Abstract
The tyrosine kinase Tec belongs to a new group of structurally related nonreceptor tyrosine kinases that also includes Btk and Itk. Previous studies have suggested that these kinases have lineage-specific roles, with Tec being involved mainly in the regulation of cytokine-mediated myeloid cell growth and differentiation. In this study, we investigated expression and activation of Tec in human B-lymphoid cell lines representing different stages of B-cell maturation, including pro-B (RS4;11, 380, REH), pre-B (NALM6), and mature B (Ramos, and one Epstein-Barr virus [EBV]-transformed lymphoblastoid line) cells. Like Btk, Tec protein was expressed in all B-cell lines tested. Tec was also highly expressed in two EBV-transformed lymphoblastoid lines derived from patients with X-linked agammaglobulinemia (XLA) lacking Btk expression, as well as in tonsillar lymphoid cells. In surface immunoglobulin-positive B cells (Ramos), ligation of the B-cell antigen receptor (BCR) with anti-IgM antibodies caused marked tyrosine phosphorylation of Tec and increased Tec tyrosine kinase activity. Likewise, cross-linking of CD19 with a monoclonal antibody in BCR-negative pro-B (RS4;11, 380) and pre-B (NALM6) cells induced Tec tyrosine phosphorylation and increased Tec autophosphorylation, as well as Btk activation. Tyrosine phosphorylation of Tec, but not of Btk, was detectable in RS4;11 cells after CD38 ligation, suggesting that these kinases are regulated differently. We conclude that Tec is expressed and can be stimulated throughout human B-cell differentiation, implying that this tyrosine kinase plays a role in B-cell development and activation.
GROWTH AND differentiation of hematopoietic cells is regulated by the interaction of surface receptors with their ligands. While some of these receptors mediate signaling events via their own tyrosine kinase activity, others use cytoplasmic tyrosine kinases to transduce signals.1,2Recently, the Tec/Btk/Itk subfamily has emerged as a new group of nonreceptor tyrosine kinases that typically have an amino-terminal pleckstrin-homology domain, a Tec-homology (TH) domain, an Src-homology (SH)-3 domain, an SH2 domain and a kinase domain.3-9 The biologic importance of this kinase subfamily is underscored by the finding that Btk function is essential for B-cell development,10,11 and that mutations in Btk cause X-linked agammaglobulinemia (XLA) in humans and the xid B-cell deficient phenotype in mice.5,6,12 13
It has been suggested that the functions of Btk and Itk are primarily related to B- and T-lymphoid development,5-7,10-15 while the other prototype molecule of this family, Tec, mainly participates in signaling pathways regulating myeloid growth and differentiation.8,16-19 In these cells, Tec is tyrosine phosphorylated following cell stimulation by a variety of hematopoietic growth factors, such as interleukin-3 (IL-3),17 stem-cell factor (SCF),18 and granulocyte colony-stimulating factor (G-CSF).19 Tec tyrosine phosphorylation is accompanied by enhanced kinase activity and increased association with the adaptor molecule Vav via the TH domain.20 It has remained unclear whether Tec participates in signaling in other hematopoietic lineages (eg, lymphoid cells), or whether it can be activated by stimuli other than hematopoietic growth factors.
In this study, we investigated whether Tec is a component of signal transduction in human B-lymphoid cells at different stages of maturation. We found that Tec was expressed in human pro-B, pre-B, and B-lymphoid cells, and even in B cells lacking Btk expression. Ligation of the B-cell antigen receptor (BCR) in mature B cells, and of the functional surface molecules CD19 and CD38 in immature B cells, induced Tec tyrosine phosphorylation and enhanced Tec kinase activity. Direct comparison of Tec and Btk activation by identical stimuli showed similarities and differences between these tyrosine kinases that suggest a complementary role in B-cell development.
MATERIALS AND METHODS
Reagents and cells.
Rabbit antisera to Tec and Btk were previously described.17,18 21 Goat antisera to Tec and Btk were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD38 monoclonal antibodies were T16 (immunoglobulin [Ig] G1; Immunotech Inc, Westbrook, ME) and THB7 (IgG1; American Type Culture Collection [ATCC], Rockville, MD). Anti-CD19 monoclonal antibody was HD37 (IgG1; Dako, Carpinteria, CA). Goat antisera to human and mouse IgM were from Southern Biotechnology Associates (Birmingham, AL). Goat antiserum to mouse Ig was from Jackson Immunoresearch Laboratories (West Grove, PA). For cell stimulation, monoclonal antibodies were used at a concentration of 5 to 10 μg/mL; goat antisera to human IgM and mouse Ig were used at 30 μg/mL. Isotype- and species-matched control antibodies were used at equivalent concentrations. Monoclonal antibody to phosphotyrosine (4G10) was purchased from UBI (Lake Placid, NY). Rabbit antisera to Lyn, Fyn, Blk, and Lck were from Santa Cruz. All other reagents were purchased from Sigma (St Louis, MO).
Human immature B-cell lines RS4;11, 380, REH, and NALM6, and the mature B-cell line Ramos were available from the St. Jude Children's Research Hospital cell bank or were purchased from ATCC. Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines were generated as described from one normal individual and from two patients with XLA and absent Btk expression. In one patient, a 4-bp deletion at codons 76 and 77 of the Btk gene resulted in a frameshift and a premature stop codon at codon 120. In the other, a base pair substitution caused a premature stop codon at codon 520.22 23 All cell lines express CD19 and CD38. The RS4;11, 380, and REH cells lack surface and cytoplasmic Ig (pro-B), NALM6 expresses cytoplasmic μ heavy chains (pre-B), while the remaining cell lines are surface-Ig positive (mature B). Cell lines were maintained in RPMI-1640 (Whittaker Bioproducts Inc, Walkersville, MD) with 10% fetal calf serum (Whittaker), L-glutamine, and antibiotics. Tonsils were from routine tonsillectomies; thymus glands were removed during cardiac surgery. Cell suspension were prepared by teasing the tissue with forceps and scalpels. The cells' viability consistently exceeded 90% by trypan-blue dye exclusion assay.
Immunoprecipitation, electrophoresis, Western blotting, and kinase assay.
Immunoprecipitation was performed as described.21 24Briefly, after stimulation, cells were lysed in 1 mL of ice-cold lysis buffer (50 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 1% [vol/vol] Triton X-100, 5 μg/mL aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 1 mmol/L EDTA, and 1 mmol/L Na3VO4); insoluble material was removed by centrifugation at 20,000g for 20 minutes at 4°C. Supernatants were cleared with protein A-Sepharose (20 μL of 50% slurry) for 1 hour. Antibodies were then added before a 1- to 2-hour incubation at 4°C. The immune-complexes were separated by using protein A-Sepharose.
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), samples were resuspended in sample buffer (10% [vol/vol] glycerol, 5% 2-mercaptoethanol, 3% [wt/vol] SDS, 65 mmol/L Tris-HCl [pH 6.8], and 0.002% [wt/vol] bromophenol blue) and separated on a 7.5% acrylamide gel.21 24 After protein transfer, nitrocellulose filters were incubated for 2 hours in 5% bovine serum albumin in TBS-T (20 mmol/L Tris [pH 7.6], 137 mmol/L NaCl, 0.1% Tween 20), and then with primary antibodies for 1 hour. After washing in TBS-T, the filters were incubated for 1 hour with horseradish peroxidase-conjugated sheep antimouse Ig, donkey antirabbit Ig (Amersham, Arlington Heights, IL), or donkey antigoat Ig (Santa Cruz) and washed again. Antibody binding was visualized by the enhanced chemiluminescence detection system (Amersham); densitometric analysis was performed with an AlphaImager densitometer (Alpha Innotech, San Leandro, CA). In some experiments, the filters were stripped, reblocked, washed, and reprobed.
For in vitro kinase assay, proteins immunoprecipitated with anti-Tec or anti-Btk antibodies were washed with kinase buffer (20 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 10 mmol/L MgCl2, 10 mmol/L MnCl2 for Tec; 40 mmol/L HEPES [pH 7.4], 10 mmol/L MgCl2, 3 mmol/L MnCl2 for Btk) and incubated for 5 to 20 minutes at 30°C in 30 μL of kinase buffer containing 10 μCi of [γ-32P] adenosine triphosphate (ATP). The reaction was stopped by adding 1 mL of ice-cold lysis buffer. After extensive washing, proteins were eluted with SDS-PAGE sample buffer (see above) and separated on 7.5% acrylamide gel. 32P-labeled proteins were visualized by autoradiography. All experiments were repeated at least three times.
RESULTS
Expression of Tec in human B cells at various stages of maturation.
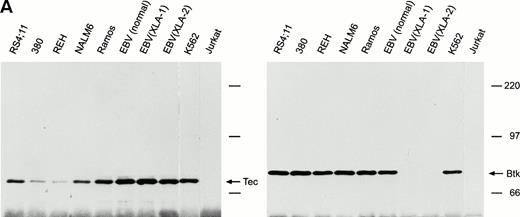
We assessed expression of Tec protein in cell lines representative of different stages of B-cell differentiation. To determine whether Tec expression in human B cells is interrelated with that of its family member Btk, we included two EBV-transformed B-lymphoblastoid cell lines derived from patients with XLA who had no Btk protein expression.23 From cell lysates containing equal amounts of protein (1 mg), we immunoprecipitated Tec with saturating concentrations of a rabbit antiserum to Tec. In all of the B-cell lines, a protein of 70 kD, corresponding to the Tec protein, reacted with the anti-Tec antisera of either goat or rabbit origin (Fig 1A), in line with the findings of Sato et al4 at the mRNA level. Tec expression appeared to be lower, albeit clearly detectable, in 380 and REH pro-B cells. Notably, the two XLA cell lines with impaired Btk expression had a level of Tec similar to that of Btk-positive mature B-cell lines (Fig1A). By densitometry, we determined that RS4;11 and Ramos cells expressed levels of Tec identical to those measured in the myeloid cell line K562, while 380 cells had approximately 30% expression, REH 10%, and NALM6 70%; EBV-transformed cell lines had 120% to 150% Tec expression. Levels of Btk expression were nearly identical in all B-cell lines studied.
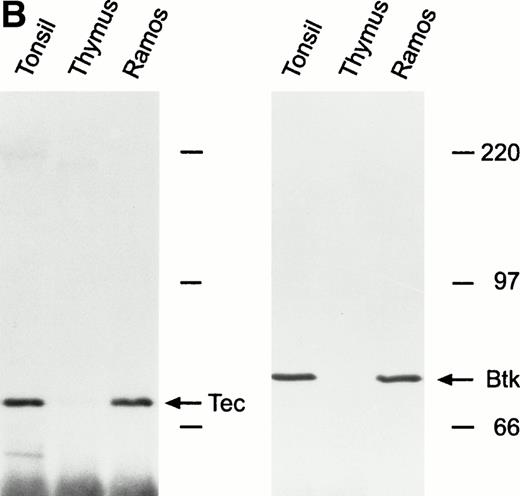
Expression of Tec and Btk in human B cells. After immunoprecipitation with rabbit antisera to Tec or Btk, Western blots were probed with goat antisera against these proteins. (A) Tec was expressed in all B-cell lines studied representative of different stages of maturation, including two EBV-transformed lymphoblastoid cell lines derived from patients with XLA and lacking Btk expression. The myeloid cell line K562 and the T-lymphoid cell line Jurkat were used as a positive and negative control, respectively. (B) Tec and Btk were also expressed in tonsillar lymphoid cells, but not in thymocytes. Molecular mass markers (in kD) are indicated. The intense band of approximately 50 kD corresponds to the Ig heavy chain of the antibody used for immunoprecipitation.
Expression of Tec and Btk in human B cells. After immunoprecipitation with rabbit antisera to Tec or Btk, Western blots were probed with goat antisera against these proteins. (A) Tec was expressed in all B-cell lines studied representative of different stages of maturation, including two EBV-transformed lymphoblastoid cell lines derived from patients with XLA and lacking Btk expression. The myeloid cell line K562 and the T-lymphoid cell line Jurkat were used as a positive and negative control, respectively. (B) Tec and Btk were also expressed in tonsillar lymphoid cells, but not in thymocytes. Molecular mass markers (in kD) are indicated. The intense band of approximately 50 kD corresponds to the Ig heavy chain of the antibody used for immunoprecipitation.
To corroborate results obtained with cell lines, we examined Tec and Btk expression in human tonsillar and thymic lymphoid cells. Both kinases were highly expressed in tonsil cells, at levels similar to those found in Ramos cells, but were not expressed in thymocytes (Fig1B).
Ligation of the B-cell antigen receptor activates Tec in mature B cells.
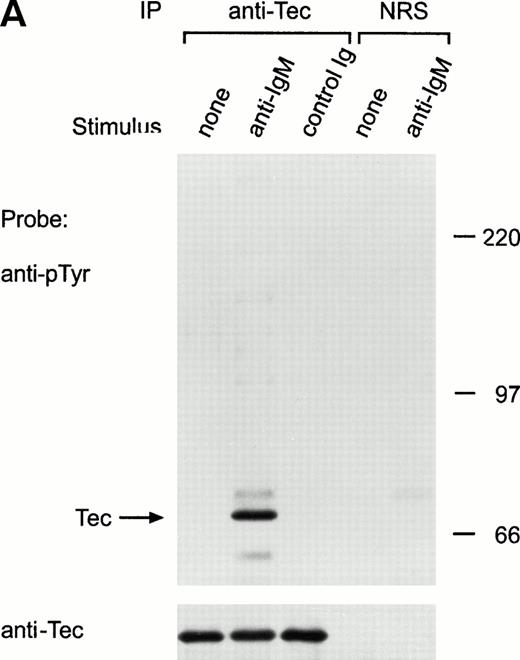
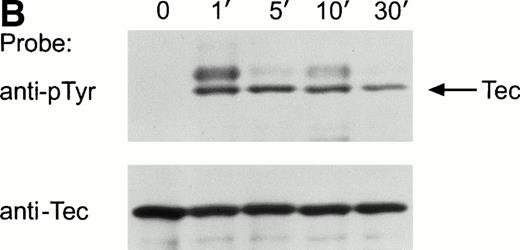
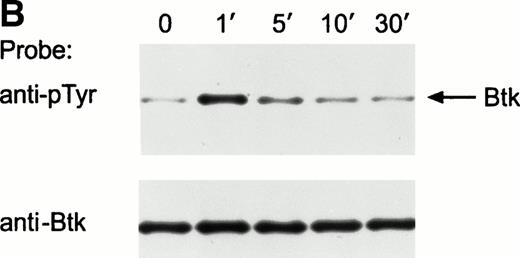
Ligation of the BCR induces tyrosine phosphorylation of several proteins.25-27 To determine whether Tec was activated by BCR signaling, we examined Tec tyrosine phosphorylation in the mature human B-cell line Ramos after cross-linking the BCR with a polyclonal antiserum to human IgM. Exposure to anti-IgM antibody caused marked tyrosine phosphorylation of Tec (Fig 2A). In time-course experiments, Tec tyrosine phosphorylation was rapid and was maximal 1 minute after ligation of BCR; levels of Tec tyrosine phosphorylation remained high for at least 10 minutes, but decreased by 30 minutes after BCR cross-linking (Fig 2B). Tyrosine phosphorylation of Tec was also detected in the murine B-cell line, WEHI-231, after ligation of surface BCR (not shown). In Ramos cells, a tyrosine phosphorylated protein of approximately 62 kD coprecipitated with Tec after BCR ligation (Fig 2A). This band was the only one consistently present in Tec immunoprecipitates. It is likely to correspond to the previously described, but as yet unidentified, protein of a similar molecular weight that coprecipitates with Tec in cells exposed to SCF or IL-6.18 28
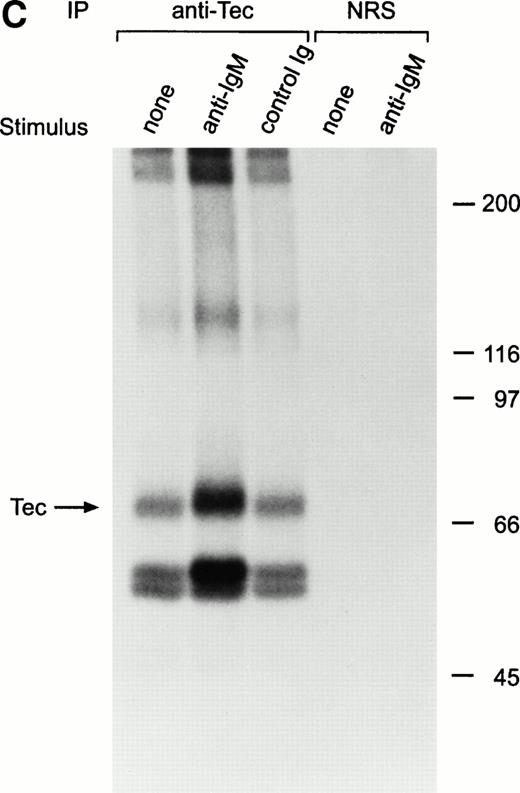
Ligation of BCR induces tyrosine phosphorylation and activation of Tec. (A) Ramos cells were incubated with anti-IgM antiserum or control serum for 5 minutes. Proteins immunoprecipitated with anti-Tec rabbit antibody or normal rabbit serum (NRS) were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). Molecular mass markers (in kD) are indicated. The position of Tec is indicated by an arrow. A tyrosine phosphorylated protein of approximately 62 kD, coprecipitated with Tec, is also noticeable. The band above Tec (approximately 74 kD) is likely due to nonspecific reactivity, as it was also detected after BCR ligation in immunoprecipitates obtained with NRS. (B) Kinetics of BCR-mediated tyrosine phosphorylation of Tec. Ramos cells were incubated with anti-IgM antiserum for the times indicated (minutes). Tyrosine phosphorylation of Tec was assessed above. (C) BCR ligation increases Tec kinase activity. Ramos cells were stimulated as above for 5 minutes and Tec kinase activity was assessed by in vitro kinase assay. The position of Tec is indicated by the arrow.
Ligation of BCR induces tyrosine phosphorylation and activation of Tec. (A) Ramos cells were incubated with anti-IgM antiserum or control serum for 5 minutes. Proteins immunoprecipitated with anti-Tec rabbit antibody or normal rabbit serum (NRS) were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). Molecular mass markers (in kD) are indicated. The position of Tec is indicated by an arrow. A tyrosine phosphorylated protein of approximately 62 kD, coprecipitated with Tec, is also noticeable. The band above Tec (approximately 74 kD) is likely due to nonspecific reactivity, as it was also detected after BCR ligation in immunoprecipitates obtained with NRS. (B) Kinetics of BCR-mediated tyrosine phosphorylation of Tec. Ramos cells were incubated with anti-IgM antiserum for the times indicated (minutes). Tyrosine phosphorylation of Tec was assessed above. (C) BCR ligation increases Tec kinase activity. Ramos cells were stimulated as above for 5 minutes and Tec kinase activity was assessed by in vitro kinase assay. The position of Tec is indicated by the arrow.
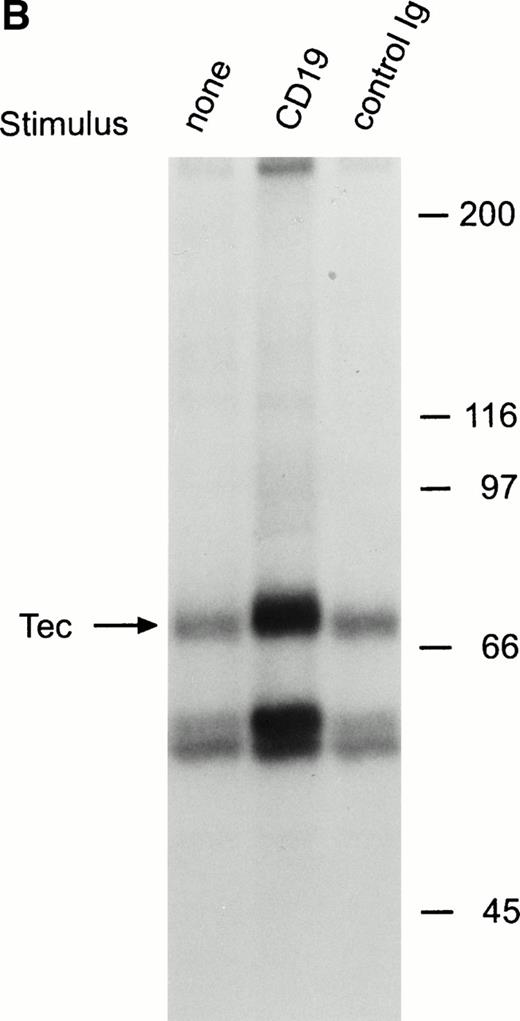
BCR ligation in Ramos cells also induced a distinct increase in Tec autophosphorylation (Fig 2C). In addition, other phosphorylated proteins coprecipitated with Tec, including two prominent proteins of approximately 56 and 59 kD.18 These proteins had a mobility different than that of the Ig heavy chain of the antibody used for immunoprecipitation, as assessed by comparisons with the Coomassie blue stained gel (not shown). Because Tec is associated with Lyn in myeloid cells,29 we determined whether the 56- and 59-kD proteins corresponded to Lyn, or to other Src-family kinases known to be activated by BCR cross-linking, such as Fyn, Blk, and Lck.25-27 In side-by-side electrophoresis and autoradiography experiments, none of the four Src-family kinases had the same mobility that the 56- and 59-kD proteins coprecipitated with Tec.
CD19 ligation activates Tec in immature B cells.
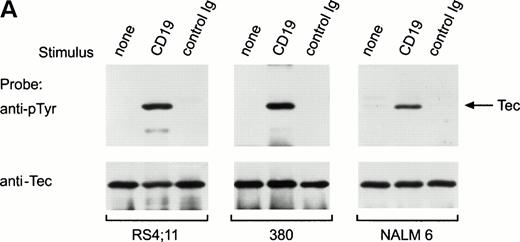
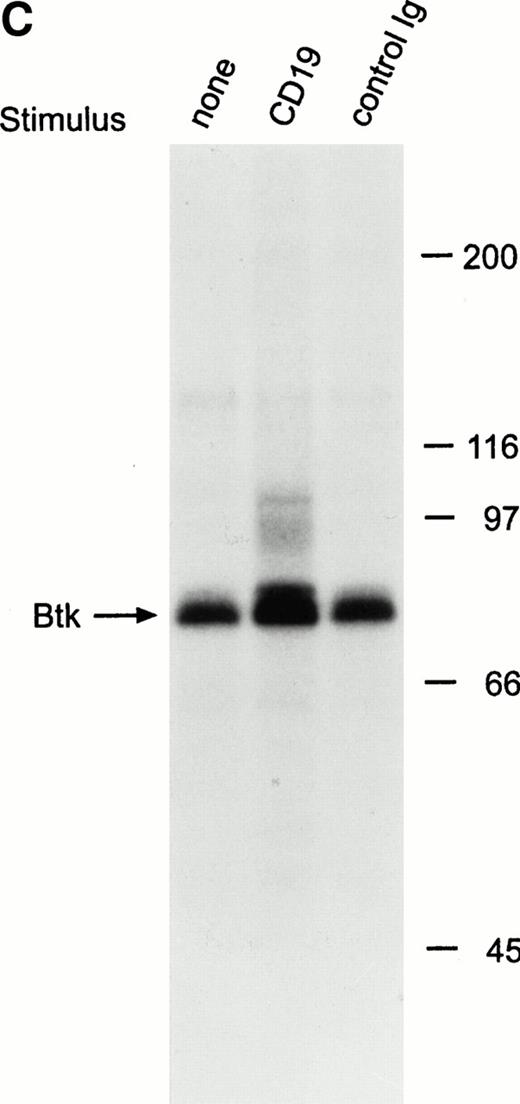
To determine whether Tec is involved in signaling pathways in immature B cells that do not express surface BCR, we stimulated cells by cross-linking CD19, a 95-kD transmembrane molecule expressed throughout all stages of B-cell differentiation.30 31 We incubated BCR-negative immature B cells (RS4;11, 380 and NALM6) with anti-CD19 monoclonal antibody for 15 minutes at 4°C, followed by cross-linking with goat antimouse antiserum for 1 minute at 37°C. This procedure caused marked tyrosine phosphorylation of Tec in all three cell lines (Fig 3A). As in Ramos cells after BCR ligation, a 62-kD tyrosine phosphorylated protein coprecipitated with Tec in RS4;11 and 380 cells after CD19 cross-linking (Fig 3A). CD19 ligation also induced a marked increase in Tec autophosphorylation (Fig 3B). Thus, Tec kinase is activated by CD19 oligomerization in immature B cells.
Ligation of CD19 in immature B cells induces tyrosine phosphorylation and activation of Tec. (A) Immature B-cell lines, RS4;11, 380, and NALM6 were incubated with anti-CD19 monoclonal antibody or isotype-matched nonreactive Ig for 15 minutes at 4°C followed by goat antimouse Ig antiserum for 1 minute at 37°C. Proteins immunoprecipitated with anti-Tec rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). The position of Tec is indicated. (B) CD19 cross-linking in immature B cells increases Tec kinase activity. RS4;11 cells were stimulated as above and Tec kinase activity was assessed by in vitro kinase assay. Molecular mass markers (in kilodaltons) are indicated. The position of Tec is indicated by the arrow.
Ligation of CD19 in immature B cells induces tyrosine phosphorylation and activation of Tec. (A) Immature B-cell lines, RS4;11, 380, and NALM6 were incubated with anti-CD19 monoclonal antibody or isotype-matched nonreactive Ig for 15 minutes at 4°C followed by goat antimouse Ig antiserum for 1 minute at 37°C. Proteins immunoprecipitated with anti-Tec rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). The position of Tec is indicated. (B) CD19 cross-linking in immature B cells increases Tec kinase activity. RS4;11 cells were stimulated as above and Tec kinase activity was assessed by in vitro kinase assay. Molecular mass markers (in kilodaltons) are indicated. The position of Tec is indicated by the arrow.
Ligation of CD19 in the BCR-positive cell line Ramos induced tyrosine phosphorylation of several proteins, including CD19 itself.32 However, we could not detect clear tyrosine phosphorylation of Tec in these cells after exposure to anti-CD19 (not shown).
Activation of Btk in immature and mature B cells.
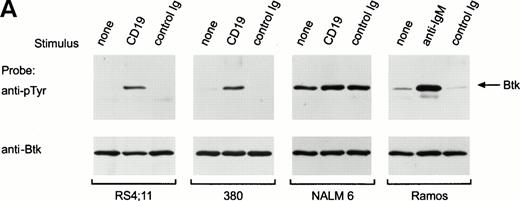
Previous studies have reported that BCR ligation in mature B cells induces activation of Btk.33-35 In Ramos cells, BCR ligation did induce tyrosine phosphorylation of Btk (Fig 4A). To determine whether Btk is activated in immature B cells after CD19 ligation, we cross-linked CD19 in RS4;11, 380 and NALM6 cells. This procedure caused marked tyrosine phosphorylation of Btk in RS4;11 and 380 (Fig 4A), indicating that Btk can be activated by CD19 oligomerization in immature B cells. In NALM6, Btk appeared to be substantially tyrosine phosphorylated before stimulation, and there was no net increase in signal after CD19 cross-linking (Fig 4A). In time course experiments, maximal tyrosine phosphorylation of Btk was observed after 1 minute of CD19 cross-linking (Fig 4B). Cross-linking of CD19 also appeared to enhance the autophosphorylation of Btk (Fig 4C).
Ligation of CD19 in immature B cells induces tyrosine phosphorylation and activation of Btk. (A) Immature B-cell lines, RS4;11, 380, and NALM6 were incubated with anti-CD19 monoclonal antibody or isotype-matched nonreactive Ig for 15 minutes at 4°C followed by goat antimouse Ig antiserum for 1 minute at 37°C. Proteins immunoprecipitated with anti-Btk rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Btk goat antibody (lower panel). The position of Btk is indicated. In RS4;11 and 380 cells, cross-linking of CD19 induced tyrosine phosphorylation of Btk, as 5-minute exposure to anti-IgM antibody did in Ramos cells.33-35 In NALM6 cells, Btk appeared to be strongly tyrosine phosphorylated before CD19 ligation and no increase in phosphorylation was detected. (B) Kinetics of CD19-mediated tyrosine phosphorylation of Btk. CD19 was cross-linked on RS4;11 cells for the times indicated (minutes). Tyrosine phosphorylation of Btk was assessed as above. (C) CD19 ligation increases Btk kinase activity. The 380 cells were stimulated as above for 1 minute and Btk kinase activity was assessed by in vitro kinase assay. Molecular mass markers (in kilodaltons) are indicated. The position of Btk is indicated by the arrow.
Ligation of CD19 in immature B cells induces tyrosine phosphorylation and activation of Btk. (A) Immature B-cell lines, RS4;11, 380, and NALM6 were incubated with anti-CD19 monoclonal antibody or isotype-matched nonreactive Ig for 15 minutes at 4°C followed by goat antimouse Ig antiserum for 1 minute at 37°C. Proteins immunoprecipitated with anti-Btk rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Btk goat antibody (lower panel). The position of Btk is indicated. In RS4;11 and 380 cells, cross-linking of CD19 induced tyrosine phosphorylation of Btk, as 5-minute exposure to anti-IgM antibody did in Ramos cells.33-35 In NALM6 cells, Btk appeared to be strongly tyrosine phosphorylated before CD19 ligation and no increase in phosphorylation was detected. (B) Kinetics of CD19-mediated tyrosine phosphorylation of Btk. CD19 was cross-linked on RS4;11 cells for the times indicated (minutes). Tyrosine phosphorylation of Btk was assessed as above. (C) CD19 ligation increases Btk kinase activity. The 380 cells were stimulated as above for 1 minute and Btk kinase activity was assessed by in vitro kinase assay. Molecular mass markers (in kilodaltons) are indicated. The position of Btk is indicated by the arrow.
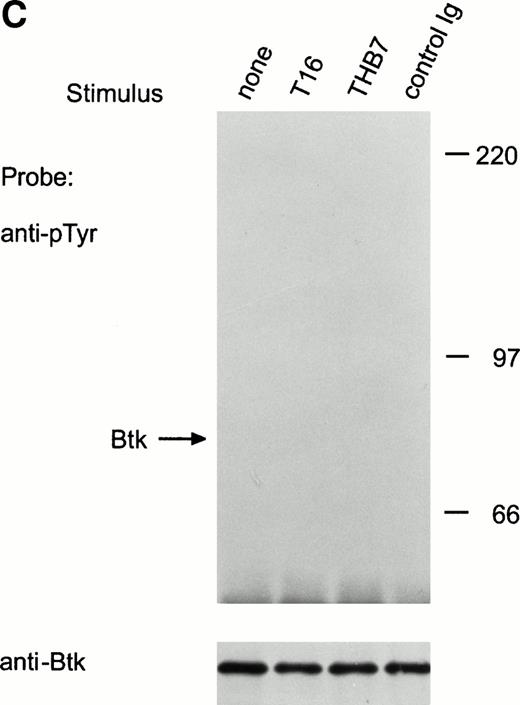
CD38 ligation in RS4;11 immature B cells activates Tec, but not Btk.
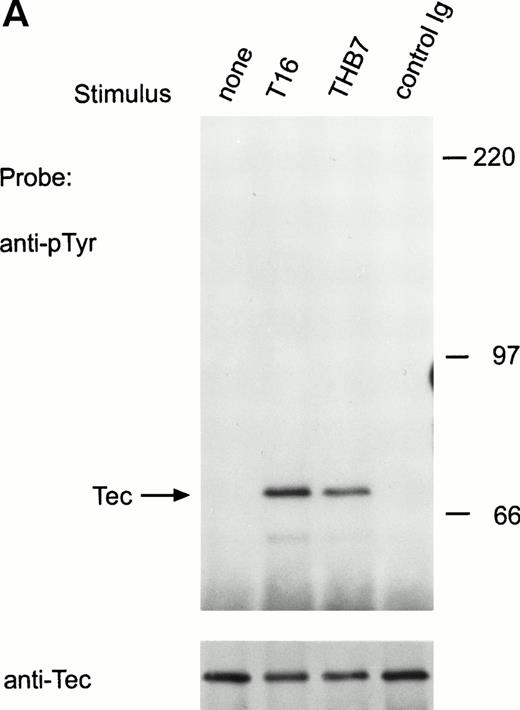
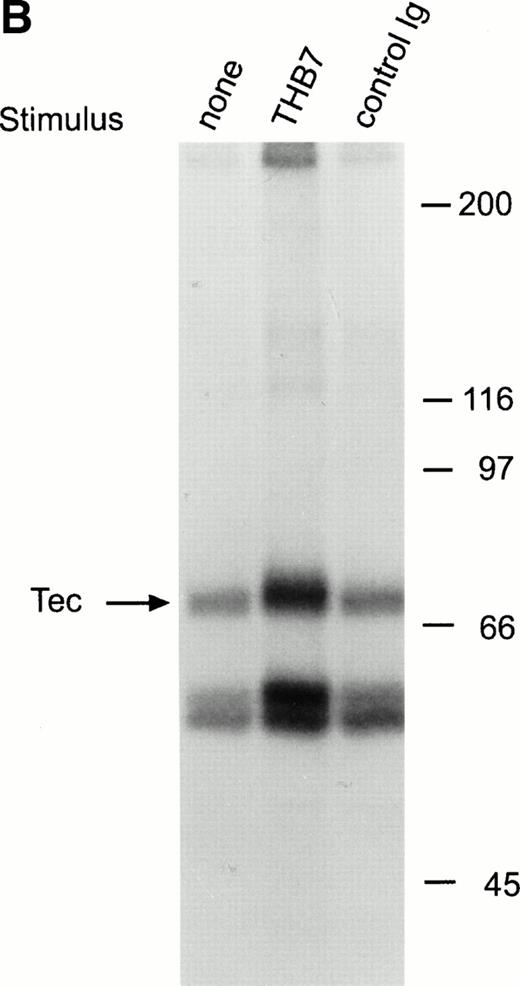
CD38 is a 45-kD transmembrane glycoprotein highly expressed on immature lymphoid cells.36 Ligation of CD38 suppresses cell growth and induces apoptosis in both normal and leukemic immature B cells.36 This cellular effect is accompanied by induction of tyrosine kinase activity.21,24,37 To date, the only identified tyrosine kinase activated by CD38 stimulation in human immature B cells is Syk.21 In the immature B-cell line, RS4;11, a 5-minute exposure to anti-CD38 monoclonal antibody (T16 or THB7) caused distinct tyrosine phosphorylation of Tec (Fig 5A). As in the case of BCR and CD19 ligation, CD38 stimulation promoted the association of Tec with a tyrosine phosphorylated protein of approximately 62 kD (Fig 5A), and caused an increase in Tec autophosphorylation in RS4;11 cells (Fig 5B). CD38 ligation in the same cells failed to induce tyrosine phosphorylation of Btk (Fig 5C).
Ligation of CD38 in RS4;11 cells induces tyrosine phosphorylation and activation of Tec, but not of Btk. (A) RS4;11 cells were incubated with anti-CD38 monoclonal antibodies, T16 and THB7, and isotype-matched nonreactive Ig for 5 minutes. Proteins immunoprecipitated with anti-Tec rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). Molecular mass markers (in kilodaltons) are indicated. The position of Tec is indicated by an arrow. (B) CD38 ligation increases Tec kinase activity. RS4;11 cells were stimulated with THB7 for 5 minutes and Tec kinase activity was assessed by in vitro kinase assay. (C) RS4;11 cells were stimulated with anti-CD38 monoclonal antibodies as in (A). Tyrosine phosphorylation of Btk was examined as above. The position of Btk is indicated by an arrow. The presence of Btk in the immunoprecipitates was confirmed by stripping the blot and reprobing with anti-Btk goat antibody (lower panel).
Ligation of CD38 in RS4;11 cells induces tyrosine phosphorylation and activation of Tec, but not of Btk. (A) RS4;11 cells were incubated with anti-CD38 monoclonal antibodies, T16 and THB7, and isotype-matched nonreactive Ig for 5 minutes. Proteins immunoprecipitated with anti-Tec rabbit antibody were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with antiphosphotyrosine antibody (pTyr; upper panel), then stripped and reprobed with anti-Tec rabbit antibody (lower panel). Molecular mass markers (in kilodaltons) are indicated. The position of Tec is indicated by an arrow. (B) CD38 ligation increases Tec kinase activity. RS4;11 cells were stimulated with THB7 for 5 minutes and Tec kinase activity was assessed by in vitro kinase assay. (C) RS4;11 cells were stimulated with anti-CD38 monoclonal antibodies as in (A). Tyrosine phosphorylation of Btk was examined as above. The position of Btk is indicated by an arrow. The presence of Btk in the immunoprecipitates was confirmed by stripping the blot and reprobing with anti-Btk goat antibody (lower panel).
DISCUSSION
B-cell development proceeds through discrete stages of clonal selection, proliferation, and differentiation influenced by microenvironmental signals. These events are modulated through ligation of surface receptors, with specific cellular effects that depend on the level of cell maturation. These signaling cascades entail early induction of tyrosine kinase activity, with consequent rapid and transient tyrosine phosphorylation of intracellular substrates. The tyrosine kinases most consistently reported to be activated by ligation of B-cell surface receptors include the Src-family kinases (Lyn, Fyn, Blk, and Lck), Syk and Btk.21,25-27,30,31,38 We have shown that Tec is activated in signaling pathways triggered by three independent B-cell surface receptors. Although previous studies suggested that Tec is preferentially involved in signaling mechanisms regulating myeloid development,8 16-19 our results imply that Tec participates directly in signaling that regulates human B-cell differentiation. Tec's function in B cells is likely to extend to other species, as we found that BCR ligation also causes Tec tyrosine phosphorylation in the murine B-cell line, WEHI-231.
Studies of Tec/Btk/Itk kinases have suggested that these molecules play a role in transducing stimuli leading to cell differentiation and/or proliferation.8 Our findings indicate that activation of Tec and Btk may result in either growth-inducing or growth-suppressing signals, as ligation of either BCR in Ramos and WEHI-231 cells or CD38 in RS4;11 cells suppresses cell growth and induces apoptosis.36,39,40 Previous studies have suggested that Tec tyrosine kinase has a specialized role in signaling pathways generated by growth factor receptors. Tec activation was detected following cell exposure to IL-3,17 SCF,18G-CSF,19 erythropoietin,20 IL-6,28and thrombopoietin.41 Tec was shown to be associated with gp130, the signal transducing unit for the IL-6 cytokine family, which includes, among others, IL-6, IL-11, leukemia-inhibitory factor, and oncostatin M.28 Tec was also shown to be associated with c-kit,18 further implying its involvement in signaling mechanisms triggered by hematopoietic growth factors. Tec's role in human B-lymphoid cells, as a component of signaling triggered by ligation of nonkinase receptors, which do not function as cytokine receptors, is a novel one for this tyrosine kinase.
The precise molecular interactions that precede and follow Tec activation in B cells are still unclear. Tec appears to be constitutively bound to Lyn tyrosine kinase through the Tec-homology domain.29,42 Thus, after cross-linking of B-cell surface receptors, Tec could be tyrosine phosphorylated after activation of Lyn. It has been reported that tyrosine phosphorylation of Tec promotes its association with the adaptor proteins Shc and Vav.17,20Thus, events downstream of Tec activation could ultimately lead to activation of the Ras/mitogen-activated protein (MAP) kinase pathway. Activation of this pathway is known to follow BCR and CD19 ligation,43 44 and we have observed that CD38 dimerization in immature B cells causes tyrosine phosphorylation of Shc and its association with Grb2 (A. Kitanaka et al, unpublished observations, September 1996). Therefore, Tec may represent a central link between early signaling events and the Ras/MAP kinase pathway in B-lineage cells.
In summary, we found that Tec is activated in both mature and immature human B-lymphoid cells by a variety of stimuli, suggesting that this tyrosine kinase plays an important role in B-cell development. It has been postulated that expression of Tec in myeloid progenitors could be responsible for the preservation of normal myeloid development in patients with XLA.8 45 Although this may be true, it is clear that Tec cannot compensate for defects in Btk in B cells, indicating that the two kinases do not have completely overlapping functions. This hypothesis is supported by our observation that stimulation of the pro-B cell line RS4;11 with CD38 could activate Tec, but not Btk. Other stimuli that are crucial for B-cell development may activate Btk, but not Tec. Future studies should clarify the importance of Tec activation for normal B-cell development and its role in the pathogenesis of B-cell immunodeficiency.
ACKNOWLEDGMENT
We thank Dr Ken Sato for helpful discussions and Sharon Naron for editorial assistance.
Supported by Grants No. RO1-CA58297 and P30-CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Dario Campana, MD, PhD, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal