Abstract
Phosphatidylinositol 3-kinase (PI3K) is a heterodimer lipid kinase consisting of an 85-kD subunit bound to a 110-kD catalytic subunit that also possesses intrinsic, Mn2+-dependent protein serine kinase activity capable of phosphorylating the 85-kD subunit. Here, we examine the Mn2+-dependent protein kinase activity of PI3Kα immunoprecipitated from normal resting or thrombin-stimulated platelets, and characterize p85/p110 phosphorylation, in vitro. Phosphoamino acid analysis of phosphorylated PI3Kα showed p85 and p110 were phosphorylated on serine, but in contrast to previous results, were also phosphorylated on threonine and tyrosine. Wortmannin and LY294002 inhibited p85 phosphorylation; however, p110 phosphorylation was also inhibited suggesting p110 autophosphorylation on serine/threonine. The protein tyrosine kinase inhibitor, erbstatin analog, partially inhibited p85 and p110 phosphorylation but did not appear to affect PI3K lipid kinase activity. The in vitro phosphorylation of p85α or p110α derived from thrombin-stimulated platelets was no different than that of resting platelets, but we confirm that in thrombin receptor-stimulated platelets enhanced levels of p85α and PI3K lipid kinase activity were recovered in antiphosphotyrosine antibody immunoprecipitates. These results suggest PI3Kα can autophosphorylate on serine and threonine, and both p85α and p110α are substrates for a constitutively-associated protein tyrosine kinase in platelets.
PHOSPHATIDYLINOSITOL 3-kinase (PI3K) is a heterodimer phospholipid kinase composed of an 85 kD (p85) regulatory subunit and a 110 kD (p110) catalytic subunit which specifically phosphorylates the D-3 hydroxyl position of membrane phosphoinositides.1,2 Multiple isoforms of each subunit have been discovered, as well as a family of proteins homologous to the p110 subunit.3-8 Numerous studies have shown PI3K activity is induced after extracellular ligation of a variety of cell-surface growth-factor or hormone receptors with their respective ligands. In many cases, receptor-induced tyrosine phosphorylation of intracellular proteins appears to play a requisite role in the transmembrane signaling of PI3K activity.9-11 The physical association of PI3K with either autophosphorylated receptor protein tyrosine kinases (PTK)9,12 or with certain members of the nonreceptor src-family of PTKs has been a recurring theme in the study of receptor-induced PI3K signaling. Interactions with receptor PTKs occur as a result of an affinity of specific regions in p85, termed src-homology-2 (SH2) domains, for specific phosphotyrosine-containing amino acid sequences (pYMXM, pYXXM, X = any amino acid).9,11,13 Interactions of p85 with the nonreceptor src-family tyrosine kinases have been reported to occur through binding of p85 polyproline motifs to SH3 domains contained in src-kinases.14,15 In T lymphocytes, interleukin-2 (IL-2) receptor ligation with the T-cell growth factor, IL-2, induces enhanced PI3K activity in antiphosphotyrosine immunoprecipitates as well as association of PI3K with the p59fyn src-family kinase.16 Thrombin receptor activation of platelets results in intracellular 3-phosphoinositide synthesis17 and the association of PI3K with the p60scr or p59fyn kinases.18 Based on these studies, it is evident protein tyrosine kinases serve as important intermediaries in PI3K signaling.
It is still unclear what role phosphorylation of PI3K itself serves in regulating postreceptor PI3K activity. The p85 subunit of PI3K was shown to be a substrate for a serine/threonine kinase.19Recently, p85 was found to be phosphorylated, in vitro, on serine/threonine residues by serine/threonine kinase activity copurifying with PI3K from rat liver.20 Dhand et al,21 have shown with baculoviral-expressed p85 and p110 in insect cells the p110 subunit is actually a dual specificity enzyme with intrinsic, Mn2+-dependent protein serine kinase activity capable of phosphorylating the p85 subunit.21Their study indicated when p85 and p110 were coexpressed in insect cells, the p85 subunit was phosphorylated on both threonine and serine, in vivo, but was phosphorylated only on serine, in vitro.21In addition, the p110 subunit also became phosphorylated on serine, in vivo, but was not phosphorylated in vitro. Serine phosphorylation of p85 by p110 results in a decrease of in vitro PI3K lipid kinase activity,21 indicating the intrinsic serine kinase activity of PI3K may be important in regulating its lipid kinase activity. Tyrosine phosphorylation of PI3K has been a more controversial area of investigation. Several groups have reported tyrosine phosphorylation of p85,10,19,22,23 and one study has shown p85 and p110 phosphorylation on tyrosine presumably by the activated platelet-derived growth factor receptor (PDGFR) in PDGFR immunoprecipitates of platelet-derived growth factor (PDGF)-stimulated fibroblasts.24 Recently, the tyrosine phosphorylation of p85 in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation of neutrophils has been shown.25Despite these findings, a functional role for tyrosine phosphorylation of either p85 or p110 is still unclear.
In previous studies showing the intrinsic protein serine kinase activity of PI3K, experiments were done with either highly purified PI3K from rat liver,20 or with baculoviral-expressed p85 and p110 in insect cells.20,21 Under these experimental conditions, PI3K was relatively free of associated proteins. Because platelets are a rich source of PI3Kα, which is rapidly activated on stimulation of the thrombin receptor,17,26-28 we chose to investigate the Mn2+-dependent, intrinsic protein kinase activity of PI3Kα immunoprecipitated from lysates of resting or thrombin-stimulated platelets, and characterize the phosphorylation profile of the p85 and p110 subunits. In this study, we confirm p85α is phosphorylated on serine, in vitro, as previously shown,20 21 but we also show it is phosphorylated on tyrosine and threonine. P85α serine/threonine phosphorylation was inhibitable by LY294002, a specific inhibitor of PI3K. We show p110α was phosphorylated on serine, threonine, and tyrosine, part of which was inhibitable by LY294002. Threonine phosphorylation of p110α has not been previously observed. These in vitro studies suggest that p110α is capable of autophosphorylation on serine and threonine, that p110α can phosphorylate p85α on serine and threonine, and both p85α and p110α are substrates for a constitutively-associated protein tyrosine kinase in platelets. The tyrosine phosphorylation of p85 or p110, however, did not appear to affect the lipid kinase activity of PI3K.
MATERIALS AND METHODS
Reagents and antibodies.
The murine monoclonal anti-PI3K (p85α) antibody, AB6,29against human p85 was the generous gift of Dr Shinya Tanaka (Hokkaido University, Sapporo, Japan), and was also obtained from Upstate Biotechnology Inc (Lake Placid, NY). Polyclonal antibody (C-17) to the p110α subunit of PI3K was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine antibody, 4G10, was obtained from Upstate Biotechnology Inc. α-Actinin monoclonal antibody was obtained from ICN Biomed (Costa Mesa, CA). IgG1 (MOPC-21) antibody was from Cappel (West Chester, PA). The thrombinreceptor- activating peptide (TRAP), SFLLRNPNDKY,30 was synthesized on a Milligen (Framingham, MA) model 9050 PepSynthesizer. Phosphatidylinositol (PtdIns) and phosphatidylserine were from Avanti Polar Lipids (Alabaster, AL). Erbstatin analog (methyl 2,5-dihydroxycinnamate), PtdIns, PtdIns(4)P, and PtdIns(4,5)P2 were from Calbiochem (San Diego, CA). Wortmannin and thrombin were from Sigma Chemical Co (St Louis, MO). LY294002 was from BIOMOL (Plymouth Meeting, PA). All other reagents were obtained from Sigma Chemical Co, unless specified otherwise.
Platelet isolation and lysis.
Human platelets were isolated from platelet-rich plasma (PRP) from healthy male volunteer donors (The Blood Center of Southeastern Wisconsin, Milwaukee, WI). PRP was supplemented with 25 ng/mL Prostaglandin E1 and 1 mmol/L acetylsalicylic acid, and subjected to centrifugation at 120g for 20 minutes to sediment contaminating blood cells. Platelets were then procured from the remaining PRP by centrifugation at 500g for 20 minutes. The platelet pellet was resuspended in “platelet buffer” containing 5 mmol/L piperazine-N,N'-bis-[2-ethanesulfonic acid] (PIPES) [pH 6.8], 145 mmol/L NaCl, 4 mmol/L KCl, 0.5 mmol/L Na2HPO4, 1 mmol/L MgCl2, 0.1% glucose, 0.1% bovine serum albumin (BSA), and 1 U/mL apyrase, immediately loaded onto a Sepharose CL-2B (Sigma, St Louis, MO) gel column, and eluted with platelet buffer. Gel-filtered platelets were then counted with a Coulter Counter (Hialeah, FL) and adjusted to the appropriate concentration with platelet buffer. Equivalent amounts of platelets (1 to 2 × 109) were used per sample in all assays. Gel-filtered platelets were treated at 25°C with the appropriate stimulus concentration and solubilized in lysis buffer (137 mmol/L NaCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 20 mmol/L Tris [pH 8], 10% glycerol, 1% Triton X-100) supplemented with 20 μg/mL aprotinin, 20 μg/mL leupeptin, 1 mmol/L Na3VO4, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). The Triton-insoluble fraction was pelleted by centrifugation at 15,000g for 4 minutes at 4°C, or for cytosol preparation, at 130,000 g for 45 minutes at 4°C. The supernatants were isolated and used where indicated.
Immunoprecipitation.
Platelet lysates (equivalent to 1 or 2 × 109platelets) were incubated with equal amounts of the appropriate antibodies for 2 hours at 4°C, and the antibody immunoprecipitates were collected on Protein G Agarose for an additional hour. Immune complexes were washed twice with lysis buffer, twice with 100 mmol/L Tris (pH 7.6)/500 mmol/L LiCl, and twice with kinase buffer (50 mmol/L HEPES [pH 7.4], 20 mmol/L MnCl2, 5 mmol/L EDTA, 150 mmol/L NaCl, 10% glycerol, and 0.02% Triton X-100). All washes were supplemented with 20 μg/mL aprotinin, 20 μg/mL leupeptin, 1 mmol/L Na3VO4, and 1 mmol/L PMSF. Immune complexes were solubilized in 2×-concentrated sodium dodecyl sulfate (SDS)-containing sample buffer and frozen until examined by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) as described below.
Phosphatidylinositol 3-kinase assay.
Phosphatidylinositol 3-kinase activity was determined as described elsewhere.16 Briefly, washed immunoprecipitates were incubated on ice for 10 minutes in 20 μL of a sonicated substrate mixture containing either PtdIns or PtdIns(4,5)P2, and phosphatidylserine (1:1) at a concentration of 0.5 mg/mL in 20 mmol/L HEPES (pH 7.4). Reactions were initiated by addition of 20 μL of kinase buffer containing 20 mmol/L HEPES (pH 7.4), 50 mmol/L MgCl2, 50 μmol/L adenosine triphosphate (ATP), 20 μCi of γ-32P-ATP (specific activity, 6,000 Ci/mmole; Dupont-NEN Research Products, Boston, MA). After 10 minutes at 37°C, the reactions were terminated with 200 μL of 1 N HCl. Four hundred microliters of CHCl3/CH3OH (1:1) was added and the phospholipids were extracted. The aqueous layer was aspirated and the CHCl3 layer was washed once with 160 μL of CH3OH/1 N HCl (1:1). The resulting CHCl3layer was dried with nitrogen gas and the phospholipid residues were solubilized in CHCl3/CH3OH (2:1). Radiolabeled PtdIns(3,4,5)P3 was separated from PtdIns(4,5)P2, PtdIns(4)P, and PtdIns standards by thin-layer chromatography (TLC). Briefly, samples were spotted onto diaminocyclohexane tetra-acetic acid (CDTA)–treated aluminum-backed Silica Gel 60 plates (250 μm, Merck, Darmstadt, Germany)31 and developed in a mobile phase composed of n-propanol:2 N acetic acid (65:35).32 Radiolabeled PtdIns(3)P was separated from PtdIns(4)P as previously described.3132P-radiolabeled phosphoinositides were visualized by autoradiography.32Pi incorporated into PtdIns(3)P or PtdIns(3,4,5)P3 was quantitated directly on the TLC plates with an automated microbiological imaging system (AMBIS) computerized imaging/radioscanning system (CSP Inc, Billerica, MA). Authentic PtdIns, PtdIns(4)P, and PtdIns(4,5)P2 standards were chromatographed in parallel lanes and visualized by spraying with 10% H2SO4 and heating to 100°C.
One- and two-dimensional gel electrophoresis and immunoblotting.
Proteins were separated by SDS-PAGE as previously described.16 For immunoblotting, proteins were electrophoretically transferred to Immobilon-P membrane (Millipore Corp, Bedford, MA) in 25 mmol/L Tris/192 mmol/L glycine at 300 mA for 30 minutes, then stepped to 800 mA for 1 hour. The membrane was incubated for 1 hour in blocking solution (Tris-buffered saline [TBS] in 3% BSA) and incubated overnight with the appropriate antibody in TBS/1%BSA/0.05% Tween-20. The blots were washed with TBS/0.2% Tween-20/0.5% BSA, incubated for 1 hour in 1 μg/mL horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL), and washed extensively. Immunoreactive proteins were detected by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL) according to the manufacturer's protocol. For two-dimensional electrophoresis, proteins were solubilized in urea sample buffer (9.5 mol/L urea, 4% Triton X-100, 2% ampholines [pH 3-10], 5% 2-mercaptoethanol) and loaded onto 1.5 mm, prefocused (200 V, 1 hour), isoelectric-focusing tube gels containing ampholines (pH 3-10). Proteins were focused to equilibrium at 400 V for 15 hours. Resolution of the proteins in the second dimension was carried out by SDS-PAGE in 8.75% gels as described above.
In vitro kinase assay.
Kinase assays were performed on washed immune complexes and initiated in 20 μL of kinase buffer (50 mmol/L HEPES [pH 7.4], 20 mmol/L MnCl2, 5 mmol/L EDTA, 150 mmol/L NaCl, 10% glycerol, and 0.02% Triton X-100) containing 50 μmol/L ATP supplemented with 20 μCi γ-32P-ATP. Wortmannin stocks were in dimethyl sulfoxide (DMSO) and erbstatin analog and LY294002 stocks were in ethanol; each were diluted with reaction buffers to desired concentrations for use in experiments. After 15 minutes at 25°C, the beads were washed twice with phosphate-buffered saline (PBS) containing 0.5 mmol/L EDTA and the immunoprecipitated proteins were solubilized in boiling SDS-containing sample buffer and separated by SDS-PAGE.
Phosphoamino acid analysis.
32P-radiolabeled proteins were separated by SDS-PAGE and identified by autoradiography. Bands of interest were excised, rehydrated in 0.05 mol/L NH4HCO3 containing 0.1% SDS and 30% β-mercaptoethanol, and extracted from the gel. The proteins were precipitated with trichloroacetic acid using BSA as carrier. The labeled proteins were hydrolyzed at 110°C in 6 N HCl and nitrogen gas for 1 hour. The hydrolysate was lyophylized and the residue was reconstituted in water and spotted onto thin-layer cellulose plates. 32P-labeled phosphoamino acids were resolved electrophoretically in the first dimension in pH 1.9 buffer containing 88% HCOOH, glacial acetic acid, H2O (50:156:1,794) at 1,000 V for 25 minutes. Resolution in the second dimension was performed in pH 3.5 buffer containing pyridine, 100 mmol/L EDTA, acetic acid, H2O (10:10:100:1,880) at 500 V for 25 minutes. Phosphotyrosine, phosphoserine, and phosphothreonine standards were run concurrently and detected with 0.2% ninhydrin in acetone. Radiolabeled amino acids were detected by autoradiography.
RESULTS
Manganese-dependent phosphorylation of the p85 and p110 subunits of PI3K, in vitro.
The protein kinase activity of PI3K has largely been studied in systems using either purified PI3K, or baculoviral-expressed PI3K from insect cells.20,21 Here, we examine the Mn2+-dependent, in vitro protein kinase activity of PI3K immunopurified from platelets. PI3K was immunoprecipitated from the Triton X-100–soluble portion of lysates prepared from resting or thrombin-treated platelets using an antibody to the p85α subunit of PI3K. It should be noted, under our lysis conditions p110 remained bound to p85 during immunoprecipitation as evidenced by coprecipitation of both subunits (see Fig 2B) as well as PI3K lipid kinase activity (see Fig 7 and Fig 8B). p110 protein kinase activity was then examined by in vitro kinase assay in the presence of Mn2+ and γ-32P-ATP as previously described.21 Labeled proteins were separated by SDS-PAGE and subjected to autoradiography. An 85-kD band immunoprecipitated from either resting or thrombin-aggregated platelet lysates was heavily phosphorylated (Fig 1, lanes 2 and 4). A 110-kD band was also very heavily phosphorylated in this reaction. The p85 and p110 in vitro phosphorylation was Mn2+-dependent (occurring at 50 μmol/L Mn2+) and was not simply due to a nonspecific divalent cation effect because it did not occur in the presence of Ca2+ alone, and was only slightly observable in the presence of Mg2+ alone (unpublished data). Similar results were obtained when PI3K was immunoprecipitated from post-130,000g supernatants of platelet lysates (see Fig 2A).
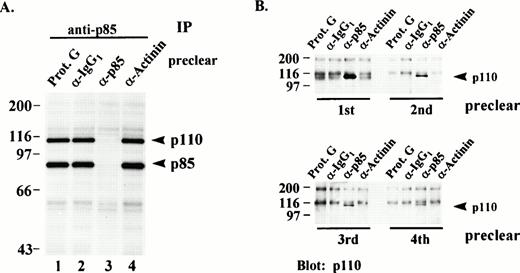
(A) In vitro phosphorylation of PI3K from immunodepleted lysates. Post-130,000g cytosolic fractions of lysates from resting platelets were serially immunodepleted (preclear) four times with either Protein G beads alone (lane 1), isotype-matched IgG1 antibody (lane 2), p85 antibody (lane 3), or an irrelevant antibody (lane 4). All of the precleared lysates were then re-immunoprecipitated (IP) with equal amounts of PI3K antibody and the immune complexes subjected to an in vitro kinase assay with γ-32P-ATP and Mn2+ as described in Materials and Methods. Immune complex proteins were eluted, separated by SDS-PAGE and visualized by autoradiography. Molecular mass markers in kilodaltons are indicated at the left. (B) The immune complexes immunoprecipitated from each lane in (A) were eluted from the Protein G beads, separated by SDS-PAGE and immunoblotted for p110 content with a p110-specific antibody. Numbers below the lanes indicate the particular preclearing step. Molecular mass markers in kilodaltons are indicated at the left.
(A) In vitro phosphorylation of PI3K from immunodepleted lysates. Post-130,000g cytosolic fractions of lysates from resting platelets were serially immunodepleted (preclear) four times with either Protein G beads alone (lane 1), isotype-matched IgG1 antibody (lane 2), p85 antibody (lane 3), or an irrelevant antibody (lane 4). All of the precleared lysates were then re-immunoprecipitated (IP) with equal amounts of PI3K antibody and the immune complexes subjected to an in vitro kinase assay with γ-32P-ATP and Mn2+ as described in Materials and Methods. Immune complex proteins were eluted, separated by SDS-PAGE and visualized by autoradiography. Molecular mass markers in kilodaltons are indicated at the left. (B) The immune complexes immunoprecipitated from each lane in (A) were eluted from the Protein G beads, separated by SDS-PAGE and immunoblotted for p110 content with a p110-specific antibody. Numbers below the lanes indicate the particular preclearing step. Molecular mass markers in kilodaltons are indicated at the left.
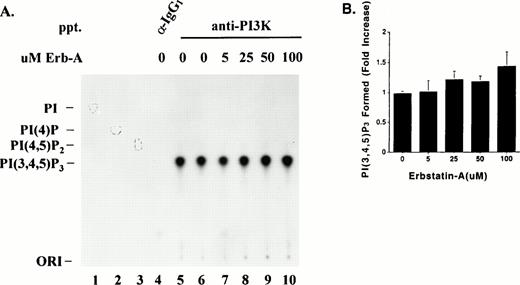
Effect of Erb-A on PI3K lipid kinase activity. (A) Triton-soluble fractions of resting platelet lysate were immunoprecipitated with equal amounts of either PI3K antibody (lanes 5 through 10) or an isotype-matched antibody (lane 4). The washed immune complexes were subjected to a “cold” in vitro kinase assay in the presence of ATP/Mn2+, and in the absence or presence of the indicated concentrations of Erb-A. Control reactions containing no Erb-A were incubated with either buffer alone (lanes 4 and 5) or ethanol/buffer vehicle (lane 6). The phosphorylated immune complexes were washed and incubated for 10 minutes in the presence of γ-32P-ATP, Mg2+, and PI(4,5)P2substrate. Radiolabeled phosphoinositides were extracted and separated by TLC as described in Materials and Methods. Migrations of authentic PI(4,5)P2, PI(4)P, or PI standards are indicated by outlined circles. PI; phosphatidylinositol. (B) Direct quantitation of radiolabeled spots from lanes 6 through 10 in (A). Each bar represents the fold-increase of PI(3,4,5)P3 formed (net counts per minute) relative to the buffer-only control (A, lane 5). Results are presented as the mean of three experiments ± standard error.
Effect of Erb-A on PI3K lipid kinase activity. (A) Triton-soluble fractions of resting platelet lysate were immunoprecipitated with equal amounts of either PI3K antibody (lanes 5 through 10) or an isotype-matched antibody (lane 4). The washed immune complexes were subjected to a “cold” in vitro kinase assay in the presence of ATP/Mn2+, and in the absence or presence of the indicated concentrations of Erb-A. Control reactions containing no Erb-A were incubated with either buffer alone (lanes 4 and 5) or ethanol/buffer vehicle (lane 6). The phosphorylated immune complexes were washed and incubated for 10 minutes in the presence of γ-32P-ATP, Mg2+, and PI(4,5)P2substrate. Radiolabeled phosphoinositides were extracted and separated by TLC as described in Materials and Methods. Migrations of authentic PI(4,5)P2, PI(4)P, or PI standards are indicated by outlined circles. PI; phosphatidylinositol. (B) Direct quantitation of radiolabeled spots from lanes 6 through 10 in (A). Each bar represents the fold-increase of PI(3,4,5)P3 formed (net counts per minute) relative to the buffer-only control (A, lane 5). Results are presented as the mean of three experiments ± standard error.
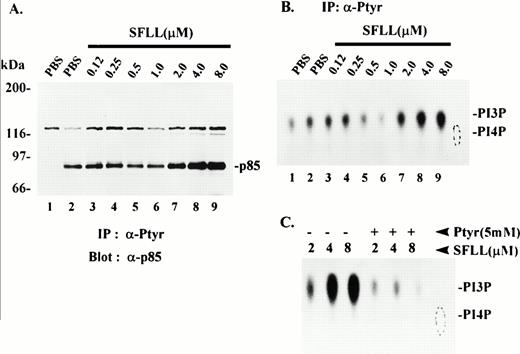
Recovery of p85 and PI3K lipid kinase activity in anti-Ptyr immune complexes from thrombin receptor-activated platelets. Platelets were treated for 1 minute with either PBS vehicle or the indicated concentrations of SFLLRNPNDKY (SFLL) and lysed in 1% Triton-containing buffer. (A) Triton-solubilized protein was immunoprecipitated with either no antibody (lane 1) or with anti-Ptyr antibody (lanes 2 through 9). Immune complexes were washed and solubilized in SDS-PAGE sample buffer. Proteins were separated by SDS-PAGE and immunoblotted with anti-p85 monoclonal antibody. Molecular mass markers in kilodaltons are indicated at the left. IP; immunoprecipitation. (B) Triton-solubilized protein was immunoprecipitated with either no antibody (lane 1) or with anti-Ptyr antibody (lanes 2 through 9). Anti-Ptyr immune complexes were incubated with phosphatidylinositol (PI) and assayed for PI3K lipid kinase activity as described in Materials and Methods. Phosphorylated products were separated by TLC and visualized by autoradiography. The relative migration of PI4P standard (dotted oval) and PI3P are indicated at the right. The radioactivity (net counts) present in each spot was quantitated directly on the TLC plate and was as follows: lane 1, 9,713; lane 2, 11,216; lane 3, 13,226; lane 4, 13,324; lane 5, 7,995; lane 6, 4,830; lane 7, 18,036; lane 8, 24,355; lane 9, 29,064. Results are representative of several independent experiments. (C) Platelets were treated with the indicated concentrations of SFLLRNPNDKY (SFLL) and lysed. The Triton-solubilized lysates were immunoprecipitated with anti-Ptyr antibody in the absence (−) or presence (+) of excess (5 mmol/L) phosphotyrosine (Ptyr). The anti-Ptyr immune complexes were examined for PI3K activity as in (B).
Recovery of p85 and PI3K lipid kinase activity in anti-Ptyr immune complexes from thrombin receptor-activated platelets. Platelets were treated for 1 minute with either PBS vehicle or the indicated concentrations of SFLLRNPNDKY (SFLL) and lysed in 1% Triton-containing buffer. (A) Triton-solubilized protein was immunoprecipitated with either no antibody (lane 1) or with anti-Ptyr antibody (lanes 2 through 9). Immune complexes were washed and solubilized in SDS-PAGE sample buffer. Proteins were separated by SDS-PAGE and immunoblotted with anti-p85 monoclonal antibody. Molecular mass markers in kilodaltons are indicated at the left. IP; immunoprecipitation. (B) Triton-solubilized protein was immunoprecipitated with either no antibody (lane 1) or with anti-Ptyr antibody (lanes 2 through 9). Anti-Ptyr immune complexes were incubated with phosphatidylinositol (PI) and assayed for PI3K lipid kinase activity as described in Materials and Methods. Phosphorylated products were separated by TLC and visualized by autoradiography. The relative migration of PI4P standard (dotted oval) and PI3P are indicated at the right. The radioactivity (net counts) present in each spot was quantitated directly on the TLC plate and was as follows: lane 1, 9,713; lane 2, 11,216; lane 3, 13,226; lane 4, 13,324; lane 5, 7,995; lane 6, 4,830; lane 7, 18,036; lane 8, 24,355; lane 9, 29,064. Results are representative of several independent experiments. (C) Platelets were treated with the indicated concentrations of SFLLRNPNDKY (SFLL) and lysed. The Triton-solubilized lysates were immunoprecipitated with anti-Ptyr antibody in the absence (−) or presence (+) of excess (5 mmol/L) phosphotyrosine (Ptyr). The anti-Ptyr immune complexes were examined for PI3K activity as in (B).
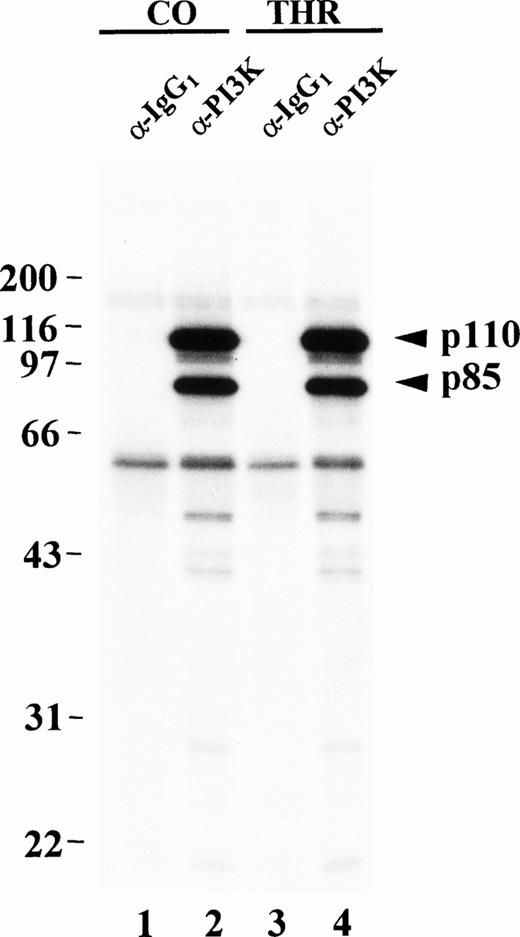
In vitro phosphorylation of PI3K from resting or thrombin-stimulated platelets. Control (CO) or thrombin-treated (THR) platelets were lysed in 1% Triton-containing lysis buffer and Triton-soluble fractions were immunoprecipitated with equal amounts of either p85 antibody (lanes 2 and 4) or an isotype-matched control antibody (lanes 1 and 3). The washed immune complexes were incubated in the presence of γ-32P-ATP and Mn2+ in an in vitro kinase assay as described in Materials and Methods. Phosphorylated proteins were eluted, separated by SDS-PAGE and detected by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
In vitro phosphorylation of PI3K from resting or thrombin-stimulated platelets. Control (CO) or thrombin-treated (THR) platelets were lysed in 1% Triton-containing lysis buffer and Triton-soluble fractions were immunoprecipitated with equal amounts of either p85 antibody (lanes 2 and 4) or an isotype-matched control antibody (lanes 1 and 3). The washed immune complexes were incubated in the presence of γ-32P-ATP and Mn2+ in an in vitro kinase assay as described in Materials and Methods. Phosphorylated proteins were eluted, separated by SDS-PAGE and detected by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
To show the p85 and p110 bands corresponded to the PI3K heterodimer, equivalent amounts of post-130,000g, cytosolic fractions were serially precleared four times with either Protein G beads alone, an isotype-matched antibody, p85 antibody, or an irrelevant antibody. All of the precleared lysates were then reimmunoprecipitated with p85 antibody and the washed immune complexes subjected to an in vitro kinase assay with γ-32P-ATP and Mn2+. Lysates from resting platelets precleared four times with either Protein-G beads alone, IgG1, or antibody to the cytoskeletal protein, α-actinin, followed by immunoprecipitation with p85 antibody showed extensive p85 and p110 phosphorylation in the in vitro kinase assay (Fig 2A, lanes 1, 2, and 4). Lysates precleared four times with p85 antibody were completely depleted of p85 and p110 resulting in the absence of phosphorylated p85 and p110 in the in vitro kinase assay (lane 3). Figure 2B confirms the PI3K heterodimer was specifically precleared by the p85 antibody. The four immune complexes precleared from each lane in Fig 2A were eluted from the Protein G beads, separated by SDS-PAGE, and immunoblotted for p110 content with a p110α-specific antibody. As is clearly shown, proteins eluted from beads of the first anti-p85 preclearing step show a considerable amount of p110 (lane 3), followed by a diminishing amount with each successive preclearing step. p110 did not appear when the preclearing was done with beads alone, anti-IgG1, or with antiactinin antibodies. These results, clearly show the p110 band coprecipitating with p85 is the p110 subunit of PI3K.
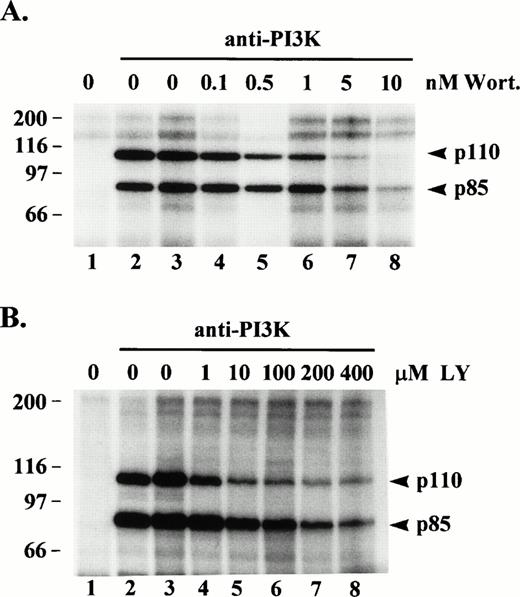
Wortmannin is a potent, irreversible inhibitor of PI3K catalytic activity. Because p110 has been shown to phosphorylate p85 on serine 608,21 we would expect to see inhibition of p85 phosphorylation in the presence of wortmannin, but we were also interested in whether p110 phosphorylation would be inhibited. Anti-PI3K immune complexes from, post-130,000g, cytosolic fractions of resting platelet lysates were preincubated in the absence or presence of various concentrations of wortmannin and then subjected to an in vitro kinase assay. Figure 3A shows heavy phosphorylation of p85 and p110 occurred in anti-p85 immune complexes incubated with buffer only (lane 2) or with buffer plus DMSO vehicle only (lane 3). Anti-p85 immune complexes preincubated in the presence of 5 or 10 nmol/L wortmannin showed a significant decrease in phosphorylation of p85 showing the protein kinase activity of p110 was indeed inhibited (lanes 7 and 8). Wortmannin, also inhibited the phosphorylation of p110 (lanes 7 and 8). The same experiment conducted with the specific PI3K inhibitor, LY29400233 (1 to 400 μmol/L), gave similar results but even at the higher concentrations some residual phosphorylation of p85 and p110 remained (Fig 3B). These data provide additional evidence the p85 and p110 bands phosphorylated in the in vitro kinase assay are PI3K, and indicate a good portion of p110 phosphorylation, in vitro, may be due to autophosphorylation.
Effect of wortmannin or LY294002 on protein kinase activity of PI3K. (A) PI3K was immunoprecipitated from, post-130,000g, cytosolic fractions of platelet lysates (lanes 2 through 8). Lane 1 consisted of Protein G beads alone incubated with lysate. The washed immune complexes were incubated for 20 min with either kinase buffer alone (lanes 1 to 2), buffer/dimethylsulfoxide vehicle (lane 3), or the indicated wortmannin (Wort) concentrations (lanes 4 through 8). (B) Same as in (A) except washed immune complexes were incubated with either kinase buffer alone (lanes 1 and 2), buffer/ethanol vehicle (lane 3), or the indicated LY294002 (LY) concentrations (lanes 4 through 8), and lane 1 represents an IgG1 control antibody immunoprecipitate. Incubations were then subjected to an in vitro kinase assay as described in Fig 1.32P-labeled proteins were eluted, separated by SDS-PAGE and detected by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
Effect of wortmannin or LY294002 on protein kinase activity of PI3K. (A) PI3K was immunoprecipitated from, post-130,000g, cytosolic fractions of platelet lysates (lanes 2 through 8). Lane 1 consisted of Protein G beads alone incubated with lysate. The washed immune complexes were incubated for 20 min with either kinase buffer alone (lanes 1 to 2), buffer/dimethylsulfoxide vehicle (lane 3), or the indicated wortmannin (Wort) concentrations (lanes 4 through 8). (B) Same as in (A) except washed immune complexes were incubated with either kinase buffer alone (lanes 1 and 2), buffer/ethanol vehicle (lane 3), or the indicated LY294002 (LY) concentrations (lanes 4 through 8), and lane 1 represents an IgG1 control antibody immunoprecipitate. Incubations were then subjected to an in vitro kinase assay as described in Fig 1.32P-labeled proteins were eluted, separated by SDS-PAGE and detected by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
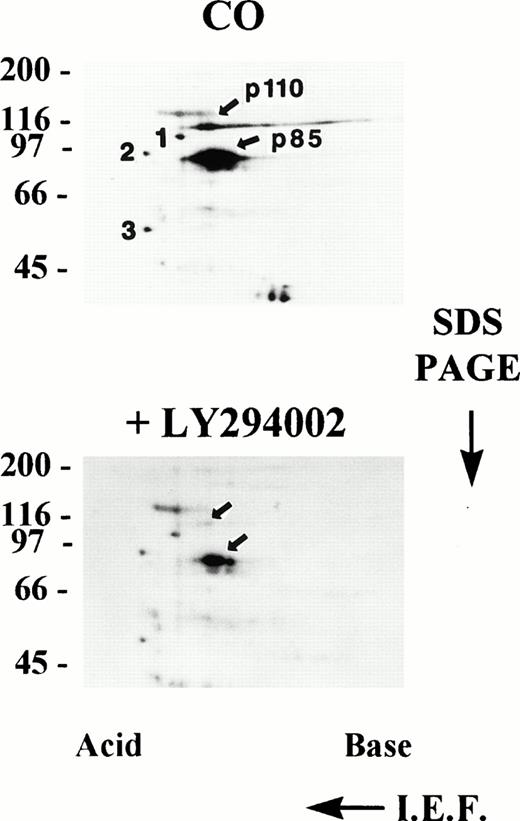
To analyze for the possibility of multiple bands migrating at 85 and 110 kD, in vitro-phosphorylated PI3K immune complexes from resting platelet cytosol were analyzed by two-dimensional electrophoresis involving isoelectric focusing (pH 3-10) in the first dimension followed by SDS-PAGE in the second dimension. Figure 4, upper panel, illustrates two-dimensional electrophoresis of in vitro phosphorylated PI3K immune complexes from resting (CO) platelet cytosol showing heavily phosphorylated, discrete spots at 85 and 110 kD localizing at acidic isoelectric points. There was some streaking of p110, which did not always occur. Two-dimensional analysis of PI3K immune complexes phosphorylated in vitro in the presence of the specific PI3K inhibitor LY294002 showed greatly diminished phosphorylation (Fig 4, lower panel). P85 and p110 phosphorylation was inhibited by 88% and 87%, respectively, as determined by AMBIS scan quantitation of radioactivity in each spot. This was a specific decrease in p85 and p110 because the decrease in intensity of three consistently seen unidentified spots in the lower panel was only 28%, 25%, and 33% respectively, relative to the same spots labeled 1, 2, and 3 in the upper panel. From these results it appears that multiple proteins were not present in the 85-kD and 110-kD bands and the majority, if not all, of the bands consisted of the PI3K heterodimer.
Two-dimensional electrophoresis of PI3K immune complexes phosphorylated, in vitro. PI3K was immunoprecipitated from, post-130,000g, cytosolic fractions of resting platelet lysates and the immune complexes subjected to an in vitro protein kinase assay in the presence of Mn2+ and γ-32P-ATP in the absence (CO) or presence of 30 μmol/L LY294002. Radiolabeled proteins were eluted with urea sample buffer and analyzed by two-dimensional electrophoresis as described in Materials and Methods. Spots 1, 2, and 3 are unidentified reference points used for relative quantitation (see Results). Molecular mass markers in kilodaltons are indicated at the left. IEF; isoelectric focusing.
Two-dimensional electrophoresis of PI3K immune complexes phosphorylated, in vitro. PI3K was immunoprecipitated from, post-130,000g, cytosolic fractions of resting platelet lysates and the immune complexes subjected to an in vitro protein kinase assay in the presence of Mn2+ and γ-32P-ATP in the absence (CO) or presence of 30 μmol/L LY294002. Radiolabeled proteins were eluted with urea sample buffer and analyzed by two-dimensional electrophoresis as described in Materials and Methods. Spots 1, 2, and 3 are unidentified reference points used for relative quantitation (see Results). Molecular mass markers in kilodaltons are indicated at the left. IEF; isoelectric focusing.
Both p85 and p110 are phosphorylated in vitro on serine, threonine, and tyrosine.
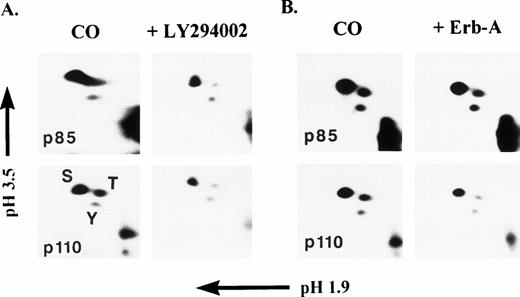
To determine the nature of p85 and p110 phosphorylation, phosphoamino acid analysis was performed on each phosphoprotein. PI3K was immunoprecipitated from the cytosolic fraction of resting platelet lysates and subjected to an in vitro kinase assay with γ-32P-ATP. The p85 and p110 bands were separated by SDS-PAGE, localized by autoradiography, extracted from the gel, and subjected to two-dimensional phosphoamino acid analysis as described in Materials and Methods. Figure 5A and B, left panels, clearly show that amino acid hydrolysates of in vitro phosphorylated p85 and p110 consisted of primarily phosphoserine (pS), but also significant phosphothreonine (pT) and phosphotyrosine (pY). Threonine phosphorylation of p110 has not been previously observed. Figure 5A, right panels, show that addition of LY294002 to the in vitro kinase assay caused a significant decrease in p85 pS (41%) and pT (82%) and a slight decrease in pY (10%). For p110, a significant decrease in pS (55%) and pT(71%) occurred, but a modest decrease in pY (29%) also occurred. These results show that LY294002 primarily inhibited serine and threonine phosphorylation of p85 and p110, which can be expected, with only a modest effect on tyrosine phosphorylation, indicating again, that the serine/threonine phosphorylation of p85 and p110 was due to p110 catalytic activity. Erbstatin analog (Erb-A), a stable analog of erbstatin, is a protein tyrosine kinase inhibitor capable of inhibiting tyrosine phosphorylation of the epidermal growth-factor receptor (EGFR) tyrosine kinase (Ki = 3.3 μmol/L).34,35 Erb-A has also been shown to inhibit autophosphorylation of the p60src tyrosine kinase.36 Figure 5B, right panels, shows that the presence of 5 mmol/L Erb-A caused a 27% decrease in p85 pS, an 18% decrease in pT, and a modest 21% decrease in pY. For p110, a 36% decrease in pS, a 56% decrease in pT, and a 24% decrease in pY was noted. Although both p85 and p110 tyrosine phosphorylation was inhibited by Erb-A, it appears that serine and threonine phosphorylation, primarily of p110, was also sensitive to the effects of Erb-A.
Two-dimensional phosphoamino acid analysis of p85 and p110 phosphorylated, in vitro. PI3K was immunoprecipitated from, post-130,000g cytosolic fractions of platelet lysates and the immune complexes subjected to an in vitro protein kinase assay in the presence of Mn2+ and γ-32P-ATP in the absence or presence of either 30 μmol/L LY294002 (A), or 5 μmol/L erbstatin-A (B). Radiolabeled proteins were eluted and separated by SDS-PAGE. p85 and p110 bands were subjected to two-dimensional phosphoamino acid analysis as described in Materials and Methods. Phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y) standards were run concurrently with the p85 and p110 amino acid hydrolysates and stained with ninhydrin. Migration of phosphoamino acid standards was coincident with the radiolabeled spots.
Two-dimensional phosphoamino acid analysis of p85 and p110 phosphorylated, in vitro. PI3K was immunoprecipitated from, post-130,000g cytosolic fractions of platelet lysates and the immune complexes subjected to an in vitro protein kinase assay in the presence of Mn2+ and γ-32P-ATP in the absence or presence of either 30 μmol/L LY294002 (A), or 5 μmol/L erbstatin-A (B). Radiolabeled proteins were eluted and separated by SDS-PAGE. p85 and p110 bands were subjected to two-dimensional phosphoamino acid analysis as described in Materials and Methods. Phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y) standards were run concurrently with the p85 and p110 amino acid hydrolysates and stained with ninhydrin. Migration of phosphoamino acid standards was coincident with the radiolabeled spots.
Erb-A attenuates PI3K phosphorylation, in vitro.
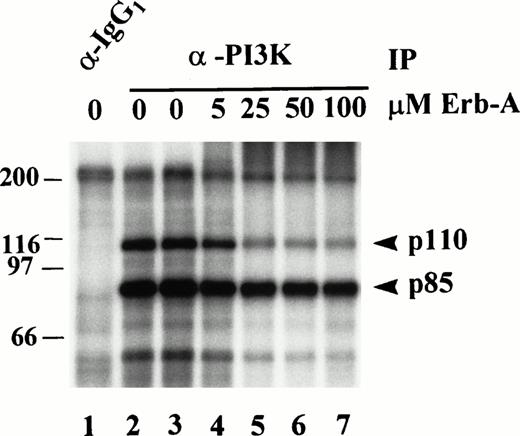
The p110 subunit of PI3K has not been shown to possess intrinsic tyrosine kinase activity. Therefore, phosphorylation of p85 and p110 on tyrosine in the in vitro kinase assay suggests they are substrates for a tyrosine kinase intimately associated with the PI3K immune complex. To substantiate this, anti-PI3K immunoprecipitates of platelet lysates were subjected to in vitro kinase assays in the absence or presence of various concentrations of Erb-A (Fig 6). Relative to the “ethanol only” control in lane 3, AMBIS scan quantitation of radioactivity incorporated into p85 and p110 shows p85 phosphorylation was inhibited 27% by 5 μmol/L Erb-A (Fig 6, lane 4), inhibited 52% by 25 μmol/L Erb-A (lane 5), and inhibited 57% and 65% in the presence of 50 and 100 μmol/L Erb-A, respectively (lanes 6 and 7). Phosphorylation of p110 was inhibited 40% by 5 μmol/L Erb-A (lane 4), inhibited 78% by 25 μmol/L Erb-A (lane 5), and inhibited 85% and 90% in the presence of 50 and 100 μmol/L Erb-A, respectively (lanes 6 and 7). These results show that both p85 and p110 phosphorylation was decreased 27% and 40%, respectively, by 5 μmol/L Erb-A, a concentration around the Ki for EGFR kinase inhibition. The reason for the significant inhibition at the higher Erb-A concentrations is not understood, but may reflect competition of high erbstatin concentrations with ATP.34
Effect of erbstatin analog on p85 and p110 phosphorylation, in vitro. PI3K was immunoprecipitated (IP) from Triton-soluble fractions of resting platelet lysates. Isotype-matched IgG1 antibody was used as a control (lane 1). Washed immune complexes were incubated in the absence or presence of the indicated concentrations of Erb-A for 15 minutes, and subjected to an in vitro kinase assay in the presence of γ-32P-ATP, and Mn2+ as described in Materials and Methods. In the absence of erb-A, kinase buffer alone (lanes 1 and 2), or ethanol/buffer vehicle (lane 3) were used as controls.32P-labeled proteins were eluted, separated by SDS-PAGE, and identified by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
Effect of erbstatin analog on p85 and p110 phosphorylation, in vitro. PI3K was immunoprecipitated (IP) from Triton-soluble fractions of resting platelet lysates. Isotype-matched IgG1 antibody was used as a control (lane 1). Washed immune complexes were incubated in the absence or presence of the indicated concentrations of Erb-A for 15 minutes, and subjected to an in vitro kinase assay in the presence of γ-32P-ATP, and Mn2+ as described in Materials and Methods. In the absence of erb-A, kinase buffer alone (lanes 1 and 2), or ethanol/buffer vehicle (lane 3) were used as controls.32P-labeled proteins were eluted, separated by SDS-PAGE, and identified by autoradiography. Molecular mass markers in kilodaltons are indicated at the left.
Erb-A does not affect PI3K lipid kinase activity.
Reports have shown PI3K lipid kinase activity is attenuated by serine phosphorylation of p85 by p110.21 It was of interest to know whether PI3K lipid kinase activity was affected by p110 tyrosine phosphorylation. To determine this, PI3K was immunoprecipitated from platelet lysates, using equal amounts of p85 monoclonal antibody, and subjected to a cold in vitro protein kinase assay with ATP in the absence or presence of various concentrations of Erb-A. The phosphorylated immune complexes were washed extensively and subjected to a PI3K lipid kinase assay using phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) as substrate in the presence of γ-32P-ATP and Mg2+ as described in Materials and Methods. PtdIns(4,5)P2 was used as a substrate to ensure that the product of the reaction, PtdIns(3,4,5)P3, was labeled only on the 3 position. Labeled phospholipids were extracted and separated by TLC in a mobile phase, which separates phosphoinositides based on their level of phosphorylation.32Figure 7A illustrates the separation of32P-PtdIns(3,4,5)P3 from PtdIns(4,5)P2, PtdIns(4)P, and PtdIns standards. PtdIns(3,4,5)P3 was formed only on p85 antibody immunoprecipitates (Fig 7A, lanes 5 through 10), and not on the control isotype-matched IgG1 immune complexes (Fig 7A, lane 4) indicating the specificity of the assay. The same profile of lipid separation was seen when PtdIns(4,5)P2 was used as a substrate in a PI3K lipid kinase assay performed on antiphosphotyrosine immune complexes from PDGF-stimulated fibroblast lysates (data not shown). Figure 7B illustrates quantitation of the radiolabel in spots shown in Fig 7A, and shows PI3K subjected to in vitro phosphorylation in the presence of 5 to 100 μmol/L Erb-A (lanes 7 through 10) was no different in its ability to convert PtdIns(4,5)P2 to PtdIns(3,4,5)P3 than in the presence of the vehicle (buffer/ethanol) alone (lane 6).
Recovery of p85 and PI3K activity in anti-Phosphotyrosine immune complexes from SFLLRNPNDKY-treated platelets.
To determine whether PI3K levels and PI3K enzymatic activity were recoverable in anti-Ptyr antibody complexes from agonist-activated platelets, platelets were treated with either PBS or a range of suboptimal (<2 μmol/L) to optimally-activating (2 to 8 μmol/L) concentrations of SFLLRNPNDKY (SFLL) peptide and immediately lysed. Triton-soluble proteins were then immunoprecipitated with the 4G10 anti-Ptyr antibody. The immune complexes were then analyzed for either p85 levels (Fig 8A), or PI3K lipid kinase activity (Figs 8B and C). In platelets stimulated with activating concentrations of peptide (aggregation observed, data not shown), increased levels of anti-Ptyr-recoverable p85 were found (Fig 8A, lanes 7 through 9) when compared to controls (lanes 1 and 2). Although p85 was observed in anti-Ptyr immune complexes from untreated platelets (lane 2) or from platelets treated with concentrations of peptide that were suboptimal in producing aggregation (lanes 3 through 6), no p85 was present in the sample in which no anti-Ptyr antibody was present (lane 1). These results suggest that p85, or a complex containing p85 tightly-associated with a phosphotyrosyl protein, was specifically immunoprecipitated by anti-Ptyr antibody.
Based on the results above and Fig 2B, it would be predicted that increased levels of p85 in anti-Ptyr immunoprecipitates would parallel elevated PI3K enzymatic activity. To confirm this, platelets were treated as described in Fig 8A and platelet lysates were immunoprecipitated with anti-Ptyr antibody. The immune complexes were then examined for PI3K enzymatic activity as described in Materials and Methods. Figure 8B illustrates a stimulus-dependent increase in anti-Ptyr-recoverable PI3K lipid kinase activity that correlates well with that of p85 levels shown in Fig 8A. Figure 8B, lane 1, shows that in the control immunoprecipitation performed in the absence of anti-Ptyr antibody there was slight PI3K activity nonspecifically bound to the beads. In the absence of stimulation (lane 2) or in the presence of suboptimal agonist concentrations (lanes 3 through 6), anti-Ptyr-recoverable PI3K activity remained at or below control levels. This activity was increased fourfold to eightfold in a concentration-dependent manner by optimal agonist concentrations (Fig8B, lanes 7 through 9). Identical results were obtained when thrombin was used as agonist (data not shown). The anti-Ptyr recoverable PI3K activity was specifically phosphotyrosine dependent because it could be effectively competed away when phosphotyrosine was present in excess (5 mmol/L) during the antiphoshotyrosine immunoprecipitation (Fig 8C).
DISCUSSION
The PI3K p110 subunit possesses a second catalytic function as a Mn2+-dependent protein kinase and can phosphorylate the p85 subunit of PI3K on serine 608 in vitro and in vivo.21 In vivo, serine and threonine phosphorylation of p85 as well as serine phosphorylation of p110 has also been shown in32Pi-labeled insect cells cotransfected with p85 and p110.21 When expressed in insect cells, p85 and p110 were shown to be unassociated with other proteins or kinases.21 Others have shown in vitro serine phosphorylation of p85 and, to a lesser extent, of p110 purified from rat liver.20 However, the PI3K heterodimer normally binds to a variety of intracellular signaling molecules, many of which are tyrosine kinases,12,18,37 which could potentially regulate the activity or trafficking of PI3K. Our results indicate when PI3Kα is immunopurified from platelets, p85 is phosphorylated on serine, in vitro, in a Mn2+-dependent manner as previously shown,20 21 but is also phosphorylated heavily on threonine and tyrosine. More surprisingly, p110 is heavily phosphorylated in vitro on serine, and also significantly on threonine and tyrosine; however, the in vitro phosphorylation of the isolated PI3K complex does not appear to be affected by thrombin activation and aggregation of platelets.
We provide four lines of evidence showing the p85α and p110α bands observed to be phosphorylated in vitro are indeed PI3K; (1) these bands are phosphorylated only in anti-PI3K immune complexes; Protein G beads alone or IgG1 immunoprecipitation show very little to no nonspecifically-bound or autophosphorylated proteins and no phosphorylated PI3K, (2) serial immunodepletion of PI3K from platelet lysates followed by anti-PI3K immunoprecipitation and in vitro kinase assay results in a complete loss of phosphorylated 85- and 110-kD bands; immunoblotting the cleared anti-p85 immune complexes with a p110α antibody confirms the presence of p110α, (3) incubation of anti-PI3K immune complexes with the irreversible PI3K inhibitor, wortmannin, or with the reversible but specific inhibitor LY294002, greatly inhibits the in vitro phosphorylation of p85α and p110α indicating that p110 catalytic activity is responsible for substantial phosphorylation of both p85 and p110, and (4) two-dimensional electrophoresis of phosphorylated PI3K immune complexes derived from post-130,000g cytosolic fractions show discrete spots for both p85 and p110, the intensities of which are attenuated 88% and 87%, respectively, in LY294002-treated samples, with no evidence for multiple proteins comigrating with either p85 or p110.
Phosphoamino acid analysis of in vitro phosphorylated PI3K immunoprecipitated from platelets confirmed both p85α and p110α are phosphorylated on serine, threonine and tyrosine. Because p110 has been shown to be a serine kinase it is also possible that it phosphorylates threonine residues. The significant inhibition of both p85 and p110 phosphorylation by wortmannin or LY294002 treatment would strongly argue that a portion of the in vitro phosphorylation of p110 on serine and threonine is likely due to autophosphorylation. P110α is already known to phosphorylate p85α on serine, but our results indicate p85 threonine phosphorylation also appears to be due to p110α catalytic activity. Although this is the most likely explanation, our data do not rule out the possibility of PI3K association with another serine/threonine kinase. Our observed in vitro tyrosine phosphorylation of p85α and p110α from resting platelet cytosol would suggest the presence of a tyrosine kinase constitutively associated with the PI3K immune complex. Many reports show the association of PI3K with a variety of tyrosine kinases, including src-family kinases.14,15,18 Many of these interactions, especially those with tyrosine phosphorylated receptor tyrosine kinases such as the prototypic PDGFR, are activation-induced and occur via phosphotyrosine binding to SH2 domains of p85 serving to activate PI3K catalytic activity.12,37,38 Although one study has shown both p85α and p110α subunits are substrates for the activated PDGFR,24 the lack of evidence showing a transmembrane receptor tyrosine kinase in platelets capable of docking PI3K, in conjunction with our evidence for significant tyrosine phosphorylation of p85 and p110, would argue for an intimate association between PI3Kα and a nonreceptor tyrosine kinase in platelets. It is unclear whether PI3K tyrosine phosphorylation exerts a regulatory effect on p110 lipid kinase activity as does p85 serine phosphorylation.20 21 Our data show p110α lipid kinase activity towards PtdIns(4,5)P2 is not affected after PI3K immune complexes are subjected to Mn2+-dependent in vitro phosphorylation either in the absence or presence of Erb-A. This suggests PI3K tyrosine phosphorylation does not appear to influence p110 lipid kinase activity.
The SH3 domains of certain src-family kinases can bind the polyproline motifs of p85,14,15 and because these interactions are not phosphotyrosine dependent they may not be contingent on an activation stimulus. We consistently observe a protein in the molecular mass range of 60 to 63 kD, which becomes phosphorylated in the in vitro kinase assay performed on anti-PI3K immune complexes (Figs 1, 2, and 6). The in vitro phosphorylation of this protein was inhibited by Erb-A (see Fig 6). This may be one of several nonreceptor src-family kinases, such as p60src, p59fyn, or p62yes, found in platelets. We also observe another autophosphorylated band at approximately 70 to 72 kD, which consistently coprecipitates specifically with PI3K. One possible candidate for this band is p72syk, a tyrosine kinase that has been shown to bind to PI3K in platelets.39If the p110 subunit of PI3K does not possess intrinsic tyrosine kinase activity, then an associated tyrosine kinase must be responsible for tyrosine phosphorylation of p85α and p110α in vitro. Data from our experiments using Erb-A support this argument. At 5 μmol/L Erb-A, which approximates the Ki (3.3 μmol/L) for inhibition of epidermal growth factor receptor (EGFR) phosphorylation, in vitro p85α phosphorylation was inhibited by 27% and p110α phosphorylation was decreased by 40%. Our attempts to immunoblot proteins derived from anti-PI3K immune complexes with antibodies to either p72syk or the src-family kinases p60src, p59fyn, or p62yes were negative. It is possible only a small percentage of PI3K may associate with these kinases, which would make detection difficult. A constitutively-associated tyrosine kinase might easily phosphorylate p85 or p110 in vitro after cell lysis, as we have observed, especially a src-family kinase which may become dephosphorylated at its C-terminal regulatory phosphotyrosine by a phosphatase. In vivo, a tyrosine kinase constitutively associated with PI3K may be “off” until an activation stimulus triggers translocation of the cytosolic PI3K/tyrosine kinase complex to the membrane/cytoskeleton where the kinase may become activated to phosphorylate p85 or p110. Tyrosine phosphorylation may then serve to recruit SH2-containing proteins which may either regulate PI3K activity or bind downstream signaling molecules.
Whether PI3K phosphorylation is an activation-dependent, biologically significant event, in vivo, remains an unanswered question. Recently, the tyrosine phosphorylation of p85 in response to GM-CSF stimulation of neutrophils has been shown.25 We have been unable to detect thrombin-dependent phosphorylation of either p85α or p110α in platelets, either in vitro or in vivo. We are able to immunoprecipitate from Triton-soluble fractions of lysates from TRAP-stimulated platelets, enhanced amounts of PI3K and PI3K lipid kinase activity with antiphosphotyrosine antibody, as shown in Fig 8. These results confirm that increased levels of the p85 regulatory subunit of PI3K can be recovered in anti-Ptyr antibody complexes from thrombin receptor-stimulated platelets, and this recovery is dependent on stimulus concentrations that induce aggregation. Moreover, the relative increase in PI3K enzymatic activity recovered in the anti-Ptyr immune complexes paralleled the increase in p85 recovered (compare Fig 8A and B), indicating that the catalytic p110α subunit was also present in the anti-p85 immune complexes. It is not known, however, whether the recovery of PI3K by anti-Ptyr antibody is due to tyrosine phopshorylation of PI3K itself or of an intimately associated protein.
Based on our data examining the in vitro phosphorylation of PI3K immunopreciptiated from post-130,000g cytosolic fractions of platelet lysates, our results suggest that in resting platelets PI3K is constitutively associated with a non-receptor protein tyrosine kinase which can use p85α and p110α as substrates, although a direct association with a kinase has not been confirmed. We have not ruled out the possibility that the PI3K heterodimer has intrinsic protein tyrosine kinase activity, but based on previous results21this does not appear to be likely. We show that p110α is also threonine phosphorylated which has not been previously observed, and in the context of our studies with wortmannin and LY294002, a considerable portion of p110α serine/threonine phosphorylation appears to be a result of autophosphorylation. Recently, a newly discovered leukocyte-specific PI3K designated p110δ was shown to autophosphorylate on serine in a Mn2+-dependent manner, and was inhibited by wortmannin.8 However, this same group was unable to show autophosphorylation of p110α alone.8 In all of our studies p110α was always bound to p85 and our data show p110α does autophosphorylate, in vitro. This indicates that the physical binding of p85α to p110α may permit p110α autophosphorylation on serine and threonine.
ACKNOWLEDGMENT
We thank Shinya Tanaka for providing p85 monoclonal antibody. We also thank Peter J. Newman and Robert T. Abraham for critical comments and suggestions.
Supported by Public Health Service grant HL-51413 (J.A.A.) from the National Institutes of Health (Bethesda, MD).
Address reprint requests to James A. Augustine, PhD, Blood Research Institute, 8727 Watertown Plank Rd, Milwaukee, WI 53226-3548.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal