Abstract
Human γδ T lymphocytes represent a minor subset of T cells in the peripheral blood, which exhibit a limited diversity and a tissue-restricted repertoire in contrast to their broad specificity. Most postthymic neoplasms that arise from this T-cell subpopulation belong to the hepatosplenic γδ lymphoma entity. Only a few cases of nonhepatosplenic γδ lymphomas have been described in detail previously. This study presents the clinicopathologic features of 11 consecutive cases of nonhepatosplenic γδ lymphoma. All were characterized by mucosal or skin initial involvement: nasal cavity (n = 3), gastrointestinal tract (n = 3), skin (n = 3), lung (n = 1), larynx (n = 1). Most patients presented with B symptoms (eight of 11), without peripheral lymphadenopathy and bone marrow involvement. A past history of chronic antigen exposure was noted in six cases, and four patients had features of immune deficiency. On histology, they were classified as pleomorphic tumors. Features of epitheliotropism and angiocentrism was observed in most cases. Tumor cells had a CD2+, CD3+, T-cell receptor (TCR)δ−1+, βF1− phenotype. They were CD5− (9 of 10) and CD4−/CD8− (9 of 10) or CD8+(1 of 10). A clonal γ-chain gene rearrangement was detected in all tested cases (9/9). All cases had an activated cytotoxic T-cell intracellular antigen-1 (TIA-1)+, Granzyme B+ phenotype. Epstein-Barr virus (EBV) sequences were detected in six cases by in situ hybridization (ISH). Despite an aggressive clinical course, complete remission was obtained in three patients, and one of the latter required a peripheral blood stem-cell transplantation. Nonhepatosplenic γδ peripheral T-cell lymphoma can be regarded as a model of activated cytotoxic lymphoma, occurring in mucosae or skin. These appear to be derived from the subpopulation of tissue-restricted γδ lymphocytes, which are involved in the host epithelial surface surveillance. The role of chronic antigen exposure in the pathogenesis of these rare lymphomas can be suggested, in view of the past history observed in at least some patients.
LYMPHOCYTES THAT EXPRESS the γδ T-cell receptor (TCR) represent a small subset compared with T cells that bear the αβ TCR.1,2 Although the overall structure of the γδ TCR is similar to the αβ TCR with variable, joining, and constant regions (γ-chain) and additional diversity regions in the δ chain, γδ T cells exhibit a more limited diversity and a tissue-restricted repertoire.1-4 Human γδ T cells account for 1% to 15% of peripheral blood lymphocytes (PBL) and, throughout the peripheral lymphoid tissues, show a predilection for the red pulp of the spleen (up to 30% of the whole T-cell population) and the gastrointestinal tract.1,2,4-6 The precise functions of γδ T cells are not completely understood. However, mouse models indicate a role for the γδ T-cell subset in host epithelial surface control and early-stage engagement in immune response against pathogens (viruses, bacteria, and parasites), before the recruitment of αβ T cells.4,7-9 γδ T cells are able to react with antigens both in a major histocompatibility complex (MHC)-restricted and MHC-unrestricted fashion, and have a broad antigen specificity from heat-shock proteins to nonpeptidic phosphorylated metabolites.8,10,11 Thus, γδ T cells appear to be early effectors in the immune response, providing a first line of defense in the epidermal and epithelial linings.8,11 These functions are mediated by cytokine production, and include the capacity to stimulate B cells for immunoglobulin production and to develop direct cytolytic activity, as well as antibody-dependent cellular cytotoxicity.8 11
γδ T cells have rarely been implicated in neoplastic lymphoproliferative disorders. Among peripheral T-cell lymphomas, we have identified hepatosplenic γδ T-cell lymphoma as a distinct entity,12,13 which is now recognized by others.14-16 This lymphoma is a rare disease with distinctive clinicopathologic features characterized by its extranodal hepatosplenic presentation and the sinusal/sinusoidal tropism of the γδ neoplastic T cells. Clinically, typical presentation consists in young men with hepatosplenomegaly and frequent systemic symptoms in contrast to the absence of peripheral lymphadenopathy. Initial examination of the blood smears often reveals a decreased platelet count without circulating lymphoma cells. Survival analysis of the rare reported cases reflects the aggressive course and the poor prognosis of this disease.12,15 Several cases of nonhepatosplenic γδ T-cell lymphoma have recently been reported.15,17-26 Most of them are cutaneous γδ T-cell lymphomas with predominant epidermal and/or subcutaneous tissue involvement.15,17-22 Rare cases of nasal γδ T-cell lymphoma have also been reported,23,24 as well as an individual case of γδ T-cell lymphomas of the thyroid gland.25 Only one patient with a nodal presentation has been described.26
In the present study, we report 11 consecutive cases of peripheral T-cell lymphomas that displayed a TCR γδ phenotype. All were characterized by an initial site of involvement in mucosal tissues or skin: nasal cavity (n = 3), gastrointestinal tract (n = 3), skin (n = 3), lung (n = 1), and larynx (n = 1). These lymphomas probably originate from the normal γδ T cells that are present or can be induced by inflammatory states in different mucosa-associated lymphoid tissues (MALT), as well as in the skin. Given the potential role of the γδ T cells in the immune response, their capacity for developing cytolytic activity, and their peculiar relationship with epithelial cells, some mechanisms of tumorigenesis are discussed.
MATERIALS AND METHODS
Patient selection.
This series includes all patients with nonhepatosplenic γδ peripheral T-cell lymphoma diagnosed between February 1986 and February 1997, using immunohistochemistry on frozen material, in three Departments of Pathology (Hôpital Henri Mondor, Créteil; Hôpital Lariboisière, Paris; and Institut Gustave Roussy, Villejuif, France). All biopsy specimens had the histology of lymphoma on hematoxylin-eosin–stained sections. On frozen sections, lymphoma cells expressed at least the CD2 and CD3 T-cell antigens, and all were positive with δTCR-1 antibody, but negative with βF1. The clinical and biologic records of each patient were reviewed for types and duration of symptoms, initial and other sites of involvement found during the course of disease, and outcome. Parts of clinical and phenotypic features of two patients (cases no. 1 and 4), as well as the cytotoxic profile in six cases, have been reported elsewhere.13,23 27-29
Tissue specimens.
Biopsy specimens from initial sites of involvement (sinonasal tract, skin, stomach, lung, larynx, and intestine) were fixed in buffered formaldehyde or Bouin's fixatives and paraffin-embedded for histopathologic analysis. A portion of each biopsy was snap-frozen in liquid nitrogen for phenotypic and genotypic studies.
Histologic studies.
Paraffin-embedded tissue sections were stained with hematoxylin-eosin, periodic acid–Schiff (PAS), and/or Giemsa for histologic studies. Lymphomas were classified according to the updated Kiel classification.30 Each case was independently examined by two pathologists. The presence of necrosis, epitheliotropism, angiocentrism, and angioinvasive features was carefully investigated.
Immunohistochemical staining.
Cryostat sections were evaluated for T-, natural killer (NK)-, and B-cell differentiation antigens using the alkaline phosphatase/anti–alkaline phosphatase (APAAP) method.31The following mouse monoclonal antibodies were used: Leu-5/CD2, Leu-4/CD3 and Leu-3/CD4, Leu-1/CD5, Leu-9/CD7, Leu-2/CD8 (Becton Dickinson, Mountain View, CA), Ber-H2/CD30, LMP-1 (Dako SA, Glostrup, Denmark), CD19, NKH1/CD56 (Coulter, Hialeah, FL), βF1 and δTCR-1, δTCS-1, Vδ2, and Vδ3 (T-Cell Diagnostics, Woburn, MA). βF1 recognizes a nonpolymorphic epitope of the β chain of the αβ TCR heterodimer. δTCR-1 recognizes a nonpolymorphic epitope of the δ chain of the γδ TCR heterodimer. δTCS-1 antibody is directed against a conformational epitope of the human Vδ1/Jδ1 junction, whereas Vδ2 and Vδ3 monoclonal antibodies recognize an epitope of the human Vδ2 and Vδ3 regions of the δ chain, respectively. CD3ε polyclonal, L26/CD20, and Ber-H2/CD30 monoclonal antibodies (all from Dako SA) were also used in paraffin-embedded sections. Cytotoxic cell proteins were detected using monoclonal antibodies that recognize T-cell intracellular antigen-1 (TIA-1) (clone TIA-1; Coulter), perforin (clone delta G9; T-Cell Diagnostics), and Granzyme B (clone GrB-7; Monosan, Uden, The Netherlands). Expression of perforin was evaluated on frozen tissue sections, whereas the mouse monoclonal antibodies TIA-1 and GrB-7, which recognize formalin-resistant epitopes of TIA-1 and Granzyme B, respectively, were used in paraffin-embedded tissue sections. For a better detection of TIA-1 and Granzyme B, a pretreatment with microwave oven heating (two cycles of 5 minutes in 0.01 mol/L citrate buffer, pH 6) was performed, as previously described.29 Optimum labeling for granzyme B was obtained by twice repeating the bridge and APAAP complex. Rabbit antimouse immunoglobulin and APAAP complexes were obtained from Dako.
Genomic study.
DNA was extracted from lymphoma tissues by a standard proteinase K digestion, phenol/chloroform precipitation procedure; 250 ng was used for each experiment. TCR γ-chain gene rearrangements were studied using a guanine-cytosine (GC) clamp multiplex polymerase chain reaction (PCR)/denaturing gradient gel electrophoresis (DGGE) procedure, as previously described.32 The use of oligonucleotides that match all of the Vγ and Jγ functional segments, combined with DGGE, allowed the achievement of a migration profile specific to each T-cell clone. The highly heterogeneous PCR γ products of polyclonal reactive T cells result in a smear on DGGE, whereas a clonal rearrangement of TCR γ genes results in one (monoallelic) or two (biallelic) bands on DGGE.
In situ hybridization study.
The in situ hybridization (ISH) procedure for the detection of EBER transcripts was performed using the fluorescein-conjugated Epstein-Barr virus (EBV; EBERs 1 and 2) oligonucleotides (Dako) complementary to nuclear RNA portion of the EBER 1 and 2 genes that are actively transcribed in latency infected cells. Details of the procedure have been previously reported.23 Briefly, deparaffinized sections were rehydrated and preparated with proteinase K, dehydrated, air-dried, and hybridized for 2 hours at 37°C with the fluorescein isothiocyanate–conjugated (FITC) EBER oligonucleotides in hybridization solution. After washing in Tris-buffered saline (TBS) that contained 0.1% Triton X-100, the following immunohistochemical detection method was used: mouse anti-FITC, rabbit antimouse immunoglobulin, and APAAP complexes (Dako). Visualization of the reaction was performed by incubation in a solution that contained bromochloroindolylphosphate (BCIP) and nitroblue tetrazolium (NBT) (Dako). The slides were briefly counterstained with methyl green. As positive controls for ISH, one case of EBV-positive Burkitt lymphoma was run in parallel.
RESULTS
Clinical features.
The main clinical features are summarized in Table1. The median age of the patients was 48 years (range, 14 to 88). Eight of 11 patients were male and three were female. All patients presented initially with mucosa or skin involvement of the nasal cavity (three cases), the gastrointestinal tract (small bowel in cases no. 10 and 11, stomach in case no. 9), the skin (cases no. 6, 7, and 8), the lung (case no. 4) and the larynx (case no. 5). B symptoms were present at presentation in eight patients. Initial staging was similar in the 11 patients, since none of them had lymph node, liver, spleen, or bone marrow involvement. Biologic findings on peripheral blood were unremarkable, except for mild anemia and lymphopenia in case no. 4. Platelet count was normal in all patients at presentation. Laboratory liver tests were also unremarkable. The lactic dehydrogenase (LDH) level was elevated in four patients (cases no. 2, 3, 7, and 8). Hypogammaglobulinemia was noted in three patients (cases no. 2, 4, and 7). Antibodies against human immunodeficiency virus (HIV) and human T-cell lymphotrophic virus type 1 (HTLV-1) were not detectable in all but one patient (no. 9), who had an HTLV-1–positive serology. In this case, HTLV-1 was not clonally integrated into the malignant cells.
Clinical Features of Patients With Nonhepatosplenic γδ T-Cell Lymphomas
| Case No. . | Age/ Sex . | Past History . | Initial Symptoms . | Initial Site of Involvement . | Initial Staging . | Sites of Involvement During Clinical Course . | Treatment/ Outcome . | |
|---|---|---|---|---|---|---|---|---|
| H/S . | LN/BM . | |||||||
| 1 | 30/M | RMS | Ulcerative lesions of the upper respiratory tract, fever | Nasal cavity | −/− | −/− | Nasal recurrence liver, spleen, cervical LN | CT (CHOP) DOD after 5 mo (hepatic failure) |
| 2 | 62/F | RMS | Nasal obstruction, periorbital edema, fever | Nasal cavity | −/− | −/− | Nasal recurrence bone marrow | CT (CHOP + RT) DOD after 12 mo |
| 3 | 56/M | RMS | Nasal obstruction, epistaxis | Nasal cavity | −/− | −/− | — | CT (ACVBP) NOD after 21 mo |
| 4 | 65/F | Pneumocystis, CMV infection, UCID | Cough, fever, pulmonary opacities | Lung | −/− | −/− | Pulmonary recurrence | CT (CHOP) DOD after 12 mo |
| 5 | 88/M | — | Dysphonia, weight loss | Larynx | −/− | −/ND | Skin, liver, spleen abdominal LN | Oral CT (CLB) DOD after 10 mo |
| 6 | 45/F | CHP | Cutaneous nodule of the left thigh, fever | Skin | −/− | −/− | Skin recurrence, lung, liver, spleen | CT (CHOP) DOD after 17 mo |
| 7 | 14/M | CHP | Cutaneous nodule of the left buttock, fever, weight loss | Skin | −/− | −/− | Local subcutaneous progression | CT (CHOP) Alive after 1 mo |
| 8 | 41/M | Spontaneous regressive skin eruptions | Multiple ulcerative necrotic cutaneous nodules | Skin | −/− | −/− | Skin recurrence | CT (Pro-Mace Cyta bom), RT, alive after 8 years |
| 9 | 53/M | Resistant strongyloidiasis, HTLV-1+ | Epigastric pain, fever | Stomach | −/− | −/− | Gastric recurrence liver, spleen, abdominal LN | CT (CHOP, DHAP), PSC transplantation NOD after 24 mo |
| 10 | 43/M | — | Peritonitis (intestinal perforation), fever, weight loss | Small bowel | −/− | −/− | — | CT (CHOP) DOD after 2 mo |
| 11 | 38/M | Celiac sprue | Abdominal pain | Small bowel | −/− | −/− | — | CT (AVmCP) Alive after 3 mo |
| Case No. . | Age/ Sex . | Past History . | Initial Symptoms . | Initial Site of Involvement . | Initial Staging . | Sites of Involvement During Clinical Course . | Treatment/ Outcome . | |
|---|---|---|---|---|---|---|---|---|
| H/S . | LN/BM . | |||||||
| 1 | 30/M | RMS | Ulcerative lesions of the upper respiratory tract, fever | Nasal cavity | −/− | −/− | Nasal recurrence liver, spleen, cervical LN | CT (CHOP) DOD after 5 mo (hepatic failure) |
| 2 | 62/F | RMS | Nasal obstruction, periorbital edema, fever | Nasal cavity | −/− | −/− | Nasal recurrence bone marrow | CT (CHOP + RT) DOD after 12 mo |
| 3 | 56/M | RMS | Nasal obstruction, epistaxis | Nasal cavity | −/− | −/− | — | CT (ACVBP) NOD after 21 mo |
| 4 | 65/F | Pneumocystis, CMV infection, UCID | Cough, fever, pulmonary opacities | Lung | −/− | −/− | Pulmonary recurrence | CT (CHOP) DOD after 12 mo |
| 5 | 88/M | — | Dysphonia, weight loss | Larynx | −/− | −/ND | Skin, liver, spleen abdominal LN | Oral CT (CLB) DOD after 10 mo |
| 6 | 45/F | CHP | Cutaneous nodule of the left thigh, fever | Skin | −/− | −/− | Skin recurrence, lung, liver, spleen | CT (CHOP) DOD after 17 mo |
| 7 | 14/M | CHP | Cutaneous nodule of the left buttock, fever, weight loss | Skin | −/− | −/− | Local subcutaneous progression | CT (CHOP) Alive after 1 mo |
| 8 | 41/M | Spontaneous regressive skin eruptions | Multiple ulcerative necrotic cutaneous nodules | Skin | −/− | −/− | Skin recurrence | CT (Pro-Mace Cyta bom), RT, alive after 8 years |
| 9 | 53/M | Resistant strongyloidiasis, HTLV-1+ | Epigastric pain, fever | Stomach | −/− | −/− | Gastric recurrence liver, spleen, abdominal LN | CT (CHOP, DHAP), PSC transplantation NOD after 24 mo |
| 10 | 43/M | — | Peritonitis (intestinal perforation), fever, weight loss | Small bowel | −/− | −/− | — | CT (CHOP) DOD after 2 mo |
| 11 | 38/M | Celiac sprue | Abdominal pain | Small bowel | −/− | −/− | — | CT (AVmCP) Alive after 3 mo |
Abbreviations: M, male; F, female; RMS, recurrent maxillary sinusitis; DOD, dead of disease; NOD, no evidence of disease; CT, chemotherapy; RT, radiotherapy; UCID, unclassified immunodeficiency; H/S, hepatosplenic; LN/BM, lymph node/bone marrow; ND, not done; PSC, peripheral stem cell; C, cyclophosphamide; H,A doxorubicin; O,V, vincristine; P, prednisone; B, bleomycin; CLB, chlorambucil; CHP, cytophagic histiocytic panniculitis.
A significant past history was present in six patients: chronic sinusitis in the three patients with nasal lymphoma (cases no. 1, 2, and 3), opportunistic pulmonary infections (cytomegalovirus,Pneumocystis carinii pneumonia) related to hypogammaglobulinemia and T-cell deficiency in patient no. 4 with pulmonary lymphoma, chronic Strongyloidiasis and Helicobacter pylori–associated gastritis in an African HTLV-1–seropositive patient (case no. 9) with gastric lymphoma, and celiac sprue disease in patient no. 11 with intestinal lymphoma. Ten patients received an anthracycline-containing polychemotherapy regimen, whereas patient no. 5, an elderly male, received oral chemotherapy. In seven patients, the clinical outcome consisted of poor response or refractory state. Most patients had an early aggressive course, with local recurrence (n = 5) and/or systemic (n = 5) or other mucosal (n = 2) localizations. The median follow-up duration was 18 months (range, 1 to 96). Six patients died of disease within 2 to 17 months. Two patients (no. 3 and 9) with nasal and gastric lymphoma, respectively, responded well to first-line (case no. 3) or second-line (case no. 9) chemotherapy. Patient no. 9, who received a second-line rescue regimen and a peripheral blood stem-cell transplantation following total-body irradiation and high-dose cytarabine with melphalan, has no evidence of disease 24 months after diagnosis. Patient no. 8, with epidermotropic γδ T-cell lymphoma who received polychemotherapy and radiotherapy, was alive after 8 years, with skin recurrence.
Histopathology.
The histologic findings are summarized in Table2. All cases disclosed a diffuse infiltrate of atypical lymphoid cells, and were classified as pleomorphic medium and large-cell (PML) (seven cases), pleomorphic large-cell (PL) (two cases), and pleomorphic small-cell lymphomas (PSC) (two cases).
Histologic Features of Nonhepatosplenic γδ T-Cell Lymphomas
| Case No. . | Site of Biopsy . | Angiocentrism . | Angioinvasion . | Epitheliotropism . | Necrosis . | Histologic Type* . |
|---|---|---|---|---|---|---|
| 1 | Nasal | + | − | + | + | PML |
| 2 | Nasal | + | + | + | + | PML |
| 3 | Nasal | + | + | + | + | PL |
| 4 | Lung | + | + | + | + | PL |
| 5 | Larynx | + | − | + | + | PML |
| 6 | Skin | + | + | + | + | PML |
| 7 | Skin | + | − | − | + | PSC |
| 8 | Skin | + | − | + | + | PML |
| 9 | Antrum | + | − | − | − | PSC |
| 10 | Ileum | + | + | + | + | PML |
| 11 | Ileum | + | − | + | + | PML |
| Case No. . | Site of Biopsy . | Angiocentrism . | Angioinvasion . | Epitheliotropism . | Necrosis . | Histologic Type* . |
|---|---|---|---|---|---|---|
| 1 | Nasal | + | − | + | + | PML |
| 2 | Nasal | + | + | + | + | PML |
| 3 | Nasal | + | + | + | + | PL |
| 4 | Lung | + | + | + | + | PL |
| 5 | Larynx | + | − | + | + | PML |
| 6 | Skin | + | + | + | + | PML |
| 7 | Skin | + | − | − | + | PSC |
| 8 | Skin | + | − | + | + | PML |
| 9 | Antrum | + | − | − | − | PSC |
| 10 | Ileum | + | + | + | + | PML |
| 11 | Ileum | + | − | + | + | PML |
Abbreviations: PML, pleomorphic medium and large; PL, pleomorphic large; PSC, pleomorphic small cell.
According to the Updated Kiel classification.
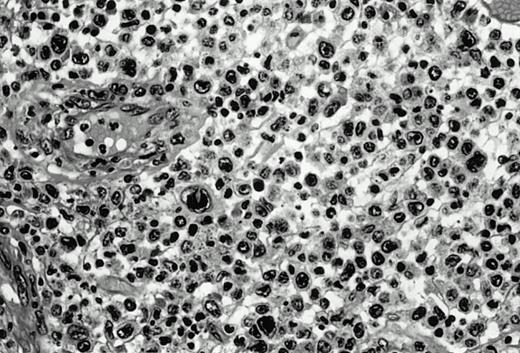
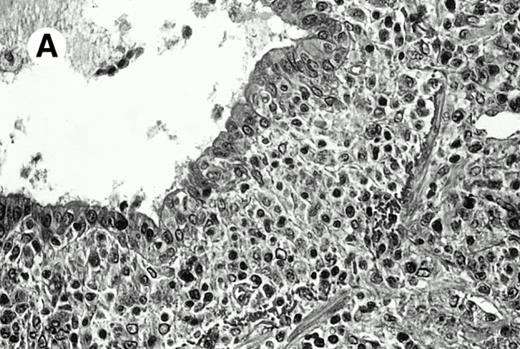
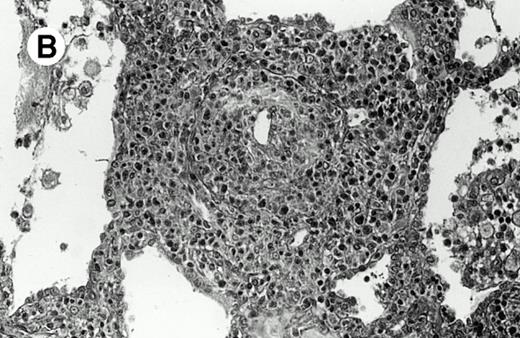
Three cases (cases no. 1 to 3) were located in the nasal cavity. The neoplastic infiltrate consisted of medium and/or large pleomorphic lymphoid cells (Fig 1)intermingled with variable numbers of small lymphoid cells, plasma cells, eosinophils, and histiocytes. Features of angiocentrism and angioinvasion, epitheliotropism, and areas of necrosis were observed in all three cases. Patient no. 4 presented with disease restricted to the lung and an open-lung biopsy was available. The neoplastic infiltrate was composed of large atypical lymphoid cells and was predominantly interstitial without extensive destruction of the normal architecture. Features of angiocentrism, angioinvasion, and epitheliotropism were present (Fig 2A and B). Case no. 5 involved the larynx, extending to the piriform sinus, the ventricle, and the arytenoid. One month later, the lymphoma disseminated to the subcutaneous tissue in the spinal region and biopsy specimens from both sites were available. The neoplastic cells were medium and large, with abundant clear cytoplasm and slightly irregular nuclei with dense chromatin. Epitheliotropism was present in the larynx mucosa. Small foci of necrosis and angiocentrism were seen without angioinvasion. Three patients presented with cutaneous disease. Cases no. 6 and 7 were comparable, as both histories started with necrotic subcutaneous nodules of the thigh, which on histologic analysis were characteristic of cytophagic histiocytic panniculitis without evidence of lymphoma (Fig 3A). Second excisional biopsies of cutaneous nodules obtained 7 and 5 months later, respectively, showed characteristic features of lymphoma in both cases (Fig 3B). However, in case no. 6, skin biopsy specimens showed an epidermotropic PML lymphoma extending to the dermis and the subcutaneous tissue, whereas in case no. 7, the neoplastic infiltrate consisted of a PSC confined to the subcutaneous tissue, admixed with benign histiocytes that showed phagocytosis. Patient no. 8 presented with recurrent skin lesions with spontaneous remission over a period of 10 years. The initial skin biopsies demonstrated an intense epidermotropic neoplastic infiltrate that extended to the superficial and deep dermis composed of atypical PML cells. All cutaneous cases displayed features of angiocentrism, but angioinvasion was observed only in case no. 6 and foci of necrosis in cases no. 6 and 8.
Pleomorphic large-cell γδ T-cell lymphoma (case no. 3) (nasal biopsy specimen with hematoxylin-eosin stain).
Pleomorphic large-cell γδ T-cell lymphoma (case no. 3) (nasal biopsy specimen with hematoxylin-eosin stain).
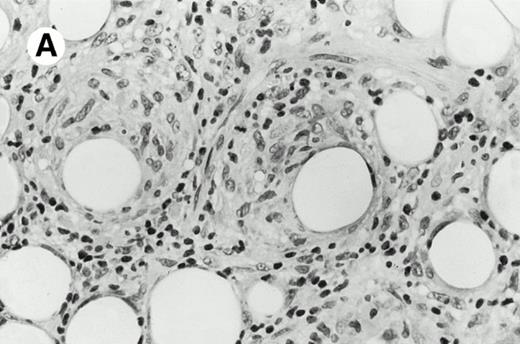
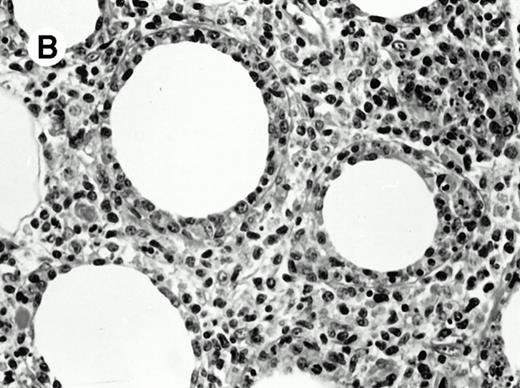
Pulmonary pleomorphic large-cell γδ T-cell lymphoma (case no. 4). (A) Atypical lymphoid cells involve the bronchiolar wall and epithelium; (B) features of angioinvasion; note the preservation of alveolar structures (lung biopsy specimen with hematoxylin-eosin stain); (C) neoplastic cells strongly express TCR γδ. (Immunohistochemical staining on frozen sections with anti-δTCR1 antibody, APAAP technique.)
Pulmonary pleomorphic large-cell γδ T-cell lymphoma (case no. 4). (A) Atypical lymphoid cells involve the bronchiolar wall and epithelium; (B) features of angioinvasion; note the preservation of alveolar structures (lung biopsy specimen with hematoxylin-eosin stain); (C) neoplastic cells strongly express TCR γδ. (Immunohistochemical staining on frozen sections with anti-δTCR1 antibody, APAAP technique.)
Subcutaneous γδ T-cell lymphoma (case no. 6). (A) Initial biopsy sample shows characteristic features of cytophagic histiocytic panniculitis without evidence of lymphoma cells; (B) second biopsy performed 7 months later demonstrates involvement of the subcutaneous tissue by a pleomorphic γδ T-cell lymphoma (hematoxylin-eosin stain).
Subcutaneous γδ T-cell lymphoma (case no. 6). (A) Initial biopsy sample shows characteristic features of cytophagic histiocytic panniculitis without evidence of lymphoma cells; (B) second biopsy performed 7 months later demonstrates involvement of the subcutaneous tissue by a pleomorphic γδ T-cell lymphoma (hematoxylin-eosin stain).
The last three cases involved the gastrointestinal tract. In case no. 9, the tumor was initially localized to the antrum of the stomach and consisted of a diffuse lymphomatous infiltrate that involved the lamina propria and was composed of atypical PSC without epitheliotropism.H pylori–like microorganisms were found. Cases no. 10 and 11 were located in the ileum, and both cases displayed features of PML that involved the entire mucosa, intermingled with variable numbers of eosinophils and plasma cells. In case no. 10, a small-cell component confined to the lamina propria was observed, which displayed some degree of epitheliotropism. Although patient no. 11 had celiac disease, no villous atrophy could be seen, but a marked increase of intraepithelial lymphocytes was present. All gastrointestinal cases displayed features of angiocentrism, but angioinvasion was found only in case no. 10 and necrosis in cases no. 10 and 11.
Immunophenotypic studies.
Complete immunophenotypic characterization was available for all cases on frozen and paraffin sections (Table 3).By definition, all cases expressed γδ TCR (δTCR1+) (Fig 2C), but were negative for TCRαβ (βF1−). In all cases, the neoplastic cells expressed the T-cell–associated markers CD2 and CD3, and most cases lacked the CD5, CD7, CD4, and CD8 antigens. Only one case (no. 9) demonstrated CD5 positivity, which was confirmed by immunostaining on paraffin sections. An additional case was positive for CD7. One case expressed the T-cell suppressor-cell antigen CD8, and the remaining cases were CD4−/CD8− or not interpretable for CD4. Ten cases were studied using the anti-δTCS1, anti-Vδ2, and anti-Vδ3 monoclonal antibodies, which react with variable epitopes of the δ chain. The tumor cells were shown to express the Vδ2-encoded epitope in seven cases, whereas two gastrointestinal cases expressed the Vδ3-encoded epitope and the pulmonary case expressed the variable Vδ1-encoded epitope. In these 10 cases, the patterns of δTCR1 and Vδ epitopes stainings were similar. Expression of the CD30 activation marker was observed on a variable proportion of neoplastic cells in seven cases, all of which were PML or PL lymphomas. Only two cases, both located in the nasal mucosa, expressed the NK marker CD56. All cases were negative for B-cell–associated markers tested either on paraffin or frozen sections.
Immunohistochemical Data, Genomic Data, and EBV Status in Patients With Skin-Mucosa-Associated γδ T-Cell Lymphomas
| Case No. . | CD2 . | CD3 . | CD5 . | CD7 . | CD4 . | CD8 . | β F1 . | δ TCR1 . | Vδ2 . | Vδ3 . | Vδ1* . | CD30 . | CD56 . | TiA-1 . | Granzyme B . | Perforin . | LMP-1 . | EBER . | TCR γ Gene . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | NI | − | NI | − | − | + | + | − | − | +/− | + | + | + | + | +/− | + | R |
| 2 | + | + | − | − | − | − | − | + | + | − | − | +/− | + | + | +/− | + | +/− | + | R |
| 3 | + | + | − | + | − | − | − | + | + | − | − | +/− | − | + | + | + | +/− | + | ND |
| 4 | + | + | − | − | − | − | − | + | − | − | + | +/− | − | + | + | + | − | − | ND |
| 5 | + | + | − | − | − | − | − | + | + | − | − | − | − | + | +/− | +/− | − | + | R |
| 6 | + | + | − | − | − | − | − | + | + | − | − | − | NI | + | + | + | − | − | R |
| 7 | + | + | NI | − | − | + | − | + | + | − | ND | − | − | + | + | ND | − | − | R |
| 8 | + | + | ND | − | − | − | − | + | ND | ND | ND | +/− | ND | + | +/− | ND | − | − | R |
| 9 | + | + | + | − | − | − | − | + | − | + | − | − | − | + | +/− | − | − | +/− | R |
| 10 | + | + | − | + | − | − | − | + | + | − | − | +/− | − | + | +/− | NI | +/− | + | R |
| 11† | + | + | − | − | − | − | − | + | − | + | − | +/− | − | + | + | +/− | − | − | R |
| Case No. . | CD2 . | CD3 . | CD5 . | CD7 . | CD4 . | CD8 . | β F1 . | δ TCR1 . | Vδ2 . | Vδ3 . | Vδ1* . | CD30 . | CD56 . | TiA-1 . | Granzyme B . | Perforin . | LMP-1 . | EBER . | TCR γ Gene . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | NI | − | NI | − | − | + | + | − | − | +/− | + | + | + | + | +/− | + | R |
| 2 | + | + | − | − | − | − | − | + | + | − | − | +/− | + | + | +/− | + | +/− | + | R |
| 3 | + | + | − | + | − | − | − | + | + | − | − | +/− | − | + | + | + | +/− | + | ND |
| 4 | + | + | − | − | − | − | − | + | − | − | + | +/− | − | + | + | + | − | − | ND |
| 5 | + | + | − | − | − | − | − | + | + | − | − | − | − | + | +/− | +/− | − | + | R |
| 6 | + | + | − | − | − | − | − | + | + | − | − | − | NI | + | + | + | − | − | R |
| 7 | + | + | NI | − | − | + | − | + | + | − | ND | − | − | + | + | ND | − | − | R |
| 8 | + | + | ND | − | − | − | − | + | ND | ND | ND | +/− | ND | + | +/− | ND | − | − | R |
| 9 | + | + | + | − | − | − | − | + | − | + | − | − | − | + | +/− | − | − | +/− | R |
| 10 | + | + | − | + | − | − | − | + | + | − | − | +/− | − | + | +/− | NI | +/− | + | R |
| 11† | + | + | − | − | − | − | − | + | − | + | − | +/− | − | + | + | +/− | − | − | R |
Abbreviations: NI, not interpretable; ND, not done; R, clonal rearrangement of TCR γ gene; +, all, or virtually all neoplastic cells positive; −, all neoplastic cells negative; +/−, only a variable proportion of neoplastic cells positive.
Using δTCS1 antibody.
This case was also CD103 (HML-1)–positive.
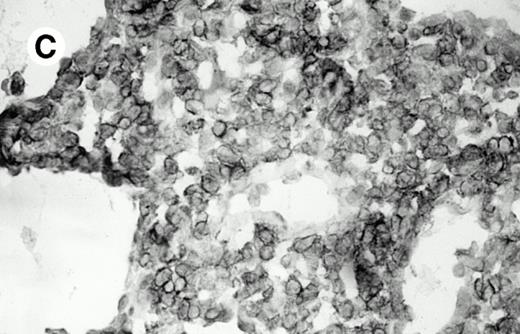
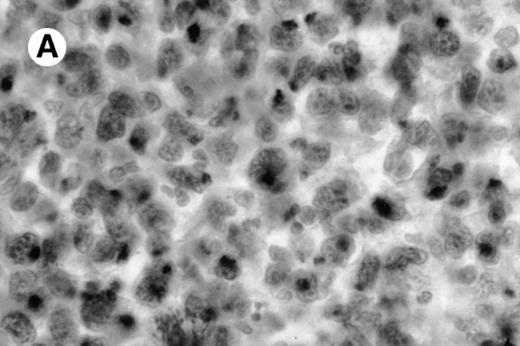
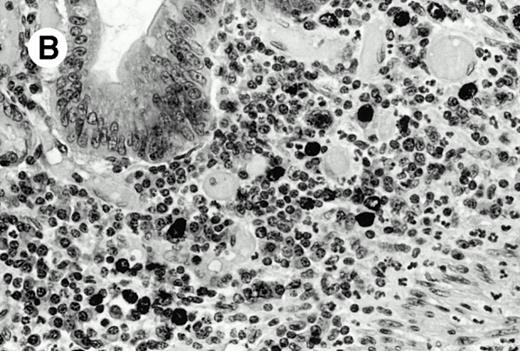
All cases were studied for cytotoxic markers. All of them demonstrated a strong granular cytoplasmic positivity for the cytotoxic granule-associated protein TIA-1. A variable proportion of neoplastic cells were also found positive with Granzyme B (Fig 4A and B) in all cases. Staining for perforin was performed on frozen sections in nine cases, of which seven were positive, one was negative, and the remaining was not interpretable.
Granzyme B expression. (A) Strong paranuclear staining of neoplastic cells in a nasal lymphoma (case no. 3); (B) strong cytoplasmic staining of a few neoplastic cells surrounding intestinal glands in an enteropathy-associated γδ T-cell lymphoma (case no. 11). (Paraffin-embedded section, APAAP technique.)
Granzyme B expression. (A) Strong paranuclear staining of neoplastic cells in a nasal lymphoma (case no. 3); (B) strong cytoplasmic staining of a few neoplastic cells surrounding intestinal glands in an enteropathy-associated γδ T-cell lymphoma (case no. 11). (Paraffin-embedded section, APAAP technique.)
EBV status.
ISH studies with the EBER probes demonstrated two patterns of EBER positivity in the tumor cells. Five cases, including the three nasal cases, the case involving the larynx, and case no. 10 involving the ileum, were considered as EBV-associated lymphomas, as more than 80% of tumor cells displayed an intense nuclear staining.33Case no. 9, which involved the stomach, displayed a pattern of positivity similar to what has been described previously as EBV-positive lymphomas, as only a small proportion of tumor cells (<10%) were found to be positive. Expression of the EBV-encoded latent membrane protein (LMP)-1 was studied by immunohistochemistry in all cases, and was found to be positive in the three cases located in the nasal mucosa (cases no. 1 to 3) and the one case that involved the ileum (case no. 10).
Genomic study.
All nine studied cases demonstrated TCR γ-chain gene rearrangement, which provides evidence of T-cell lineage and monoclonality.
DISCUSSION
Among T-cell neoplasms, the proportion of TCR αβ versus TCR γδ malignancies differs according to the stage of differentiation of the tumor cells. Thus, a significant proportion of thymic (T-acute lymphoblastic leukemia and T-lymphoblastic lymphoma) neoplasms express γδ TCR, rather than αβ TCR, whereas only a small percentage of postthymic peripheral T-cell lymphomas are of γδ origin.13,34 Among them, hepatosplenic γδ T-cell lymphoma is a distinct clinical, morphologic, and immunologic entity, with specific sinusal/sinusoidal localization of malignant cells in spleen, liver, and bone marrow.12-16
In the present study, we report 11 consecutive patients with a nonhepatosplenic peripheral T-cell lymphoma of γδ phenotype. Interestingly, none of them had a nodal presentation. In contrast, they all presented with an initial involvement of skin- or mucosa-associated tissue. Individual cases of nonhepatosplenic γδ T-cell lymphomas, mainly occurring in the skin, have been previously reported.15,17-26 Two patients in the present series had the morphologic features of subcutaneous T-cell lymphoma resembling panniculitis.35 The αβ or γδ T-cell phenotype of such lymphomas has not been extensively investigated. However, several cases have been demonstrated to have a γδ T-cell origin.17-20 These were characterized by a single lesion at presentation, a long time between initial lesions and diagnosis, and histopathologic features of cytophagic histiocytic panniculitis preceding the development of a morphologically detectable neoplastic infiltrate. Thus, it appears that the so-called cytophagic histiocytic panniculitis may represent, at least in some cases, a prelymphomatous state of subcutaneous γδ lymphomas. Another patient of the present series disclosed marked epidermal infiltration, which resembled pagetoid reticulosis and was consistent with the rare reported cases of γδ epidermotropic T-cell lymphoma.15,21,22 Three patients had a sinonasal presentation, thus corresponding to the rare reported cases of nasal γδ T-cell lymphomas.23 24 The other patients had a primary laryngeal, pulmonary, or gastrointestinal γδ T-cell lymphoma, which, to the best of our knowledge, have not yet been described.
The 11 γδ T-cell lymphomas of this series displayed histologic features of pleomorphic tumors. In some cases, the lymphoid infiltration of the epithelial structures resulted in features resembling lymphoepithelial lesions, as observed in B-cell MALT lymphomas36 and in the few reported cases of skin-associated lymphoid tissue (SALT) lymphomas.37Immunohistochemical analysis demonstrated the γδ T-cell derivation. Most cases were double-negative (CD4−/CD8−), a common phenotype observed in most γδ T cells, although a minor subpopulation of γδ T cells in peripheral lymphoid tissues expresses the CD8 antigen.38Most cases lacked CD5, a common finding in γδ peripheral T-cell lymphomas, regardless of their site of origin.12-15,17,18,24 PCR analysis for the TCR γ gene, which allows the detection of clonal γ gene rearrangements in most T-cell lymphomas32 whatever their αβ or γδ phenotype, disclosed a clonal T-cell population in all cases tested. In addition, an exclusive Vδ chain of the γδ TCR was demonstrated in the 10 cases tested. A majority of them were positive for the Vδ2 chain, which is also found in the majority of the peripheral blood γδ T cells in humans.1 3
Clinical outcome was associated with short survival in most patients. A durable remission was only observed in the three patients treated with a heavy regimen of chemotherapy. These results are consistent with the aggressivity of γδ T-cell lymphoma known in the hepatosplenic entity.12-15 Another interesting observation in the present series was the propensity of the neoplastic γδ cells to be localized at presentation or at relapse in skin or mucosal sites, as observed in eight patients. This suggests a preferential homing to the skin- and mucosa-associated lymphoid tissues, which parallels that of normal γδ T cell subsets and may be related to their functions in immune surveillance.2,6,8,11,38 Four patients had a progression with secondary hepatosplenic involvement, consistent with the other characteristic homing of γδ tumor cells in the spleen, as observed in hepatosplenic γδ T-cell lymphomas12-15 and in normal spleen.1,2 5
It is noteworthy that a past history of chronic tissue-restricted antigen exposure was observed in six patients. The skin, pulmonary, upper respiratory, and gastrointestinal tracts are continuously exposed to a wide variety of antigens from living microorganisms to other environmental components. Therefore, the role of antigenic stimulation in the development of these lymphomas may be hypothesized. This is also consistent with the putative role of normal γδ cells in immune surveillance of host epithelial surfaces for infected and damaged cells.8 11
The role of EBV in the pathogenesis of at least some of these γδ T-cell lymphomas can be questioned. Indeed, the pattern of EBER gene expression by ISH with EBV genomes found in virtually all tumor cells (EBV-associated pattern) was shown in the three nasal and in the laryngeal lymphomas, as well as in one intestinal T-cell lymphoma, and is similar to that observed in sinonasal NK lymphomas, as well as in a few nonhepatosplenic γδ T-cell lymphomas.23,24,39Although a causal relationship between EBV and γδ T-cell lymphomagenesis is elusive, chronic EBV infection might have contributed to one step of proliferation and/or transformation in at least the five EBV-associated lymphomas.33 In one of the gastrointestinal lymphomas, the peculiar pattern of EBER genes expression with EBV genomes found in only a few scattered large neoplastic γδ cells, suggests that EBV infected tumor cells after transformation. In addition, the absence of EBV in skin and lung γδ lymphomas indicates, in keeping with previous studies, that the putative role of EBV in T-cell lymphomagenesis is site-dependent, probably related to the sites of normal reservoir of the virus.33
γδ T cells are cytotoxic T lymphocytes40,41 that are known to act as a first line of defense against pathogens, before the recruitment of αβ cells.7,9,11,42-44 Interestingly, we extend, in the present series, results of a previous study29 that showed the activated cytotoxic phenotype of nonhepatosplenic γδ lymphomas, since tumor cells expressed the cytotoxic effector TIA-1 and Granzyme B proteins in all cases, with expression of perforin in most cases. These results are in accordance with the cytotoxic activity exhibited by normal γδ T cells with constitutive expression of TIA-1, Granzyme B, and perforin proteins.40,41 The activated cytotoxic profile of nonhepatosplenic cases contrasts to the finding of a nonactivated cytotoxic profile of most hepatosplenic γδ tumors.15 29
In the present series, at least some cases are likely to belong to different clinicopathologic entities of extranodal lymphomas,16 which appear to be derived from cytotoxic lymphocytes.29,45 Concerning the nasal T/NK lymphoma entity included in the angiocentric category of the Revised European and American Lymphoma (REAL) classification,16 we and others have shown that most cases are of NK origin,23,24,46,47although rare cases have a γδ phenotype.23,24 It appears from the present study and from the literature that nasal T/NK lymphomas, whatever their NK- or γδ T-cell origin, have common clinical and histopathologic features, are associated with EBV, and are derived from activated cytotoxic cells, and thus most probably belong to the same clinicopathologic entity. Intestinal T-cell lymphomas, with or without evidence of enteropathy, are most often derived from cytotoxic cells.45 Most of them are derived from αβ T cells.48 To our knowledge, a single case of γδ gastrointestinal lymphoma has been mentioned in one report, without precise localization.45 We show here that intestinal T-cell lymphomas, including some with enteropathy due to gliadin hypersensitivity, can also derive, although rarely, from γδ T cells. These findings are in accordance with the fact that intraepithelial lymphocytes are mostly derived from αβ T cells, but also include a significant proportion of γδ T-cells (5% to 15%).6,49 Several studies have shown that intraepithelial lymphocytes are cytotoxic cells that are in a resting state in normal human intestine,50 and are increased and activated in celiac disease.51-53
In the reported cases, chronic antigen exposure was associated with a dysregulation of the immune response in six cases: hypogammaglobulinemia, T-cell deficiency, HTLV-1 infection, and celiac sprue. In addition, EBV infection found in six patients may also reflect a latent immune disorder. Impairment of the immune response following antigen-driven stimulation could have favored the proliferation of the reactive γδ T cells. Indeed, expansion of γδ T cells has been found in autoimmune diseases such as celiac sprue, in PBL of patients with primary immunodeficiency, and in patients with HTLV-1 infection, accounting for a proliferation of γδ T-cell subsets in patients with an immune defect.54,55 It is noteworthy that expansion of γδ T cells has been also reported in renal allograft recipients56 and that hepatosplenic lymphoma may occur in such patients.57 Additional genetic alterations may contribute to full neoplastic transformation. Further studies are needed to investigate the presence of recurrent cytogenetic abnormalities in these lymphomas, such as the iso 7q chromosome, as recently described in hepatosplenic γδ T-cell lymphoma.15 58
In conclusion, nonhepatosplenic γδ T-cell lymphomas can be regarded as a model of activated cytotoxic lymphoma, preferentially occurring in mucosae or skin. These appear to be derived from tissue-restricted γδ lymphocytes implicated in immune reactions and in epithelial surface surveillance. Further studies are needed to investigate the putative role of chronic antigen stimulation in the pathogenesis of these lymphomas as it has been shown, using in vitro studies, in B-cell gastric MALT lymphomas related to H pylori–mediated antigen stimulation,34 and to understand the molecular mechanisms of their neoplastic transformation.
ACKNOWLEDGMENT
We gratefully acknowledge the following physicians who provided clinical data: Bertrand Godeau, Marine Divine, Corinne Haioun, Olivier Hermine, Yves Bouhnik, Remy Marianowski, Jean-Marc Zini, Marie-France Avril, Jose Pico, and Jean-Nicolas Munck. We are also indebted to Odile Casiraghi, Claire Legendre for providing histopathologic findings, to Panagiotis Kanavaros for helpful discussion, and to Marie-Laure Boulland for kind technical assistance.
Supported in part by Grant No. CRC95241 from the Délégationà la Recherche Clinique.
Address reprint requests to Philippe Gaulard, MD, Département de Pathologie, Hôpital Henri Mondor, 94010 Créteil Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal