Abstract
Graft-versus-host disease (GVHD) and infections are two major complications of allogeneic bone marrow transplantation (BMT). In the course of GVHD, one of the pathways that activated cytotoxic T cells use to execute their killing mechanisms is the Fas/Fas ligand pathway. This killing mechanism might be accompanied by the release of soluble Fas (sFas) in the circulation. To examine the association of serum sFas levels and post-BMT complications, we have analyzed sFas levels in sera of bone marrow recipients with and without GVHD. Postallogeneic BMT sFas levels were significantly increased during clinically relevant acute GVHD (aGVHD; P = .002). However, during infections sFas levels tended to decrease (P = .088). Yet, the simultaneous occurrence of GVHD and infections resulted in extreme high sFas levels. These results suggested that sFas release may be correlated with the amount of tissue damage, because aGVHD induces more damage than infections. The presence of significantly increased sFas levels during aGVHD provides new insights into the GVHD pathogenesis.
HEMATOLOGICAL MALIGNANCIES can be treated by bone marrow transplantation (BMT). However, allogeneic BMT can be complicated by graft-versus-host disease (GVHD) and infections, because BMT recipients are immunosuppressed.1-3 Moreover, GVHD enhances the susceptibility for infections and, although they occur simultaneously, GVHD may be masked.4
Induction of apoptosis is an important T-cell effector mechanism that is mediated by the interaction of the Fas/APO-1 molecule with its ligand (FasL).5-7 Fas is a member of the tumor necrosis factor/nerve growth factor receptor (TNFR/NGFR) superfamily.8-10 Soluble receptors have been described for other members of the TNFR/NGFR family, which are mainly derived by proteolytic cleavage.11-14 Soluble forms of the TNF receptor type I and II are present in human serum and are able to inhibit TNFα activity.15-19
A soluble splice variant of Fas (sFas) has been identified in the serum of healthy individuals, of patients with autoimmune disease,20-22 and of patients with B- and T-cell leukemias.23 Also B- and T-cell lines and activated peripheral blood mononuclear cells were shown to produce sFas.23-26 Alternative splice variants of the Fas-gene have been identified, indicating that sFas is generated by alternative splicing rather than proteolytic cleavage.20,23,25,27 sFas has been shown to inhibit apoptosis induction in vitro.20,24,26,27 Studies in Fas-deficient lpr mice and in FasL-lacking gld mice indicated that Fas-mediated cytotoxicity is an important effector mechanism in GVHD.28-34
To our knowledge nothing is known about the production of sFas during GVHD or organ transplant rejection in man. We questioned whether there exists a causal relationship between the putative increased T-cell activity during GVHD and sFas levels in BMT recipients. Because T cells are also involved in the immune response during infections, we have analyzed sFas levels in BMT recipients during acute GVHD (aGVHD) and infections.
MATERIALS AND METHODS
Patients.
Fifty-two adult patients who underwent BMT between 1978 and 1990 in the Leiden University Hospital were included in this study. Thirty-nine patients received bone marrow from a human leukocyte antigen (HLA)-identical sibling, 1 patient received bone marrow from her HLA-identical father, and 12 patients received autologous bone marrow. Underlying diseases were acute myeloid leukemia (n = 33), chronic myeloid leukemia (n = 6), non-Hodgkin's lymphoma (n = 4), aplastic anemia (n = 4), morbus Hodgkin's (n = 3), and acute lymphoblastic leukemia (n = 2). Patients were conditioned with cyclophosphamide (Cy) and total body irradiation (TBI; n = 37); Cy and total lymph node irradiation (n = 4); Cy, campath, and busulphan (n = 3); Cy, BCNU, and etoposide (n = 3); Cy, TBI, ATG, and Ara-C (n = 2); Cy, BCNU, etoposide, and Ara-C (n = 2); or Cy, TBI, and campath (n = 1). Cyclosporin A (n = 18) or methotrexate (n = 22) was given as GVHD prophylaxis. Of the patients receiving allogeneic bone marrow (19 women and 21 men) the mean age was 30 years (range 17 to 47). The mean age of the patients receiving autologous bone marrow (5 women and 7 men) was 37 (range 20 to 58). Normal levels of sFas were determined in sera taken from bone marrow donors (n = 41) and healthy blood donors (n = 15) designated as healthy controls.
Complications after BMT.
aGVHD was diagnosed according to clinical and histopathological criteria.35 In the assessment of GVHD, a grade of 0 or I was considered to indicate absent or clinically unrelevant disease and a grade of II or higher the presence of clinically relevant disease.
Viral infections (CMV, VZV, and HSV) and fungal infections were diagnosed on the basis of culture, histopathology, and specific antibody tests and bacterial infections (pneumonia and sepsis) were diagnosed on the basis of an infiltrate on x-ray and/or positive bacterial culture from sputum, blood, or bronchoalveolar lavage.
Sera.
Serum samples were collected before BMT and after BMT. Post-BMT serum samples were collected systematically for the first 3 months post-BMT and thereafter incidentally up to 3 years post-BMT. Serum samples were also collected from BMT donors and from healthy blood donors. All sera were stored at −30°C until further use. For this study we have striven to include at least one sample for every 10-day period post-BMT until day 100 and during complications.
Serum sFas measurements.
Serum sFas levels were assessed by sandwich enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies CLB-CD95/2 and CLB-CD95/6. Maxisorp microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 100 μL/well CLB-CD95/2 (2 μg/mL) in 0.1 mol/L NaHCO3/Na2CO3 buffer (pH = 9.6) at room temperature and blocked with 100 μL/well phosphate-buffered saline (PBS)/2% whole milk for 30 minutes at room temperature. Samples were diluted 10 times in high performance ELISA buffer (HPE; CLB, Amsterdam, The Netherlands). A twofold dilution of the standard was made in HPE, ranging from 1,000 pg/mL to 2 pg/mL. One hundred microliters of samples and standards and 10 μL biotinylated CLB-CD95/6 (10 μg/mL) were pipetted into each well and the plate was incubated for 2 hours at room temperature. After washing vigorously 100 μL/well streptavidine poly-horseradish peroxidase (1:10,000 diluted in PBS/2% milk) was added to the plate, incubated for 30 minutes at room temperature, and washed vigorously. The ELISA was developed using 0.1 mg/mL 3,5,3′,5′-tetramethylbenzidine (Merck, Darmstadt, Germany) and 0.003% H2O2 in 0.11 mol/L NaAc (pH = 5.5) for 10 minutes. The color reaction was stopped with 100 μL 2M H2SO4 and plates were read at 450 nm in a Titertek Multiskan reader (Labsystems Multiskan Multisoft, Helsinki, Finland).
sFas levels and liver involvement.
Sixteen patients suffered from moderate to severe GVHD without liver involvement, and 15 patients suffered from moderate to severe GVHD with liver involvement. To evaluate the contribution of liver damage to increased sFas levels, total blood bilirubin levels were used as a marker for the occurrence of liver damage36 after exclusion of other causes of hyperbilirubinemia.
Statistical analysis.
For statistical analysis, sFas levels were transformed to their10log value. To test for differences in sFas levels of healthy controls and pre-BMT sFas levels of BMT recipients unpairedt-tests were used. Differences between autologous BMT patients and allogeneic patients on the changes in sFas levels from pre- to post-BMT were determined with the repeated measurements multivariate analysis of variance (MANOVA). For this analysis sFas levels determined in sera, taken from autologous bone marrow recipients (n = 12) and from allogeneic bone marrow recipients without complications (n = 26) before BMT and within 30 days post-BMT, were used. The factor “patients” is included in the analysis to correct for general patient levels.
To determine the correlation of aGVHD or infections with changes in sFas levels post-BMT within the allogeneic BMT group, these complications were coded into the following dichotomous variables: “aGVHD” = grade 0 to I (0) versus grade II to IV (1) and “infection” = absence (0) versus bacterial, viral, or fungal infection (1). The statement used in the MANOVA is the following: sFas BY patients (1 40) WITH aGVHD, infection. Thus, only changes within patients are used and not the differences between patient groups. This analysis can be seen as an extension of the well-known paired t-test. The correlation between changes in sFas levels and changes in bilirubin levels within selected patients was obtained in a truly multivariate MANOVA with “patients” as factor and 10log(bili) and sFas as outcome variables.P values less than .05 were accepted as significant.
RESULTS
sFas levels in healthy controls and in BMT recipients without complications.
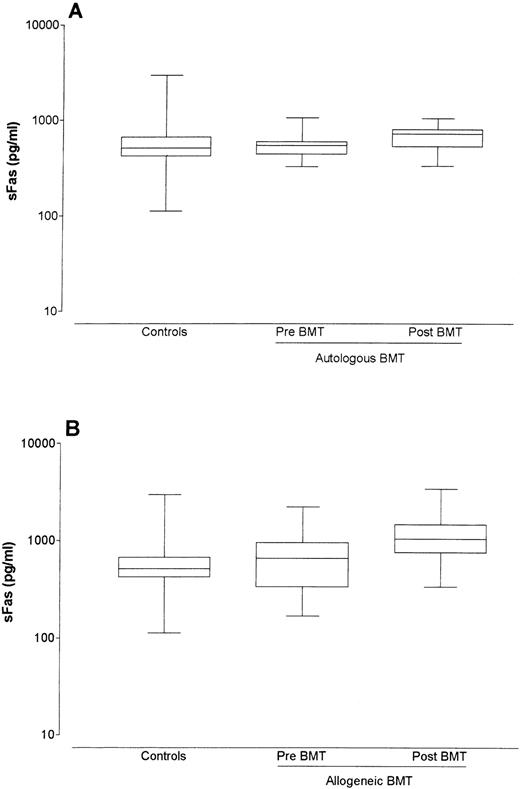
Control serum sFas levels were determined in healthy controls and ranged from 112.2 to 2,951.2 pg/mL (median 512.9 pg/mL). The pre BMT sFas levels of 12 patients receiving autologous BMT ranged between 323.6 and 1,047.1 pg/mL (median 549.5 pg/mL) and did not differ significantly from sFas levels from healthy controls (P = .92). sFas levels in autologous bone marrow recipients rose slightly in the first 30 days post-BMT, although this increase was not significant (median pre-BMT level = 549.5 pg/mL; median post-BMT level = 724.4 pg/mL; P = .122; Fig 1A).
Box-whisker plots of sFas levels in sera of controls, of autologous bone marrow recipients pre- and post-BMT (A), and of allogeneic bone marrow recipients pre- and post-BMT (B). The box represents the 25% and 75% percentiles, with a line indicating the median value. The whiskers show minimum and maximum values.
Box-whisker plots of sFas levels in sera of controls, of autologous bone marrow recipients pre- and post-BMT (A), and of allogeneic bone marrow recipients pre- and post-BMT (B). The box represents the 25% and 75% percentiles, with a line indicating the median value. The whiskers show minimum and maximum values.
The pre-BMT levels of 40 allogeneic BMT patients ranged between 166.0 and 4,570.9 pg/mL (median 660.7 pg/mL) and did not differ significantly from sFas levels of healthy controls (P = .16) or of autologous patients pre-BMT (P = .32). Allogeneic BMT recipients without complications showed a significant increase in sFas levels after BMT (median pre-BMT level = 660.7 pg/mL; median post-BMT level = 1,047.1 pg/mL; P < .001; Fig 1B). sFas levels in both autologous and allogeneic BMT recipients without complications did not stay elevated, but returned shortly post-BMT to normal or to slightly elevated levels (data not shown).
Post-BMT levels during complications in allogeneic BMT recipients.
In patients suffering from complications sFas levels reached higher levels than in patients without complications. To analyze the effects of aGVHD and infections on sFas levels, the absence or presence of each complication was recorded for each sample date. Table 1 shows the descriptive statistics of the sFas levels measured in the presence of no to mild aGVHD (grade 0-I) or clinically relevant aGVHD (grade II-IV) and in the absence or presence of infections. Of each group the number of samples measured and quartiles (median, 25% percentile and 75% percentile) are given. Median sFas levels increase during relevant aGVHD but not during infections. However, in the presence of both relevant GVHD and infections sFas levels are strongly elevated. The effect of both aGVHD and infections on sFas levels were analyzed in the repeated measurements MANOVA analysis as described in the Materials and Methods section. Statistical analysis of the different complications showed that during aGVHD (grade II-IV) sFas levels are significantly increased (P = .002), whereas sFas levels tend to decrease during infections (P = .088; Table 2).
Post BMT sFas Levels With or Without Severe aGVHD or Infections
| aGVHD Grade . | No Infection . | Infection . | ||||
|---|---|---|---|---|---|---|
| # . | Median . | 25%-75% . | # . | Median . | 25%-75% . | |
| 0-I | 261 | 1,174.9 | 851.1-1,621.8 | 103 | 1,148.2 | 776.2-1,737.8 |
| II-IV | 83 | 1,445.4 | 955.0-1,995.3 | 32 | 1,862.1 | 1,071.5-2,511.9 |
| aGVHD Grade . | No Infection . | Infection . | ||||
|---|---|---|---|---|---|---|
| # . | Median . | 25%-75% . | # . | Median . | 25%-75% . | |
| 0-I | 261 | 1,174.9 | 851.1-1,621.8 | 103 | 1,148.2 | 776.2-1,737.8 |
| II-IV | 83 | 1,445.4 | 955.0-1,995.3 | 32 | 1,862.1 | 1,071.5-2,511.9 |
In each group the number of post-BMT samples (#) and the quartiles (median, 25% percentile and 75% percentile in pg/mL) are given. Grade 0-I = no or mild aGVHD, grade II-IV = clinically relevant aGVHD. Infection comprises viral, bacterial, and fungal infections.
Results of the Repeated Measurements MANOVA
| Complication . | B . | SE . | Pvalue . |
|---|---|---|---|
| aGVHD | 0.061 | 0.019 | .002 |
| Infections | −0.028 | 0.016 | .088 |
| Complication . | B . | SE . | Pvalue . |
|---|---|---|---|
| aGVHD | 0.061 | 0.019 | .002 |
| Infections | −0.028 | 0.016 | .088 |
sFas values were transformed to their 10log value for statistical analysis. For each, complication, regression coefficient (B), standard error (SE) and P values are given. In this analysis, B represents the effect of the presence versus the absence of a complication on sFas levels. Thus, a positive B indicates an increasing effect, whereas a negative B indicates a decreasing effect on sFas levels.
Correlation of sFas and liver GVHD.
To investigate whether liver damage is correlated with increased sFas levels, total blood bilirubin levels were used as a marker for liver damage. Sixteen patients suffered from moderate to severe GVHD without liver involvement, and 15 patients suffered from moderate to severe GVHD with liver involvement. MANOVA analysis showed a significant correlation between bilirubin levels and sFas levels (r = .443,P < .001), indicating that sFas levels during GVHD with liver involvement were significantly different from sFas levels during GVHD without liver involvement.
DISCUSSION
In experimental GVHD, Fas-mediated apoptosis is an important effector mechanism.34,37 A soluble form of Fas (sFas) can be detected in human serum and is able to inhibit Fas-mediated apoptosis in vitro.20,26 27 Because activated lymphocytes produce sFas and lymphocyte activity plays an important role in GVHD, we questioned whether sFas would play a role in the GVHD pathogenesis in humans.
Our results showed that BMT treatment already caused an early and temporary increase in sFas levels. This increase was not correlated to the occurrence of complications and was more pronounced in allogeneic BMT recipients than in autologous BMT recipients. Immunologic disparities between donor and host in allogeneic BMT may cause immunoreactivity early after allogeneic BMT, which will subside when the graft is accepted. Thus, the conditioning regimen itself causes an increase in sFas levels, which is reinforced by immunoreactivity in allogeneic BMT.
Furthermore, a significant correlation between increased sFas levels and aGVHD grade II-IV is found but not with infections. In the presence of both aGVHD and infections, strongly increased sFas levels were found, indicating that the enhancing effect of aGVHD on sFas levels dominates over the leveling effect of infections. Increased sFas levels coincided and in some patients preceded aGVHD episodes.
The source of increased levels of sFas found during GVHD is unknown. sFas can be produced by activated immune cells and, because Fas has a wide tissue distribution, it may also be released by damaged target cells.23-26 Hepatocytes constitutively express Fas and are a ready target for Fas-mediated apoptosis.38,39 Because the liver is one of the target organs of GVHD, sFas release by damaged hepatocytes may contribute significantly to the enhanced serum levels found during GVHD. Our study showed a significant correlation between bilirubin levels and sFas levels indicating that liver damage, as delineated by serum hyperbilirubinemia, is associated with increased sFas levels. However, because Fas is also expressed in the skin and gastrointestinal tract, sFas may also be released by these tissues during GVHD.31
The presence of elevated levels of sFas in the sera of GVHD patients prompted us to hypothesize on the role of sFas in the pathogenesis of GVHD. Fas/FasL-induced apoptosis normally serves as a mechanism for the regulation of an immune response via activation-induced cell death. Activated lymphocytes express both Fas and FasL and can induce apoptosis in other activated lymphocytes (“fratricide”) or in themselves (“suicide”).40-47 sFas molecules are able to block Fas/FasL interaction and, thus, prevent apoptosis induction.20,24,26 Although the levels found in serum are probably too low to play a role in the prevention of apoptosis, local levels at the site of the graft-versus-host reaction may be much higher.20 sFas may play a dual role in GVHD. On the one hand sFas may inhibit Fas-related cytotoxicity of the effector T cells on the target cells during GVHD. However, because elevated levels of sFas are found during active GVHD, this explanation does not seem plausible. On the other hand sFas may prevent Fas-mediated apoptosis of the effector T cells themselves. This latter event would result in a non–self-limiting immune response and may lead to a prolonged graft-versus-host reaction, because effector T cells will still be able to kill through the perforin/granzyme pathway.33 Support for such a mechanism was described in patients with systemic lupus erythematosus.20
In contrast to the high serum sFas levels found during GVHD, sFas levels tend to decrease during infections. This may imply that regulation of the immune response via Fas/FasL during infections is not blocked by high levels of sFas as opposed to the regulation of the immune response during GVHD. Recent observations suggest that membrane-expressed Fas and sFas can be differentially regulated.23 25 Whereas the regulation of Fas and sFas expression during infections may reflect the normal immune response, high levels of sFas found during GVHD may not only result from release by damaged cells but also by abnormal expression of the sFas splice variant.
In conclusion, we have shown that increased serum sFas levels in BMT patients correlate significantly with aGVHD and are further increased in those cases in which GVHD and infections occur simultaneously. In cases of solely infections, sFas levels tend to decrease in BMT patients. The latter finding may have also been of great importance for solid organ transplantation, because diagnosis between rejection and infection is difficult and requires, more than in BMT, examination of biopsy specimens of relevant organs. Furthermore, high local sFas levels may inhibit Fas-mediated regulation of the immune response, thereby facilitating development of GVHD.
ACKNOWLEDGMENT
The authors thank Dr R. Willemze, Dr A. Brand, and Mrs L. Huige for the collection of serum samples of hematological patients. We thank Prof J.J. van Rood and Drs A. Brand, J. Bruning, F. Claas, and M. Oudshoorn for critical reading of the manuscript.
L.M.L. and T.v.L. contributed equally to this report.
Supported by the JA Cohen Institute of Radiopathology and Radiation Protection (IRS), by EC Grant No. BIO2 CT92 0300, by the MACROPA Foundation and by Grant No. 95CR841 from the Dutch League Against Rheumatism.
Address reprint requests to Linda M. Liem, PhD, Department of Immunohematology and Blood Bank, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal