Abstract
Defects of the common gamma chain subunit of the cytokine receptors (γc) or of Jak3, a tyrosine kinase required for γc signal transduction, result in T−B+ severe combined immunodeficiency (SCID). However, atypical cases, characterized by progressive development of T lymphocytes, have been also reported. We describe a child with SCID caused by Jak3 gene defects, which strongly but not completely affect Jak3 protein expression and function, who developed a substantial number (>3,000/μL) of autologous CD3+CD4+ T cells. These cells showed a primed/activated phenotype (CD45R0+ Fas+HLA-DR+ CD62Llo), defective secretion of T-helper 1 and T-helper 2 cytokines, reduced proliferation to mitogens, and a high in vitro susceptibility to spontaneous (caused by downregulation of bcl-2 expression) as well as activation-induced cell death. A restricted T-cell receptor repertoire was observed, with oligoclonal expansion within each of the dominant segments. These features resemble those observed in γc-/y and in Jak3−/−mice, in which a population of activated, anergic T cells (predominantly CD4+) also develops with age. These results suggest that residual Jak3 expression and function or other Jak3-independent signals may also permit the generation of CD4+ T cells that undergo in vivo clonal expansion in humans; however, these mechanisms do not allow development of CD8+ T cells, nor do they fully restore the functional properties of CD4+ T lymphocytes.
JAK3, A MEMBER of the Janus family tyrosine kinase,1,2 is an intracellular kinase required for signaling through the common gamma chain (γc),3-5 a subunit of the cytokine receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15.6
Functional integrity of the γc/Jak3 system is essential for lymphoid development,7-9 signaling,10 and survival.11 In humans, genetic defects affecting the IL-2RG gene (encoding for γc) or the Jak3 gene result in X-linked12-14 or in autosomal recessive15-19severe combined immunodeficiency (XL-SCID or AR-SCID), respectively, with lack of circulating T cells and a normal or increased number of B lymphocytes that are, however, functionally defective.20
Disruption of the IL-2RG or the Jak3 genes in mice also results in SCID, but with a distinct biological phenotype, characterized by a severe defect of T-, B-, and natural killer (NK)-cell differentiation, that allows an age-dependent increase of activated and anergic CD4+ T cells in the periphery.13,14,17-19 Jak3 protein expression in the thymus is essential to reconstitute thymocyte development (including γδ and NK cells); furthermore, maintenance of peripheral expression of Jak3 is required to correct the immunological abnormalities (T-cell anergy) observed in Jak3−/− mice.21
In humans, atypical cases of XL-SCID, characterized by development of T lymphocytes, have been recently described.4,22-24 With the exception of the case reported by Stephan et al, in which development of autologous T lymphocytes resulted from a spontaneous reversion of the genetic defect at the IL-2RG gene locus,24 T cells remained genetically and functionally defective in other patients with atypical XL-SCID.4,22 23
We report the case of an infant with T−B+ SCID caused by Jak3 mutations, which strongly but not completely affect Jak3 protein expression and function, who developed early in life a substantial number of autologous, activated, and oligoclonal CD3+CD4+ T cells that remained genetically and functionally defective.
MATERIALS AND METHODS
Case report.
The infant is the only son of nonconsanguineous parents. The diagnosis of SCID was serendipitously obtained at birth, when profound abnormalities of lymphocyte subpopulations (Table 1) were disclosed during blood testing for an episode of intestinal subobstruction that resolved spontaneously at 1 week of age. The infant was admitted to the Bone Marrow Transplantation Unit of the Department of Pediatrics, University of Brescia, at 1 month of age. At that time, chest radiograph examination revealed mild interstitial pneumonia. He was hospitalized in a laminar flow unit, and he was treated with intravenous immunoglobulins, antibiotics, and acyclovir until the age of 3 months, when he received haploidentical bone marrow transplantation (BMT) with T-depleted marrow from the father following conditioning with busulphan and cyclophosphamide. Before BMT, an increase of T-cell count was detected in the periphery (Table 1).
Immunophenotypical Analysis
| . | At Birth . | At 2.5 Months . | At 3 Months . | Healthy Controls (1 to 4 mo) . |
|---|---|---|---|---|
| PBL/μL | 3,026 | 4,253 | 8,890 | 4,100 ± 1,500 |
| CD3 (%) | 2 | 24 | 41 | 71 ± 7 |
| CD4 (%) | 2 | 24 | 41 | 40 ± 7 |
| CD8 (%) | 1 | 1 | 1 | 25 ± 5 |
| CD19 (%) | 79 | 48 | 44 | 18 ± 6 |
| CD16 (%) | 13 | 5 | 5 | 11 ± 5 |
| . | At Birth . | At 2.5 Months . | At 3 Months . | Healthy Controls (1 to 4 mo) . |
|---|---|---|---|---|
| PBL/μL | 3,026 | 4,253 | 8,890 | 4,100 ± 1,500 |
| CD3 (%) | 2 | 24 | 41 | 71 ± 7 |
| CD4 (%) | 2 | 24 | 41 | 40 ± 7 |
| CD8 (%) | 1 | 1 | 1 | 25 ± 5 |
| CD19 (%) | 79 | 48 | 44 | 18 ± 6 |
| CD16 (%) | 13 | 5 | 5 | 11 ± 5 |
The molecular defect in the Jak3 gene and the biochemical analysis of the Jak/STAT signaling pathway have been reported in detail elsewhere, together with other Jak3-deficient SCID patients (patient LE25). Briefly, the patient carries distinct mutations on the two Jak3 alleles: an intragenic deletion that results in several mutant splicing products, including an in-frame deleted cDNA form lacking exons 10 to 12, and a de novo A to G transition at the nucleotide 1537, leading to a glutamic acid to glycine substitution at codon 481 (E481G). Both mutant forms of the Jak3 protein were expressed in lymphoblastoid cell lines, albeit in trace amounts, as detected by Western blotting. Furthermore, residual preservation of the function of the E481G Jak3 mutant was shown, based on reduced but detectable phosphorylation of Jak3 and STAT5 proteins in response to IL-2.25 Molecular analysis of the patient's peripheral blood T cells revealed that they were autologous (as detected by highly polymorphic DNA markers D1S80 and DQ alpha) and genetically defective, as shown by mutation analysis at the Jak3 locus (data not shown).
After BMT, the patient developed hemolytic anemia, associated with the presence of autologous and donor-derived T cells in the periphery.
Lymphocyte surface phenotype analysis.
Evaluation of lymphocyte surface membrane expression was performed on heparinized whole blood samples with the following monoclonal antibodies (MoAbs): fluorescein-isothiocyanate (FITC)-conjugated OKT3/CD3, FITC-OKT8/CD8, FITC- or phycoerytrin (PE)-conjugated OKT4/CD4, PE-OKDR/HLA DR (Ortho, Raritan, NJ), PE-Leu11c/CD16, PE-Leu45RO/CD45RO (Becton Dickinson, Mountain View, CA), FITC-B4/CD19, PE-2H4/CD45RA (Coulter Immunology, Hialeah, FL), PE-DREG-56/CD62L, and FITC-DX2/CD95 (Pharmingen, San Diego, CA).
After two washes in phosphate-buffered saline (PBS)-1% fetal calf serum (FCS), samples were analyzed with a flow cytometer equipped with an argon-ion laser (488 nm; Cytoron Absolute, Ortho), using the ABS software. Gates were selected on lymphoid cells as determined by forward and right-angle scatter properties; dead cells were excluded by setting an appropriate threshold trigger on the low forward light scatter parameter; and nonspecific staining was assessed using FITC-conjugated nonimmune mouse IgG (Coulter). Data were acquired and stored in list mode. Fluorescence analysis was performed on a log scale.
Preparation of lymphocytes.
Mononuclear cells (PBMC) were separated from the peripheral blood by Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation and washed three times in RPMI-1640 (GIBCO Laboratories, Grand Island, NY).
Lymphocyte activation.
All experiments were performed in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 1% glutamin, and antibiotics. Proliferative responses were measured in triplicates of 200 μL containing 1 × 105 total PBMC using various combinations of the following stimulating agents: immobilized anti-CD3 (OKT3) MoAb, 200 ng/mL, with or without IL-2, 20 U/mL (Biosource International, Camarillo, CA); phytoemoagglutinin (PHA), 1.2 μg/mL (Irvine Scientific, Santa Ana, CA); phorbol 12-myristate 13-acetate (PMA), 5 ng/mL (Sigma, St Louis, MO) plus ionomycin, 500 ng/mL (Sigma); and 1 × 105irradiated (2,000 rads) allogeneic PBMC from a pool of healthy donors. After 72 hours of culture (or 120 hours for the mixed lymphocyte culture) at 37°C, 5% CO2, the cells were pulsed overnight with 1 μCi (3H)-thymidine and were obtained subsequently. Incorporated radioactivity was measured by liquid scintillation counting (Beckman, Fullerton, CA), and the results were expressed as mean counts per minute of triplicates.
Apoptosis assay.
PBMC, cultured with or without the stimulating agents in the conditions indicated above, were also evaluated for apoptosis.
After 72 hours of culture, cells were washed and the percentage of hypodiploid nuclei (less than diploid DNA content) was determined with a slight modification of the method of Nicoletti et al,26as described.27 Briefly, 1 × 106 PBMC were fixed in 1 mL of cold 70% ethanol at 4°C for 20 minutes. Cells were then washed, incubated at room temperature for 1 minute with RNase (0.5 mg/mL; Boehringer GmbH, Mannheim, Germany), for 15 minutes with propidium iodide (100 mg/mL; Sigma), and immediately analyzed. The correct threshold was selected experimentally using the model system of apoptosis induced in murine thymocytes by 72 hours culture with dexamethasone 10−7 mol/L. Apoptotic cell nuclei, easily distinguishable from debris owing to the condensation of nuclear chromatin, emitted red fluorescence in the RD-FL channels 46 to 146. As there was no overlap between apoptotic nuclei and debris, the small percentage of residual low-fluorescence detritus (<1% in normal cells) was eliminated by gating the red fluorescence scale at channel 45. All measurements were performed with the same instrument setting.
Analysis of intracellular bcl-2 expression.
A total of 5 × 105 PBMC were permeabilized using Permeafix (Ortho) for 40 minutes, before an incubation with a rabbit polyclonal antibody to bcl-2 (Santa Cruz Biotechnology Inc, Santa Cruz, CA), or with polyclonal nonimmune rabbit IgG. After 30 minutes, cells were washed and incubated with FITC-conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology Inc). Quantitation of positivity for bcl-2 was performed using QuickCal beads (Flow Cytometry Standards Corp, San Juan, PR), and results were expressed as specific molecule equivalents of soluble fluorochrome (MESF), after subtracting the values obtained for nonspecific fluorescence (always <2,000 MESF).
Cytokine production and measurement.
For cytokine production, 1 × 106 PBMC were cultured at 37°C, 5% CO2 in 24-well flat-bottomed plates in a final volume of 1 mL with PMA (5 ng/mL) plus ionomycin (500 ng/mL). After 72 hours, supernatants were collected and frozen at −20°C until use. Sandwich enzyme-linked immunosorbent assay (ELISA) commercial kits were used to measure IL-2 (Genzyme, Cambridge, MA), IL-4 (BioSource International), IL-5 (CytImmune Sciences, College Park, MD), and interferon (IFN)-γ (BioSource International).
Analysis of the T-cell receptor repertoire diversity.
One microgram of the total RNA, prepared with the guanidinium thiocyanate-phenol-chloroform method from PBMC, was used to synthesize the first strand of TCRB chain cDNA using a specific TCRBC primer (βcDNA: 5′ GGG CTG CTC CTT GAG GGG CTG CGG 3′) and the RiboClone cDNA Synthesis System (Promega Corporation, Madison, WI). cDNA was subjected to enzymatic amplification using a second human TCRBC primer (βAI: 5′ CCC ACT GTG CAC CTC CTT CC 3′) and a TCRBV degenerated primer (Vβd: 5′ ACG TGA ATT CT(GT) T(ACT)(CT) TGG TA(CT) (AC)(AG)(AT) CA 3′). Forty cycles of polymerase chain reaction (PCR) were performed under the following conditions: denaturation at 94°C for 1 minute, annealing at 52°C for 1 minute, and extension at 72°C for 1 minute; the last cycle extension was performed at 72°C for 7 minutes. The specificity of the total amplified products was analyzed using a colorimetric method and biotinylated TCRBV-specific probes.28 Subsequently, the TCRBV chains of interest were amplified by 35 cycles of PCR, using TCRBV-specific family primers (TCRBV2: 5′ TCA TCA ACC ATG CAA GCC TGA CCT 3′; TCRBV4: 5′ GCC CAA ACC TAA CAT TCT CAA CTC 3′; TCRBV5S1: 5′ ATA CTT CAG TGA GAC ACA GAG AAA 3′; TCRBV6: 5′ AGG CCT GAG GGA TCC GTC TC 3′; and TCRBV14: 5′ GTC TCT CGA AAA GAG AAG AGG AAT 3′) and the TCRBC oligonucleotide βAI. For heteroduplex analysis, 20 μL of TCRBV-specific products were heated to 95°C for 5 minutes and cooled to 50°C for 1 hour. The annealed samples were kept on ice and then run for 5 to 6 hours at 200 V, at room temperature, on a 12% nondenaturing polyacrylamide gel (PAGE; 29:1 acrylamide/bisacrylamide) performed in 1× TBE buffer (0.089 mol/L Tris-borate and 0.002 mol/L EDTA, pH 8.0). DNA Molecular Weight V (Boehringer Mannheim) was used as size marker. The gel was stained for 30 to 60 minutes, at room temperature, in the dark, in a solution containing 0.75 μg/mL ethidium bromide in 200 mL of 1× TBE and photographed under ultraviolet light. TCRBV8-specific amplified products from the T-cell line J77 and from PBMC stimulated with an anti-TCRBV8 MoAb were used as monoclonal and polyclonal controls, respectively.29 TCRBV2 and TCRBV5S1 products, obtained from a new amplification performed with specific primers, were purified, cloned, and sequenced as recently described.30 Sequences were compared with published data relative to TCRBV, TCRBD, TCRBJ, and TCRBC segments.31 32
RESULTS
Immunophenotypical and molecular studies.
Cytofluorimetric analysis performed on PBMC collected from the patient at the time of birth revealed a typical T−B+ SCID phenotype, with a severely reduced proportion of peripheral blood T lymphocytes and an increased proportion of B cells (Table 1).
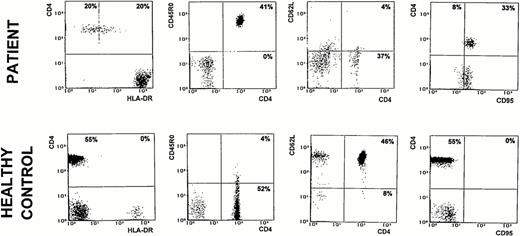
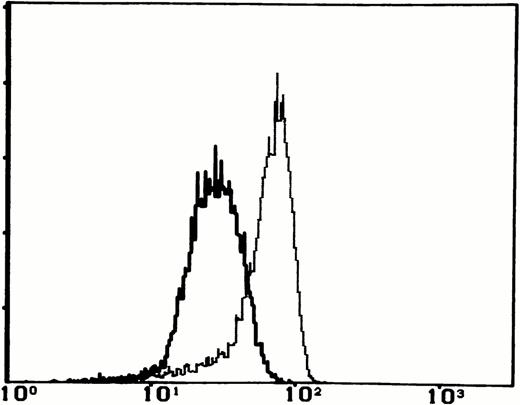
Interestingly, at 3 months of age the patient developed autologous (as assessed by HLA-typing) CD3+CD4+ cells that increased to 41% just before undergoing haploidentical BMT from his father (Table 1). However, these lymphocytes were phenotypically abnormal, as they predominantly coexpressed the activation marker HLA-DR, were almost exclusively CD45R0+ and CD95/Fas+, and showed a reduced density of CD62L (Fig 1). These features are typical of primed/activated T cells.
Two-color analysis of activation/memory markers expression by peripheral CD4+ lymphocytes from the patient at 3 months of age and from one representative age-matched healthy control. Data are presented as relative log fluorescence intensity.
Two-color analysis of activation/memory markers expression by peripheral CD4+ lymphocytes from the patient at 3 months of age and from one representative age-matched healthy control. Data are presented as relative log fluorescence intensity.
Normal expression of the γc was found on the lymphocyte surface, as detected with the rat TUGh4 MoAb (a kind gift of Dr K. Sugamura, Department of Microbiology, Tohoku University School of Medicine, Japan; data not shown).
TCRBV repertoire diversity.
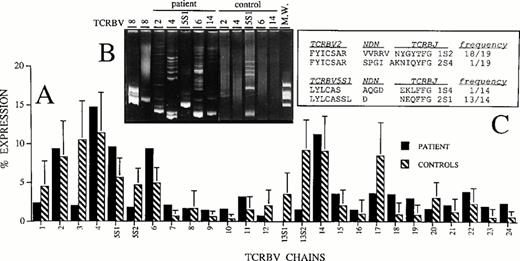
The analysis of the TCRBV usage was performed on patient's PBMC at 3 months of age and compared with that of age-matched healthy controls. Figure 2A shows that all TCRBV transcripts, with the only exception of TCRBV13S1 chain, can be amplified from the patient's lymphocytes without evidence for over-representation of single TCRBV population. On the contrary, the most notable difference is the greatly reduced percentage of TCRBV3, TCRBV13S1, and TCRBV13S2 chains in the patient as compared with controls. However, because we have previously found that the level of TCRBV diversity is not necessarily related to the relative percentage of TCRBV expression,33 we also performed a heteroduplex analysis of TCRBV2, TCRBV4, TCRBV5S1, TCRBV6, and TCRBV14 transcripts, which were expressed at highest levels in the patient's T cells. This technique is based on the different ability of amplified TCRBV segments to migrate in a polyacrylamide gel, depending on their monoclonal or polyclonal composition.29 As shown in Fig 2B, all the PCR products obtained with the 5 TCRBV family-specific primers generated homoduplex bands that are indicative of predominant homogeneous molecular species in the context of a background of heteroduplex bands or of smears, respectively, representing minor oligoclonal or polyclonal expansions. On the contrary, the amplified products of the same TCRBV segments prepared from PBMC of an age-matched healthy control and chosen among those analyzed in Fig 2A generated smears or faint heteroduplex bands. This result is consistent with our previous observations suggesting that, in the absence of antigenic stimulation, most of the TCRBV segments expressed by PBMC from normal individuals are largely polyclonal.34
The strongest evidence for oligoclonal expansion that confirmed the results obtained with the heteroduplex analysis was provided by sequencing of TCRBV2 and TCRBV5S1 transcripts. In fact, in both groups of sequences the dominance of a single clone was detected (Fig 2C). We did not observe, however, amino acid similarities in the NDN regions of the two major groups of clones.
Functional studies.
In accordance with the lack of T cells at birth, in vitro response of PBMC to T-cell activating agents (PHA, anti-CD3, and anti-CD3 plus IL-2) was abolished, whereas stimulation with PMA plus ionomycin, which can also elicit B-cell activation, resulted in a substantial proliferative response (Table 2).
Proliferative Response (cpm)
| . | Patient at Birth . | Healthy Control (newborn) . | Patient at 3 Months . | Healthy Control (3 mo) . |
|---|---|---|---|---|
| Medium | 2,700 | 670 | 3,150 | 950 |
| CD3 | 6,700 | 19,150 | 4,200 | 33,150 |
| PHA | 5,500 | 75,100 | 6,900 | 85,000 |
| PMA plus Ionomycin | 46,950 | 53,100 | 33,700 | 47,500 |
| CD3 plus IL-2 | 8,550 | 44,900 | 36,200 | 45,550 |
| IL-2 | 5,950 | 3,100 | 17,600 | 2,450 |
| MLC (vpooled unrelated donors) | ND | ND | 18,250 | 72,250 |
| . | Patient at Birth . | Healthy Control (newborn) . | Patient at 3 Months . | Healthy Control (3 mo) . |
|---|---|---|---|---|
| Medium | 2,700 | 670 | 3,150 | 950 |
| CD3 | 6,700 | 19,150 | 4,200 | 33,150 |
| PHA | 5,500 | 75,100 | 6,900 | 85,000 |
| PMA plus Ionomycin | 46,950 | 53,100 | 33,700 | 47,500 |
| CD3 plus IL-2 | 8,550 | 44,900 | 36,200 | 45,550 |
| IL-2 | 5,950 | 3,100 | 17,600 | 2,450 |
| MLC (vpooled unrelated donors) | ND | ND | 18,250 | 72,250 |
Abbreviations: MLC, mixed lymphocyte culture; ND, not done.
A defect of the proliferative response to PHA, anti-CD3, and pooled unrelated cells was also observed after appearance of circulating autologous T lymphocytes. This defect was too profound to be explained solely by the reduction of the T-cell number as compared with the controls' (Table 2). A moderate increase of proliferation was obtained after the addition of exogenous IL-2 to both unstimulated and stimulated cultures, in keeping with the strongly reduced, but detectable, levels of Jak3 and STAT5 phosphorylation observed after the stimulation with IL-2.25 These observations suggested a defective IL-2 production following in vitro culture with the activating stimuli mentioned above.
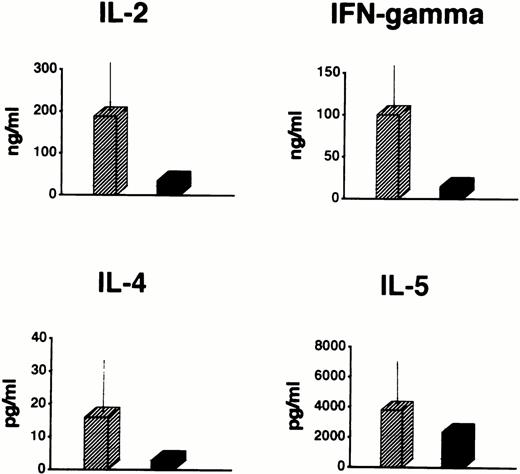
In fact, even under powerful stimulating conditions (PMA plus ionomycin for 72 hours), patient PBMC produced markedly lower levels of IL-2 and IFN-γ than in the normal controls (Fig3). On the other hand, no increase in the production of IL-4 and IL-5 was observed (Fig 3), thus ruling out the possibility that the defective proliferative response was accounted for by a shift toward Th2-type cytokine secreting cells. In view of the substantial number of T cells in the periphery, this defect was also too profound to be explained solely by the reduction of the T-cell number as compared with the controls'.
Cytokine production by total PBMC from the patient at the age of 3 months (▪) and from 10 age-matched healthy controls (▨) in response to PMA (5 ng/mL) plus ionomycin (500 ng/mL). Data of healthy controls are expressed as mean ± 1 SD.
Cytokine production by total PBMC from the patient at the age of 3 months (▪) and from 10 age-matched healthy controls (▨) in response to PMA (5 ng/mL) plus ionomycin (500 ng/mL). Data of healthy controls are expressed as mean ± 1 SD.
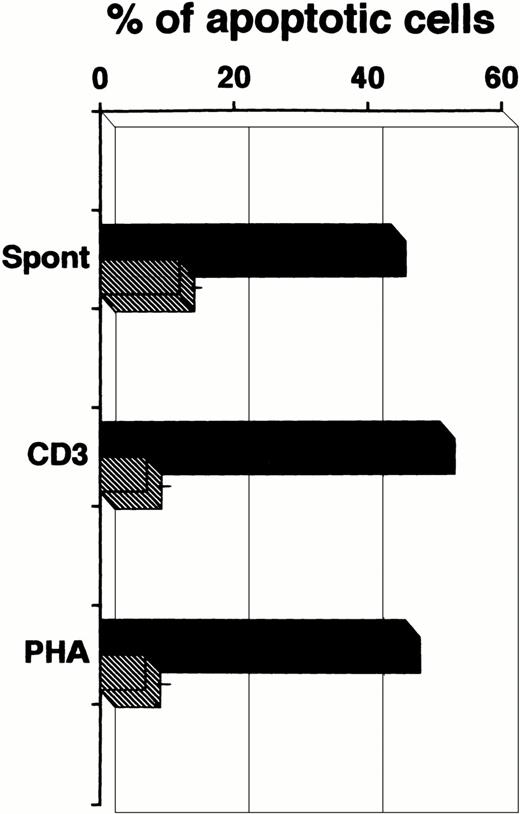
We considered the possibility that defective lymphocyte proliferation and the reduced cytokine production were caused by increased cell death during the culture. Indeed, an increased proportion of apoptotic cells was detected in the patient, both in unstimulated and stimulated cultures (Fig 4).
Increased rates of apoptotic cells in unstimulated and stimulated cultures of PBMC of the patient (▪) compared with 10 age-matched healthy controls (▨). Data of healthy controls are expressed as mean ± 1 SD. All cultures were performed for 72 hours.
Increased rates of apoptotic cells in unstimulated and stimulated cultures of PBMC of the patient (▪) compared with 10 age-matched healthy controls (▨). Data of healthy controls are expressed as mean ± 1 SD. All cultures were performed for 72 hours.
Because the products of bcl-2 gene are known to modulate the susceptibility of T cells to spontaneous apoptosis,11cytoplasmic levels of this protein were evaluated by flow cytometry. The intensity of staining for bcl-2, expressed as MESF, was significantly reduced after 48 hours of unstimulated culture of lymphocytes from the patient, as compared with that observed in three age-matched healthy controls (4,592 v 14,750 ± 555; Fig 5). Taken together, these data suggest that the raised number of spontaneously dying PBMC in the patient is accounted for by downmodulation of bcl-2 gene product expression.
The expression of bcl-2 on PBMC from the patient (continuous lines) and one representative healthy control (dashed lines) after 48 hours of unstimulated culture.
The expression of bcl-2 on PBMC from the patient (continuous lines) and one representative healthy control (dashed lines) after 48 hours of unstimulated culture.
DISCUSSION
We report a case of a child with an AR-SCID, caused by Jak3 mutations, presenting at birth a typical T−B+phenotype, who developed autologous CD3+CD4+ T cells after the first few months of life. These lymphocytes showed a primed/activated phenotype; had a severely defective proliferative response to anti-CD3, PHA, and allogeneic cells; and secreted very low levels of Th1 and Th2 cytokines, as compared with controls. Functional defects, including reduced proliferative response and defective cytokine production, were accounted for by the large number of cells dying either spontaneously (as a consequence of bcl-2 downregulation) or after activation in vitro. These data are in keeping with the notion that the response induced in T lymphocytes by antigen stimulation is influenced by their activation status, because triggering of TCR/CD3 complex of recently activated lymphocytes leads to apoptosis rather than proliferation.35-37
Our report is similar to other atypical presentations of XL-SCID, in which the presence of autologous, poorly functioning T lymphocytes was documented.4,22-24 In particular, the patient reported by DiSanto et al22 carried a splice site mutation in the γc gene leading to two alternative transcripts, only one being functional, although present in limited amounts. Furthermore, Russell et al4 reported another XL-SCID patient with a missense mutation in the cytoplasmic region of the γcprotein that substantially diminished, but did not abrogate, its association with Jak3. In this latter case, the patient at birth had no T lymphocytes and developed CD3+CD4+ cells at the age of 19 weeks.38 These results suggest that reduced but detectable expression of γc4,22 or Jak3 (present case) proteins may be enough to generate a limited number of peripheral T-cell clones. Alternatively, other hematopoietic growth factors that do not use Jak3 (eg, IL-3, Thymic Stromal Lymphopoietin,39 or stem cell factor through its receptor c-kit40) might substitute for some of the functions of Jak3-dependent cytokines allowing the generation of T cells. However, in the absence of γc/Jak3 signals the action of these cytokines might be insufficient for the correct activation and function of these lymphocytes. In particular, IL-7 receptor signaling has been recently shown to upregulate bcl-2 expression in developing thymocytes and in mature peripheral T cells,41,42 thus maintaining their viability and function.41 42
Interestingly, murine models of defective Jak3 function (Jak3−/− mice) also have a similar, abnormal T-cell development. T-cell differentiation in the thymus is partially conserved, despite severe cell depletion. Furthermore, an age-dependent increase of CD4+ lymphocytes is observed in the periphery. These cells have an activated/memory phenotype, fail to respond to mitogens, and show a reduced IL-2 secretion following stimulation with anti-CD3 plus anti-CD28.17-19
The observation that, like in Jak3−/− mice, preferential accumulation of CD4+ lymphocytes was observed in our patient suggests that cytokine signaling through the γc/Jak3 pathway may be more critical for the maturation or survival of CD8 than of the CD4 lineage T cells.
In our experience, oligoclonal, in vivo-activated, poorly functioning T cells were observed in other immunodeficiencies. In Omenn's syndrome, a rare inherited immunodeficiency, peripheral blood T cells, usually present in normal number, are oligoclonal43 and show an activated phenotype and a defective proliferative response to mitogens.44 As in the case described here, we have recently shown that functional T-cell defects observed in Omenn's syndrome might be explained, at least in part, by excessive cell death resulting from two main mechanisms: spontaneous apoptosis in unstimulated cultures, associated with reduced expression of bcl-2 gene product, and susceptibility to activation-induced cell death through a Fas/Fas ligand-mediated mechanism, after restimulation in vitro with mitogens.45 Activated, anergic T cells have been also detected in combined immunodeficiency caused by functional defects (unpublished observation, February 1997), in early phases of immune reconstitution following BMT,46-48 and in trans-placental engraftment of maternal T cells occurring in some cases of SCID T−B+/−.49
In all cases, the unknown developmental pathways used by T lymphocytes seem to be antigen driven; furthermore, they lead to terminally differentiated T cells that are functionally defective because of high susceptibility to undergo cell death.
On the other hand, not much is known about the origin of these activated and anergic T lymphocytes. Refilling of a depleted T-cell compartment may arise from two main potential ways: thymopoiesis, which creates new T cells from progenitors and maintains or increases the potential diversity of T-cell repertoire, and peripheral expansion, which probably restricts that repertoire. It is unlikely that defective thymopoiesis alone may account for the aberrant T-cell phenotype observed in our patient, as in this case accumulation of “naı̈ve” T cells would be expected. Based on recent findings in murine models, indicating that the lack of expression of Jak3 in the periphery is associated with accumulation of activated, anergic CD4+ T cells in the periphery,21 it is likely that defective immune homeostasis in the periphery accounts for the development of oligoclonal, activated, and anergic T cells in our patient.
Accordingly, the data relative to the TCR diversity further support the hypothesis of an ongoing clonal expansion. The TCRBV repertoire of this patient was not simply restricted in terms of TCRBV genes usage; each of the dominant TCRBV families expressed were found to be oligoclonal. The presence of a limited number of available TCRBV chain molecules may limit antigenic recognition and thus contribute to immunodeficiency.
Finally, the observation that our patient developed hemolytic anemia after BMT, when a persistence of autologous T cells was detected, is in agreement with the observation that deletion of self-reactive T cells in the thymus and in the periphery is defective in Jak3-deficient mice,50 and thus adds to the key role played by the γc/Jak3 signaling pathway in T-lymphocyte differentiation and function.
Partially supported by grants from Telethon Italy (grant A.42 to L.D.N.). This paper is manuscript no. 12 of the Genoma 2000/ITBA Project, funded by CARIPLO.
Address reprint requests to Luigi D. Notarangelo, MD, Clinica Pediatrica, Spedali Civili Brescia, Piazza Spedali Civili 1, 25123 Brescia, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal