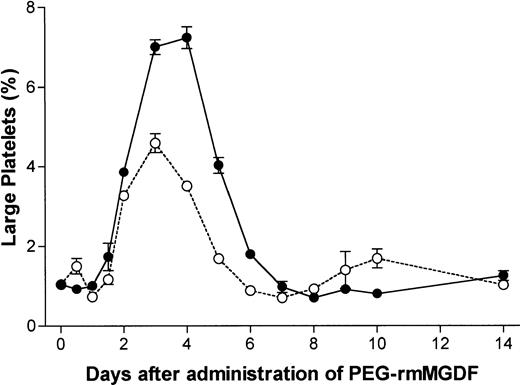
Because of the concern that MPV measurement may not be sensitive enough to detect minor increases in platelet size due to small populations of newly released large platelets, we further examined platelet size by analyzing the percentage of large platelets using flow cytometry, as described in the Materials and Methods section. Flow cytometric analyses of platelets were performed concurrently at all time points where MPVs were measured. After administration of 250 μg/kg of PEG-rmMGDF, the percentage of large platelets was first increased on day 2 (P = .0001), was maximal on day 4, and was decreased to below baseline on day 8 (P = .0001) (Figs 3 and4), consistent with the changes in MPV documented above in response to this dose (Fig 2). The corrected α for analysis of data shown in Fig 4 was .004. In contrast, administration of 25 μg/kg of MGDF resulted in an initial minor increase in the percentage of large platelets detected at 12 hours (P = .0003) followed by a slight decrease at 24 hours (P = .0003), preceding the pronounced increase that began on day 2 (P < .0001) (Fig 4). Subsequently, except for the earlier peak in the percentage of large platelets noted on day 3, the changes in platelet size in response to the lower dose resembled those detected by MPV. These results suggest that flow cytometry is more sensitive than MPV in detecting minor fluctuations in platelet size and that the effect of PEG-rmMGDF on production of large platelets is dose-related.
Shown are representative flow cytometric profiles of platelet size on day 4 after carrier or 250 μg/kg of PEG-rmMGDF. Platelets were labelled with FITC-4A5 monoclonal antibody and analyzed by flow cytometry. A vertical gate was set to detect the percentage of large platelets, defined to be larger than 99% of platelets of control mice. Note the higher percentage of large platelets in the MGDF-treated mice compared with control mice.
Shown are representative flow cytometric profiles of platelet size on day 4 after carrier or 250 μg/kg of PEG-rmMGDF. Platelets were labelled with FITC-4A5 monoclonal antibody and analyzed by flow cytometry. A vertical gate was set to detect the percentage of large platelets, defined to be larger than 99% of platelets of control mice. Note the higher percentage of large platelets in the MGDF-treated mice compared with control mice.
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on the percentage of large platelets defined by flow cytometry to be larger than 99% of platelets of control mice. A major increase in the percentage of large platelets was noted on day 2 after PEG-rmMGDF, with peaks occurring on day 3 and days 3 to 4 after the 25 and 250 μg/kg doses, respectively. Each data point represents the mean ± SEM of the percentage of large platelets, for the 25 μg/kg dose, n = 4 to 7 mice per time point for the MGDF group and n = 12 for controls, and for the 250 μg/kg dose, n = 5 to 6 mice per time point for the MGDF group and n = 15 for controls. Data from control mice were pooled to represent the day 0 data point.
Serial effects of a single intravenous dose, 25 μg/kg (○) or 250 μg/kg (•) of PEG-rmMGDF administered on day 0 on the percentage of large platelets defined by flow cytometry to be larger than 99% of platelets of control mice. A major increase in the percentage of large platelets was noted on day 2 after PEG-rmMGDF, with peaks occurring on day 3 and days 3 to 4 after the 25 and 250 μg/kg doses, respectively. Each data point represents the mean ± SEM of the percentage of large platelets, for the 25 μg/kg dose, n = 4 to 7 mice per time point for the MGDF group and n = 12 for controls, and for the 250 μg/kg dose, n = 5 to 6 mice per time point for the MGDF group and n = 15 for controls. Data from control mice were pooled to represent the day 0 data point.
Electron microscopy.
Platelets were examined by transmission electron microscopy on day 4 after PEG-rmMGDF (250 μg/kg), the time point of maximal increase in platelet size. Platelets had normal ultrastructure, with the exception of frequent endoplasmic reticulum and Golgi Complex profiles in platelet sections, suggesting their premature release from stimulated megakaryocytes (Fig 5).
Transmission electron micrographs of platelets from mice on day 4 after receiving carrier only (a and c) or a single intravenous dose of 250 μg/kg of PEG-rmMGDF (b, d, and e). Platelets produced in response to PEG-rmMGDF had normal ultrastructure, with the exception of frequent endoplasmic reticulum (arrowheads in [d] and [e]) and Golgi Complex profiles in platelet sections, suggesting their premature release. db, dense bodies; g, alpha granule. Magnification in (a) and (b) × 13,000, and in (c), (d), and (e) × 34,000.
Transmission electron micrographs of platelets from mice on day 4 after receiving carrier only (a and c) or a single intravenous dose of 250 μg/kg of PEG-rmMGDF (b, d, and e). Platelets produced in response to PEG-rmMGDF had normal ultrastructure, with the exception of frequent endoplasmic reticulum (arrowheads in [d] and [e]) and Golgi Complex profiles in platelet sections, suggesting their premature release. db, dense bodies; g, alpha granule. Magnification in (a) and (b) × 13,000, and in (c), (d), and (e) × 34,000.
Dose-Response Effects of Single Dose PEG-rmMGDF
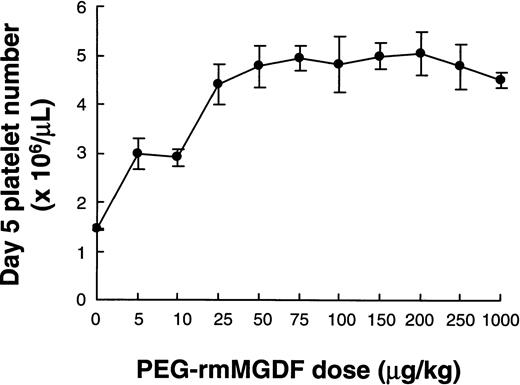
We determined platelet number on days 5 and 6 following a range of single intravenous doses (5 to 250 μg/kg) of PEG-rmMGDF injected into mice. These specific time points were selected based on the kinetic results, which showed that maximal platelet production occurred on day 5 after PEG-rmMGDF administration (Fig 1). The second lot of PEG-rmMGDF, of lower potency, was used in this and subsequent experiments. In no case was the platelet number on day 6 (data not shown) higher than that on day 5 at α = .05. A single injection of MGDF potently stimulated platelet production even at the 5 μg/kg dose, the lowest dose given (Fig 6). Peak platelet number increased in a dose-dependent manner with a plateau at 25 μg/kg (Kruskal-Wallis comparing values at doses 10 to 250 μg/kg,P = 0.03, α = 0.05; Kruskal-Wallis comparing values at doses 25 to 250 μg/kg, P = .925, and Wilcoxon-Mann-Whitney comparing values at 10 and 25 μg/kg, P = .016, corrected α = .025). Increasing the dose above 25 μg/kg, using this batch of PEG-rmMGDF, did not result in further enhancement of the platelet number response. This plateau effect could have possibly resulted from a complete saturation of the c-mpl receptors of megakaryocytes and/or to the production of larger platelets by the higher doses. Very large platelets were noted by phase-contrast microscopy with 1,000 μg/kg of PEG-rmMGDF, however, quantitation of platelet size was not performed.
Dose-response effects of single intravenous injection of PEG-rmMGDF on platelet number in mice on day 5 after PEG-rmMGDF. PEG-rmMGDF potently stimulated platelet production in a dose-dependent manner. Each data point represents the mean ± SEM of the platelet numbers. Number of mice injected at each dose level = 5.
Dose-response effects of single intravenous injection of PEG-rmMGDF on platelet number in mice on day 5 after PEG-rmMGDF. PEG-rmMGDF potently stimulated platelet production in a dose-dependent manner. Each data point represents the mean ± SEM of the platelet numbers. Number of mice injected at each dose level = 5.
Single Versus Daily Dosing of PEG-rmMGDF
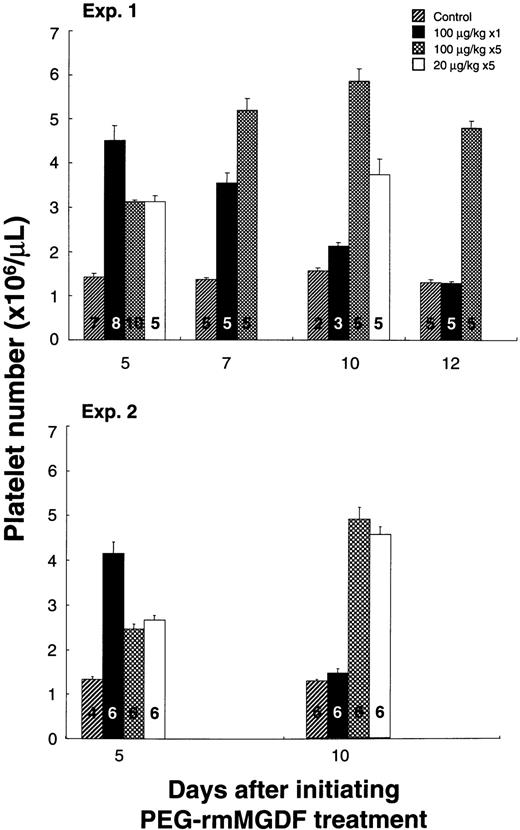
We next examined whether the daily administration of MGDF was more efficacious in elevating platelet number than single dosing. Our aim was to determine whether the thrombopoietic response to a single dose could be enhanced by daily administration of the same dose or by dividing the dose over several days. Based on the dose-response curve of the second lot of PEG-rmMGDF, we chose a 100 μg/kg dose to ensure that maximal response to single dosing would be achieved. Specifically, we compared the thrombopoietic responses to 100 μg/kg of PEG-rmMGDF when given as a single injection on day 0, versus when given daily for 5 days (100 μg/kg daily on days 0 through 4), versus when divided equally over 5 days (20 μg/kg daily on days 0 through 4). As depicted in Fig7, single injection of 100 μg/kg of PEG-rmMGDF produced a higher platelet number on day 5, when compared with daily injection for 5 days of 100 μg/kg (P < .0001 and P = .002 for experiments 1 and 2, respectively) or 20 μg/kg (P = .003 andP = .002 for experiments 1 and 2, respectively); corrected α = .008. By day 7, platelet number after the single injection declined (P = .036; α = .05), whereas, it continued to increase after daily injection of 100 μg/kg (P = .008; α = .05). On day 10, platelet number after the single dose returned to baseline; however, it remained significantly increased after daily administration of 20 or 100 μg/kg of PEG-rmMGDF compared with control (P = .002 for experiment 2; corrected α = .008). This prolonged elevation did not reach statistical significance in experiment 1, most likely due to the small number of mice in the control group. On day 12, platelet number after daily administration of 100 μg/kg remained substantially elevated compared with control (P = .008, corrected α = .017).
Comparison of platelet number response to PEG-rmMGDF administered intravenously as a single dose of 100 μg/kg versus daily at 100 μg/kg or 20 μg/kg for 5 days. Single dose administration of PEG-rmMGDF resulted in a higher elevation in platelet number on day 5, whereas daily dose administration produced a more prolonged thrombocytosis. No data were collected on days 7 and 12 for the 20 μg/kg dose in experiment 1 and for all doses in experiment 2. Bars represent the mean ± SEM of the platelet numbers. Numbers displayed in bars indicate number of mice in each group.
Comparison of platelet number response to PEG-rmMGDF administered intravenously as a single dose of 100 μg/kg versus daily at 100 μg/kg or 20 μg/kg for 5 days. Single dose administration of PEG-rmMGDF resulted in a higher elevation in platelet number on day 5, whereas daily dose administration produced a more prolonged thrombocytosis. No data were collected on days 7 and 12 for the 20 μg/kg dose in experiment 1 and for all doses in experiment 2. Bars represent the mean ± SEM of the platelet numbers. Numbers displayed in bars indicate number of mice in each group.
The hematocrits of the mice with repeated bleeding were not reduced by the small collected blood volume (10 μL).
Examination of MPV on day 5 showed a greater increase in MPV after daily administration of 100 μg/kg than after single dosing (P= .008 and P = .002 in experiments 1 and 2, respectively; corrected α = .017) (Table 2). Similarly, a greater increase in MPV was documented on day 5 after daily administration of 20 μg/kg of PEG-rmMGDF (P = .002; corrected α = .008). This finding indicated that the lower platelet number on day 5 after multiple dosing was not due to suppression of thrombopoiesis, but rather to the production of larger platelets.
Effect of Single Versus Daily Dose Administration of PEG-rmMGDF on Day 5 MPV
| Group . | MPV (fL) . | |
|---|---|---|
| Experiment 1 . | Experiment 2 . | |
| Control | 4.26 ± 0.12 | 5.02 ± 0.08 |
| (4) | (4) | |
| MGDF 100 μg/kg × 1 | 5.00 ± 0.10 | 5.31 ± 0.04 |
| (5) | (6) | |
| MGDF 100 μg/kg daily × 5 | 7.52 ± 0.15* | 7.81 ± 0.09† |
| (5) | (6) | |
| Group . | MPV (fL) . | |
|---|---|---|
| Experiment 1 . | Experiment 2 . | |
| Control | 4.26 ± 0.12 | 5.02 ± 0.08 |
| (4) | (4) | |
| MGDF 100 μg/kg × 1 | 5.00 ± 0.10 | 5.31 ± 0.04 |
| (5) | (6) | |
| MGDF 100 μg/kg daily × 5 | 7.52 ± 0.15* | 7.81 ± 0.09† |
| (5) | (6) | |
Values shown represent mean ± SEM. Numbers in parentheses indicate number of mice per group.
Significantly different from MPV of mice treated with MGDF 100 μg/kg × 1, P = .008, Wilcoxon-Mann-Whitney.
Significantly different from MPV of mice treated with MGDF 100 μg/kg × 1, P = .002, Wilcoxon-Mann-Whitney.
DISCUSSION
The long search for a platelet growth factor has recently culminated in the discovery and cloning of the c-mpl ligand. The availability of recombinant forms of this vital cytokine, with its potent stimulatory action on megakaryocytopoiesis and platelet production, has provided a promising thrombopoietic agent for use in clinical trials. Ideally, trials designed to determine the optimal dosing of this cytokine should be based on the kinetic response to single dose administration. However, to date, no such detailed analysis has been performed. In the present study, we have shown that intravenous administration of a single dose of PEG-rmMGDF to mice results in a marked increase in platelet number and a moderate increase in platelet size. The degree of thrombocytosis induced by single injection was dose-dependent. When compared with daily dosing, single PEG-rmMGDF administration stimulated a higher early (day 5), but less sustained increase in platelet number.
Our kinetic data indicate that single intravenous injection of 25 or 250 μg/kg of PEG-rmMGDF into mice induces a dose-dependent increase in platelet number, with maxima of 2.7-fold and 5.7-fold of normal on day 5 after the 25 and 250 μg/kg doses, respectively. Our kinetic analysis of the megakaryocytic response to the above doses of PEG-rmMGDF,24 showed that the markedly higher peak in platelet number with the higher dose is the consequence of a greater increase in megakaryocyte frequency, rather than a higher stimulation of either megakaryocyte polyploidization or megakaryocyte size. That is, the maximal increase in the frequency of immature megakaryocytes (2N/4N cells) observed on day 4 after the higher dose was twice that after the lower dose, whereas, the increase of megakaryocyte polyploidization and size were similar with both doses. Further, the greater expansion in the pool of immature megakaryocytes following the 250 μg/kg dose, translated into a sustained peak in the frequency of polypoid megakaryocytes (8N-128N cells), thus accounting for the greater and more prolonged effect on platelet production seen after this dose.
A 2-day delay before an increase in platelet number was observed after the single administration of either 25 or 250 μg/kg of PEG-rmMGDF, indicating that MGDF does not act immediately to release platelets from mature megakaryocytes. A similar delay before an increase in platelet number was also observed in other studies after daily administration of this cytokine.20,26,35 Our examination of the megakaryocytic response to single administration of PEG-rmMGDF,24 and its consequences on platelet production reported herein, shows that the initial response to single PEG-rmMGDF administration is a right shift in megakaryocyte DNA ploidy at 12 hours, followed by an increase in megakaryocyte frequency and size on day 2, which precede the increase in platelet number on day 3. These in vivo megakaryocytic changes are in agreement with in vitro data36 showing that c-mpl ligand has both a proliferative and differentiative effect on megakaryocyte progenitors and explain the delay before the increase in platelet number, the time elapsing between stimulation of immature megakaryocytes and platelet release. This mechanism of action of MGDF also explains why intensifying the dose by increasing it or administering it daily, does not result in an earlier response in platelet number. Interestingly, a similar delay in platelet recovery was observed after induction of immune thrombocytopenia by antiplatelet antisera in experimental animals.3 37
Similar to its effect on platelet number, we found that PEG-rmMGDF causes a dose-dependent increase in platelet size, documented both by MPV measurement and analysis of large platelets by flow cytometry. With both doses (25 and 250 μg/kg) of PEG-rmMGDF, the initial and maximal increase in platelet size preceded the initial and maximal increase in platelet number. Prematurely released platelets, with otherwise normal ultrastructure, were noted on electron microscopy performed on the day of maximal stimulation of platelet size. This suggests that the large platelet size may denote premature release of platelets from MGDF-stimulated megakaryocytes, whose cytoplasm did not yet undergo complete maturation. Also, based on our observation that the increase in platelet size occurred concurrently with the profound increase in the proportion of 64N-128N megakaryocytes,24 one could postulate that platelets derived from unusually high ploidy and large megakaryocytes induced by PEG-rmMGDF, are larger. Of note, we observed no increase in platelet size at 36 hours after PEG-rmMGDF, the time at which modal megakaryocyte ploidy was increased to 32N. Interestingly, platelets of C3H mice, whose modal megakaryocyte ploidy is 32N, also do not show an increase in platelet size,38 suggesting that only higher ploidy megakaryocytes are responsible for releasing large platelets after PEG-rmMGDF. MPV rapidly declined at the peak in platelet number, and subsequently was downregulated to below baseline, reflecting a feedback regulation by the profound thrombocytosis on platelet production. A similar downregulation of platelet size was observed following rebound thrombocytosis after experimental immune thrombocytopenia.39 Of relevance, the proportion of 64N-128N megakaryocytes has markedly decreased on day 5 after PEG-rmMGDF, with subsequent downregulation of megakaryocyte ploidy,24 further supporting the hypothesis that the changes in platelet size after MGDF are related to the alterations in megakaryocyte ploidy.
We observed a 2-day delay before platelet size increased after 25 or 250 μg/kg of PEG-rmMGDF. In addition, no change in MPV was noted at 24 hours after increasing the dose to 500 μg/kg. These findings are in contradistinction to changes observed after induction of immune thrombocytopenia in rodents where an increase in platelet size was detected as early as 8 to 12 hours after administration of platelet antisera.3-5 Our data suggest that thrombopoietin acting alone, is not responsible for the early release of large platelets noted after experimental immune thrombocytopenia. However, thrombopoietin acting in concert with other cytokines that may be elevated in acute thrombocytopenia, may still play a role in the early release of large platelets, as c-mpl ligand activity was detected as early as 8 hours after the administration of platelet antisera.14 Nevertheless, based on the time lag before the increase in platelet number and size after c-mpl ligand, which acts primarily on immature megakaryocytes, one could postulate that the release of large platelets after induction of experimental immune thrombocytopenia, occurs independently of this cytokine and could be attributed to another thrombopoietic factor with platelet releasing ability, which is yet to be characterized.
In contrast to the effect of PEG-rmMGDF we observed on platelet size, Ulich et al20 have reported a dose-dependent decrease in MPV after daily intraperitoneal injection of 1 to 100 μg/kg of nonpegylated recombinant human MGDF (rHuMGDF) into mice. Harker et al26,40 also have shown a decrease in MPV after daily subcutanous injection of low doses (0.05 to 2.5 μg/kg) of PEG-rHuMGDF in nonhuman primates. However, using a wider range of MGDF doses, Ulich et al41 showed that, although a low dose of 1 μg/kg of pegylated-rHuMGDF injected daily intraperitoneally into mice causes a decrease in MPV, high doses of 50 to 500 μg/kg cause an increase in MPV, while an intermediate dose of 10 μg/kg causes an initial increase followed by a decrease in MPV. Therefore, considering the 10-fold to 20-fold higher potency of the pegylated product21 26 and the 10-fold higher potency of the murine versus human protein in mice, the opposite effects of MGDF on MPV could be dose-related, explaining the increase in platelet size we observed. The reason for the different responses in platelet size to different doses of MGDF remains unclear.
Platelets produced in response to single PEG-rmMGDF administration have normal characteristics. This was evidenced by the absence of ultrastructural abnormalities, and the apparent normal life span,34 reflected by the rate of decline in platelet number from peak values.
The thrombopoietic stimulation resulting from single intravenous PEG-rmMGDF injection in our study was comparable in magnitude to the maximal increase in platelet number reported after daily subcutanueous or intraperitoneal injection of this cytokine for 5 to 28 days into mice or baboons.20,21,26,35,40 41 This profound thrombopoietic response to the single intravenous injection does not seem to depend on the route of administration, as we observed a similar degree of thrombocytosis after single subcutanous injection of PEG-rmMGDF (data not shown). Although this finding could still be related to the use of nonspecies specific recombinant c-mplligand forms in the other studies, we questioned whether daily administration of this cytokine has a suppressive effect on thrombopoiesis.
To evaluate the necessity of the daily administration schedule of recombinant c-mpl ligand that has been widely adopted in earlier preclinical studies, we compared the thrombopoietic responses to PEG-rmMGDF administered as a single dose versus daily for 5 days. Surprisingly, the platelet increase on day 5 after injection, in response to the single dose was higher than that following the daily administration of the same dose or one fifth of the dose for 5 days. Although single dosing resulted in an earlier higher response, the thrombocytosis was more prolonged after daily dosing. Examination of platelet size showed that the smaller platelet number increase on day 5 after daily dosing reflected the production of larger platelets, rather than suppression of thrombopoiesis. In early clinical trials, administration of recombinant forms of human thrombopoietin in single42 or daily43 dosing was shown to be safe and effective in ameliorating chemotherapy-induced thrombocytopenia in patients with cancer.
In summary, our data indicate that a single dose of PEG-rmMGDF, by stimulating megakaryocyte progenitor proliferation and subsequent differentiation, substantially stimulates platelet production in mice, causing an increase in both platelet number and platelet size. Single dose administration of PEG-rmMGDF is more effective in elevating platelet number early compared with daily administration, but results in a less sustained thrombocytosis. This, in conjunction with the pharmacokinetics of this cytokine, suggests that optimal dosing of this cytokine in future clinical trials can be achieved by its intermittent administration every 2 to 4 days.
ACKNOWLEDGMENT
We thank Jim Houston and Roseann Lambert (recently deceased) for providing their technical expertise in the flow cytometric analyses of platelets, and Amgen Inc for their generous supply of PEG-rmMGDF.
Supported in part by P30CA21765 Cancer Center Support Grant and P01CA201180 from the National Cancer Institute, Public Health Services, Department of Health and Human Service (Bethesda, MD) and by the American Lebanese Syrian Associated Charities (Memphis, TN).
Address reprint requests to Najat C. Daw, MD, Department of Hematology/Oncology, St. Jude Children's Research Hospital, 332 N. Lauderdale, Memphis, TN 38105.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Transmission electron micrographs of platelets from mice on day 4 after receiving carrier only (a and c) or a single intravenous dose of 250 μg/kg of PEG-rmMGDF (b, d, and e). Platelets produced in response to PEG-rmMGDF had normal ultrastructure, with the exception of frequent endoplasmic reticulum (arrowheads in [d] and [e]) and Golgi Complex profiles in platelet sections, suggesting their premature release. db, dense bodies; g, alpha granule. Magnification in (a) and (b) × 13,000, and in (c), (d), and (e) × 34,000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.466/3/m_blod4021205.jpeg?Expires=1767818980&Signature=j3cpjcyNXEvFgvRzUY6w2qYMYNsMd7QvuEkxAD7uHEldhKJGMvbQ3kcp1JIJfhSGax5ZvHRF3oanYvdnlxpiAwlkROxrh3x~9jqxhORzxtBjJ3FjhKRjV36dOTm5N7Z~G7~qbXTBY-sJ5J5M64TOknyV2sJuY83Nxt4uPc9UoDwYqSn0m69JC6jAaafj1sFX9a8bMDW8AYUXuKjGyrwrzOlZNNYVpkRGs08H4ZoAF2pD~W3ybHSGWwcu9NNZgTJHfh8KHNKwH7p~kIgZ9N5pqM3HRNNf4BwVCp1Gkb0wTx3DygjNGrwpuq-4XtqDgCf8utiJICfqvX0aXA3EzH8AaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal