ALTHOUGH MANY ASPECTS of the development of malignant tumors are still incompletely understood, conditions have been identified under which malignancies develop at a higher frequency than in the population at large.1-3 These include, for example, actinic exposure of the skin and the mutagenic effect of UV light; genetic disorders such as Fanconi anemia, ataxia telangiectasia, and immunodeficiency syndromes, which are associated with chromosome fragility, defects of repair enzymes, or cellular immune defects; a high incidence of malignancies has also been observed in patients receiving immunosuppressive therapy after solid organ transplantation; long-term studies in survivors of the atomic bomb explosions at Hiroshima and Nagasaki have yielded a wealth of data on the effect of various doses and qualities (gamma rays or neutrons) of radiation on the development of malignancies, in particular of the blood-forming organs.4 Similar observations have been made in patients who received irradiation for medical indications, eg, acne, ankylosing spondylitis, and other disorders.5-7 Secondary malignancies are a well-recognized complication in patients with Hodgkin's disease or non-Hodgkin's lymphoma treated with chemotherapy or combined modality treatment.8-10 Certain viruses, such as Epstein-Barr virus (EBV), which is used in the laboratory to immortalize cell lines, can transform cells in vivo, which then may show uncontrolled growth and evolve into malignancies.11-14Experiments in the 1960s and 1970s in murine models suggested, furthermore, that a graft-versus-host reaction after allogeneic spleen cell transplantation could transform from an immunologic to a neoplastic disorder, ie, the development of lymphoma.15
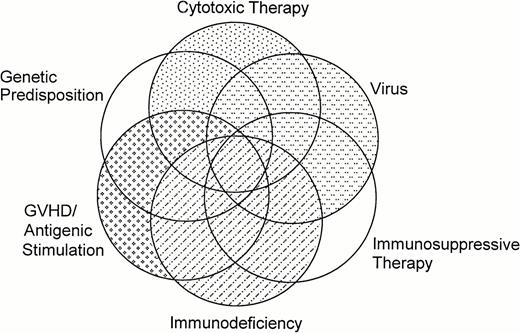
Finally, marrow transplant studies in rhesus monkeys and dogs in the 1970s and 1980s showed a significant increase in the incidence of malignancies relative to controls in radiation chimeras, ie, in animals irradiated with lethal doses of total body irradiation (TBI) and infused with autologous or allogeneic marrow cells (reviewed in Deeg et al,16 Broerse et al,17 and Kolb et al18). Thus, it should not be surprising that new malignancies occur in patients after hematopoietic stem cell transplantation, in which one or several of these risk factors are present. The potential overlapping effects of various factors are shown schematically in Fig 1. The major categories of posttransplant malignancies are listed in Table 1.
Overlap and interactions of factors that may contribute to the development of new malignancies after hematopoietic stem cell transplantation.
Overlap and interactions of factors that may contribute to the development of new malignancies after hematopoietic stem cell transplantation.
New Malignancies After Marrow or Blood Stem Cell Transplantation
| 1. PTLD (A) B-cell PTLD (B) T-cell lymphoma (C) Hodgkin's disease |
| 2. Hematologic malignancies (A) Recurrence of leukemia in donor cells (B) New leukemia in host cells (C) MDS |
| 3. Solid tumors (A) Carcinomas (B) Sarcomas (C) CNS tumors |
| 1. PTLD (A) B-cell PTLD (B) T-cell lymphoma (C) Hodgkin's disease |
| 2. Hematologic malignancies (A) Recurrence of leukemia in donor cells (B) New leukemia in host cells (C) MDS |
| 3. Solid tumors (A) Carcinomas (B) Sarcomas (C) CNS tumors |
POSTTRANSPLANT LYMPHOPROLIFERATIVE DISORDERS (PTLD) AND LYMPHOMAS
The majority of cases of PTLD after hematopoietic stem cell transplantation have been observed in allogeneic (rather than autologous) recipients.19 Most of these PTLD are best classified as B-cell PTLD rather than non-Hodgkin's lymphoma.2,11 20-24 In addition, some T-cell PTLD have been reported. Thirdly, lymphomas with clinical and biological characteristics typical for non-Hodgkin's lymphoma or Hodgkin's disease as seen in nontransplanted patients have occurred after stem cell transplantation. Although lymphoproliferative disorders do not represent a frequent posttransplant complication, important insights have been gained into the pathophysiology and considerable progress has been made in regards to treatment.
B-Cell PTLD
Incidence.
B-cell PTLD are clinically and morphologically heterogeneous; usually they are associated with T-cell dysfunction and the presence of EBV. B-cell PTLD have been observed with almost any organ transplant.12,25-31 Cohen11 recently reviewed 100 well-documented cases, including 32 in marrow transplant recipients. Additional cases have been described since then, bringing the total number of PTLD after hematopoietic transplants to about 70 to 100.32-40 In Cohen's review,11 the mean interval from transplantation to the development of B-cell PTLD was 5 months, with most being diagnosed within 3 months. It appears that patients transplanted for congenital immunodeficiencies are at a particularly high risk for PTLD, presumably due to the underlying immunodeficiency and T-cell depletion of the donor graft generally used for these diseases (see risk factors). Because the diagnostic criteria may differ from study to study (eg, a nonlethal infectious mononucleosis-like syndrome may resolve spontaneously, whereas acute-onset extensive disease may be diagnosed only at autopsy), the true incidence of B-cell PTLD after hematopoietic stem cell transplantation is difficult to determine. In a large single-center survey (1,400 allografted patients), the cumulative incidence of B-cell PTLD reached a plateau of 1.6% by 4 years after transplantation; other published data range from 0.6% to 10%.41
Clinical features.
The most frequent presentation of PTLD is with fever and lymphadenopathy. Intra-abdominal lymphadenopathy, splenomegaly, or hepatomegaly may cause symptoms such as abdominal pain, vomiting, or diarrhea. Extrahematopoietic organ involvement, including lungs, kidneys, and the central nervous system (CNS), is frequent. CNS involvement is of particular concern, because it has been associated with a dismal prognosis. The differential diagnosis in a symptomatic patient should include PTLD a priori in high-risk situations such as in recipients of T-cell–depleted or HLA-nonidentical transplants. Early diagnosis has become important since powerful therapeutic instruments (see below) have been developed. Early diagnosis can be established and the effect of therapy can now be monitored by semiquantitative polymerase chain reaction (PCR) of the EBV DNA (see pathogenesis and treatment).
Pathology.
B-cell PTLD occurring after allogeneic hematopoietic stem cell transplantation are almost always of donor origin and associated with EBV-genomic DNA integration. Biopsies show monomorphic or polymorphic, diffuse large-cell lymphoma of B-cell origin. However, whereas the morphology of B-cell PTLD occurring after solid organ transplantation has been described extensively, few studies have examined in detail the histologic features of PTLD in hematopoietic stem cell recipients.37-39,42,43 Those reports show that, whereas some of these PTLD are histopathologically similar to the polymorphic PTLD described in solid organ transplant recipients, as many as half of the cases after stem cell transplantation show aggressive features of immunoblastic lymphoma.23 Also, in contrast to PTLD after organ transplantation, most B-cell PTLD occurring after stem cell transplantation are oligoclonal or monoclonal, as determined by analysis of Ig gene rearrangements and fused termini of episomal EBV DNA,13,23,44-46 although some discrepancies between these two methods (tumors appearing monoclonal on the basis of EBV genomic analysis and polyclonal by analysis of Ig gene rearrangement) have been observed.44,47,48 PTLD express the full array of latent EBV antigens, including EBNA-1, -2, -3, -4, -5, and -6 and LMP1.14,23,49-52 Karyotypic analyses have identified nonconsistent cytogenetic abnormalities, more frequently in monoclonal lesions of more aggressive histology. However, with the exception of two cases of B-cell PTLD developing in heart transplant recipients,53 the characteristic translocation of Burkitt's lymphoma has not been observed in lymphoproliferative disorders developing after marrow (or solid organ) transplantation.
In a recent report of 10 cases of PTLD in marrow transplant recipients, Orazi et al43 attempted to correlate morphology with clonality (based on Ig chain gene rearrangement and immunochemistry), proliferative activity as measured by immunostaining for the proliferating cell nuclear antigen (PCNA), and presence of p53 overexpression. The cases included seven polymorphic B-cell lymphomas and three immunoblastic lymphomas. Ig heavy chain gene rearrangement analysis showed B-cell clonality in three of seven polymorphic lymphomas and in all three immunoblastic lymphomas. The EBV genome, the expression of the EBV latent membrane protein, or both were found in all 10 cases. High proliferative activity as assessed by the expression of the PCNA antigen was found in all cases, and five specimens were p53+.
Risk factors.
B-cell PTLD were the first posttransplant malignancies for which risk factors were identified.21,38,39,54 In 1989, Witherspoon et al54 showed in multivariate analysis that treatment of acute graft-versus-host disease (GVHD) with either antithymocyte globulin or monoclonal anti-CD3 antibody, total body irradiation, T-cell depletion of donor marrow, and HLA nonidentity between donor and recipient were risk factors for PTLD. A more recent survey by Bhatia et al41 showed the following factors to be associated with an increased risk of B-cell PTLD: T-cell depletion of the graft (relative risk [RR] = 11.9), HLA mismatch (RR = 8.9), use of antithymocyte globulin for acute GVHD prophylaxis (RR = 5.9) or in the preparative regimen (RR = 3.1), and primary immune deficiency disease (RR = 2.5). The cumulative risk of developing a B-cell PTLD in patients with primary immune deficiency who received a T-cell–depleted HLA-mismatched transplant was 64.8% ± 17.7% at 4 years, compared with 0.9% ± 0.2% (P < .001) in patients who received an HLA-matched transplant with no in vitro manipulation of the graft. The role of HLA-mismatching in the pathogenesis of B-cell PTLD is not clear but may consist in chronic antigenic stimulation or delayed immune reconstitution. In unrelated transplants, the National Marrow Donor Program (NMDP) reported an incidence of PTLD of 2% overall, 5% in patients receiving a T-cell depleted marrow, and 1% for those receiving a T-replete graft.55 However, available data suggest that the risk is not uniform but depends on the method of T-cell depletion and the type of additional immunosuppression used in the posttransplantation period. Although in patients transplanted with marrow depleted of T cells with specific monoclonal antibodies the incidence of EBV-positive PTLD ranged from 11% to 25%, the incidence was less than 1% with techniques removing both T and B lymphocytes (eg, soybean agglutinin or Campath-1), possibly reflecting the 2 to 3 log reduction in B lymphocytes associated with these procedures.23,56 However, when additional posttransplant immunosuppression with steroids and antithymocyte globulin was administered after HLA-matched or mismatched related transplants or transplants from unrelated donors using soybean agglutination/E-rosetting for T-cell depletion, the incidence of PTLD increased to 6% to 18%.23 Finally, even in the absence of in vitro T-cell depletion, the use of intensive in vivo immunosuppressive prophylaxis or therapy of GVHD, especially with anti–T-cell agents such as OKT3 antibody or antithymocyte globulin, is associated with the development of B-cell PTLD.3 57
Pathogenesis.
B-cell PTLD are thought to develop because of depressed EBV-specific cellular immunity and the inherent transforming capacities of EBV. EBV is a ubiquitous herpes virus that infects 95% of individuals by adulthood. The virus persists as a latent infection in certain epithelial cells, where reactivation and replication may occur intermittently, and in B lymphocytes.58 EBV type A and type B have been defined on the basis of sequence divergence in the EBNA-2 gene. In a recent series of 27 solid organ transplant recipients who developed PTLD, type A EBV was present in 24 of 27 cases (89%) by PCR amplification of EBNA-2 and EBNA-3c regions. In addition, there was polymorphism at the EBER locus documenting the presence of four different type A EBV strains. None of the 27 cases harbored type B EBV.59 Whether the same applies to marrow transplant recipients remains to be determined.
Among the 80 to 100 EBV-encoded proteins, the latent membrane protein 1 (LMP-1) plays an essential role in B-cell immortalization. LMP-1 has recently been shown to induce the expression of bcl-2, which inhibits programmed death of the infected cells. LMP-1 is also considered an oncogene because of its ability to transform rodent fibroblasts. Deletions near the 3′ end of the LMP-1 gene, in a region that affects the half-life of the LMP-1 protein, have been reported in some EBV-related lymphoproliferative disorders60,61; B-cell PTLD after marrow or stem cell transplantation have not been analyzed yet.
Infection of B cells by EBV also induces high levels of interleukin-1 (IL-1), IL-5, IL-6, IL-10, CD23, and tumor necrosis factor (TNF). The cellular IL-10 and the EBV-induced BCRF1, a homolog of IL-10, act as autocrine growth factors, stimulating the proliferation of EBV-transformed B cells and inhibiting their susceptibility to apoptosis. Much of the initial work investigating anti-EBV cellular responses was performed in patients with acute infectious mononucleosis (reviewed in O'Reilly et al23). Early in the course of the disease, natural killer cells and cytotoxic and suppressor T cells reactive against EBV emerge. Using standard assays of cell-mediated cytolysis, Crawford et al62 found that, in recipients of unmodified marrow, 7 of 10 patients studied had defective killing of autologous targets at 3 months posttransplant, but all were normal by 6 months.
In a recent study, investigators at Memorial Sloan-Kettering Cancer Center explored whether deficiencies of EBV-specific cellular immunity contribute to EBV-PTLD susceptibility.63,64 They performed limiting dilution analysis to quantify anti-EBV specific cytotoxic T-lymphocyte precursor (CTLp) frequencies in 26 recipients of unmodified or T-cell–depleted grafts from EBV-seropositive donors. At 3 months, only 5 of the 26 patients had EBV CTLp frequencies in the normal range of seropositive controls, whereas at 6 months, 9 of 13 patients were within the normal range. This time interval of low CTLp frequency corresponds to the period in which B-cell PTLD are observed. The same investigators showed that EBV-specific cytotoxic T lymphocytes home preferentially to and induce selective regression of autologous EBV-induced B-cell lymphoproliferative lesions in xenografted SCID mice.65 These studies have led to clinical trials (see below) on the role of EBV-specific T lymphocytes in controlling EBV-induced B-cell proliferation. Rather definitive proof has been provided by the St Jude group using adoptive transfer of gene-modified EBV-specific T lymphocytes.66 Preliminary clinical results67 showed that adoptive transfer of EBV-specific cytotoxic T lymphocytes offered effective therapy for B-cell PTLD. The investigators showed long-term persistence of gene-marked EBV-specific cytotoxic T lymphocytes in vivo. These cells not only restored cellular immunity against EBV, but also provided a population of CTLps that responded to in vivo or ex vivo challenge with the virus for as long as 18 months.
Prophylaxis and treatment.
Because various recognized risk factors such as initial diagnosis (primary immune deficiency syndrome) or type of donor (HLA-nonidentical) cannot be changed and others (eg, GVHD prophylaxis) are considered an integral part of the overall treatment regimen, it has been proposed to use early identification of EBV-associated PTLD as an indication for therapy rather than apply true prophylaxis. The St Jude group used both the outgrowth of transformed B lymphocytes ex vivo and detection of EBV DNA by a PCR method as tools to detect EBV-associated lymphoproliferation before clinical disease developed.68 A semiquantitative PCR assay is used to assist in the detection of EBV DNA in peripheral blood and in monitoring the effect of therapy.67 69-71
Complete regression of B-cell PTLD has been reported in 40% of patients after reduction or discontinuation of immunosuppressive therapy, particularly in renal transplant recipients.72Immunosuppression is intrinsic to marrow transplantation, and discontinuation of immunosuppression is likely to result in flares of GVHD and a further delay in recovery of T-cell–mediated immunity. EBV-transformed B cells contain a circular viral DNA that is not susceptible to inhibition by thymidine kinase (TK) inhibitors. Nevertheless, anecdotal reports suggest tumor regression with either acyclovir and ganciclovir therapy (reviewed in Benkerrou et al72 and Sullivan et al73). Chemotherapy and irradiation have been useful in selected cases, and in a recent series of cardiac transplant recipients, among 19 consecutive patients with PTLD, 6 of 8 treated with aggressive chemotherapy are surviving in complete remission, at a median follow-up of 38 months.74Surgical resection has proven effective when the PTLD was limited to single sites in solid organ transplant recipients.11
More recently, three approaches have shown promise in the treatment of B-cell PTLD in marrow transplant recipients: α interferon, B-cell–specific monoclonal antibodies, and cellular therapy. A combination of α interferon and intravenous Ig was first reported in 1988 by the Minneapolis group to be effective in B-cell PTLD. Remissions were maintained in several patients.75 In a recent update, three of seven patients receiving α interferon achieved a complete remission (Gross and Filipovich, personal communication, July 1997).
Two anti–B-cell antibodies (anti-CD21 and anti-CD24) were used by Alain Fischer's group and by one of the authors (G.S.) in a multicenter trial.36,76,77 Among 19 marrow transplant recipients, 10 had a complete remission and 6 survived at a median follow-up of 20 months.72 The survivors in this series all were patients with oligoclonal disease. Studies in a SCID mouse model78 show that, after initial remission, with such an approach 30% to 50% of mice relapsed within 30 to 70 days, providing a very strong indication that persistence of residual B cells can provoke a second tumor in the absence of efficient cytotoxic T cells. Currently, the anti-CD21 and CD24 antibodies used in these studies are not available for clinical use (Alain Fischer, personal communication, July 1997). Based on in vitro data showing an antitumor effect of anti–IL-6 antibody in neutralizing the IL-6–dependent proliferative loop,79 80 the same investigators are now testing this antibody in patients with PTLD (Alain Fischer, personal communication, July 1997).
In 1994, Papadopoulos et al47 first reported therapeutic efficacy of the infusion of donor leukocytes in five patients who developed a B-cell PTLD after T-cell–depleted allogeneic marrow transplantation. Unirradiated donor leukocytes were infused at doses calculated to provide 1.0 × 106 CD3+ T cells/kg of body weight. All five patients had complete pathologic or clinical responses. Three of the five patients developed chronic GVHD and two died of respiratory failure with no evidence of PTLD at autopsy. Subsequently, Rooney et al67 reported on the use of gene-marked EBV-specific T lymphocytes to control or prevent B-cell PTLD in 10 patients. Three of the patients had shown signs of EBV reactivation, with or without overt lymphoproliferation, and 7 received T-cell infusions as prophylaxis. In the 3 patients with EBV reactivation, EBV DNA levels that had increased 1,000-fold or more returned to control levels within 3 to 4 weeks of immunotherapy. In a recent update, the Sloan-Kettering team reported data on 15 patients with eradication of B-cell PTLD in 14; GVHD occurred in 6 among the 12 evaluable patients.81 The St Jude team described the prophylactic use of EBV-specific T-cell clones in 25 high-risk patients, none of which developed PTLD. Among 6 patients who either refused CTL therapy or were ineligible for treatment, 2 developed lymphomas that were successfully treated with CTL.82Bordignon's group most recently reported on the use of HSV-TK gene transfer in donor lymphocytes infused to control B-cell PTLD in two patients. One of these patients subsequently developed GVHD that was successfully treated with ganciclovir by way of activating the HSV-TK suicide gene.83
Thus, promising approaches have been developed for the treatment of B-cell PTLD in high-risk marrow84 and solid organ transplant recipients.33 However, the numbers of patients treated are still limited. Also, the use of cellular therapy may induce GVHD if non–EBV-specific CTL are used and still requires high-level biotechnology laboratories to provide either EBV-specific CTL clones or HSV-TK–transduced T lymphocytes.
T-Cell Lymphoproliferative Disorders
Besides the well-defined B-cell PTLD, an entity of T-cell proliferative disorders without EBV association has been reported both after solid organ and marrow transplantation. After solid organ transplantation these disorders have occurred predominantly at extranodal sites and were monoclonal.85 86 After marrow transplantation, only three such cases have been reported87; two occurred late after transplant and may be included in the late-onset lymphoma category (see below). None of the cases was associated with human T-cell lymphotropic virus type 1 (HTLV1), human immunodeficiency virus (HIV), or human herpes virus 6 (HHV6) infection.
Late-Onset Lymphoma
Some 20 cases of late occurring lymphomas have been reported in the literature.21,88-94 At least two have been linked to EBV infection (just as early onset PTLD) and three were associated with T-cell depletion of the graft. These cases presented like ordinary non-Hodgkin's lymphoma with lymph node enlargement with or without generalized symptoms; one of these patients has been reported to be disease-free after chemotherapy. At least two of the late lymphomas were Hodgkin's disease. At Hôpital Saint Louis in Paris, such a late occurrence of EBV-related Hodgkin's disease in donor cells was observed in a patient transplanted 8 years before for chronic myelogenous leukemia.89 Although more work is needed, ongoing studies seem to support the notion that these late-occurring lymphomas represent an entity distinct from the early occurring B-cell PTLD (R. Curtis, personal communication, November 1997).
MYELODYSPLASTIC SYNDROME (MDS) AND ACUTE LEUKEMIA
MDS and Acute Leukemia After Allogeneic Transplantation
In the early 1970s, Fialkow et al95 and Thomas et al96 reported on two patients with acute lymphoblastic leukemia receiving TBI and transplanted with marrow from an HLA-identical sibling donor, who within 2 to 4 months experienced what appeared to be a relapse of their original disease. However, further studies using cytogenetic analysis showed that the leukemic cells were donor-derived. Both donors continued to be healthy. Several similar cases, including patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML), were subsequently reported from other institutions (reviewed in Deeg et al97). Conditioning regimens in those patients consisted of chemotherapy only or chemotherapy plus TBI, and the diagnosis of recurrent leukemia in donor cells was made 6 months to more than 3 years after transplantation. Boyd et al98estimated that as many as 3% to 5% of leukemia recurrences may in fact be new leukemias in donor cells. However, no molecular tools were used in that study.
The mechanism that would lead to leukemia in previously healthy transplanted cells was not clear. Several hypotheses have been proposed. Donor cells may have been transformed by antigenic stimulation through host tissue,95,99,100 as observed in murine models of marrow transplantation.101 However, if this was the case, one would expect a higher frequency of this event. Alternatively, the recipient lymphohematopoietic environment in which the original leukemia had developed might trigger a similar development in donor cells.95 Furthermore, fusion of normal cells with leukemic cells still residing in the recipient or transfection of an etiologic agent (virus/oncogene) might have transformed donor cells.102-104 Although these possibilities are conjectural, the clinical observations are of interest in the context of leukemogenesis in general.
More recent studies have used refined molecular biology tools (eg, variable number tandem repeat [VNTR] analysis) to determine the origin (host v donor) of normal or abnormal cells in patients posttransplant. As determined by microsatellite analysis, disease reappearance in donor-derived cells is infrequent.105 A rare case of transplantation of leukemia from the donor into the patient has been reported.106
MDS, of some concern in autologous transplant recipients (see below), has occurred extremely infrequently after allogeneic transplantation (even in patients with Fanconi anemia in whom MDS develops frequently if not transplanted with normal cells). This observation provides indirect support for the notion that MDS after autologous transplantation is related to pretransplant factors rather than the transplant itself.
MDS and AML After Autologous Stem Cell Transplantation
High-dose chemotherapy and autologous stem cell transplantation are used with increasing frequency in the treatment of non-Hodgkin's lymphoma, Hodgkin's disease, breast cancer, and other indications. Recent randomized trials have shown that this approach is more effective than conventional chemotherapy in patients with chemotherapy-sensitive relapse107 and in some patients with high-risk non-Hodgkin's lymphoma.108,109 Secondary MDS and AML have been observed after conventional chemotherapy and to a lesser extent radiotherapy for Hodgkin's disease and non-Hodgkin's lymphoma.8,110-113 Alkylating agents, epipodophylotoxins, combined modality therapy, and splenectomy have been implicated as risk factors.110 Clearly, therefore, this complication does occur in patients who have not been transplanted, and a thorough evaluation of all transplant candidates, particularly in regard to cytogenetic abnormalties, before autologous transplantation is mandatory.114
Nevertheless, beginning in 1993, several studies reported the development of secondary MDS and AML in patients with Hodgkin's disease and non-Hodgkin's lymphoma who had undergone autologous transplantation at a frequency that appeared unusually high (reviewed in Socié,88 Blume,115Kumar,116 Taylor et al,117Rohatiner,118 and Stone119). Marolleau et al120 first reported three cases of AML among 168 patients treated with autologous transplants for advanced lymphomas (median follow-up, 3 years). In 1994, the University of Nebraska team121 reported its experience in a case-control study. Twelve cases of MDS/AML occurred in 511 patients after autologous transplants for Hodgkin's disease (n = 249) or non-Hodgkin's lymphoma (n = 262). The cumulative incidence at 5 years was estimated to be 4% (11% and 12% for the two groups, respectively, among patients alive at 5 years). Age greater than 40 years at the time of transplant and the use of TBI were risk factors. Among 262 patients receiving autologous transplants for non-Hodgkin's lymphoma at the Dana Farber Cancer Center, 12 developed MDS/AML for a 6-year cumulative incidence of 18% ± 9%.122 Pretreatment variables predictive (in univariate analysis) for the development of MDS included prolonged interval between initial treatment and transplantation, duration of exposure to chemotherapy (alkylating agents), and use of radiotherapy, especially pelvic irradiation. The Minneapolis team123reported on 206 patients with either Hodgkin's disease (n = 68) or non-Hodgkin's lymphoma (n = 138) who showed a 5-year cumulative incidence of MDS of 14.5% ± 11.6%. Recipients of peripheral blood transplants had an apparent higher risk than marrow transplant recipients (31% ± 33% v 10.5% ± 12%, respectively;P = .0035). In these three series combined, the elapsed time between transplant and diagnosis of MDS/AML ranged from 30 to 103 months. In a study at City of Hope Medical Center, clonal chromosomal abnormalities were detected in 10 of 275 patients after autologous transplant for Hodgkin's disease or non-Hodgkin's lymphoma.124 125 The diagnosis was made 1.8 to 6.5 years after chemotherapy and 0.5 to 3.1 years after transplantation, respectively. In nine patients the abnormalities involved chromosome 5, 7, 11q23, 21q22, or combinations thereof. Five patients had morphologic evidence of MDS or AML. The cumulative probability of developing clonal chromosomal abnormalities reached 9% ± 4.7% at 3 years after transplantation.
The Minneapolis team recently updated their results.41Among 258 patients receiving autologous transplants for Hodgkin's disease or non-Hodgkin's lymphoma, 10 developed MDS/AML, for a cumulative probability of 13.5% ± 4.8% at 6 years. In multivariate analysis, the use of peripheral blood stem cells (RR = 5.8) and age over 35 years at transplant (RR = 3.5) were associated with an increased risk of MDS/AML. A French study of 467 patients also observed a higher incidence of MDS after peripheral blood than after marrow stem cell transplantation.126
MDS/AML have also been reported after transplantation for breast cancer.127 Although studies are less extensive than in patients with Hodgkin's disease or non-Hodgkin's lymphoma, there is evidence that in particular after accelerated dose adjuvant therapy, the incidence of MDS may be high.
These observations are of interest and raise several questions. Is MDS/AML after transplantation related to pretransplant chemoradiotherapy administered as primary or salvage therapy? Among 188 patients who underwent transplant for multiple myeloma at the University of Arkansas,128 71 were enrolled in a total therapy program and received no more than one course of standard chemotherapy (median, 7.6 months of treatment), whereas 117 patients had received more prolonged treatment courses before transplantation (median, 24 months). Seven patients developed MDS, all in the group of patients who had received prolonged treatment, leading the investigators to conclude that pretransplant therapy was the major risk factor for MDS after autologous transplantation. A closely related question is whether MDS/AML arises from the infused marrow (or peripheral blood) stem cells or from residual cells in the patient. If the disease develops from reinfused stem cells, then it is unlikely that TBI administered in preparation for transplant is a risk factor—unless we postulate that TBI modifies the microenvironment in a way that enhances the risk of leukemogenesis. However, if the development of MDS/AML is related to the transplant procedure, we need to ask the following questions. Is it the procedure itself or, eg, the status of immunoincompetence following the transplant that contributes to the development of MDS? Do peripheral blood stem cells modify the milieu in a way different from marrow? Investigations into the function of growth factor mobilized peripheral blood stem cells show indeed cellular function (T cells and monocytes) and cytokine patterns different from marrow.129 In fact, the term disordered engraftment has been proposed to describe the hematopoiesis in these patients.119
SOLID TUMORS
Observations in animal models suggested that posttransplant (or postirradiation) solid tumors occurred with considerable delay, ranging from 7.5 to 15 years (median, 11.5 years) in x-irradiated and 4 to 15 years (median, 8 years) in rhesus monkeys irradiated with fission neutrons.17 The time interval in γ-irradiated dogs was 1.6 to 10.5 years (median, 8 years).16 Extrapolation to humans with a longer expected life span would suggest that solid tumors might develop a decade or more after transplantation. This appears to be born out by the actual data.1-3
Solid Tumors After Allogeneic Transplants
Initial reports, generally on small numbers of patients who had undergone allogeneic (or syngeneic) marrow transplantation, documented the development of some adenocarcinomas of the rectum, brain tumors (glioblastomas) particularly in patients who had also received cranial irradiation (1,800 to 2,400 cGy) before transplantation, squamous cell carcinomas of the skin, and cancers of the oropharyngeal mucosa.97,130 In contrast to PTLD, which generally were diagnosed within 2 to 4 months of transplantation, these solid tumors were observed at 1 to 5 years.1-3
In the first larger series, analyzing results in 2,145 patients transplanted from 1970 through 1987 in Seattle, Witherspoon et al54 found 35 new malignancies; 13 of these were solid tumors, including glioblastoma, melanoma, squamous cell carcinoma, adenocarcinoma, hepatoma, and basal cell carcinoma. These tumors were diagnosed between 2.5 months and 14 years (median, 4.6 years) after transplantation. Although TBI was a significant risk factor when all malignancies were considered, only the use of antithymocyte globulin as an immunosuppressive agent was identified as a significant risk factor for solid tumors. Subsequent analysis of the results in patients with aplastic anemia transplanted in Seattle and at Hôpital Saint Louis in Paris, as well as reports from other European centers, showed that irradiation, in particular total lymphoid or thoraco-abdominal irradiation (as compared with conditioning regimens that did not involve irradiation), was a significant risk factor for the development of solid tumors.131 A combined analysis of results in 700 patients with aplastic anemia transplanted at the Fred Hutchinson Cancer Research Center or Hôpital Saint Louis suggested that, in addition to irradiation (RR [RR] = 3.9), treatment of chronic GVHD with azathioprine (RR = 7.5) and older age (RR = 1.1) increased the risk of a posttransplant malignancy.131 Not surprisingly, the highest incidence of malignancy was observed in patients in whom the etiology of marrow failure was Fanconi anemia (Kaplan-Meier estimate at 15 years, ∼40%). However, it is of note that no hematologic malignancies (MDS, etc) were observed in either idiopathic or Fanconi-associated aplastic anemia, an indication that the transplanted (allogeneic) stem cells were able to develop and differentiate normally in the patient's marrow microenvironment.
Bhatia et al41 summarized observations in patients transplanted in Minneapolis. Among 2,150 patients, 15 developed a solid tumor (8 in 1,400 allogeneic and 7 in 750 autologous transplant recipients)41 for a cumulative probability of 5.6% at 13 years. Irradiation was the major risk factor (RR = 6; P = .008). Kolb et al132 determined the incidence of posttransplant malignancies in 1,211 patients who had survived at least 5 years after transplantation at 45 European centers. Forty-seven patients developed malignancies, including squamous cell carcinoma, breast cancer, glioblastoma, lymphoma, and others. In comparison to normal controls, the incidence rates were increased significantly for malignancies of the oral cavity, skin, esophagus, uterine cervix, and brain. In univariate analysis, donor age, chronic GVHD, and treatment of GVHD with cyclosporine, thalidomide, azathioprine, or methotrexate, and the number of agents used were found to be significant. In multivariate analysis using a Cox model, donor age above 30 years and chronic GVHD were significant risk factors, but the use of irradiation for conditioning was not.
In a large collaborative study, Curtis et al133 analyzed results in 19,220 patients (97.2% allogeneic and 2.8% syngeneic recipients) transplanted between 1964 and 1992 at 235 centers. There were 80 solid tumors for an observed/expected (O/E) ratio of 2.7 (P < .001). In patients surviving at least 10 years after transplantation, the risk was increased eightfold. The cumulative incidence of tumors was 2.2% at 10 years and 6.7% at 15 years. The risk was increased significantly for melanoma (O/E = 5.0), cancers of the oral cavity (11.1), liver (7.5), CNS (7.6), thyroid (6.6), bone (13.4), and connective tissue (8.0). The risk was highest for the youngest patients and declined with age (P for trend, <.001). Other risk factors are summarized in Table2. Most striking was the link of squamous cell carcinoma with chronic GVHD and male gender. The underlying diagnosis was important insofar as the risk of solid tumors was higher for patients with acute leukemia and lower in patients with lymphoma or aplastic anemia. The risk associated with TBI decreased if irradiation was administered with a fractionation regimen, but increased with the total cumulative dose administered. This analysis strongly suggests that reduced doses of TBI, the omission of limited field irradiation, and the prevention of GVHD, in particular chronic GVHD, should reduce the risk of posttransplant solid tumors.
Most Frequent Solid Tumor Malignancies and Significant Variables
| Tumor . | RR by Variable . | ||||||
|---|---|---|---|---|---|---|---|
| High-Dose TBI . | Limited Field Irradiation . | GVHD . | T-Cell Depletion . | HLA Nonidentity . | Male Gender . | ||
| Acute (II-IV) . | Chronic . | ||||||
| Squamous cell carcinoma | |||||||
| Buccal cavity (n = 14) | 3.0 | 136* | 1.7 | 6* | 0 | 0 | 9.7* |
| Skin (n = 8) | 0.2 | 0 | 2.1 | 22* | 0 | 0 | ∞* |
| Thyroid CA (n = 7) | 5.8 | 0 | 1.9 | 0 | 4.9 | 0 | 0.4 |
| Bone or connective tissue (n = 8) | 0.6 | 0 | 0.4 | 0.6 | 0 | 2.2 | 1.2 |
| Brain or other CNS (n = 10) | 4.3 | 0 | 0.4 | 0 | 0 | 0.6 | 2.5 |
| Melanoma, skin (n = 9) | 8.2 | 0 | 0.6 | 0.4 | 4.5 | 0 | 0.4 |
| Tumor . | RR by Variable . | ||||||
|---|---|---|---|---|---|---|---|
| High-Dose TBI . | Limited Field Irradiation . | GVHD . | T-Cell Depletion . | HLA Nonidentity . | Male Gender . | ||
| Acute (II-IV) . | Chronic . | ||||||
| Squamous cell carcinoma | |||||||
| Buccal cavity (n = 14) | 3.0 | 136* | 1.7 | 6* | 0 | 0 | 9.7* |
| Skin (n = 8) | 0.2 | 0 | 2.1 | 22* | 0 | 0 | ∞* |
| Thyroid CA (n = 7) | 5.8 | 0 | 1.9 | 0 | 4.9 | 0 | 0.4 |
| Bone or connective tissue (n = 8) | 0.6 | 0 | 0.4 | 0.6 | 0 | 2.2 | 1.2 |
| Brain or other CNS (n = 10) | 4.3 | 0 | 0.4 | 0 | 0 | 0.6 | 2.5 |
| Melanoma, skin (n = 9) | 8.2 | 0 | 0.6 | 0.4 | 4.5 | 0 | 0.4 |
Data from Curtis et al.131
P < .01.
Solid Tumors After Autologous Transplants
Although studies to date have focused on allogeneic transplant recipients, there is evidence for an increased incidence of new malignancies in autologous patients as well. A French study analyzed results in patients with Hodgkin's disease, 467 of whom had received an autologous stem cell transplant and 3,855 had been treated with conventional therapy.126 Among the transplanted patients, 18 developed a new malignancy for an the incidence of 8.9% at 5 years. The incidence was particularly high in patients above the age of 35 years and in patients who had received peripheral blood (rather than marrow) stem cells. Whereas the incidences of MDS were similar in transplanted and nontransplanted patients, transplanted patients were at a higher risk of solid tumors (P = .039). As noted before, a recent analysis of results in patients transplanted in Minneapolis also showed seven solid tumors in 750 autologous transplant recipients. Unpublished Seattle data show 6 solid tumors among 684 autologous transplant recipients conditioned with a radiation-containing or chemotherapy-only regimen (R.P. Witherspoon, personal communication, November 1997). Further observations in autologous transplant recipients will be of great interest because etiologic factors, such as chronic alloantigenic stimulation and GVHD, can basically be excluded.
Pathogenesis of Solid Posttransplant Tumors
Much less is known about the pathogenesis of solid tumors than of PTLDs. However, the interaction of various factors, as shown in Fig 1, appears to apply to these malignancies as well. Using a PCR technique, Socié et al (unpublished observations) found evidence for involvement of human papilloma virus (HPV) 13, 15, or 16 in three of eight tumors examined; HHV8 was detected in one tumor. In addition, the pattern of p53 expression suggested mutations of this gene in all eight tumors studied. Mutations might be induced by cytotoxic therapy, and suppressed immunity would interfere with a normal surveillance. Clearly, considerable work is needed for a better understanding of those questions.
Therapy
Therapy of solid tumors after transplantation has followed the standards used in nontransplant patients. Experimental studies suggest that selective immunostimulation and measures aimed at scavenging free radicals may be beneficial in preventing tumor development.
STATISTICAL CONSIDERATIONS IN EVALUATING THE RISK OF NEW MALIGNANCIES AFTER STEM CELL TRANSPLANTATION
The development of new malignancies has long been recognized as a potential complication of cytotoxic therapy, either with chemotherapeutic agents or irradiation. An increased incidence has been observed, eg, in patients treated for Hodgkin's disease, acute leukemia, or solid tumors in childhood. Intensive cytotoxic conditioning therapy is also used in preparation for stem cell transplantation to eradicate the underlying disease. Furthermore, in allogeneic transplants, the conditioning regimen provides immunosuppression, thereby assuring sustained engraftment of donor-derived cells. As a result, transplantation is followed by a period of severe immunodeficiency that is further enhanced, in allogeneic transplants, by immunosuppressive agents administered for prophylaxis or therapy of GVHD. These and other factors (including the primary disease and treatment administered pretransplant) contribute to the development of second malignancies after stem cell transplantation.
The data reviewed here raise questions about the best approach to estimate risks and to provide information for physicians and patients about the excess risk of second malignancy after stem cell transplantation. The most commonly used method is the standardized incidence ratio (SIR), ie, the ratio of observed (O) incidence of malignancies in the patient cohort compared with the expected (E) incidence of these malignancies in the general population of the same age and gender. High SIR, or RRs, in cohorts of young patients must be viewed within the context of the frequency of events in the comparable general population at similar ages. For example, few cases of a new acute leukemia in a cohort lead to a high SIR because of the rarity of this disease in the general population, whereas a substantial number of second breast cancers is needed before the O/E ratio becomes significant because of the relative frequency of this tumor type within the general population.
Another commonly used method is actuarial risk estimates (using Kaplan-Meier method, eg). These actuarial estimates often lead to alarming figures once the interval after treatment exceeds 5 to 10 years, due to the fact that data for most of the study population are censored at follow-up intervals shorter than those at which second malignancies are recognized. As a result, this method magnifies the percentage of change caused by any event. This problem should be kept in mind when comparing actuarial estimates provided by two different studies (ie, 5% and 15% actuarial incidences of second malignancies at 10 years might not be different, due to large confidence interval limits). These methodologic aspects have been reviewed in a recent editorial on second cancers after Hodgkin's disease in childhood treated with conventional chemoradiotherapy.134
Finally, in the context of hematopoietic stem cell transplantation, one has to ask whether the general population is the best reference group. Because other conventional (standard chemotherapy) treatment is administered for some (if not all) diseases that are also treated with transplantation, it would be important to compare the risk of second malignancy (and survival!) in patients receiving transplants versus conventional therapy rather than transplanted patients versus the healthy population.
CONCLUSIONS
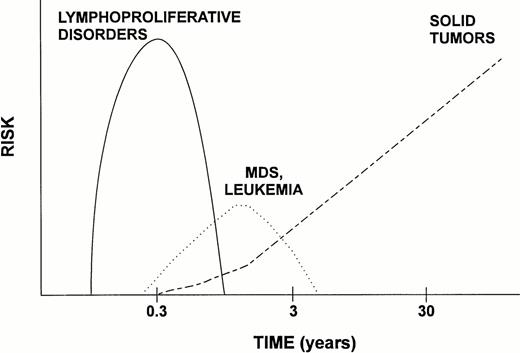
Hematopoietic stem cell transplantation offers curative therapy for many patients with otherwise incurable disease. Currently about 20,000 transplants are performed annually and most patients who do not experience a recurrence of their underlying disease within 1 or 2 years of transplantation do well and lead productive lives. However, some complications do occur, one of them being the development of a new malignancy. The incidence of posttransplant malignancies appears to be low overall, although some high-risk situations have been recognized, including an underlying diagnosis of immunodeficiency or other genetic defects, high-dose irradiation for conditioning, T-cell depletion of the marrow, HLA nonidentity of the donor, and chronic GVHD. Although we have begun to develop a good understanding of the mechanism involved in and the frequency of PTLD, information on hematopoietic disorders and solid tumors is much more rudimentary. The time course of development of the various malignancies varies (Fig 2), and longer observation is required before the full extent of the risk of solid tumors can be assessed. Thus, many questions remain. Nevertheless, available data provide a basis on which to develop approaches that may be associated with lower risks.
Scheme of time course and RR of the major categories of posttransplant malignancies. Whereas lymphoproliferative disorders (PTLD) occur almost exclusively in allogeneic transplant recipients, solid tumors are observed in both allogeneic and autologous patients. MDS and leukemia have been reported more frequently after autologous transplantation. (Note logarithmic scale of time axis.)
Scheme of time course and RR of the major categories of posttransplant malignancies. Whereas lymphoproliferative disorders (PTLD) occur almost exclusively in allogeneic transplant recipients, solid tumors are observed in both allogeneic and autologous patients. MDS and leukemia have been reported more frequently after autologous transplantation. (Note logarithmic scale of time axis.)
ACKNOWLEDGMENT
The authors thank H.J. Kolb, R.P. Witherspoon, and R. Curtis for their continuing contributions; R. Storb and E. Gluckman for support; A. Fischer and A. Fillipovich for communication of yet unpublished results; B. Larson and H. Childs for typing the manuscript; and E.D. Thomas for providing critical comments.
Supported by Public Health Services Grants No. CA18029, CA18221, CA15704, and HL36444 and by Contract No. NCI N01-CP-51027.
Address reprint requests to H. Joachim Deeg, MD, Clinical Research Division, Fred Hutchinson Cancer Research Center and University of Washington, 1124 Columbia St, M318, Seattle, WA 98104.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal