Abstract
Seroepidemiology and polymerase chain reaction studies have strongly suggested that human herpesvirus type 8 (HHV-8) is associated with Kaposi's sarcoma, Castleman's disease, and body cavity-based lymphoma. The genome of HHV-8 harbors a viral analogue of the interleukin-6 (IL-6) gene. The amino acid sequence of the viral IL-6 (vIL-6) protein is 24.7% identical to human IL-6 (hIL-6). IL-6 as a B-cell growth and differentiation factor is known to play an essential role in the pathophysiology of B-cell tumors. Thus, it seems possible that virus-encoded IL-6 contributes to malignant growth of HHV-8–positive B-cell lymphatic tumors. We have tested a preparation of HHV-8–derived IL-6 for the ability to promote the proliferation of the human myeloma cell line INA-6, which is strictly dependent on exogenous IL-6 for growth and survival. Viral IL-6 significantly induced DNA synthesis of INA-6 cells, but required much more protein on a weight basis when compared with hIL-6 for maximal proliferation. The proliferative effect of vIL-6 was almost completely inhibited by a combination of anti–IL-6 receptor (IL-6R) and anti-gp130 antibodies or IL-6R superantagonist Sant7 and anti-gp130 antibodies. This report demonstrates that vIL-6 has proliferative activity on human cells and that the IL-6R and gp130 are involved in vIL-6 signaling in the myeloma cell line INA-6.
HUMAN HERPESVIRUS type 8 (HHV-8) or Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) was first identified by Chang et al1 in KS tissues of acquired immunodeficiency syndrome patients and appears to be the first human member of the γ-2 herpesviruses (rhadinoviruses) with herpesvirus saimiri as a well-characterized prototype of this group. The viral genome consists of linear double-stranded DNA of about 165 kb in length and, like other rhadinoviruses, contains numerous open reading frames with homology to known cellular genes.2,3 However, several of the homologous genes encoded by HHV-8 are not shared by other rhadinoviruses or any other viruses as far as it is known. One of these genes encodes a structural equivalent of interleukin-6 (IL-6) with striking similarity to human and murine IL-6.4,5 IL-6 has long been suggested to be involved in the pathogenesis of a variety of diseases, including KS, body cavity-based lymphoma (BCBL), and Castleman's disease (CMD).6,7 Interestingly, HHV-8 sequences have been consistently detected in human immunodeficiency virus (HIV)-related and HIV-unrelated forms of these diseases. The detection of an IL-6 gene homologue in HHV-8 and its respective gene product led to the suggestion that viral IL-6 (vIL-6) may play a functional role in the pathogenesis of KS, BCBL, CMD, and possibly other lymphoproliferative diseases. Recently, Rettig et al8reported to have found HHV-8 in dendritic cells cultured from the bone marrow of patients with multiple myeloma. IL-6 has long been known to be a growth factor for plasma cells and a central role in the pathophysiology of multiple myeloma has been suggested.7 9-11
Whereas the predicted proteins derived from the viral gene homologues were named without reference to their potential in vivo functions, the vIL-6 gene product has already been shown to support the in vitro proliferation and to prevent apoptosis of the IL-6–dependent mouse hybridoma cell line B9 in a dose-dependent manner.4 We have tested a recombinant protein preparation of HHV-8 IL-6 on its ability to bind to and promote proliferation of the IL-6–dependent human myeloma cell line INA-6. This is the first report demonstrating biologic activity of HHV-8 IL-6 on human myeloma cells.
MATERIALS AND METHODS
Cell lines and culture.
The human myeloma cell line INA-6 was established from the pleural effusion of a patient with (IgG)-κ plasma cell leukemia and is strictly dependent on exogeneous IL-6 for growth and survival.12 The B9 cell line was kindly provided by Dr L.A. Aarden (Amsterdam, The Netherlands).13 The cells were routinely maintained in RPMI 1640 medium supplemented with 20% or 10% heat-inactivated fetal calf serum (FCS; GIBCO/BRL, Eggenstein, Germany), 2 mmol/L L-glutamine, antibiotics, 50 μmol/L 2-β-mercaptoethanol and 500 (INA-6) or 20 (B9) U/mL human IL-6 (hIL-6) at 37°C in a humidified atmosphere with 6% CO2.
Reagents.
Recombinant human (rh) IL-6 (specific activity, 2 to 4 × 108 U/mg) was purchased from Pharma Biotechnologie Hannover (Hannover, Germany). Neutralizing polyclonal goat anti–IL-6, monoclonal antihuman IL-6R (CD126; clone 17506.1), and antihuman gp130 (CD130; clone 28126.111) antibodies were purchased from R&D Systems (Minneapolis, MN). Monoclonal antibodies IKR6 (anti–IL-6R) and IKR3 (anti-gp130) were from ImmunoKontact (Frankfurt, Germany) and correspond to clones B-R6 and B-R3.14 The IL-6R superantagonist Sant7 was prepared as described.15
Preparation of HHV-8 IL-6.
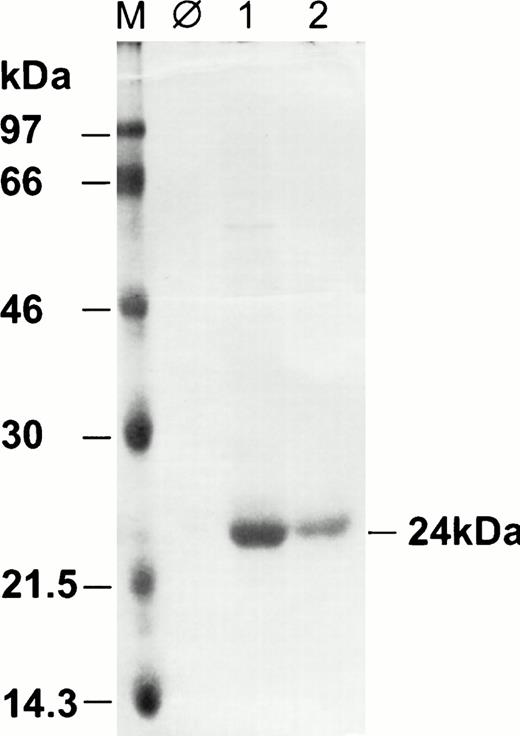
A genomic fragment of HHV-8 vIL-6 was amplified using synthetic oligonucleotides vIL6-5H-Bam (AGCTGGATCCAAGTTGCCGGACGCCCCCGAGTTTG) and vIL6-3-Hind (AGCTAAGCTTATCGTGGACGTCAGGAGTCAC). After digestion withBamHI and HindIII, the polymerase chain reaction product was ligated into expression vector pQE9 (Quiagen Inc, Hilden, Germany). The resulting expression plasmid (pQEvIL-6) codes for an amino terminal tag of 6 histidin residues (MRGSHHHHHHGS) and amino acids 23-204 of vIL-6. It does thus not include the putative amino terminal signal peptide.5 The recombinant protein has a calculated relative molecular weight of 22.6 kD. It was expressed inEscherichia coli strain JM109 and purified under denaturating conditions according to the manufacturer's instructions (Quiagen). Briefly, 500 mL of LB medium containing 100 μg ampicillin/mL was inoculated with 20 mL of an overnight culture of E coli JM109 harboring expression plasmid pQEvIL-6. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 2.5 mmol/L at an optical density at 600 nm of 0.5, and the bacteria were grown for another 3 hours. Cells were harvested by centrifugation at 4,000g and lysed in 30 mL of 6 mol/L guanidinum rhodanide/10 mmol/L Tris, pH 8.0. The lysate was cleared by centrifugation at 10,000g and applied immediately to a column of 5 mL Ni-NTA resin (Quiagen). The column was rinsed with 5 vol of wash-buffer (8 mol/L urea, 100 mmol/L sodium phosphate, 10 mmol/L Tris/HCl, pH 6.3). Wash-buffer containing increasing amounts of imidazole (10 to 400 mmol/L) was applied to the column to elute recombinant protein. Collected fractions were checked for the presence of recombinant protein by electrophoretic separation on a 12% polyacrylamide gel and staining with Coomassie blue. Recombinant vIL-6 was eluted at 200 mmol/L imidazole (Fig 1, lane 1). Recombinant vIL-6 was then renaturated by dialysis against 20 mmol/L HEPES, pH 8.0, 1 mmol/L MgCl2, 20 mmol/L KCl, 0.5 mmol/L dithiotreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.1 mmol/L EDTA, and 10% glycerol. An aliquot of the renaturated recombinant vIL-6 was loaded on a 12% polyacrylamide gel and checked for the absence of degradation (Fig 1, lane 2). The protein concentration of the renaturated recombinant vIL-6 was determined by the colorimetric bicinchonic acid assay as described by the manufacturer (Pierce Inc, Rockford, IL).
Coomassie brilliant blue-stained polyacrylamide gel of procaryotically expressed vIL-6. The apparent molecular weight of vIL-6 observed here is 24 kD, which is in agreement with the calculated molecular weight of 22.6 kD. M, molecular weight marker; lane 1, 4 μg vIL-6, eluted from Ni-NTA with 200 mmol/L imidazole; lane 2, 2 μg of the same protein preparation as lane 1 after dialysis.
Coomassie brilliant blue-stained polyacrylamide gel of procaryotically expressed vIL-6. The apparent molecular weight of vIL-6 observed here is 24 kD, which is in agreement with the calculated molecular weight of 22.6 kD. M, molecular weight marker; lane 1, 4 μg vIL-6, eluted from Ni-NTA with 200 mmol/L imidazole; lane 2, 2 μg of the same protein preparation as lane 1 after dialysis.
Cell proliferation assay.
INA-6 and B9 cells were washed three times in media without IL-6, resuspended, and cultured in flat-bottom microtiter plates at a density of 104 cells per well in a final volume of 200 μL culture media containing 10% FCS for 72 hours. Cytokines, Sant7, or antibodies were added as indicated. Antibodies were used at a final concentration of 10 μg/mL. Cells were pulsed with [3H]-thymidine (TdR; 1 μCi/well; specific activity, 2.0 Ci/mmol/L; Amersham, Braunschweig, Germany) 6 hours before harvesting and counted in a β-scintillation counter (1205 Betaplate; LKB Wallac, Turku, Finland). Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures.
RESULTS
Effects of vIL-6 on the human myeloma cell line INA-6.
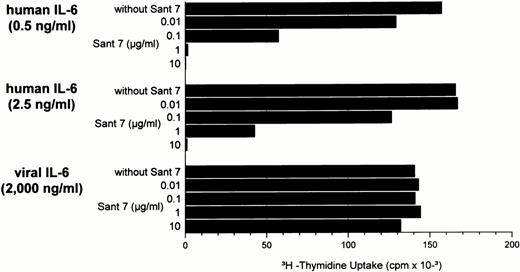
Based on the sequence similarity of HHV-8 IL-6 to hIL-6 and regarding its functional activity on murine B9 cells, binding of vIL-6 to the human IL-6R appeared possible. Therefore, we looked for the ability of HHV-8 IL-6 to support the proliferation of the human myeloma cell line INA-6 developed in our laboratory, which is strictly dependent on IL-6 for proliferation and survival.12 IL-11, oncostatin M (OM), leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (CNTF) in concentrations up to 100 ng/mL have no effect on the proliferation of INA-6 cells. As shown in Fig 2, vIL-6 induced DNA synthesis of INA-6 cells in a dose-dependent manner and to an extent comparable to human IL-6. However, whereas maximal proliferation with hIL-6 was achieved at 5 ng/mL, 20 μg/mL of the vIL-6 preparation, a 4,000-fold larger amount of protein, was necessary to yield maximal proliferation. The same difference in activity between human and viral IL-6 was seen in proliferation assays using another human IL-6–responsive (but not human IL-6–dependent) plasma cell line, JK-6.12 With the murine B9 cell line, which is about 100 times more sensitive to IL-6 than the human INA-6 cell line, a similar magnitude of difference between vIL-6 and hIL-6 activity was seen, with maximal DNA synthesis at 200 ng/mL vIL-6 and 0.05 to 0.2 ng/mL hIL-6. Moreover, a different batch of vIL-6 was used and yielded the same results.
Effects of human and viral IL-6 at different concentrations on the proliferation of the IL-6–dependent human myeloma cell line INA-6. [3H]-thymidine was measured during the last 6 hours of 72 hours of incubation. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures.
Effects of human and viral IL-6 at different concentrations on the proliferation of the IL-6–dependent human myeloma cell line INA-6. [3H]-thymidine was measured during the last 6 hours of 72 hours of incubation. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures.
Effect of antibodies specific for hIL-6, hIL-6R and gp130 on hIL-6– and vIL-6–induced proliferation.
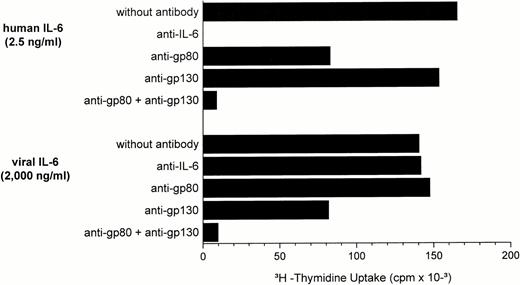
Stimulation of INA-6 myeloma cells by vIL-6 was not inhibited by a polyclonal antihuman IL-6 antiserum (Fig3). Whereas 2.5 ng/mL hIL-6 were completely neutralized (Fig 3), 200 ng/mL hIL-6 could not be blocked (data not shown). The proliferative activity of hIL-6 as well as vIL-6 on INA-6 cells was almost completely inhibited by a combination of neutralizing antibodies specific for the human IL-6R (gp80) and the gp130 molecule (Fig 3), the latter forming a complex with the IL-6R on the cell surface necessary for signal transduction. Contrary to the situation with hIL-6, the anti-gp130 antibody alone led to significant inhibition of vIL-6–induced INA-6 proliferation, whereas anti-gp80 had little effect. These results suggest that, although both IL-6R and gp130 seem to be involved in vIL-6 function, the relative interaction and affinity of the two receptor chains for human and viral IL-6 are likely to be substantially different.
Human and viral IL-6–induced DNA synthesis of INA-6 cells in the absence or presence of antibodies specific for hIL-6, hIL-6R (gp80), and gp130. A polyclonal antiserum was used for neutralization of IL-6 protein. Monoclonal antibodies 17506.1 and 28126.111 were used as IL-6R– and gp130-specific reagents. All antibodies were added at initiation of cultures at a final concentration of 10 μg/mL.
Human and viral IL-6–induced DNA synthesis of INA-6 cells in the absence or presence of antibodies specific for hIL-6, hIL-6R (gp80), and gp130. A polyclonal antiserum was used for neutralization of IL-6 protein. Monoclonal antibodies 17506.1 and 28126.111 were used as IL-6R– and gp130-specific reagents. All antibodies were added at initiation of cultures at a final concentration of 10 μg/mL.
Effect of vIL-6 on hIL-6–induced proliferation.
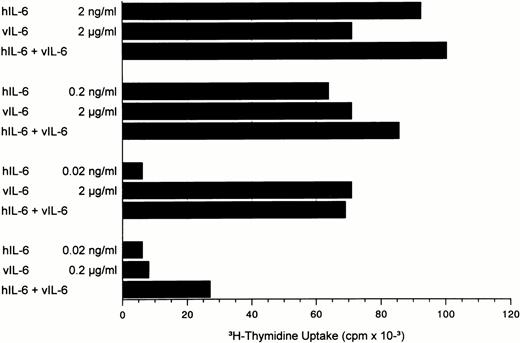
Because large quantities of vIL-6 are necessary to exert similar effects on cell proliferation as do small amounts of hIL-6, vIL-6 might lead to receptor blockade. Therefore, various concentrations of hIL-6 and vIL-6 were tested in different combinations. When both cytokines were used at concentrations that yield almost maximal proliferation of INA-6 cells (2 ng/mL hIL-6 and 2 μg/mL vIL-6), no inhibitory effects were seen (Fig 4). The proliferative effect of intermediate doses of hIL-6 could be marginally increased with the addition of high quantities of vIL-6. However, this additive effect was clearly seen using suboptimal quantities of hIL-6 and vIL-6 (0.02 ng/mL and 0.2 μg/mL, respectively).
Effects of human and viral IL-6 alone and in different combinations on the proliferation of human INA-6 cells. IL-6 concentrations in the combination were the same as indicated for each hIL-6 and vIL-6 alone.
Effects of human and viral IL-6 alone and in different combinations on the proliferation of human INA-6 cells. IL-6 concentrations in the combination were the same as indicated for each hIL-6 and vIL-6 alone.
Effect of IL-6R superantagonist Sant7 on hIL-6– and vIL-6–induced proliferation.
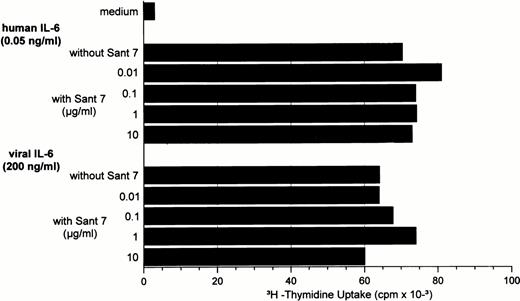
IL-6R antagonists have already been shown to efficiently inhibit IL-6–induced proliferation of malignant plasma cells and to induce apoptosis.15-17 Superantagonist Sant7 is one of several IL-6 variants that has increased affinity for the IL-6R but has completely lost the ability to bind gp130.15 Sant7, which specifically binds to the human IL-6R, was not able to inhibit hIL-6– as well as vIL-6–induced proliferation of B9 cells and, thus, is not active in the mouse system (Fig 5). This finding is in line with lack of Sant7 binding in vitro to recombinant mouse IL-6R (G. Ciliberto, unpublished observations). However, when tested in different concentrations on the human INA-6 cell line, Sant7 was able to inhibit hIL-6–induced proliferation in a dose-dependent manner (Fig 6). The effects of 0.5 ng/mL hIL-6 were blocked completely by 1 μg/mL Sant7; 10 μg/mL Sant7 was required when 2.5 ng/mL hIL-6 were present. In line with previously published results,15 a 2,000- to 4,000-fold excess of Sant7 was necessary for the inhibition of hIL-6 activity. A total of 2 μg/mL of vIL-6, the quantity required to achieve significant proliferation of INA-6 cells, could not be blocked by 10 μg/mL Sant7. In an experiment with the same quantity of hIL-6, which is a 1,000-fold more than what is required for maximal DNA synthesis, 10 μg/mL Sant7 could only partially block proliferation (data not shown).
Human IL-6R superantagonist Sant7 up to a concentration of 10 μg/mL does not inhibit human and viral IL-6–induced proliferation of the B9 mouse cell line. Sant7 was added at initiation of cultures together with IL-6.
Human IL-6R superantagonist Sant7 up to a concentration of 10 μg/mL does not inhibit human and viral IL-6–induced proliferation of the B9 mouse cell line. Sant7 was added at initiation of cultures together with IL-6.
IL-6R superantagonist Sant7 is able to inhibit proliferation of INA-6 cells induced by hIL-6 in a dose-dependent manner. Effects of 0.5 ng/mL and 2.5 ng/mL hIL-6 could be blocked by 1 μg/mL and 10 μg/mL Sant7, respectively. A total of 2 μg/mL of vIL-6 could not be blocked with up to 10 μg/mL Sant7.
IL-6R superantagonist Sant7 is able to inhibit proliferation of INA-6 cells induced by hIL-6 in a dose-dependent manner. Effects of 0.5 ng/mL and 2.5 ng/mL hIL-6 could be blocked by 1 μg/mL and 10 μg/mL Sant7, respectively. A total of 2 μg/mL of vIL-6 could not be blocked with up to 10 μg/mL Sant7.
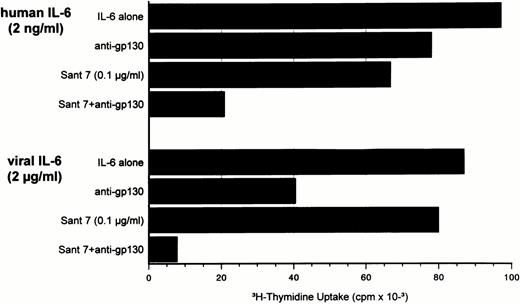
When Sant7 was administered in addition to anti-gp130 antibodies that partially inhibited INA-6 growth, a complete stop of proliferation could be achieved even with suboptimal Sant7 concentrations (Fig 7). This coblocking of signal transduction was also exerted when vIL-6 was used.
Human and viral IL-6–induced proliferation of INA-6 cells is only partially inhibited by 10 μg/mL anti-gp130 antibodies (clone IKR3). The addition of IL-6R superantagonist Sant7 at low concentrations led to a marked increase in inhibition of hIL-6 as well as vIL-6 effects. Anti-gp130 antibodies and Sant7 were added at initiation of cultures together with IL-6.
Human and viral IL-6–induced proliferation of INA-6 cells is only partially inhibited by 10 μg/mL anti-gp130 antibodies (clone IKR3). The addition of IL-6R superantagonist Sant7 at low concentrations led to a marked increase in inhibition of hIL-6 as well as vIL-6 effects. Anti-gp130 antibodies and Sant7 were added at initiation of cultures together with IL-6.
DISCUSSION
This report describes for the first time a growth-promoting activity for HHV-8 IL-6 on human cells. The inhibition studies with antibodies specific for IL-6R/gp130 as well as with IL-6R superantagonist Sant7 clearly demonstrate that the proliferative activity of vIL-6 was specific. The dose-dependent activity and the same magnitude of difference in activity between vIL-6 and hIL-6 when tested on a human and a mouse cell line argue in the same direction. It is unlikely that the comparatively low specific activity is due to incorrect folding of vIL-6 during renaturation, and avoiding this step in control batches gave similar activities. Log step differences in activity as seen with hIL-6 and vIL-6 are not unusual and have been described for IL-6, IL-11, and OM in the mouse system with the B9 cell line before.18,19 Posttranslational modifications in eukaryotic cells, particularly glycosylation, have been reported not to be important for the biologic activity of hIL-6.20 Thus, the rather low activity of vIL-6 is likely to be the result of its low binding affinity to receptor structures due to limited sequence homology. Indeed, 4 residues of hIL-6 have been found to be crucial for interaction of the cytokine with the human IL-6R21: Phe74, Phe78, Arg 179, and Arg182. Alignment of hIL-6 sequence against vIL-6 sequence shows that, although Phe78 and Arg179 are conserved, a Glycine is present in position 74 and an Aspartate is present in position 182 of vIL-6 sequence.5 Because amino acid changes of hIL-6 Phe74 and Arg182 have been shown to strongly reduce binding to human IL-6R,21 this observation furnishes an elegant molecular explanation for the postulated low binding affinity of vIL-6 to the human IL-6R.
There are at least two possible explanations for the finding that antihuman IL-6 antibodies do not inhibit vIL-6. Firstly, the quantitative amount of vIL-6 protein needed to induce proliferation may exceed the blocking capacity of the anti–IL-6 antibody used. Alternatively or in addition, structurally different vIL-6 may not be sufficiently detected by polyclonal antibodies selected for binding of hIL-6.
The results obtained with antibodies specific for the IL-6R and gp130 clearly show that both receptor chains are involved in vIL-6 binding and activity. The complete inhibition of INA-6 proliferation with a combination of IL-6R/gp130 antibodies and with IL-6R superantagonist Sant7 together with anti-gp130 antibodies confirms the important role that gp80 plays for vIL-6 signaling in plasma cells. Partial inhibition of hIL-6–induced proliferation of INA-6 cells with anti-gp80 antibodies alone is consistent with sequential binding of hIL-6 to the IL-6R with low affinity first, and then this complex associates with gp130 resulting in high-affinity binding and intracellular signaling.22 In contrast, vIL-6 activity was significantly inhibited by anti-gp130 antibodies only. This may be due to differenes in binding affinities and sequence. Another cytokine that uses gp130 as signaling subunit, OM, has been shown to bind directly to gp130 with low affinity, but without a biological response.23 When present at a high molar excess, OM acts as an IL-6 antagonist on cells that do not express a functional OM receptor. However, in our system, a 10,000-fold excess of vIL-6 on a per weight basis did not show an antagonistic effect on hIL-6–induced proliferation of INA-6 cells. On the contrary, both hIL-6 and vIL-6 have an additive effect that is best seen at suboptimal doses. Further studies with soluble IL-6R and IL-6R antagonists may further clarify the receptor binding properties of vIL-6.
The finding that HHV-8 IL-6 is functionally active on human malignant plasma cells seems particularly important, because IL-6 has been shown to be a major growth factor for human myeloma cells.10 In a study published recently, the presence of HHV-8 in dendritic cells cultured from bone marrow of patients with multiple myeloma and plasma cell leukemia was reported and a causative role for HHV-8 in the pathophysiology has been suggested.8
On the basis of proliferative activity, the INA-6 and B9 cell lines provide a functional assay allowing to dissect between cellular and viral IL-6. Whereas hIL-6 could be completely inhibited by neutralizing antibodies specific for hIL-6 and by the receptor antagonist Sant7, vIL-6 was only active in high concentrations and could not be blocked. It remains to be seen if such large quantities of vIL-6 are produced physiologically and, if so, whether this is clinically relevant. The cell line system described here should be helpful to further elucidate the role of viral IL-6 in the pathogenesis of HHV-8–associated diseases.
Supported by the Wilhelm Sander-Stiftung (Grant No. 96.019.1) and by the Dr. Mildred Scheel-Stiftung (Grant No. W134/94/FL2).
Address reprint requests to Martin Gramatzki, MD, Division of Hematology/Oncology, Department of Medicine III, University of Erlangen-Nuernberg, Krankenhausstrasse 12, D-91054 Erlangen, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Effects of human and viral IL-6 at different concentrations on the proliferation of the IL-6–dependent human myeloma cell line INA-6. [3H]-thymidine was measured during the last 6 hours of 72 hours of incubation. Values represent the mean [3H]-TdR incorporation (cpm) of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/6/10.1182_blood.v91.6.1858/2/m_blod4064302.jpeg?Expires=1766148748&Signature=m8lex0CZT9D25QQi40~9UOhBXflx4RZPV9e1jpzin7x8CXArar9D3SFD9jBvpaCphiZ5iNfTA5DBk2mRkwNTLT2fJUaPyjyiIJK9mznkaavNgd~fKYfzGUbQ~CC~uB83tytlIS-DgUzZ1hLW7LRySlzGrJlGyhE3-x518L9Q0eqTCA7qI2SKHgG8Q9MPtASk0GelPZ~~EqhrMihU-dTdNF-SnUCv8l7fLRy~juMry0MdzWz-2hEkHb-6ePyy3v2evGFgjpA-eoDUWlht9JzW9tSj3-Ap2zP8CFHlwGvg55NII0nSeoKFhUabvwPV9~OS7s9jiaDV3iGPS~91bSm2FQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal