Abstract
Recent reports have shown that leukocyte-leukocyte adhesion is dependent on L-selectin and that leukocyte recognition of L-selectin may be mediated by P-selectin glycoprotein ligand-1 (PSGL-1). We show that the specific attachment and rolling of human neutrophils and the leukemia cell lines HL-60 and U937 on immobilized, purified L-selectin under continuous shear stress is only partially inhibited by treatment with the PSGL-1 monoclonal antibody (MoAb), KPL1 (41% to 53% inhibition), suggesting that L-selectin ligand activity in addition to PSGL-1 may mediate myeloid cell rolling on L-selectin. K562 cells cotransfected with cDNAs encoding α(1,3)fucosyltransferase-VII (FucT-VII) and PSGL-1 rolled on L-selectin. Adhesion of FucT-VII-PSGL-1 transfectants to L-selectin was completely blocked by MoAb KPL1, indicating that both L-selectin and P-selectin bind similar sites on PSGL-1. In support of existence of a non–PSGL-1 L-selectin ligand activity on leukocytes, an HL-60 membrane preparation immunodepleted of PSGL-1 supported rolling of L-selectin, but not P-selectin transfectants. Treatment of HL-60 cells with O-sialoglycoprotein endopeptidase inhibited attachment and rolling on L-selectin and P-selectin. However, neuraminidase treatment completely blocked HL-60 rolling on L-selectin, but not P-selectin, suggesting L-selectin and P-selectin ligand activities have different contributions of sialic acid. These findings indicate that myeloid cells express sialylated, O-linked glycoprotein ligand activity independent of PSGL-1 that supports L-selectin–mediated rolling.
THE SELECTIN CLASS of adhesion molecules plays a critical role in the migration of leukocytes to sites of inflammation by mediating the initial attachment and rolling of leukocytes on vascular endothelium prior to integrin-dependent arrest and extravasation.1,2 L-selectin (CD62L), which is constitutively expressed by circulating leukocytes,3 serves as a homing receptor for lymphocyte binding to peripheral lymph node high endothelial venules.4 L-selectin is also required for leukocyte adhesion in response to trauma and inflammation as shown by L-selectin monoclonal antibody (MoAb) inhibition of neutrophil rolling on cytokine-stimulated endothelial monolayers in vitro5,6and leukocyte rolling in mesenteric venules in vivo.7,8Furthermore, leukocyte recruitment during inflammation is impaired in L-selectin–deficient mice.9 10
In addition to leukocyte-endothelial interactions, it also appears that L-selectin contributes to leukocyte-leukocyte adhesive interactions between leukocytes. L-selectin MoAbs inhibit formyl peptide-induced neutrophil aggregation11 and transient adhesive interactions between flowing neutrophils and adherent neutrophils in vitro.12 In addition, monolayers of KG1a hematopoietic progenitor cells support L-selectin–dependent adhesion of human peripheral blood lymphocytes.13
Leukocyte recognition of L-selectin appears to depend on the expression of sialylated and/or fucosylated glycoprotein ligands. Neuraminidase treatment of neutrophil monolayers blocks L-selectin–dependent interactions with flowing neutrophils.12 Similarly, treatment of adherent KG1a cells inhibits the ability of these cells to support adhesion of lymphocytes.13 Incubation of neutrophils with O-sialoglycoprotein endopeptidase (OSGE), which cleaves mucinlike glycoproteins containing clustered, O-linked serine or threonine residues,14 inhibits aggregation with untreated neutrophils.15 Furthermore, specific binding of recombinant L-selectin–IgM to neutrophils is blocked by treatment with neuraminidase and OSGE.16
Recent reports have suggested that the sialylated and O-glycosylated P-selectin glycoprotein ligand-1 (PSGL-1)17,18 may also serve as an L-selectin ligand. Immobilized PSGL-1 supports neutrophil adhesion under flow conditions which is completely blocked by an L-selectin MoAb.19 In addition, L-selectin–IgM binding to myeloid cells is inhibited by treatment with a polyclonal antibody against PSGL-116 and monolayers of L-selectin transfectants support HL-60 adhesion that is blocked by the PSGL-1 MoAb, PL1.20 However, the combination of an L-selectin MoAb and PL1 only partially inhibits formyl peptide-induced neutrophil aggregation.21 Similarly, PL1 only partially blocks attachment of flowing neutrophils to fixed, adherent neutrophil monolayers,19 implying the existence of leukocyte L-selectin ligands distinct from PSGL-1. Furthermore, in some assays, PSGL-1 does not appear to be required for leukocyte adhesion to L-selectin. PL1 does not disrupt L-selectin–dependent neutrophil-neutrophil interactions in flow22 and an OSGE-insensitive, non-mucinlike component of neutrophil adhesion to recombinant L-selectin–IgG under flow conditions has been shown.23
The objective of this study was to determine whether leukocyte ligand structures distinct from PSGL-1 mediate leukocyte recognition of L-selectin. By studying leukocyte adhesion directly to purified L-selectin immobilized on the wall of a flow chamber, potential artifacts associated with leukocyte-leukocyte adhesion assays, such as incomplete MoAb saturation of PSGL-1, Fc receptor cross-linking, L-selectin shedding, and the heterogeneous surface presented by adherent leukocytes could be minimized. We show that the specific binding of leukocytes to L-selectin under flow conditions appears to be dependent on the expression of mucinlike glycoprotein ligand activity and that adhesion can be mediated by both PSGL-1–dependent and –independent mechanisms.
MATERIALS AND METHODS
Antibodies and selectin chimeras.
L-selectin MoAb DREG56 (IgG1)24 was a gift of T.K. Kishimoto (Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT). P-selectin MoAb G1 (IgG1)25 and PSGL-1 MoAbs PL1 and PL2 (IgG1)26 were gifts of R.P. McEver (University of Oklahoma, Oklahoma City). Additional PSGL-1 MoAbs were KPL1 and KPL2 (IgG1) (Snapp, K.R.; Ding, H.; Atkins, K.; Luscinskas, F.W.; Warnke, R.; and Kansas, G.S.; submitted). The CD18 MoAb, TS1/18, was purified from hybridoma supernatant as described.27 CSLEX-1 (IgM)28 and recombinant human L-selectin–IgG and P-selectin–IgG chimeras29 were gifts of L.A. Lasky and S.A. Watson (Genentech, Inc, South San Francisco, CA). Fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin was purchased from Dako (Carpinteria, CA).
Isolation of L-selectin and P-selectin.
Human L-selectin was purified from human tonsil (provided by R.S. Larson, University of New Mexico, Albuquerque) homogenized in 20 mmol/L Tris, pH 8.0; 140 mmol/L NaCl; and 0.025% azide (TSA, pH 8.0) with 5 mmol/L EDTA, 10 μmol/L leupeptin, 0.1 U/mL aprotinin, and 1% Triton X-100. After centrifugation of the lysate at 500g and 100,000g, the supernatant was passed over a column of CNBr-activated Sepharose 4B (Pharmacia Biotech, Piscataway, NJ) coupled to DREG56 (2 mg/mL). The column was washed with TSA, pH 8.0; containing 1% octylglucopyranoside (OG; Sigma, St Louis, MO) and eluted with acetate buffer, pH 3.0, 1% OG. The eluate was neutralized with 1 mol/L Tris, pH 9.0; 1% OG (15% vol/vol). Human P-selectin was purified from outdated platelets (American Red Cross, Richmond, VA) as previously described.30
Cell lines and neutrophil isolation.
The human leukemia cell lines HL-60, U937, SKW3, and K562 were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (100 μg/mL) (GIBCO-BRL, Grand Island, NY). K562 cells were stably transfected with cDNA encoding for α(1,3)fucosyltransferase-VII (FucT-VII), PSGL-1, or both FucT-VII and PSGL-1.31 The murine pre-B lymphocytic cell line, 300.19, was maintained in RPMI 1640 supplemented with 10% FBS and 10 μmol/L 2-mercaptoethanol (GIBCO-BRL). 300.19 cells stably transfected with cDNA encoding human L-selectin or P-selectin have been described.32 For adhesion assays, cell lines were suspended in RPMI 1640 with 10 mmol/L HEPES, pH 7.4; and 1% human serum albumin (HSA) at room temperature. Human neutrophils were isolated from heparinized venous blood by density separation over Mono-Poly Resolving Medium (ICN Biochemicals, Aurora, OH).11 Neutrophils were suspended in Hanks' balanced salt solution (HBSS) without calcium and magnesium, supplemented with 10 mmol/L HEPES, pH 7.4; and 0.1% HSA, and placed on ice. Before use in experiments, neutrophils were washed into HBSS with 2 mmol/L CaCl2; 10 mmol/L HEPES, pH 7.4; and 0.1% HSA at room temperature.
Enzymatic digestion of cell surface glycoproteins.
O-Sialoglycoprotein endopeptidase (OSGE) (P. haemolytica), E.C. 3.4.24.57, was purchased from Accurate Chemical & Scientific Corp (Westbury, NY). Neuraminidase (V. cholerae), E.C. 3.2.1.18, was purchased from Calbiochem (La Jolla, CA). Chymotrypsin (type 1-S), E.C. 3.4.21.1 was purchased from Sigma. HL-60 cells (5 × 106cells in 1 mL) were incubated at 37°C with either 120 μg OSGE in RPMI 1640, pH 7.4; and 10% FBS for 2 hours; 200 mU neuraminidase in phosphate-buffered saline, pH 6.4; and 10% FBS for 2 hours; or 10 U chymotrypsin in RPMI 1640, pH 7.4; for 15 minutes, followed by resuspension at 0.5 × 106/mL in RPMI 1640, pH 7.4; and 1% HSA. Enzymatic activities of OSGE and neuraminidase were verified by flow cytometry using MoAbs against CD43 (IgG1) (Biodesign International, Kennebunk, ME) for OSGE-treated cells and CSLEX-1 for neuraminidase-treated cells. Cell surface CD43 and sialyl Lewisx were reduced by greater than 95% after OSGE and neuraminidase treatment, respectively (data not shown). Enzyme treatments did not reduce cell viability based on trypan blue exclusion.
Laminar flow adhesion assay.
Purified human selectins or selectin-IgG chimeras were diluted as indicated in 50 mmol/L Tris, pH 9.5; and 0.025% azide, followed by adsorption to polystyrene slides cut from bacteriological petri dishes (Falcon 1058) for 2 hours at room temperature. Site densities of purified human L-selectin (1:5) and P-selectin (1:60) were determined to be 170 and 270 sites/μm2, respectively, by radioimmunoassay as previously described using MoAbs DREG56 and G1.30 The slides were blocked with 3% HSA for 1 hour at room temperature or overnight at 4°C and fitted into a parallel plate laminar flow chamber33 which was mounted on the stage of an inverted phase-contrast microscope (Diaphot-TMD; Nikon, Garden City, NY). Adhesive interactions between cellular Fc receptors and the Fc domain of selectin-IgG were eliminated by incubation of the adsorbed selectin substrate with polyclonal goat F(ab′)2 (10 μg/mL) against human IgG Fc (Biodesign International). In some experiments, the adsorbed selectin substrate or cell suspensions were incubated with MoAbs (10 μg/mL) for 15 minutes at room temperature prior to initiation of flow. Cell suspensions (0.5-1 × 106/mL) were drawn through the flow chamber at room temperature using a syringe pump (Harvard Apparatus, South Natick, MA) and the number of bound cells quantitated from videotape recordings of 10-20 fields of view obtained while scanning the lower plate of the flow chamber using a 10× or 20× objective after 2 to 3 minutes of flow. Wall shear stress (dyne/cm2) was calculated assuming a viscosity of assay buffer equal to water at room temperature (1.0 centipoise; 24°C). For cell tracking analysis, video images were captured using public domain NIH Image v.1.57 and the displacement of individual leukocytes under shear conditions was determined at 0.03-second intervals. Critical velocity was defined as the velocity of a noninteracting leukocyte in a shear flow near the wall of the flow chamber.33 Leukocytes with a translational velocity lower than critical velocity were defined as rolling.
HL-60 lysate and immunodepletion of PSGL-1.
HL-60 cell pellets (2 × 108 cells) were incubated with ice cold lysis buffer (20 mmol/L Tris, pH 8.0; 140 mmol/L NaCl; 0.025% sodium azide; 5 mmol/L EDTA; 10 μmol/L leupeptin; 0.1 U/mL aprotinin; and 1% Triton X-100). After centrifugation of the lysate at 500g and 100,000g, the supernatant was passed over a wheat germ agglutinin (WGA)-Sepharose column prepared by coupling WGA (Pharmacia Biotech, Piscataway, NJ) at 2 mg/mL to CNBr-activated Sepharose 4B. The column was washed with lysis buffer containing 1% OG and eluted with 100 mmol/L N-acetyl-D-glucosamine (Sigma) in lysis buffer with 1% OG. Protein-containing fractions were identified under nonreducing conditions by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by silver staining. PSGL-1 was immunodepleted from the WGA affinity-purified HL-60 lysate using protein G-Sepharose (Pharmacia Biotech) saturated with KPL1 (10 mg IgG/mL). A mock immunoprecipitation was performed by incubating lysate with protein G-Sepharose alone. Immunodepletion of PSGL-1 from the lysate was verified by subjecting supernatants to 7.5% SDS-PAGE and transfer of separated proteins to nitrocellulose. The membrane was probed for PSGL-1 by incubating with KPL1 followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Pierce, Rockford, IL). The blot was developed using enhanced chemiluminescence detection reagents and exposed to Hyperfilm ECL (Amersham, Arlington Heights, IL) for 2 to 3 minutes.
RESULTS
Rolling of neutrophils and leukemia cell lines on L-selectin.
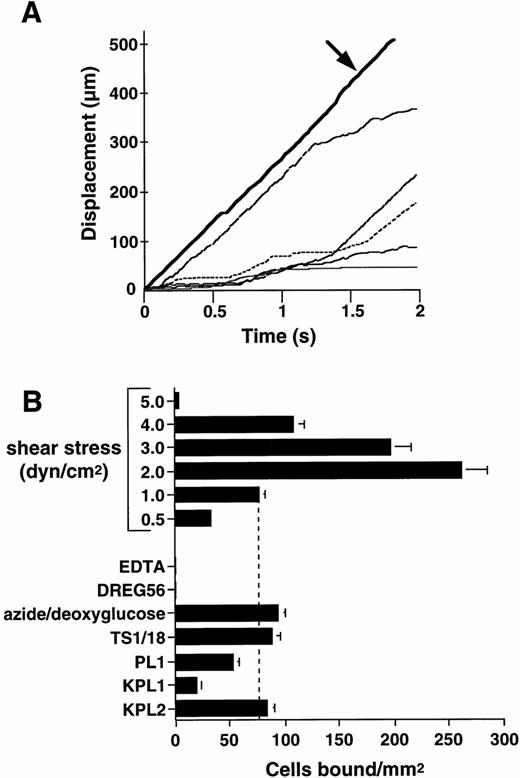
Purified human L-selectin, adsorbed to the lower wall of a parallel plate flow chamber, supported attachment and rolling of human neutrophils under flow conditions (Fig 1).Neutrophils formed stable rolling interactions with immobilized L-selectin at velocities below the critical velocity determined for nonadherent cells traveling adjacent to the lower wall of the flow chamber (Fig 1A). The ability of neutrophils to attach to L-selectin under flow was dependent on the level of wall shear stress. Neutrophil attachment significantly increased as the wall shear stress was lowered from 5.0 to 2.0 dyne/cm2 (Fig 1B). However, the efficiency of neutrophil attachment decreased as the wall shear stress was further reduced to 0.5 dyne/cm2, which is consistent with previous work showing that a threshold level of shear stress is required to support the selectin receptor-ligand interaction.30 34
Human neutrophils roll on immobilized L-selectin under flow conditions. Human neutrophils (1 × 106/mL) were infused through a parallel plate flow chamber in which dilutions of human purified L-selectin (1:5; 170 sites/μm2) were adsorbed to the lower wall. (A) Displacement as a function of time for neutrophils rolling on L-selectin under a continuous wall shear stress (1 dyne/cm2). Tracings represent positions of five independent cells measured at 0.03-second intervals. Bold solid line (arrow) represents the tracking of a noninteracting neutrophil traveling at critical velocity near the wall of the flow chamber. (B) Neutrophil attachment to purified L-selectin under continuous flow conditions is shear stress-dependent. Adhesion to L-selectin was specific based on inhibition of neutrophil attachment after inclusion of EDTA (5 mmol/L) in the perfusion media and treatment of the adsorbed L-selectin substrate with L-selectin MoAb, DREG56 (10 μg/mL). Metabolic inhibition with 0.06% azide and 50 mmol/L 2-deoxy-D-glucose for 30 minutes at room temperature or incubation of neutrophils with CD18 MoAb, TS1/18 (10 μg/mL), did not block attachment to L-selectin. Treatment of neutrophils with PSGL-1 MoAbs PL1 (Fab fragments) (10 μg/mL) or KPL1 (10 μg/mL), but not KPL2 ascites (1:500), partially inhibited attachment to L-selectin. Mean ± SEM of bound cells/mm2 in multiple fields from two to four independent experiments.
Human neutrophils roll on immobilized L-selectin under flow conditions. Human neutrophils (1 × 106/mL) were infused through a parallel plate flow chamber in which dilutions of human purified L-selectin (1:5; 170 sites/μm2) were adsorbed to the lower wall. (A) Displacement as a function of time for neutrophils rolling on L-selectin under a continuous wall shear stress (1 dyne/cm2). Tracings represent positions of five independent cells measured at 0.03-second intervals. Bold solid line (arrow) represents the tracking of a noninteracting neutrophil traveling at critical velocity near the wall of the flow chamber. (B) Neutrophil attachment to purified L-selectin under continuous flow conditions is shear stress-dependent. Adhesion to L-selectin was specific based on inhibition of neutrophil attachment after inclusion of EDTA (5 mmol/L) in the perfusion media and treatment of the adsorbed L-selectin substrate with L-selectin MoAb, DREG56 (10 μg/mL). Metabolic inhibition with 0.06% azide and 50 mmol/L 2-deoxy-D-glucose for 30 minutes at room temperature or incubation of neutrophils with CD18 MoAb, TS1/18 (10 μg/mL), did not block attachment to L-selectin. Treatment of neutrophils with PSGL-1 MoAbs PL1 (Fab fragments) (10 μg/mL) or KPL1 (10 μg/mL), but not KPL2 ascites (1:500), partially inhibited attachment to L-selectin. Mean ± SEM of bound cells/mm2 in multiple fields from two to four independent experiments.
Neutrophil attachment and rolling on L-selectin was completely calcium- and L-selectin–dependent as shown by inhibitory effects of EDTA and treatment of the adsorbed substrate with the L-selectin MoAb, DREG56 (Fig 1B). Cellular metabolism and/or β2-integrin expression were not required for rolling on L-selectin because treatment of the neutrophil suspension with the metabolic inhibitor combination azide/deoxyglucose or the function-blocking β2-integrin MoAb, TS1/18,27 did not reduce neutrophil adhesion to L-selectin (Fig 1B). Treatment of neutrophils with the PSGL-1 MoAb, KPL1, which recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks leukocyte adhesion to P-selectin (Snapp, K.R.; Ding, H.; Atkins, K.; Luscinskas, F.W.; Warnke, R.; and Kansas, G.S.; submitted), resulted in significant inhibition of neutrophil adhesion to L-selectin under continuous flow conditions (73% ± 2% inhibition; Fig 1B). In contrast, the nonblocking, isotype-matched PSGL-1 MoAb, KPL2 (Snapp, K.R.; Ding, H.; Atkins, K.; Luscinskas, F.W.; Warnke, R.; and Kansas, G.S.; submitted), failed to inhibit neutrophil rolling on L-selectin (Fig 1B). Incubation of neutrophil suspensions with Fab fragments of PL1, a PSGL-1 MoAb which blocks neutrophil rolling on P-selectin,26 also partially inhibited attachment of neutrophils to L-selectin (27% ± 5% inhibition; Fig 1B).
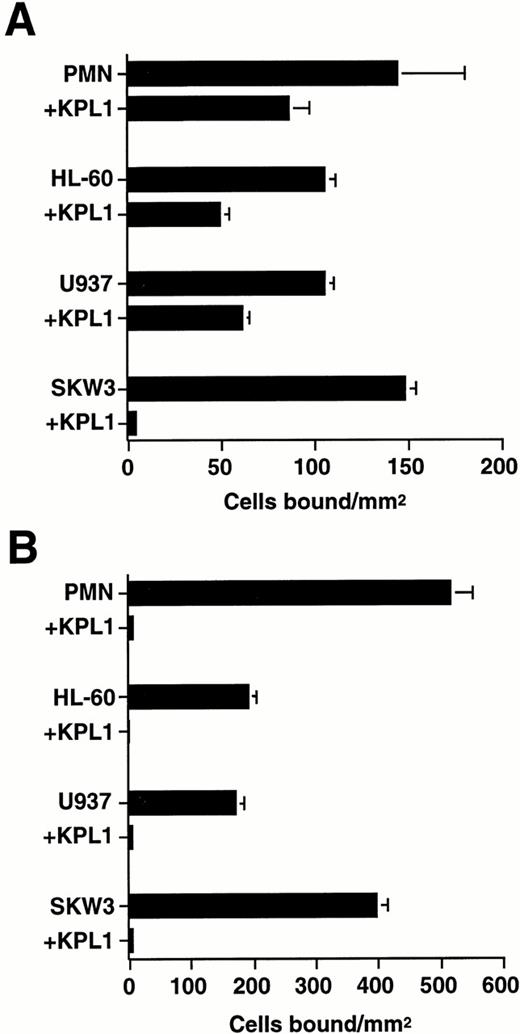
To distinguish between MoAb effects on cell attachment under flow and inhibitory effects of the PSGL-1 MoAb on rolling, neutrophils and the human leukemia cell lines HL-60, U937, and SKW3 were treated with PSGL-1 MoAbs and allowed to settle onto immobilized L-selectin before the onset of flow (Fig 2). This experimental design selectively tests for MoAb effects on leukocyte rolling independent of potential indirect effects such as activation-induced shape changes that may alter initial cell attachment.35 Incubation of cell suspensions with KPL1 resulted in a complete inhibition of SKW3 rolling, but only partial reductions in the number of rolling neutrophils (41% ± 3% inhibition), HL-60 promyelocytes (53% ± 3% inhibition), and U937 cells (42% ± 2% inhibition) on L-selectin (Fig 2A) at 1.0 dyne/cm2 wall shear stress. In contrast, KPL1 treatment completely blocked neutrophil, HL-60, U937, and SKW3 rolling on purified P-selectin, indicating the presence of functionally saturating concentrations of antibody (Fig 2B). Incubation of cell suspensions with the nonblocking, isotype-matched MoAb KPL2 failed to inhibit rolling of neutrophils and cell lines on L-selectin or P-selectin (data not shown). These results indicate that neutrophil, HL-60, and U937 cell lines express both PSGL-1–dependent and –independent L-selectin ligand activity.
Effect of PSGL-1 MoAb on human neutrophil and leukemia cell line rolling on L-selectin and P-selectin. Neutrophils (1 × 106/mL) and the leukemia cell lines HL-60, U937, and SKW3 (0.5 × 106/mL) were treated with either MoAb KPL1 (10 μg/mL) or KPL1 ascites (1:500) and allowed to bind to purified L-selectin (1:5; 170 sites/μm2) (A) or P-selectin (1:60; 270 sites/μm2) (B) under static conditions for 90 seconds, followed by initiation of flow at a wall shear stress of 1 dyne/cm2. Mean ± SEM of bound cells/mm2 in multiple fields from three independent experiments.
Effect of PSGL-1 MoAb on human neutrophil and leukemia cell line rolling on L-selectin and P-selectin. Neutrophils (1 × 106/mL) and the leukemia cell lines HL-60, U937, and SKW3 (0.5 × 106/mL) were treated with either MoAb KPL1 (10 μg/mL) or KPL1 ascites (1:500) and allowed to bind to purified L-selectin (1:5; 170 sites/μm2) (A) or P-selectin (1:60; 270 sites/μm2) (B) under static conditions for 90 seconds, followed by initiation of flow at a wall shear stress of 1 dyne/cm2. Mean ± SEM of bound cells/mm2 in multiple fields from three independent experiments.
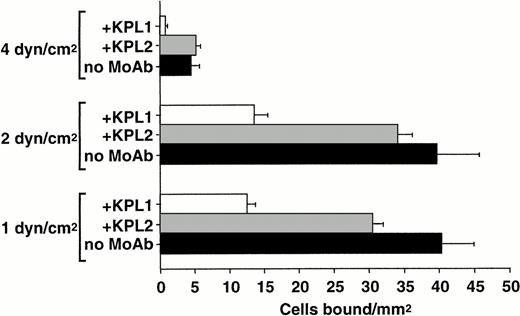
To determine the relative role of PSGL-1–independent and –dependent interactions with L-selectin at distinct wall shear stresses, neutrophils were perfused over purified L-selectin under continuous flow conditions in the presence and absence of MoAb KPL1. Neutrophil-neutrophil interactions that may influence the apparent capture rate and total accumulation of cells on L-selectin22 were minimized by using more dilute suspensions of cells and decreasing the time of flow before quantitating adhesion. PSGL-1 appears to contribute more to neutrophil capture on L-selectin as wall shear stresses are increased (Fig3). At 4.0 dyne/cm2 wall shear stress, MoAb KPL1 significantly inhibited capture of neutrophils on L-selectin, but had less inhibitory effects as shear stress was lowered to 2.0 and 1.0 dyne/cm2 (Fig 3). This finding suggests that the PSGL-1–independent L-selectin ligand activity on neutrophils may have lower association rates and may play a greater role at relatively low levels of shear stress.
Shear stress-dependent contribution of PSGL-1 to neutrophil capture by L-selectin. Neutrophils (0.5 × 106/mL) were treated with either MoAb KPL1 (10 μg/mL) or KPL2 ascites (1:500) and perfused over a range of wall shear stresses through a parallel plate flow chamber in which dilutions of human purified L-selectin (1:5; 170 sites/μm2) were adsorbed to the lower wall. Numbers of bound cells were quantitated from 30 fields of view after 1 minute of continuous flow at each shear stress. Mean ± SD of bound cells/mm2 in multiple fields from two independent experiments.
Shear stress-dependent contribution of PSGL-1 to neutrophil capture by L-selectin. Neutrophils (0.5 × 106/mL) were treated with either MoAb KPL1 (10 μg/mL) or KPL2 ascites (1:500) and perfused over a range of wall shear stresses through a parallel plate flow chamber in which dilutions of human purified L-selectin (1:5; 170 sites/μm2) were adsorbed to the lower wall. Numbers of bound cells were quantitated from 30 fields of view after 1 minute of continuous flow at each shear stress. Mean ± SD of bound cells/mm2 in multiple fields from two independent experiments.
FucT-VII/PSGL-1 transfectants roll on L-selectin.
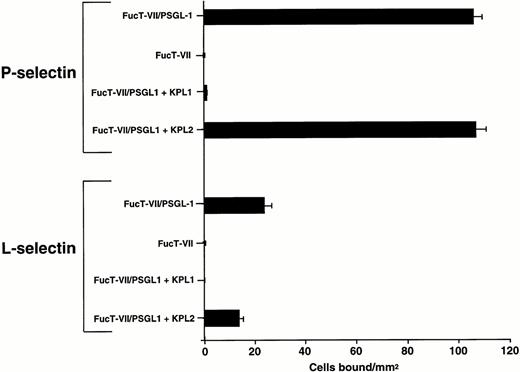
To determine whether PSGL-1 is sufficient to mediate attachment and rolling on immobilized L-selectin under flow conditions, K562 cells transfected with cDNAs encoding FucT-VII and PSGL-1 (FucT-VII/PSGL-1)31 were infused into the flow chamber containing either adsorbed purified L-selectin or P-selectin. As shown in Fig 4, FucT-VII/PSGL-1 transfectants attached and rolled on both P-selectin and L-selectin under flow conditions. Adhesion of FucT-VII/PSGL-1 transfectants on L-selectin and P-selectin was completely inhibited by MoAb KPL1 (Fig 4). K562 cells transfected with FucT-VII cDNA alone were observed to bind to purified L-selectin at a level more than 20-fold less (1 to 3 transient interactions/mm2) than FucT-VII/PSGL-1 transfectants, despite a 4- to 6-fold higher level of sialyl Lewisxexpression,31 suggesting a highly specific interaction of PSGL-1 with L-selectin (Fig 4).
FucT-VII/PSGL-1 transfectants roll on L-selectin. K562-FucT-VII and K562-FucT-VII/PSGL-1 transfectants (0.5 × 106/mL) were infused through a parallel plate flow chamber at a wall shear stress of 1 dyne/cm2 in which purified human L-selectin (1:5; 170 sites/μm2) or P-selectin (1:60; 270 sites/μm2) were adsorbed to the lower wall of the chamber. Nontransfected K562 cells did not bind to L-selectin or P-selectin. Mean ± SEM of bound cells/mm2 in multiple fields from three independent experiments.
FucT-VII/PSGL-1 transfectants roll on L-selectin. K562-FucT-VII and K562-FucT-VII/PSGL-1 transfectants (0.5 × 106/mL) were infused through a parallel plate flow chamber at a wall shear stress of 1 dyne/cm2 in which purified human L-selectin (1:5; 170 sites/μm2) or P-selectin (1:60; 270 sites/μm2) were adsorbed to the lower wall of the chamber. Nontransfected K562 cells did not bind to L-selectin or P-selectin. Mean ± SEM of bound cells/mm2 in multiple fields from three independent experiments.
An HL-60 lysate supports rolling of L-selectin transfectants.
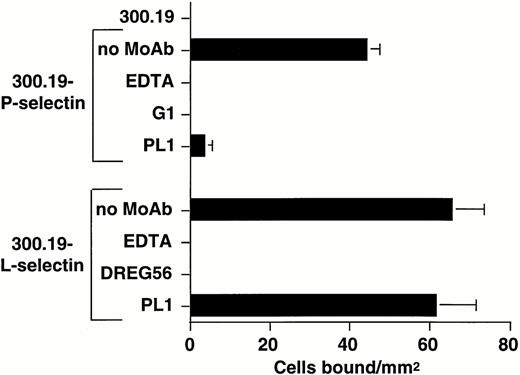
To determine whether the presentation of L-selectin on a cellular surface modifies the leukocyte L-selectin ligand specificity and to further examine the requirement for PSGL-1 expression in myeloid cell recognition of L-selectin, a reciprocal adhesion assay was designed in which an HL-60 cell lysate, partially purified by WGA-Sepharose chromatography, was incorporated as an adhesive substrate in the flow chamber. Suspensions of L-selectin and P-selectin transfectants served as specific probes for L-selectin and P-selectin ligand activity in the lysate. As shown in Fig 5, the WGA-purified HL-60 lysate supported calcium-dependent rolling of both 300.19 P-selectin and L-selectin transfectants, but not nontransfected 300.19 cells. Adhesion of P-selectin and L-selectin transfectants was completely inhibited by treatment with the P-selectin MoAb, G1,25 and DREG56, respectively (Fig 5). Incubation of the lysate with PL1 completely abolished adhesion of P-selectin transfectants, but did not significantly inhibit binding of L-selectin transfectants (Fig 5), which was consistent with the inability of PSGL-1 MoAbs to completely block rolling of neutrophils, HL-60, and U937 cells on L-selectin.
L-selectin transfectants roll on an HL-60 lysate partially purified by WGA affinity chromatography. Nontransfected 300.19 cells, 300.19 L-selectin, or 300.19 P-selectin transfectants (1 × 106/mL) were infused through a parallel plate flow chamber at a wall shear stress of 0.64 dyne/cm2 in which a dilution of an HL-60 lysate partially purified by WGA affinity chromatography was adsorbed to the lower wall of the chamber. L-selectin and P-selectin–dependent adhesion were shown by incubation of L-selectin transfectants with MoAb DREG56 (10 μg/mL) and P-selectin transfectants with MoAb G1 (10 μg/mL). PSGL-1–dependent adhesion was shown by treating the adsorbed lysate with MoAb PL1 (10 μg/mL). Mean ± SEM of bound cells/mm2 in multiple fields from two to three independent experiments.
L-selectin transfectants roll on an HL-60 lysate partially purified by WGA affinity chromatography. Nontransfected 300.19 cells, 300.19 L-selectin, or 300.19 P-selectin transfectants (1 × 106/mL) were infused through a parallel plate flow chamber at a wall shear stress of 0.64 dyne/cm2 in which a dilution of an HL-60 lysate partially purified by WGA affinity chromatography was adsorbed to the lower wall of the chamber. L-selectin and P-selectin–dependent adhesion were shown by incubation of L-selectin transfectants with MoAb DREG56 (10 μg/mL) and P-selectin transfectants with MoAb G1 (10 μg/mL). PSGL-1–dependent adhesion was shown by treating the adsorbed lysate with MoAb PL1 (10 μg/mL). Mean ± SEM of bound cells/mm2 in multiple fields from two to three independent experiments.
L-selectin transfectants roll on lysates immunodepleted of PSGL-1.
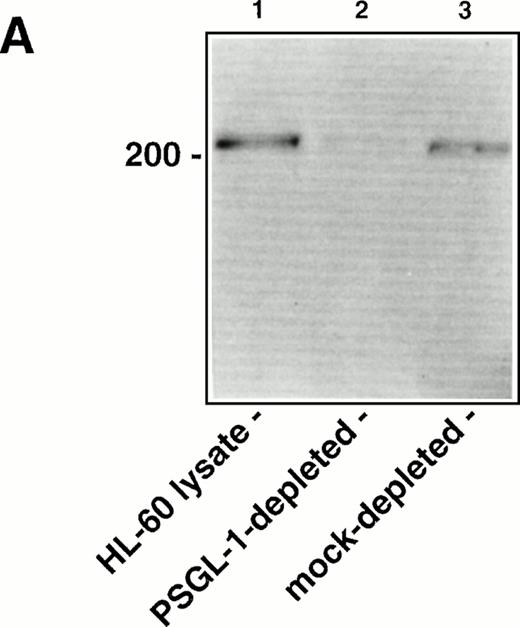
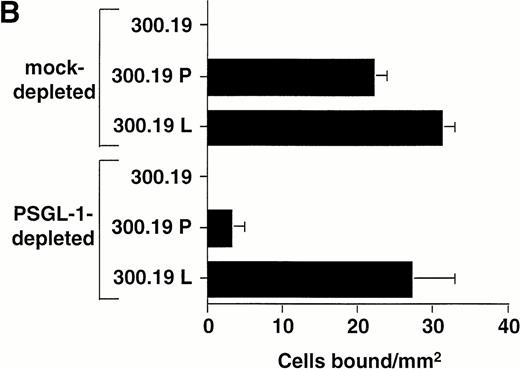
To determine the relative contribution of PSGL-1 to L-selectin transfectant rolling on the WGA-purified HL-60 lysate versus other membrane glycoproteins, the lysate was immunodepleted of PSGL-1 using KPL1 bound to protein G-Sepharose beads. Western blot analysis showed significant depletion of PSGL-1 from the lysate under these conditions, based on a reduction in intensity of the prominent 220- to 250-kD band corresponding to PSGL-1 dimer36 (Fig6A). Immunodepleted lysates were then incorporated into the flow chamber and assayed for L-selectin and P-selectin ligand activity. Compared to a mock-immunodepleted control, in which the lysate was incubated with protein G-Sepharose alone, adhesion of P-selectin transfectants was almost completely inhibited after immunodepletion of PSGL-1 (Fig 6B). Only a small number of transient attachments (1 second in duration) and no stable rolling interactions were observed. This result confirmed the requirement of PSGL-1 to support P-selectin–dependent rolling in this system. In contrast, L-selectin transfectants attached and formed stable rolling interactions on lysates immunodepleted of PSGL-1, indicating that L-selectin ligand activity independent of PSGL-1 was present in the lysate. However, the number of bound L-selectin transfectants was partially reduced relative to the mock-immunodepleted control (Fig 6B), apparently caused by the contribution of PSGL-1 to the L-selectin ligand activity in the HL-60 lysate. A second round of immunodepletion of the lysate completely eliminated residual skipping interactions with P-selectin transfectants, but only reduced adhesion of L-selectin transfectants by an additional 10% (data not shown).
Immunodepletion of PSGL-1 from the WGA affinity purified HL-60 lysate inhibits binding of P-selectin transfectants, but not L-selectin transfectants. (A) Western blot analysis showing immunodepletion of PSGL-1 from the HL-60 lysate. Lane 1: intact WGA-purified HL-60 lysate (220 to 250-kD band corresponding to PSGL-1); lane 2: lysate after immunodepletion with KPL1/protein G-Sepharose; lane 3: lysate after mock immunodepletion (incubation with protein G-Sepharose alone). PSGL-1 was detected by probing the blot with KPL1 ascites (1:2,500), followed by HRP-conjugated goat anti-mouse IgG and development using enhanced chemiluminescence. (B) Adhesion of 300.19 P-selectin or 300.19 L-selectin transfectants (1 × 106/mL) to PSGL-1–depleted HL-60 lysates under flow conditions (0.64 dyne/cm2) after immunodepletion of PSGL-1. Mean ± SD of bound cells/mm2 in multiple fields from two independent experiments.
Immunodepletion of PSGL-1 from the WGA affinity purified HL-60 lysate inhibits binding of P-selectin transfectants, but not L-selectin transfectants. (A) Western blot analysis showing immunodepletion of PSGL-1 from the HL-60 lysate. Lane 1: intact WGA-purified HL-60 lysate (220 to 250-kD band corresponding to PSGL-1); lane 2: lysate after immunodepletion with KPL1/protein G-Sepharose; lane 3: lysate after mock immunodepletion (incubation with protein G-Sepharose alone). PSGL-1 was detected by probing the blot with KPL1 ascites (1:2,500), followed by HRP-conjugated goat anti-mouse IgG and development using enhanced chemiluminescence. (B) Adhesion of 300.19 P-selectin or 300.19 L-selectin transfectants (1 × 106/mL) to PSGL-1–depleted HL-60 lysates under flow conditions (0.64 dyne/cm2) after immunodepletion of PSGL-1. Mean ± SD of bound cells/mm2 in multiple fields from two independent experiments.
To investigate the possibility that domains of PSGL-1 independent of the KPL1 epitope may have remained in the lysate after immunodepletion, Western blots were stripped and reprobed with MoAb PL2, which recognizes residues 188-235 in the consensus repeat domain of human PSGL-137 and does not block neutrophil adhesion to P-selectin.26 The resulting blot was nearly identical to the KPL1-probed blot shown in Figure 6A, in that the intensity of the band representing 220-250–kD PSGL-1 dimer in the PSGL-1–immunodepleted lysate was strongly reduced compared with the intact and mock-immunodepleted lysates (data not shown). This finding suggests that epitopes on PSGL-1 distinct from the P-selectin–binding site were not likely to be responsible for mediating the L-selectin transfectant rolling observed after immunodepletion with MoAb KPL1.
Effect of protease and neuraminidase treatment on myeloid cell L-selectin ligand activity.
The L-selectin ligand activity on myeloid cells was characterized by assessing the effect of enzyme treatments on HL-60 rolling on immobilized L-selectin or P-selectin under flow conditions. Several groups have reported conflicting observations on the protease sensitivity of L-selectin ligand activity expressed by leukocytes.13,15,22 OSGE specifically cleaves glycoproteins that contain clustered O-glycosylated serine or threonine residues,14 including PSGL-1.17 Incubation of HL-60 cells with OSGE resulted in complete inhibition of attachment and rolling on immobilized L-selectin and P-selectin (Table1), indicating that ligand activity for these selectins requires the expression of an O-glycosylated, mucinlike protein. Chymotrypsin treatment also completely blocked HL-60 rolling on L-selectin and P-selectin (Table 1), which confirmed the protease sensitivity of L-selectin and P-selectin ligand activity.
Effect of Protease and Neuraminidase Treatment on HL-60 Adhesion to L-Selectin and P-Selectin Under Flow Conditions
| Treatment . | Percent of Control Adhesion . | |
|---|---|---|
| L-Selectin . | P-Selectin . | |
| O-sialoglycoprotein endopeptidase | 0 ± 0 | 3.7 ± 3.7 |
| Chymotrypsin | 0 ± 0 | 0 ± 0 |
| Neuraminidase, V cholerae | 7.0 ± 7.0 | 70.6 ± 5.5 |
| Treatment . | Percent of Control Adhesion . | |
|---|---|---|
| L-Selectin . | P-Selectin . | |
| O-sialoglycoprotein endopeptidase | 0 ± 0 | 3.7 ± 3.7 |
| Chymotrypsin | 0 ± 0 | 0 ± 0 |
| Neuraminidase, V cholerae | 7.0 ± 7.0 | 70.6 ± 5.5 |
HL-60 cells were treated with enzymes as described in Materials and Methods and were perfused (0.5 × 106 cells/mL) through the flow chamber at a wall shear stress of 1 dyne/cm2 in which human L-selectin–IgG (2 μg/mL), P-selectin–IgG (2 μg/mL), or purified P-selectin (1:60) were adsorbed to the lower wall of the chamber. Control binding for HL-60 cells on L-selectin–IgG, P-selectin–IgG, and purified P-selectin was 43 ± 3, 218 ± 24, and 68 ± 8 cells/mm2, respectively. Mean ± SEM percent of control binding/mm2 in multiple fields from two to three independent experiments.
HL-60 cell suspensions were incubated with neuraminidase to establish whether sialylation is required for myeloid cell recognition of L-selectin. Ligand activity for P-selectin appears to be relatively resistant to neuraminidase, since radiolabeled P-selectin binding to purified PSGL-117,36 and P-selectin–IgM binding to neutrophils16 is only partially inhibited by neuraminidase treatment. As shown in Table 1, treatment of HL-60 cells with neuraminidase resulted in almost complete inhibition of attachment and rolling on L-selectin, but only a partial decrease in binding to P-selectin. The differential neuraminidase sensitivity of HL-60 cell adhesion to L-selectin and P-selectin supports the existence of distinct carbohydrate requirements for L-selectin and P-selectin ligand activity on myeloid cells.
DISCUSSION
Previous reports of L-selectin mediated leukocyte-leukocyte adhesion have produced conflicting conclusions regarding the identity of the leukocyte ligand for L-selectin. A candidate ligand for L-selectin appears to be PSGL-1, based on the demonstration of neutrophil rolling on purified PSGL-1 and its inhibition by an L-selectin MoAb.19 However, other findings suggest that PSGL-1 may be only one of several L-selectin ligands expressed by leukocytes. PSGL-1 MoAb treatment only partially inhibits formyl peptide-induced homotypic neutrophil aggregation21 and does not completely block transient neutrophil interactions with fixed neutrophil monolayers.19 Furthermore, the failure of a PSGL-1 MoAb to inhibit L-selectin–dependent neutrophil-neutrophil interactions in flow22 and the demonstration of OSGE-resistant neutrophil adhesion to L-selectin23 suggest that PSGL-1 may not always be required for leukocyte adhesive interactions with L-selectin. Overall, these observations are analogous to conflicting reports on whether PSGL-1 serves as a leukocyte ligand for E-selectin22,31 38-40 and underscore the need to further characterize leukocyte L-selectin ligand activity.
Under flow conditions, resting human neutrophils were observed to form specific and stable rolling interactions with purified native L-selectin immobilized on the wall of a flow chamber, similar to those formed by neutrophils on recombinant L-selectin–IgG.23L-selectin ligand activity on leukocytes appears to be constitutive, because treatment of neutrophils with metabolic inhibitors did not inhibit adhesion to L-selectin. In contrast to leukocyte interactions with the vascular selectins, neutrophils were observed to attach to L-selectin at shear stresses that were significantly higher than corresponding interactions reported for either P-selectin or E-selectin at similar site densities.26,39,41 The relatively high capture rate of neutrophils to purified L-selectin is consistent with its proposed role in mediating leukocyte capture from flow in vivo.42 43
In agreement with studies reporting partial inhibitory effects of PSGL-1 MoAbs in leukocyte-leukocyte adhesion assays,19,21neutrophil and myeloid cell line attachment to purified L-selectin under flow conditions was only partially inhibited after treatment with PSGL-1 MoAbs. Partial PSGL-1 MoAb inhibition of L-selectin–mediated neutrophil-neutrophil interactions under flow may be caused by either incomplete saturation of PSGL-1, a consequence of adherent neutrophil spreading,44 and/or levels of shear stress that may favor or preclude a non–PSGL-1 L-selectin interaction. PSGL-1 does appear to contribute significantly to neutrophil-neutrophil interactions under static conditions or low shear stress and when at least one of the interacting neutrophil populations has been chemotactically stimulated.16,19,21 Because of these considerations, we elected to study leukocyte adhesion directly to immobilized L-selectin, which removes complications of L-selectin shedding, PSGL-1 redistribution,44 and simplifies the flow field in which the adhesive interaction takes place. The residual binding of neutrophils, HL-60, and U937 cells to native L-selectin in the presence of shear stress and MoAb KPL1 suggests that structures in addition to PSGL-1 contribute to recognition of L-selectin under flow conditions. In contrast to the myeloid cells tested, rolling of the SKW3 lymphoid cell line on L-selectin was completely inhibited by PSGL-1 MoAb treatment, indicating that PSGL-1 serves as the principal L-selectin ligand in this cell line. Therefore, the relative contribution of PSGL-1 to L-selectin ligand activity may be at least partly dependent on leukocyte type.
To address the possibility that PSGL-1 has binding sites for L-selectin that are distinct from the P-selectin binding site, which may be the case for PSGL-1 E-selectin interactions,45 K562 cells transfected with cDNAs encoding PSGL-1 and FucT-VII were evaluated for binding to L-selectin in the presence of MoAb KPL1. MoAb KPL1 recognizes an epitope which contains a sulfated tyrosine (Snapp, K.R.; Ding, H.; Atkins, K.; Luscinskas, F.W.; Warnke, R.; and Kansas, G.S.; submitted) previously shown to be critical for binding of PSGL-1 to P-selectin.45-48 Adhesion of FucT-VII/PSGL-1 transfectants to immobilized L-selectin was completely abolished by KPL1, suggesting that PSGL-1 interacts with L-selectin at a binding site shared by P-selectin. Consistent with this observation, binding of L-selectin transfectants to PSGL-1 expressing COS cells is completely blocked by MoAb KPL1 (Snapp, K.R.; Ding, H.; Atkins, K.; Luscinskas, F.W.; Warnke, R.; and Kansas, G.S.; submitted). Therefore, the PSGL-1 recognition of L-selectin may depend on the N-terminal sulfated tyrosine domain shown to be required for recognition of P-selectin.
To characterize interactions between L-selectin ligand activity on leukocytes and L-selectin expressed on a cell surface rather than immobilized on a substrate, HL-60 cell membrane preparations were isolated using WGA-lectin affinity chromatography. Rolling of L-selectin expressing transfectants on HL-60 membrane preparations was only partially reduced after significant immunodepletion of PSGL-1 from the lysate, whereas P-selectin transfectant binding was completely inhibited. This finding showed that ligand activity reconstituted from a myeloid cell lysate supported L-selectin–dependent adhesion under conditions in which P-selectin recognition was completely abolished. The partial reduction in L-selectin binding to the PSGL-1–immunodepleted lysate appears to represent the contribution of PSGL-1 to L-selectin ligand activity in this membrane preparation.
The ligand activity mediating myeloid cell adhesion to L-selectin appears to belong to a subset of mucinlike glycoproteins bearing O-linked carbohydrate side chains, which include PSGL-1,17leukosialin (CD43),49 and CD34.50 The OSGE sensitivity of the myeloid cell L-selectin ligand activity characterized in the present study sets it apart from that of KG1a hematopoietic progenitor cells, because treatment with OSGE does not block L-selectin–dependent lymphocyte adhesion to KG1a monolayers.13 A nonmucinlike component of L-selectin ligand activity on leukocytes has also been described in a recent report showing OSGE-insensitive adhesion of myeloid cells to recombinant L-selectin.23 However, the OSGE-insensitive adhesion was only observed with high concentrations of L-selectin–IgG substrate, which may have obscured OSGE effects because of either low affinity interactions with sialylated glycoproteins29 or background adhesion mediated by Fc receptor interactions with the selectin-IgG chimera.
In contrast to the similar OSGE protease sensitivity of P-selectin and L-selectin ligand activity on myeloid cells, studies with HL-60 rolling on L-selectin and P-selectin showed a differential sensitivity to neuraminidase treatment. Myeloid cell L-selectin ligand activity appears to be considerably more dependent on the presentation of sialic acid residues than PSGL-1 binding to P-selectin. This finding is consistent with previous reports showing that neuraminidase treatment blocks L-selectin–dependent interactions between flowing neutrophils and adherent neutrophils12 and L-selectin–IgM binding to myeloid cells.16 In contrast, PSGL-1 recognition of P-selectin is relatively resistant to neuraminidase,16 17which suggests distinct structural requirements for L-selectin and P-selectin ligand activity.
There is currently a limited understanding of the physiological role of L-selectin ligand expression by leukocytes. L-selectin–dependent interactions have been proposed as an initial step in neutrophil aggregation that enhances cell-cell contact time for formation of larger, more stable aggregates through β2–integrin-mediated adhesion.21 Transient, L-selectin–dependent adhesive interactions between flowing leukocytes and leukocytes adherent to the vessel wall may serve as a mechanism to sustain leukocyte recruitment at inflammatory sites.12 In support of this hypothesis, capture of neutrophils in flow by adherent neutrophils appears to amplify neutrophil accumulation on stimulated endothelium and purified selectins in vitro.19 22 Our data show that mucinlike ligands distinct from PSGL-1 support leukocyte adhesion and rolling on L-selectin. Therefore, the expression of multiple ligand structures for L-selectin by leukocytes may be important for the efficiency of leukocyte capture from flow and the accumulation of leukocytes at inflammatory sites.
ACKNOWLEDGMENT
We thank Drs R.P. McEver and K.L. Moore for the gift of PL1 and PL2, Dr T.K. Kishimoto for the gift of DREG56, and Drs L.A. Lasky and S.R. Watson for the gifts of selectin-IgG chimeras.
Supported by National Institutes of Health (NIH) Grants No. HL54614 to M.B.L., HL54136 to K.L., and NIH NRSA postdoctoral fellowship No. HL09578 to C.L.R.; a Mitzutani Foundation grant to K.L.; and Grant No. CB-204 from the American Cancer Society to G.S.K. G.S.K. is an Established Investigator of the American Heart Association.
Address reprint requests to Michael B. Lawrence, PhD, Department of Biomedical Engineering, University of Virginia, Box 377, Health Sciences Center, Charlottesville, VA 22908.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal