Abstract
Platelet/endothelial cell adhesion molecule-1 (PECAM-1) is a 130-kD member of the Ig gene superfamily that is expressed on the surface of circulating platelets, monocytes, neutrophils, and selective T-cell subsets. It is also a major component of the endothelial cell intercellular junction. Previous studies have shown that cross-linking PECAM-1 on the surface of leukocytes results in the activation of adhesion molecules of both the β1 and β2integrin family. In addition, the process of leukocyte transendothelial migration appears to be mediated, at least in part, by homophilic adhesive interactions that take place between leukocyte and endothelial cell junctional PECAM-1 molecules. However, little is known about the functional role of this membrane glycoprotein in human platelets. In the present study, we examined the effects of PECAM-1 engagement on integrin-mediated platelet-extracellular matrix or platelet-platelet interactions. Bivalent, but not monovalent, anti–PECAM-1 monoclonal antibodies (MoAbs) specific for membrane-proximal Ig-homology domain 6 significantly augmented platelet deposition (increased surface coverage) and aggregation (increased average size) onto extracellular matrix, under both oscillatory or defined low shear flow conditions (200 s−1) in a modified cone and plate viscometer. Moreover, bivalent anti-domain 6 MoAbs were capable of serving as costimulatory agonists to markedly enhance both adenosine diphosphate (ADP)- and platelet activating factor (PAF)-induced platelet aggregation responses. These antibodies appeared to act via outside-in signal transduction through PECAM-1, as evidenced by the fact that their binding (1) led to conformational changes in the αIIbβ3 integrin complex, (2) induced surface expression of P-selectin, and (3) resulted in the tyrosine phosphorylation of PECAM-1. Together, these data support a role for PECAM-1 in cellular activation and suggest that PECAM-1 may serve as a costimulatory agonist receptor capable of modulating integrin function in human platelets during adhesion and aggregation.
PLATELET/ENDOTHELIAL cell adhesion molecule-1 (PECAM-1) is a 130-kD transmembrane glycoprotein that is expressed on the surface of circulating platelets, monocytes, neutrophils, and selected T-cell subsets. It is also a major constituent of the endothelial cell intercellular junction, where up to 106 PECAM-1 molecules concentrate after the formation of cell-cell contact (for a review, see Newman1). The 574 amino acid extracellular domain of PECAM-1 is organized into six Ig-like homology units,2 followed by a single-pass transmembrane domain, and a 118 amino acid cytoplasmic tail that contains specific sites for palmitoylation, phosphorylation, and assembly of cytosolic signaling molecules.3-6 Approximately 30% of the molecular mass of PECAM-1 is composed of carbohydrate residues whose influence on the adhesive properties of PECAM-1 is as yet unknown.
A great deal has been learned in the past several few years about the participation of PECAM-1 in the process of leukocyte transendothelial migration. The expression and/or distribution of PECAM-1 on the cell surface has been shown to be modulated in transmigrating leukocytes7-9 and on endothelial cells exposed to inflammatory cytokines.9-13 Moreover, Zocchi et al14 have shown that PECAM-1– but not ICAM-1–transfected fibroblasts support chemokine-independent transmigration of activated T lymphocytes.14 Finally, anti–PECAM-1 antibodies have been shown to inhibit leukocyte migration across an endothelial cell barrier both in vitro15 and in vivo,16-18 and the potential clinical relevance of interfering with PECAM-1 function has recently been shown in two different animal models of cardiac ischemia/reperfusion injury.19 20
The precise mechanism by which PECAM-1 mediates cell-cell interactions is not clear. Several studies have shown that PECAM-1 is capable of interacting with other PECAM-1 molecules expressed on the cell surface21-23; a homophilic process that is mediated by N-terminal Ig-homology domains 1 and 2.22,23 The integrin αvβ324,25 and a yet to be characterized 120-kD glycoprotein found on activated T lymphocytes26 have also been implicated as heterophilic counter receptors for PECAM-1. However, in addition to its intrinsic adhesive properties, a number of investigators have shown that engagement of PECAM-1 can upregulate the function of adhesion receptors other than PECAM-1, most notably members of the integrin family. Tanaka et al27 provided the first experimental evidence that PECAM-1 might be involved in transducing signals to other adhesion receptors. They showed that cross-linking of PECAM-1 on the surface of selected T-lymphocyte subsets resulted in the upregulation of β1 integrin function. T cells treated with certain bivalent anti–PECAM-1 monoclonal antibodies (MoAbs) exhibited increased adherence to plastic wells coated with fibronectin (via α5β1) or vascular cell adhesion molecule-1 (VCAM-1; via α4β1). Additional cross-linking of the anti–PECAM-1 MoAb using goat antimouse IgG further augmented adhesion, but monovalent Fab fragments were ineffective, suggesting that dimerization of PECAM-1 on the cell surface might be required for integrin affinity modulation. Similarly, Leavesley et al28 reported that the binding of a bivalent anti–PECAM-1 MoAb to CD34+ hematopoietic progenitor cells enhanced their adhesion to VCAM-1–transfected CHO cells,28a process presumably mediated by α4β1. Modulation of integrin affinity does not appear to be limited to β1 integrins, because several groups have reported that ligation of PECAM-1 also leads to upregulation of β2integrin function in lymphokine-activated killer cells,29monocytes and neutrophils,30 and natural killer cells.31 Together, these data suggest a mechanism by which dimerization or oligomerization of PECAM-1 on the cell surface results in the generation of specific signals that are capable of modulating integrin affinity.
Human platelets offer an attractive model system to further examine the relationship between PECAM-1 engagement and cellular activation. Platelets normally exist in a resting, nonadhesive state, but can be stimulated by multiple agonists, under well-controlled conditions, to undergo a series of easily measurable cell biologic and biochemical changes, including protein-tyrosine phosphorylation, α-granule release, cell-extracellular matrix interactions, and cell-cell interactions (platelet aggregation). Moreover, platelets coexpress approximately 10,000 copies of PECAM-132,33 and 80,000 copies of the well-characterized integrin, αIIbβ3.34 Finally, there are specific MoAbs that are capable of detecting subtle conformational changes in this integrin that report its conformational (affinity) state, as well as MoAbs that specifically and sensitively measured the exposure of the α-granule-specific membrane protein, P-selectin, on the platelet surface. The purpose of the present investigation, therefore, was to test the hypothesis that PECAM-1 could serve as a costimulatory agonist receptor whose engagement modulates downstream cellular responses, including platelet adhesion, platelet aggregation, and integrin affinity.
MATERIALS AND METHODS
MoAbs.
Well-characterized35,36 domain-specific MoAbs used in this study included PECAM-1.3 (directed against Ig-homology domain 1), PECAM-1.1 (domain 5), PECAM-1.2 (domain 6), and 4G6 (domain 6; kindly provided by Dr Steven Albelda, University of Pennsylvania School of Medicine, Philadelphia, PA). IV.3, a blocking MoAb specific for the human FcγIIa receptor,37 was kindly provided by Dr Clark Anderson (Ohio State University, Columbus, OH). The ligand-induced binding site (LIBS) antibody, D3, which specifically recognizes the active conformation of αIIbβ3,38 was kindly provided by Dr Lisa Jennings (University of Tennessee, Memphis, TN). The anti–P-selectin MoAb, S12,39 was kindly provided by Dr Rodger McEver (University of Oklahoma, Oklahoma City, OK). F(ab′)2 and Fab fragments were generated using immobilized pepsin or papain, respectively, according to the manufacturer's (Pierce, Rockford, IL) instructions. After overnight dialysis in phosphate-buffered saline (PBS), pH 7.4, all fragments were carefully analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under both reducing and nonreducing conditions to ensure that no intact IgG remained. Before their use, the reactivity of all PECAM-1–specific antibodies was confirmed by enzyme-linked immunosorbent assay against an immobilized recombinant protein containing the complete extracellular domain of human PECAM-1. The functional integrity of IV.3 Fab fragments was determined by measuring their ability to block FcγIIa receptor-mediated platelet activation induced by human heparin-induced thrombocytopenia (HITP) antibodies.
Platelet deposition on extracellular matrix (ECM).
ECM-coated tissue culture wells were prepared according to the method of Gospodarowicz et al.40 Briefly, bovine corneal endothelial cells were grown to confluence in 16-mm diameter tissue culture plates (Nunc, Roskilde, Denmark), washed with PBS, pH 7.4, and dissolved by exposure to 0.5% Triton X-100 and 0.1 mol/L NH4OH, followed by extensive washing with distilled water. Two hundred fifty microliters of citrated whole blood was preincubated with the indicated antibodies for 15 minutes at 37°C, and the mixture was added to the ECM-coated plates, which were then inserted into a modified rotating Teflon cone and plate viscometer described in detail by Varon et al.41 The platelets were then subjected to either low (200 s−1) or high (1,300 s−1) shear rates for 2 minutes, thoroughly washed with PBS, fixed, and stained by May-Grünwald stain. Platelet interactions with extracellular matrix and with each other were analyzed using an inverted Olympus light microscope (Olympus Corp, Lake Success, NY). The image was captured by a video camera, digitized, and quantitated using a computer-assisted image analysis system.41 Data are expressed as the percentage of surface coverage, the average size of the aggregate, and the total object number. Statistical analysis of the data (mean ± SD) was performed using a paired Student's t-test for single measurements or repeated measures of variance for a series of measurements. Pvalues less than .05 were considered significant.
Platelet aggregation.
Blood from healthy, nonaspirinated volunteer donors was collected into plastic tubes containing 3.8% (wt/vol) trisodium citrate (9:1) or acid citrate dextrose, pH 4.6 (9:1) and mixed gently by inversion. Platelet-rich plasma (PRP) was prepared by centrifugation of the blood at 140g for 15 minutes and collecting the upper layer. Platelet-poor plasma (PPP) was prepared by centrifugation of the remaining lower layer at 2,000g for 10 minutes. The concentration of platelets in PRP was adjusted with PPP to 2 × 108/mL. Aggregation studies were performed using a four channel platelet aggregometer (PAP-4 Bio-Data, Horsham, PA). Preliminary studies showed that the anti–PECAM-1 MoAbs, by themselves, failed to induce platelet aggregation (not shown), similar to previous reports of the functional effects of other anti–PECAM-1 MoAbs.32,33 42 All aggregation studies were performed in duplicate on at least three separate occasions.
Flow cytometry.
Platelets were washed twice in Ringer's citrate dextrose (RCD; 108 mmol/L NaCl, 38 mmol/L KCl, 1.7 mmol/L NaHCO3, 21.2 mmol/L Na citrate, 27.8 mmol/L glucose, and 1.1 mmol/L MgCl2, pH 6.5) containing 50 ng/mL prostaglandin E1(PGE1), diluted to a final concentration of 5 × 106/mL, and then incubated with various agonists in a microtiter well for 1 hour at room temperature in the presence of 2 mmol/L CaCl2. Control agonists included buffer alone, normal mouse IgG1 (NM IgG), 5 mmol/L RGEW peptide, or 5 mmol/L RGDW peptide. Antibody agonists were used at 10 μg/mL final concentration and included PECAM-1.3, PECAM-1.1, PECAM-1.2, and 4G6. All antibodies were used as either intact IgGs, F(ab′)2 fragments, or monovalent Fab fragments. Platelets were washed, incubated with fluorescein isothiocyanate (FITC)-conjugated D3 or S12 for 30 minutes at room temperature, washed once more, transferred to 450 μL of RCD, pH 7.4, and analyzed on a FACScan (Becton Dickinson, San Jose, CA). At least 5,000 platelets per sample were examined. Fluorescence data were displayed as logarithmic contour plots, dot plots, or logarithmic histograms using LYSYS II software (Becton Dickinson). Mean fluorescence intensity of S12 or D3 binding (minus background fluorescence observed in the presence of buffer alone) from at least three separate experiments (±SD) was then plotted versus the agonist used.
Immunoprecipitation and antiphosphotyrosine immunoblots.
Washed human platelets (1 × 109/mL) were incubated under stirring conditions with buffer, 7 μmol/L thrombin receptor-activating peptide (TRAP; having the amino acid sequence SFLLRN), NM IgG1 F(ab′)2, or PECAM-1.2 F(ab′)2 and then lysed for 1 hour at 4°C in an equal volume of 2% Triton X-100, 10 mmol/L EGTA, 15 mmol/L HEPES, 145 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, 20 μg/mL leupeptin, and 2 mmol/L sodium orthovanadate, pH 7.4. After centrifugation at 14,000 rpm for 5 minutes at 4°C, clarified supernatants were subjected to immunoprecipitation using 10 μg of NM IgG1 or PECAM-1.3. Immune complexes were collected on protein G Sepharose beads (Pharmacia Biotech AB, Uppsala, Sweden), resolved on a 12.5% SDS-polyacrylamide gel, and transferred electrophoretically to Immobilon polyvinylidene fluoride membrane (Millipore, Bedford, MA). Detection of tyrosine-phosphorylated PECAM-1 using the horseradish peroxidase (HRP)-conjugated antiphosphotyrosine MoAb, PY-20, was performed as previously described.43
RESULTS
Engagement of PECAM-1 Ig-domain 6 promotes platelet-extracellular matrix and platelet-platelet interactions.
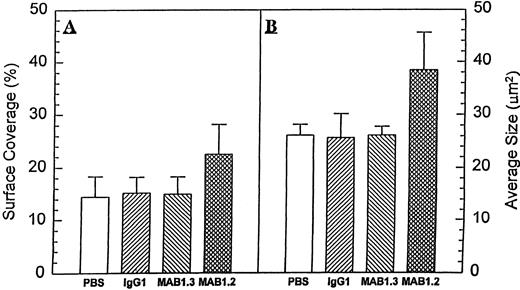
To investigate the effects of PECAM-1 engagement on the process of platelet adhesion and spreading, citrated whole blood was exposed to extracellular matrix in a modified cone and plate viscometer at a shear rate of 200 s−1, as previously described.41 Under these conditions, platelets adhere to the matrix but do not form platelet aggregates. Application of higher shear rate of 1,300 s−1 or the addition of other agonists is accompanied by more extensive adhesion as well as the formation of platelet aggregates. As shown in Fig 1, preincubation of platelets with the anti–PECAM-1 Ig-domain 6-specific antibody, PECAM-1.2, before their exposure to extracellular matrix coated surfaces increased by nearly 50% both surface coverage (from 14.47 ± 3.84 to 22.50 ± 5.66,P < .017) and the average size of the platelet aggregates (from 26.10 ± 2.05 to 38.42 ± 7.12, P < .002). The MoAb 4G6, which also maps to PECAM-1 Ig-domain 6,35 showed similar effects (not shown). NM IgG1 or the anti–PECAM-1 Ig-domain 1-specific MoAb, PECAM-1.3, failed to augment platelet deposition on extracellular matrix, suggesting that this stimulatory effect was transmitted into the cell in a PECAM-1 domain-specific manner. In addition, PECAM-1.2 significantly enhanced both adenosine diphosphate (ADP)- and platelet activating factor (PAF)-induced platelet-platelet interactions, as measured by platelet aggregometry using platelet-rich plasma (Fig 2A). This effect was not due to activation of the platelet FcγIIa receptor, because preincubation of platelets with saturating amounts of a blocking antibody to FcγRII (IV.3) did not abolish the costimulatory effect of PECAM-1.2 (Fig 2B). The effect of PECAM-1.2 on both ADP- (2 μmol/L) and PAF- (40 nmol/L) induced activation of platelets was dose-dependent at PECAM-1.2 concentrations ranging from 0.2 to 5 μg/mL (Fig 2C and D). Together, these data suggest that engagement of PECAM-1 through Ig domain 6 lowers the threshold for platelet stimulation by a variety of agonists, including ADP, PAF, and ECM under conditions of shear.
Anti–PECAM-1 antibodies promote platelet deposition on extracellular matrix. Two hundred fifty microliters of citrated whole blood was preincubated for 15 minutes at 37°C with either buffer alone or 5 μg/mL of normal mouse IgG1, PECAM-1.3, or PECAM-1.2. After 8 minutes of exposure to ECM-covered plates under conditions of low shear (200 s−1), samples were washed and stained and adherent platelets and aggregates were evaluated by image analysis as described in the Materials and Methods. Data are expressed as the percentage of ECM coverage (A) as well as average size (in square micrometers) of the aggregates formed (B). Note that both parameters were significantly increased (P = .017 andP = .02, respectively) in the presence of PECAM-1.2, but not PECAM-1.3.
Anti–PECAM-1 antibodies promote platelet deposition on extracellular matrix. Two hundred fifty microliters of citrated whole blood was preincubated for 15 minutes at 37°C with either buffer alone or 5 μg/mL of normal mouse IgG1, PECAM-1.3, or PECAM-1.2. After 8 minutes of exposure to ECM-covered plates under conditions of low shear (200 s−1), samples were washed and stained and adherent platelets and aggregates were evaluated by image analysis as described in the Materials and Methods. Data are expressed as the percentage of ECM coverage (A) as well as average size (in square micrometers) of the aggregates formed (B). Note that both parameters were significantly increased (P = .017 andP = .02, respectively) in the presence of PECAM-1.2, but not PECAM-1.3.
Anti–PECAM-1 MoAbs act as costimulatory agonists in ADP- and PAF-induced platelet aggregation. PRP was preincubated with (1) buffer, (2) normal mouse IgG1, (3) PECAM-1.3, or (4) PECAM-1.2. All antibodies were used at a final concentration of 5 μg/mL. Note that PECAM-1.2 augmented low-dose (1.25 μmol/L) ADP-induced platelet aggregation, both in the absence (A) and presence (B) of the MoAb IV.3 (a blocking MoAb specific for FcγRIIa). The degree of potentiation of aggregation induced by PECAM-1.2 varied somewhat from experiment to experiment (compare [A] with [B]), and this variation was not attributable to FcγRIIa blockade. Incubation of PRP with increasing concentrations of PECAM-1.2 (shown in micrograms per milliliter), followed by the addition of either 2 μmol/L ADP (C) or 40 nmol/L PAF (D), showed that the effects of antibody-mediated dimerization of PECAM-1 are dose-dependent.
Anti–PECAM-1 MoAbs act as costimulatory agonists in ADP- and PAF-induced platelet aggregation. PRP was preincubated with (1) buffer, (2) normal mouse IgG1, (3) PECAM-1.3, or (4) PECAM-1.2. All antibodies were used at a final concentration of 5 μg/mL. Note that PECAM-1.2 augmented low-dose (1.25 μmol/L) ADP-induced platelet aggregation, both in the absence (A) and presence (B) of the MoAb IV.3 (a blocking MoAb specific for FcγRIIa). The degree of potentiation of aggregation induced by PECAM-1.2 varied somewhat from experiment to experiment (compare [A] with [B]), and this variation was not attributable to FcγRIIa blockade. Incubation of PRP with increasing concentrations of PECAM-1.2 (shown in micrograms per milliliter), followed by the addition of either 2 μmol/L ADP (C) or 40 nmol/L PAF (D), showed that the effects of antibody-mediated dimerization of PECAM-1 are dose-dependent.
Dimerization of PECAM-1 on the platelet surface leads to conformational changes in αIIbβ3 and exposure of P-selectin on the platelet surface.
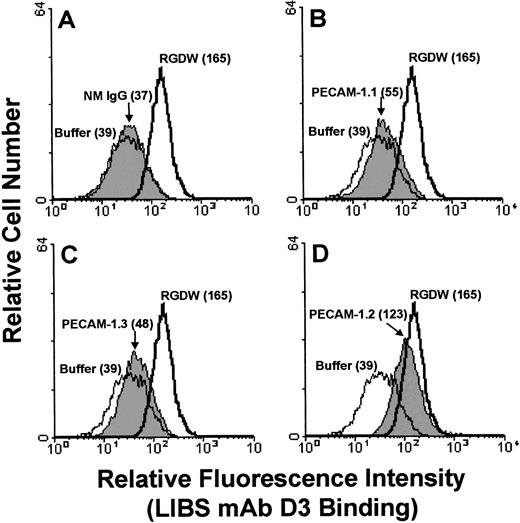
To investigate the molecular mechanism by which PECAM-1 Ig-domain 6 engagement promotes increased platelet adhesion (Fig 1) and aggregation (Fig 2), we examined the ability of PECAM-1 antibodies, in either monovalent or bivalent form, to induce (1) conformational changes in the platelet integrin αIIbβ3 and (2) α-granule secretion. As shown in Fig 3, incubation of platelets with PECAM-1.2 (directed against Ig-domain 6), but not PECAM-1.1 (Ig-domain 5) or PECAM-1.3 (Ig-domain 1), led to activation of the αIIbβ3 complex, as reported by the binding of the conformationally sensitive LIBS antibody, D3. This effect was dependent on PECAM-1 dimerization, because PECAM-1.2 F(ab′)2 fragments induced D3 binding, whereas monovalent Fab fragments did not (Fig 4A). A second Ig-domain 6-specific MoAb, 4G6, had similar effects. In addition, incubation of resting platelets with either of these two MoAbs, either as intact IgGs or F(ab′)2 fragments, led to exposure of the α-granule membrane-specific protein, P-selectin, on the platelet surface (Fig4B), indicating that PECAM-1 cross-linking also results in some degree of cellular activation. These events do not appear to be due to ADP release or thromboxane A2 generation, because, as shown in Table 1, the addition of PGE1, apyrase, indomethicin, or SQ 29.5 (a thromboxane receptor antagonist) before stimulation with PECAM-1.2 had little to no effect on D3 exposure.
Engagement of PECAM-1 Ig-domain 6 leads to conformational changes in the integrin αIIbβ3. Washed human platelets were preincubated with the FcγRIIa-specific antibody, IV.3 (to prevent possible Fc-receptor activation), before the addition of 10 μg/mL of normal mouse IgG (NM IgG), PECAM-1.1, PECAM-1.2, or PECAM-1.3. Buffer or RGDW peptide was used as negative and positive controls, respectively. After the addition of FITC-conjugated D3 for 30 minutes at room temperature, platelets were washed and transferred to 450 μL of RCD buffer, pH 7.4, and analyzed by flow cytometry. Note that PECAM-1.2 binding resulted in the exposure of the D3 epitope to almost the same extent as that induced by RGD peptide, a known modulator of integrin conformational change.62 The ability of PECAM-1.2 to induce conformational changes in αIIbβ3 varied somewhat from experiment to experiment; sometimes PECAM-1.2 was more effective than RGDW in inducing D3 binding (see Fig 4).
Engagement of PECAM-1 Ig-domain 6 leads to conformational changes in the integrin αIIbβ3. Washed human platelets were preincubated with the FcγRIIa-specific antibody, IV.3 (to prevent possible Fc-receptor activation), before the addition of 10 μg/mL of normal mouse IgG (NM IgG), PECAM-1.1, PECAM-1.2, or PECAM-1.3. Buffer or RGDW peptide was used as negative and positive controls, respectively. After the addition of FITC-conjugated D3 for 30 minutes at room temperature, platelets were washed and transferred to 450 μL of RCD buffer, pH 7.4, and analyzed by flow cytometry. Note that PECAM-1.2 binding resulted in the exposure of the D3 epitope to almost the same extent as that induced by RGD peptide, a known modulator of integrin conformational change.62 The ability of PECAM-1.2 to induce conformational changes in αIIbβ3 varied somewhat from experiment to experiment; sometimes PECAM-1.2 was more effective than RGDW in inducing D3 binding (see Fig 4).
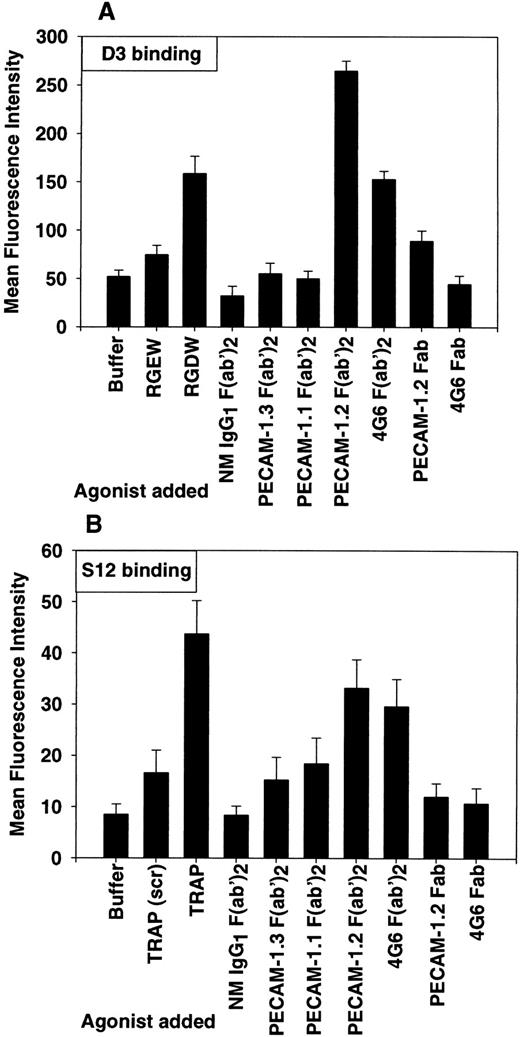
Cross-linking PECAM-1 induces conformational changes in αIIbβ3 and exposure of P-selectin on the platelet surface. Washed platelets (5 × 106/mL) were incubated with the indicated agonists as described in the Materials and Methods. All antibodies were used at a final concentration of 10 μg/mL and were used in the form of intact IgG (not shown), F(ab′)2 fragments, or as monovalent Fab fragments. After the addition of FITC-conjugated D3 (A) or FITC-conjugated S12 (B) for 30 minutes at room temperature, platelets were washed and transferred to 450 μL of RCD, pH 7.4, and analyzed by flow cytometry. Results are expressed as the mean ± SD of fluorescence intensity for three independent experiments. The observation that bivalent PECAM-1.2 or 4G6 F(ab′)2 fragments, but not their monovalent Fab counterparts, induced conformational changes in the αIIbβ3 integrin complex and exposure of P-selectin on the platelet surface suggests that receptor dimerization is required for outside/in signal transduction mediated by PECAM-1.
Cross-linking PECAM-1 induces conformational changes in αIIbβ3 and exposure of P-selectin on the platelet surface. Washed platelets (5 × 106/mL) were incubated with the indicated agonists as described in the Materials and Methods. All antibodies were used at a final concentration of 10 μg/mL and were used in the form of intact IgG (not shown), F(ab′)2 fragments, or as monovalent Fab fragments. After the addition of FITC-conjugated D3 (A) or FITC-conjugated S12 (B) for 30 minutes at room temperature, platelets were washed and transferred to 450 μL of RCD, pH 7.4, and analyzed by flow cytometry. Results are expressed as the mean ± SD of fluorescence intensity for three independent experiments. The observation that bivalent PECAM-1.2 or 4G6 F(ab′)2 fragments, but not their monovalent Fab counterparts, induced conformational changes in the αIIbβ3 integrin complex and exposure of P-selectin on the platelet surface suggests that receptor dimerization is required for outside/in signal transduction mediated by PECAM-1.
Effect of Platelet Inhibitors on LIBS Exposure by PECAM-1.2
| Agonist . | Inhibitor . | |||
|---|---|---|---|---|
| 50 ng/mL PGE1 . | 10 U/mL Apyrase . | 5 μmol/L Indomethicin . | 5 μmol/L SQ 29.5 . | |
| 2 mmol/L RGEW | 0 | 9.6 | 2.5 | 0 |
| 2 mmol/L RGDW | 138.1 | 151.8 | 136.5 | 136.5 |
| NM IgG | 6.6 | 17.4 | 0 | 0 |
| PECAM-1.1 | 5.5 | 1.3 | 2.0 | 2.1 |
| PECAM-1.3 | 0 | 0 | 4.5 | 0 |
| PECAM-1.2 | 33.5 | 46.2 | 36.6 | 25.6 |
| Agonist . | Inhibitor . | |||
|---|---|---|---|---|
| 50 ng/mL PGE1 . | 10 U/mL Apyrase . | 5 μmol/L Indomethicin . | 5 μmol/L SQ 29.5 . | |
| 2 mmol/L RGEW | 0 | 9.6 | 2.5 | 0 |
| 2 mmol/L RGDW | 138.1 | 151.8 | 136.5 | 136.5 |
| NM IgG | 6.6 | 17.4 | 0 | 0 |
| PECAM-1.1 | 5.5 | 1.3 | 2.0 | 2.1 |
| PECAM-1.3 | 0 | 0 | 4.5 | 0 |
| PECAM-1.2 | 33.5 | 46.2 | 36.6 | 25.6 |
Platelets were treated with IV.3 Fab fragments (10 μg/mL) before the addition of the indicated agonist or MoAb. MoAbs were used at a final concentration of 10 μg/mL. Numbers shown are the mean fluorescence intensity given by the binding of the LIBS antibody, D3, to platelets after platelet stimulation by the indicated agonist, in the presence of various inhibitors of platelet secondary metabolites or scavengers of ADP release. The mean fluorescence intensity of D3 binding in the presence of buffer alone has been subtracted. Data shown are representative of results obtained in three independent experiments.
Engagement of PECAM-1 induces tyrosine phosphorylation of its cytoplasmic domain.
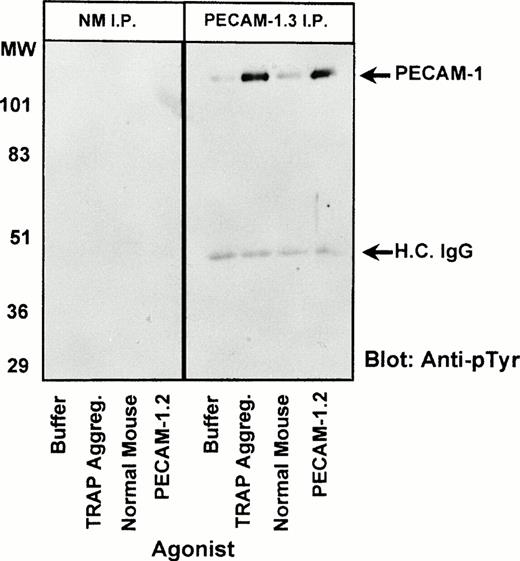
Recent studies have shown that PECAM-1 becomes tyrosine phosphorylated during platelet aggregation in an integrin-dependent process that results in the association of the SH2 domain-containing protein-tyrosine phosphatase, SHP-2, with tyrosine residues 663 and 686 of the PECAM-1 cytoplasmic domain.43 To further examine the mechanism by which PECAM-1.2 is able to act as a costimulatory agonist to modulate integrin-mediated cellular adhesion and aggregation responses (Figs 1-3), we incubated resting platelets with PECAM-1.2 F(ab′)2 fragments, solubilized the cells, immunocaptured PECAM-1, and probed Western blots of the resulting immunoprecipitated proteins with the phosphotyrosine-specific MoAb, PY-20. As shown in Fig 5, dimerization of PECAM-1 induced by PECAM-1.2 F(ab′)2 fragments initiated outside/in signal transduction, resulting in tyrosine phosphorylation of the PECAM-1 cytoplasmic domain to an extent similar to that induced by the strong platelet agonist, TRAP. These data suggest that dimerization or oligomerization of PECAM-1 on the platelet surface promotes downstream cellular events, including increased adhesion and aggregation, via the creation of specific docking sites for SH2-containing signaling molecules such as SHP-2.
PECAM-1 dimerization results in its tyrosine phosphorylation. Washed platelets (1 × 109/mL) were incubated at 37°C with the following agonists in the presence of 2 mmol/L CaCl2, 1 mmol/L MgCl2, and 100 μg/mL fibrinogen: (1) buffer (in the presence of stirring), (2) 7 μmol/L TRAP for 5 minutes under stirring conditions (fully aggregated), (3) 10 μg/mL normal mouse IgG1 F(ab′)2fragments for 30 minutes (stirred), and (4) 10 μg/mL PECAM-1.2 F(ab′)2 for 30 minutes (stirred). After detergent lysis, immunoprecipitations (IP) were performed using either normal mouse IgG1 (NM, left panel) or PECAM-1.3 (right panel). Bound proteins were resolved by 12.5% SDS-PAGE and analyzed by immunoblotting using an HRP-conjugated antiphosphotyrosine antibody (PY-20). Arrows indicate the positions of tyrosine phosphorylated PECAM-1 and the heavy chain of IgG.
PECAM-1 dimerization results in its tyrosine phosphorylation. Washed platelets (1 × 109/mL) were incubated at 37°C with the following agonists in the presence of 2 mmol/L CaCl2, 1 mmol/L MgCl2, and 100 μg/mL fibrinogen: (1) buffer (in the presence of stirring), (2) 7 μmol/L TRAP for 5 minutes under stirring conditions (fully aggregated), (3) 10 μg/mL normal mouse IgG1 F(ab′)2fragments for 30 minutes (stirred), and (4) 10 μg/mL PECAM-1.2 F(ab′)2 for 30 minutes (stirred). After detergent lysis, immunoprecipitations (IP) were performed using either normal mouse IgG1 (NM, left panel) or PECAM-1.3 (right panel). Bound proteins were resolved by 12.5% SDS-PAGE and analyzed by immunoblotting using an HRP-conjugated antiphosphotyrosine antibody (PY-20). Arrows indicate the positions of tyrosine phosphorylated PECAM-1 and the heavy chain of IgG.
DISCUSSION
In addition to serving as a homophilic cell-cell adhesion molecule,22 recent studies have shown that antibody-mediated engagement or dimerization of PECAM-1 on the cell surface can result in a number of downstream cellular events, including synthesis and release of hydrogen peroxide44 and proinflammatory cytokines45 by human monocytes, cell spreading and cytoskeletal rearrangement of natural killer cells,46 tyrosine phosphorylation of multiple cytoplasmic proteins in human T lymphocytes,47 and differentiation of cord blood progenitor cells.48 In addition, cross-linking PECAM-1 on the surface of leukocytes has been shown to result in the activation of adhesion molecules of both the β1 and β2 integrin family.27-31 The purpose of the present investigation was to examine whether PECAM-1–mediated activation of integrins could be extended to the β3integrin subfamily and to begin to understand the mechanism by which PECAM-1 engagement leads to augmentation of integrin function.
Unlike that which has been shown in other cell types, numerous PECAM-1–specific antibodies have been examined for their effects on platelet function over the past 10 years, and not one has been found, by itself, to induce or inhibit in vitro platelet aggregation, adhesion, or granule secretion. In the present report, we found that certain anti–PECAM-1 MoAbs were able to potentiate the ability of human platelets to adhere both to ECM proteins (Fig 1) and to each other (Fig 2). The findings suggest that, at least in human platelets, PECAM-1 engagement is by itself insufficient to induce a measurable cellular response and requires costimulation by either shear (Fig 1) or another soluble agonist (Fig 2). We have extended these observations by further showing that the mechanism by which this occurs appears to be related to the generation of intracellular signals that follow dimerization or oligomerization of PECAM-1 on the cell surface. Thus, intact anti–PECAM-1 IgG as well as F(ab′)2fragments, but not monovalent Fab fragments, were shown to be capable of inducing conformational changes in the platelet integrin αIIbβ3 and exposing P-selectin on the platelet surface (Figs 3 and 4). Although we do not yet fully understand the signal transduction pathway that leads from PECAM-1 engagement to integrin activation, Levine et al47have recently shown that anti–PECAM-1 MoAbs induce tyrosine phosphorylation of multiple unidentified cellular substrates.47 Our finding that PECAM-1 itself becomes tyrosine phosphorylated after PECAM-1 antibody binding (Fig 5), coupled with the recent demonstration by Jackson et al43 that tyrosine phosphorylation of the PECAM-1 cytoplasmic domain creates docking sites for cytosolic SH2-containing signaling molecules, offers hints as to the molecular mechanism of PECAM-1/integrin cross-talk and may help explain the link between PECAM-1 dimerization and β1 and β2 integrin activation seen previously by other investigators.
Stockinger et al44,45 have clearly shown that, in some instances, cellular activation induced by anti–PECAM-1 MoAbs can be entirely explained by the secondary engagement of IgG Fc receptors. In fact, the contribution of Fc receptor activation to signal transduction events attributed to PECAM-1 engagement cannot be ruled out in several other investigations in which intact anti–PECAM-1 IgG and/or secondary antibodies were used to evoke a PECAM-1–specific cellular response.28,29,49 The Fc region of IgG of many animal species, when in the correct conformation, can serve as a potent stimulatory agonist, as exemplified by the often life-threatening clinical syndrome, heparin-induced thrombocytopenia, in which human antibodies specific for heparin/platelet factor 4 complexes bind, via the Fc region of the resulting immune complex, to the platelet Fc receptor (FcγRIIa), resulting in both platelet activation (thrombosis) and clearance (thrombocytopenia).50,51 The often-employed use of nonactivating, isotype-matched MoAbs alone is an insufficient control for specificity of Fc receptor activation, because unbound antibodies do not have nearly as high an affinity for the Fc receptor as do bound antibodies,37 and many MoAbs, even when bound via their Fab regions to their target antigens, are spatially oriented in such a manner that precludes Fc receptor activation (for a more detailed treatment of the critical role of antigen topography in the interaction of antibodies with Fc receptors, see Kumpel et al,52 Horsewood et al,53 and Tomiyama et al54). In the present investigation, we attempted to carefully exclude Fc receptor-mediated cellular activation by using F(ab′)2 antibody fragments and/or by preblocking platelet FcγRIIa (the only Fc receptor for IgG present in platelets55) with saturating levels of the blocking FcγRIIa-specific MoAb, IV.3. The fact that augmentation of platelet aggregation (Fig 2), LIBS and P-selectin exposure (Figs 3 and 4 and Table 1), and PECAM-1 tyrosine phosphorylation (Fig 5) all occurred even after these precautions had been taken strongly support the notion that PECAM-1 receptor dimerization, and not secondary Fc receptor engagement, is capable of leading directly to cellular activation.
It is not clear why some anti–PECAM-1 MoAbs are able to augment integrin function and cellular activation, whereas others are not, or why, in the present study, both potentiating anti–PECAM-1 antibodies, PECAM-1.2 and 4G6, map to Ig-domain 6, the Ig-homology domain closest to the membrane. Several previous observations may be relevant. First, Ig-domain 6 contains two divalent cation binding sites,56although the structural and functional consequences of cation occupancy are not yet known. Second, Sun et al22 recently showed that the homophilic adhesive properties of PECAM-1–containing proteoliposomes could be selectively increased by Fab fragments of the same two antibodies, PECAM-1.2 and 4G6 (anti–PECAM-1 Fabs specific for other Ig homology domains did not augment adhesion), and proposed that engagement of domain 6 might induce LIBS-like long-range conformational changes in the PECAM-1 molecule. Finally, another domain 6-specific antibody, LYP21, as well as an Ig-domain 6 peptide corresponding to amino acid residues 551-574 have been shown to inhibit T-cell responses57 and delay the onset of graft-versus-host disease.58 Together with the data presented here on the effect of these antibodies on integrin activation and P-selectin exposure, it is tempting to speculate that Ig-domain 6 may play a regulatory role in PECAM-1 function, mediating both outside-out as well as outside-in signal transduction.
However, other explanations for the seemingly selective action of domain 6 antibodies on cell function are possible. It could be that MoAbs specific for Ig-domain 6 happen to bind with a topographical orientation that preferentially favors PECAM-1/PECAM-1 interactions on the cell surface. Receptor dimerization, in turn, may be all that is required to induce conformational changes in the molecule, leading to its activation and initiating intracellular tyrosine phosphorylation, augmenting integrin function, or increasing the homophilic binding properties of PECAM-1 proteoliposomes. It is notable that augmentation of β1 and β2 integrin function in leukocytes does not appear to be limited to Ig-domain 6-specific MoAbs27,35; perhaps the relative receptor density or its lateral mobility within the plane of the membrane is sufficiently different from that in platelets to permit PECAM-1 dimerization by a less-selective set of anti–PECAM-1 MoAbs. Further studies using fixed monomeric and dimeric membrane-bound forms of PECAM-1 are planned to distinguish between these two models of PECAM-1–mediated cellular activation.
Independent of the mechanism by which PECAM-1 MoAbs are exerting their effects, it is becoming clear that PECAM-1 can both initiate, as well as respond to, changes in cellular function. Osawa et al59have recently shown that PECAM-1 becomes tyrosine phosphorylated in endothelial cells subjected to mechanical shear stress and suggested that PECAM-1 may be one of the junctional receptors responsible for sensing and then communicating changes in fluid flow. Whether mechanical shear contributes to the tyrosine phosphorylation of platelet PECAM-1 that occurs in aggregating platelets43 is not known. In addition, Sagawa et al60 showed that aggregation of the high-affinity IgE receptor in rat basophilic leukemia cells also results in the tyrosine phosphorylation of PECAM-1. Finally, the recent studies of Lu et al61 suggest that the phosphorylation state of PECAM-1 may also be sensitive to cell-cell and cell matrix interactions, because integrin engagement and/or cell migration of cultured endothelial cells were shown to result in tyrosine dephosphorylation of PECAM-1. Taken together, these data implicate PECAM-1 as one of a growing number of cell surface receptors that are able to mediate bidirectional signal transduction, serving both as an agonist receptor as well as an adhesion molecule in blood and vascular cells. Studies defining the cytosolic signaling molecules that link these two interrelated cellular functions of PECAM-1 represent important areas of future investigation.
ACKNOWLEDGMENT
The authors thank Kevin Kupcho for technical support in performing flow cytometric measurements, Dr Gian Visentin for his assistance in HITP antibody-induced activation of platelets, and Dr William Campbell for providing indomethicin and SQ 29.5.
D.V. and D.E.J. contributed equally to this work.
Supported by Grants No. HL-44612 and HL-40926 (to P.J.N.) from the National Institutes of Health and a postdoctoral fellowship award to D.E.J. (#F96F-Post-34) from the Wisconsin Affiliate of the American Heart Association. D.V. and N.S. were supported by grants from the National Council for Research and Development, Israel and Deutsche Forschungsanstalt, Fuer Luft und Raumfahrt. P.J.N. is an Established Investigator of the American Heart Association.
Presented in abstract form at the 1996 European Granulocyte and Platelet Meeting in Helsinki, Finland.
Address reprint requests to Peter J. Newman, PhD, Blood Research Institute, The Blood Center of Southeastern Wisconsin, 638 N 18th St, Milwaukee, WI 53233-2121.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Anti–PECAM-1 MoAbs act as costimulatory agonists in ADP- and PAF-induced platelet aggregation. PRP was preincubated with (1) buffer, (2) normal mouse IgG1, (3) PECAM-1.3, or (4) PECAM-1.2. All antibodies were used at a final concentration of 5 μg/mL. Note that PECAM-1.2 augmented low-dose (1.25 μmol/L) ADP-induced platelet aggregation, both in the absence (A) and presence (B) of the MoAb IV.3 (a blocking MoAb specific for FcγRIIa). The degree of potentiation of aggregation induced by PECAM-1.2 varied somewhat from experiment to experiment (compare [A] with [B]), and this variation was not attributable to FcγRIIa blockade. Incubation of PRP with increasing concentrations of PECAM-1.2 (shown in micrograms per milliliter), followed by the addition of either 2 μmol/L ADP (C) or 40 nmol/L PAF (D), showed that the effects of antibody-mediated dimerization of PECAM-1 are dose-dependent.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.500/3/m_blod4022902.jpeg?Expires=1769097833&Signature=TVrdzTgaOle5NX5DzkeUr2OShXOE3zr9JnoFLXDyTtwKP87si5kTReZDdJlxpnNbRhnL4jd8mIjhiC4pTXgswk3ue1EwMfqenArQprxlMecDdCQMigrBmCLLj6uge~Qrw2ISVbCWqme0YaGRmhd7~ssduZjXdNByAygyAv0kBe4vvATbKyQpFlWPzUPSj2odIxChfkoKzvt~eLV52dsYL4vo-MrLUHIW1ZpZvI4YWroZ68aY5cgPHCdM7ZWGpLVHwr7Dcq42X0o4sMxkoMUrlTdiBa0PNOqW0N~Y~x0lpDoTwCoBbshuCkb1MVQIS2TY1KtkjDri7bFL9koH36rHPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal