Abstract
Bone marrow dendritic cells (DC) from patients with multiple myeloma (MM) were recently reported to be infected with Kaposi's sarcoma-associated herpesvirus (KSHV). Because immunotherapy strategies using DC are very promising in this disease, we looked for KSHV DNA in clinical-grade DC generated in vitro from MM patients. Adherent apheresis cells from MM patients were maintained for 7 days in clinical-grade X-VIVO 15 culture medium supplemented with granulocyte-macrophage colony-stimulating factor, interleukin-4, or interleukin-13. Tumor necrosis factor α was added for the last 2 days. We obtained a cell population with a DC phenotype able to endocytose fluorescein isothiocyanate (FITC)-dextran and efficiently activate resting allogenic T lymphocytes. To detect KSHV DNA, we used polymerase chain reaction (PCR) followed by Southern blotting of PCR product with a sensitivity detecting a few copies of viral DNA. All the PCR were repeated in a blinded fashion three times, on 1 μg and 0.2 μg of genomic DNA, in two different laboratories. Clinical-grade DC from 10 (91%) of 11 patients were not infected with KSHV. The apheresis cells and the purified CD34+ cells from the same patients were also negative. A very weak PCR band was detected with DC from one patient, but the initial apheresis cells were negative. The detection of KSHV infection in 1 (9%) of 11 MM patients probably represents background seroprevalence. It seems likely that functional and clinical-grade DC from MM patients can safely be used in clinical trials.
BONE MARROW dendritic cells (DC) from patients with multiple myeloma (MM) were found by Rettig et al1 to be infected with Kaposi's sarcoma-associated herpes virus (KSHV) in contrast to those from non-MM patients. Additionally, these cells were reported to express viral interleukin-6 (vIL-6) ,which is known to share functional homology with human IL-6.2-4vIL-6 supports the proliferation of B9 murine hybridoma cells, activates human gp130 independently of binding to IL-6Rα,5 and promotes the survival and proliferation of myeloma cells (unpublished results). The ability of KSHV to code for vIL-6 and other potential oncogenic proteins6-10 suggests that KSHV, which is involved in the pathogenesis of Kaposi's sarcoma, a subset of Castleman disease and body-cavity based lymphoma,11-13 could also be associated with MM, an IL-6–related disease.14
Several studies have demonstrated that DC efficiently induce antigen-specific antitumoral immunity in vitro and in vivo.15-18 As recently described, clinical trials with DC pulsed with monoclonal Ig gave promising results in MM.19DC from MM patients infected with KSHV is a major impediment to the future clinical use of these cells.
We recently described a simple method to obtain a virtually pure population of functional dendritic cells from apheresis cells from patients with MM using a combination of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, and tumor necrosis factor-α (TNFα).20 Several studies have shown that IL-4 and IL-13 share several biologic activities. In particular, they induce monocytes to differentiate into dendritic cells.21,22 Moroever, the IL-13 receptor, which comprises the common IL-4 receptor α chain,23 is able to transduce a signal for both cytokines.24-27 In this report, we extend our method to clinical-grade culture conditions and compare these two related cytokines, IL-4 and IL-13, for their ability to promote DC development. We demonstrate that clinical-grade functional DC from most patients with MM do not harbor KSHV DNA, making their use in vivo possible. In addition, we were unable to detect any KSHV DNA in apheresis cells or in purified CD34+ progenitors from these patients.
PATIENTS, MATERIALS, AND METHODS
Patients.
We evaluated 11 patients with MM (median age, 55 years). All patients exhibited active disease and, according to Durie and Salmon classification, there was 1 stade IA, 1 stade IIIB, and 9 stade IIIA. Five patients had an IgGκ, 2 an IgGλ, 3 an IgAκ, and 1 an IgAλ monoclonal component. After written informed consent was obtained, patients were treated with a three VAD (Vincristine, Adriamycine, Dexamethasone) chemotherapy regimen followed by high-dose cyclophosphamide (4 g/m2) and recombinant human granulocyte colony-stimulating factor (G-CSF; filgrastim; NEUPOGEN; Amgen-Roche, Neuilly sur Seine, France; daily injection of 5 μg/kg from day 2 after chemotherapy until hematologic recovery). The peripheral blood CD34+ cell count was monitored every day and peripheral blood cells were collected at the peak of CD34+ cell count during a 5-hour apheresis. The mean leucocyte count was 21.3 ± 15.2 × 103/μL (range, 2.5 to 69 × 103/μL) and the median time of collection after cyclophosphamide injection was 10 days (range, 9 to 12 days). Peripheral blood from two healthy volunteers was collected under heparin after written informed consent was obtained.
Cell lines.
Preparation and characterization of DC.
DC were generated from apheresis cells (AC) as previously described.20 Briefly, 3 × 106 AC/mL were grown in RPMI 1640 supplemented with 2 mmol/L of L-glutamine and 10% fetal calf serum (FCS) or in X-VIVO 15 serum-free medium from Biowittaker (Walkersville, MD) (ie, clinical-grade DC). After 2 hours, nonadherent cells were discarded and adherent cells were cultured in the same media with 100 ng/mL of GM-CSF (LEUCOMAX; Sandoz, Basel, Switzerland) and 25 ng/mL of IL-4 (Genzyme, Cambridge, MA) or IL-13 (Sanofi, Labège, France). After 5 days of culture, TNFα (R&D Systems, Minneapolis, MN) was added at 20 ng/mL. The same phenotypic and functional analyses were performed on DC obtained in RPMI-FCS and X-VIVO 15 media. On day 5 of culture, before TNFα addition, we studied the capacity of these cells for endocytosis through the mannose receptor using lysine-fixable fluorescein isothiocyanate (FITC)-dextran, molecular weight equal to 40,000 (Molecular Probes Inc, Eugene, OR), as previously described.30 AC-derived DC were incubated for 7, 15, and 30 minutes at 37°C with 1 mg/mL of FITC-dextran in RPMI-10% FCS-25 mmol/L HEPES. After 4 washes with cold phosphate-buffered saline (PBS)-1% FCS-0.02% NaN3 , cells were analyzed on a FACScan apparatus (Becton Dickinson, San Jose, CA). Background uptake was measured by incubating cells at 4°C. On day 7, cells maintained in the different media and cytokine conditions were collected and labeled with the following monoclonal antibodies (MoAbs): CD4 and CD83 conjugated with phycoerythrin (PE) and CD14, CD54, CD58, CD80, and HLA DR conjugated with FITC (Immunotech, Marseille, France). FITC-anti-CD1a, FITC-anti-CD40, and FITC-anti-CD86 MoAbs were purchased from Pharmingen (San Diego, CA). Intracellular staining for CD68 was performed using EBM11 MoAb (Dako, Glostrup, Denmark) and Fix and Perm kit (Caltag Laboratories, Burlingame, CA). Negative controls were performed with corresponding irrelevant matched murine MoAbs (Immunotech). Finally, these DC were used in an allogenic mixed lymphocyte reaction (MLR) to stimulate T cells purified from healthy volunteers' peripheral blood by 2 cycles of negative selection using anti-CD14, -CD19, -CD56, -CD16, and -HLA DR MoAbs (Immunotech) and goat-antimouse Ig magnetic microbeads (Dynal, Oslo, Norway) as previously reported.20 Graded numbers of irradiated DC (30 Gy) were added to 1.5 × 105 allogenic T cells in 200 μL of RPMI-5% serum AB culture medium. After 5 days of coculture, cells were pulsed with 1 μCi of tritiated thymidine (Amersham, Buckingham, UK) for 12 hours and radioactive incorporation was counted. Results are expressed as mean counts per minute (cpm) ± SD determined in sextuplet culture wells.
KSHV DNA detection.
For patients no. 1 to 11, two different cell populations were tested for KSHV infection: AC and clinical-grade DC derived from these AC. In addition, for patients no. 1 through 8, we purified CD34+cells. AC were collected after centrifugation on Ficoll-Hypaque (Biowittaker). CD34+ AC were purified by the clinical-grade methodology from Baxter (Isolex 300; Baxter International Inc, Deerfield, IL) according to the manufacturer's instructions. This allowed the selection of a highly pure population of CD34+cells (median, 92%; range, 85% to 98%) as assessed by labeling with a pool of three PE-conjugated CD34 MoAbs that recognized three different epitopes of the CD34 molecule (Immunotech). DC were collected after 7 days of culture in X-VIVO 15 medium with GM-CSF, IL-4, and TNFα . AC, CD34+ cells and clinical-grade DC were frozen and stored at −80°C until DNA extraction. DNA was extracted with a Promega kit (Madison, WI) according to the manufacturer's instructions. The positive control was genomic DNA from the BCBL-1 cell line. To assess the integrity of genomic DNA, we performed a polymerase chain reaction (PCR) with primers for β-globin (forward primer, 5′-CAACTTCATCCACGTTCACC-3′; reverse primer, 5′-GAAGAGCCA AGGACAGGTAC-3′). The 268-bp sized β-globin fragment could be detected in all the DNA samples (data not shown). For sensitivity assay, 1 μg to 0.1 pg of BCBL-1 DNA was diluted in the DNA of the KSHV-negative XG-1 MM cell line. All PCR were performed 3 times with 1 μg of DNA (corresponding to approximatively 150,000 cells) using the primers described by Chang et al11 that amplify a 233-bp DNA fragment. A total of 40 cycles of PCR amplification was used and PCR products (10 μL) were electrophoresed on a 3% agarose gel impregnated with ethidium bromide. They were then blotted onto a positive nylon membrane and hybridized, as described,11 with the 25-bp internal probe, labeled with32P using the T4 polynucleotide kinase (GIBCO BRL, Paisley, UK). Autoradiographs were developed after 2 and 6 hours of exposure; the membranes were also exposed for 24 hours to PhosphorImager to exclude the presence of weak bands. The same procedure was performed on 0.2 μg of DNA in the laboratory of Dr Chang.
vIL-6 immunohistochemistry.
Expression of KSHV vIL-6 was evaluated in two DC cultures (patients no. 1 and 10) using a polyclonal rabbit antiserum raised against vIL-6 peptides that does not cross-react with human IL-6. DC were harvested and embedded in 1% agarose plugs that were formalin-fixed and then processed in paraffin. Four-micrometer sections were cut on coated slides and immunostaining was performed with the avidin-biotin complex (ABC) method using a previously published protocol.2 BCP-1 (KSHV-infected) and P3HR-1 (EBV-infected) cell lines prepared in the same way were used as positive and negative controls, respectively.
RESULTS
Generation of clinical-grade DC from apheresis from MM patients.
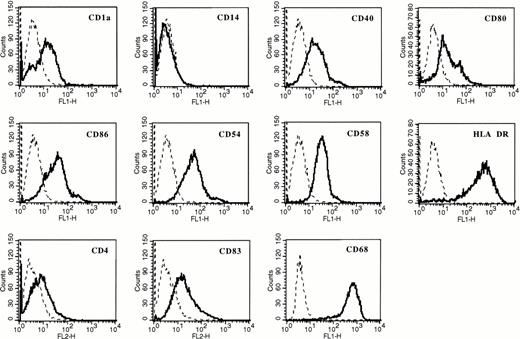
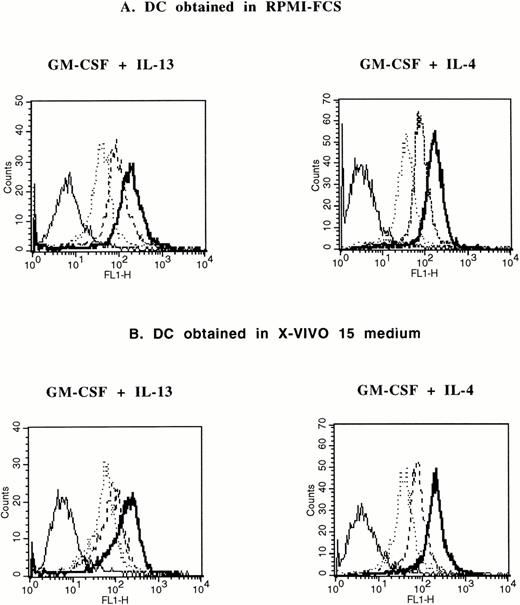
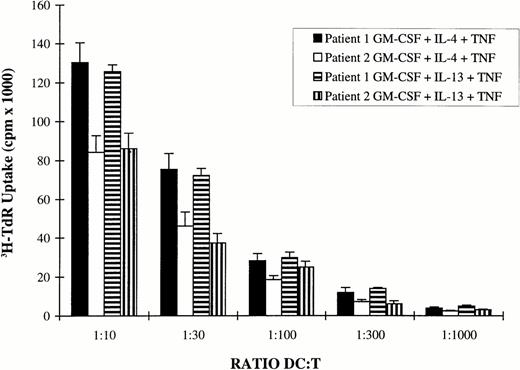
Adherent AC from patients with MM were cultured with RPMI 1640 and 10% FCS or with clinical-grade X-VIVO 15 culture medium with GM-CSF and IL-4 or GM-CSF and IL-13 for 5 days. TNFα was added on day 5 for 2 additional days. In agreement with our previous report,20the various culture conditions resulted in the generation of cells with phenotypic characteristics of DC (Table 1). In particular, these cells were always CD14− and HLA DR+. Cells cultured with X-VIVO 15 medium expressed less CD1a and CD4 than those cultured with RPMI and 10% FCS. A complete phenotypic picture of clinical-grade DC cultured with GM-CSF and IL-13 is shown in Fig 1. It was similar to the phenotype of DC cultured in X-VIVO 15 medium with GM-CSF and IL-4 we previously reported.20 Despite these phenotypic differences, cells generated in X-VIVO 15 medium have the same ability to endocytose FITC-dextran as those obtained in RPMI-FCS (Fig 2). Again, no difference was found for DC generated with GM-CSF and IL-4 or GM-CSF and IL-13 (Fig 2). Furthermore, these cells efficiently present antigens to resting allogenic T cells (Fig 3).
Phenotypic Comparison of DC Obtained From MM Patients in Different Culture Conditions
| Cell Surface Expression % (MFI) . | Culture Conditions . | |||
|---|---|---|---|---|
| RPMI 1640-10% FCS . | X-VIVO 15 . | |||
| IL-4 . | IL-13 . | IL-4 . | IL-13 . | |
| CD14 | 0 | 0 | 0 | 0 |
| HLA DR | 100 (773) | 100 (661) | 100 (412) | 100 (380) |
| CD1a | 92 (704) | 90 (589) | 41.5 (63) | 41.5 (51) |
| Cell Surface Expression % (MFI) . | Culture Conditions . | |||
|---|---|---|---|---|
| RPMI 1640-10% FCS . | X-VIVO 15 . | |||
| IL-4 . | IL-13 . | IL-4 . | IL-13 . | |
| CD14 | 0 | 0 | 0 | 0 |
| HLA DR | 100 (773) | 100 (661) | 100 (412) | 100 (380) |
| CD1a | 92 (704) | 90 (589) | 41.5 (63) | 41.5 (51) |
Apheresis cells from 11 MM patients were maintained according to the four culture conditions described in Patients, Materials, and Methods and harvested at day 7. Data represent the mean percentage of positive cells (%) and, in parentheses, the mean intensity of fluorescence (MFI) determined by flow cytometry.
Dendritic phenotype of adherent AC cultured in X-VIVO 15 medium with GM-CSF and IL-13. AC were maintained in X-VIVO 15 clinical-grade culture medium with GM-CSF and IL-13 for 5 days. TNFα was added for 2 additional days. Flow cytometric analysis was performed at day 7. Isotype-matched murine MoAbs were used as negative controls (dotted lines).
Dendritic phenotype of adherent AC cultured in X-VIVO 15 medium with GM-CSF and IL-13. AC were maintained in X-VIVO 15 clinical-grade culture medium with GM-CSF and IL-13 for 5 days. TNFα was added for 2 additional days. Flow cytometric analysis was performed at day 7. Isotype-matched murine MoAbs were used as negative controls (dotted lines).
Comparison of FITC-dextran endocytosis by DC obtained in X-VIVO 15 and in RPMI-10% FCS. DC generated after 5 days of culture in RPMI-10 % FCS (A) or in X-VIVO 15 medium (B) supplemented with GM-CSF and IL-13 or GM-CSF and IL-4 were incubated for various lengths of time in medium containing 1 mg/mL of FITC-dextran and fluorescence was analyzed by flow cytometry after extensive washing. Solid lines are the background uptake at 4°C. Dotted lines are 7 minutes at 37°C. Broken lines are 15 minutes at 37°C. Bold lines are 30 minutes at 37°C.
Comparison of FITC-dextran endocytosis by DC obtained in X-VIVO 15 and in RPMI-10% FCS. DC generated after 5 days of culture in RPMI-10 % FCS (A) or in X-VIVO 15 medium (B) supplemented with GM-CSF and IL-13 or GM-CSF and IL-4 were incubated for various lengths of time in medium containing 1 mg/mL of FITC-dextran and fluorescence was analyzed by flow cytometry after extensive washing. Solid lines are the background uptake at 4°C. Dotted lines are 7 minutes at 37°C. Broken lines are 15 minutes at 37°C. Bold lines are 30 minutes at 37°C.
DC obtained in X-VIVO 15 medium stimulate allogenic T-lymphocyte proliferation. DC were generated in clinical-grade culture medium using 7 days of culture of AC in the presence of GM-CSF and IL-4 or GM-CSF and IL-13. TNFα was added for the last 2 days. After 3 washes and irradiation (30 Gy), they were used in graded numbers as stimulator cells for 1.5 × 105 allogenic T cells. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR after 5 days of coculture. Data are expressed as the mean ± SD of sextuplet culture wells. 3H-TdR incorporation rates of irradiated DC or purified T cells were less than 600 cpm.
DC obtained in X-VIVO 15 medium stimulate allogenic T-lymphocyte proliferation. DC were generated in clinical-grade culture medium using 7 days of culture of AC in the presence of GM-CSF and IL-4 or GM-CSF and IL-13. TNFα was added for the last 2 days. After 3 washes and irradiation (30 Gy), they were used in graded numbers as stimulator cells for 1.5 × 105 allogenic T cells. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR after 5 days of coculture. Data are expressed as the mean ± SD of sextuplet culture wells. 3H-TdR incorporation rates of irradiated DC or purified T cells were less than 600 cpm.
In conclusion, clinical-grade culture medium allowed the generation of functional DC from patients with MM. In addition, IL-13 was as efficient as IL-4 in these culture conditions.
Lack of KSHV DNA in clinical-grade DC from patients with MM.
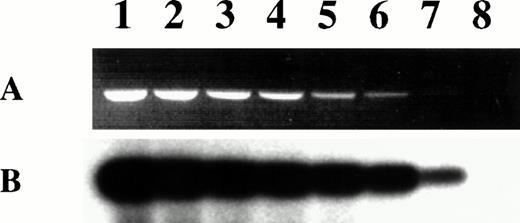
We investigated whether DC generated from MM patients in X-VIVO 15 supplemented with GM-CSF and IL-4 contained KSHV DNA. Using 40 cycles of PCR with KSHV 330 primers, Southern blotting, and hybridization with an internal radiolabeled probe, we were able to detect the presence of KSHV DNA in 1 pg of genomic DNA of the KSHV-infected BCBL-1 cell line diluted in 1 μg of genomic DNA of the KSHV-negative XG-1 myeloma cell line (Fig 4). Because every BCBL-1 cell contained an average of 30 copies of the KSHV genome,31 1 pg of BCBL-1 DNA corresponded to about 5 KSHV copies. This was in agreement with the previously reported high sensitivity of this PCR.32 We failed to detect KSHV DNA in 1 μg of DNA (ie, 150,000 cells) from MM patients' clinical-grade DC in 10 (91%) of 11 cases (Fig 5A and B). For patient no. 8, a weak band could be detected, indicating that few KSHV copies were present. All PCR were repeated with identical results 3 times in Montpellier on 1 μg of DNA and in New York using 0.2 μg of DNA. We obtained exactly the same PCR data when starting from DNA of DC obtained in RPMI-10% FCS (data not shown). In addition, none of the 11 patients' apheresis cells was positive for KSHV DNA (Fig 5C). Because CD34+ cells could also be used to generate DC in vitro,33-35 CD34+ cells from 8 of 11 patients were purified (85% to 98% of CD34+ cells; median, 92%) and tested. These cells were also negative for KSHV DNA (Fig 5D).
Sensitivity of KSHV PCR. Lanes 1 through 8 contain 10-fold dilutions of BCBL-1 DNA from 1 μg (lane 1) to 0.1 pg (lane 8). BCBL-1 DNA was diluted in the KSHV-negative XG-1 MM cell line DNA so that all the PCR were run on 1 μg of total DNA. (A) Ethidium bromide-stained agarose gel of the 233-bp amplification products. (B) Specific hybridization of the PCR products to a32P-end-labeled internal probe after transfer to a nylon membrane.
Sensitivity of KSHV PCR. Lanes 1 through 8 contain 10-fold dilutions of BCBL-1 DNA from 1 μg (lane 1) to 0.1 pg (lane 8). BCBL-1 DNA was diluted in the KSHV-negative XG-1 MM cell line DNA so that all the PCR were run on 1 μg of total DNA. (A) Ethidium bromide-stained agarose gel of the 233-bp amplification products. (B) Specific hybridization of the PCR products to a32P-end-labeled internal probe after transfer to a nylon membrane.
PCR amplification of DNA from clinical-grade DC (A and B), from corresponding AC (C), and from CD34+ cells (D) from MM patients. (A) Ethidium bromide-stained agarose gel of the 233-bp amplification products. Lane M was a molecular size marker. (B, C, and D) Specific hybridization of the PCR products to a32P-end-labeled internal probe after transfer to a nylon membrane. The positive control (lane C) was the PCR product from the BCBL-1 cell line.
PCR amplification of DNA from clinical-grade DC (A and B), from corresponding AC (C), and from CD34+ cells (D) from MM patients. (A) Ethidium bromide-stained agarose gel of the 233-bp amplification products. Lane M was a molecular size marker. (B, C, and D) Specific hybridization of the PCR products to a32P-end-labeled internal probe after transfer to a nylon membrane. The positive control (lane C) was the PCR product from the BCBL-1 cell line.
DISCUSSION
We have previously reported the generation of DC by culturing apheresis cells from patients with MM with GM-CSF and IL-4 for 7 days. These cells had the phenotype of DC (CD1+, CD4+, CD14−, HLA DR+, CD80+, CD86+), were able to endocytose FITC-dextran, and were able to present soluble antigens to naive T cells. We used RPMI 1640 culture medium and FCS and presented preliminary data showing that clinical-grade serum-free culture medium could be used.20
In the present study, we reinforce these data and show that pure and functional DC could be generated with clinical-grade serum-free culture medium. Adherent apheresis cells were collected and cultured with X-VIVO 15 culture medium for 7 days with GM-CSF and IL-4. The replacement of IL-4 with IL-13 yielded similar results. These cells had a DC phenotype with slight differences suggestive of a less mature phenotype when compared with those generated with RPMI 1640 and 10% of FCS. However, their endocytic capacity was similar. We previously reported that addition of TNFα for the last 2 days of culture was necessary to get an efficient antigen-presenting ability,20and this was confirmed in this study (data not shown). Thus, we show here that fully functional DC can be generated from MM patients.
To address the recent concern that bone marrow DC are infected with KSHV and express vIL-6 gene,1 we investigated whether DC generated from apheresis cells in our culture conditions might be infected with KSHV. Using a very sensitive PCR and Southern blotting, it is possible to amplify KSHV DNA in 1 pg of DNA from KSHV-infected BCBL-1 cells. We failed to detect any KSHV amplification in 1 μg of DNA from clinical-grade DC from 10 patients with MM. PCR was performed blindly in two independent laboratories using KS330233primers. Furthermore, we failed to find expression of vIL-6 by immunostaining with anti–vIL-6 antibody in two patients with negative PCR results (data not shown). The initial apheresis cells (n = 11) as well as pure CD34+ cells (n = 8) from which the DC precursors originated were also negative. For one patient, we found a weak PCR amplification that was seen only after Southern blotting, suggesting less than 5 KSHV copies in 1 μg of DNA. No PCR amplification was seen using 100 ng of DC DNA (data not shown). These data indicate that only a few cells of 150,000 cells were infected with KSHV. The detection of KSHV in 1 (9%) of 11 patients is within the range (0% to 25%) of background KSHV seroprevalence in several studies.36-39 It is of note that total AC and CD34+ purified cells from this patient were negative for KSHV PCR. It is possible that the rare cells infected with KSHV were retained by the adherence step used to generate DC from total AC, resulting in sufficient enrichment for the presence of KSHV DNA and detection with our very sensitive method. It has been recently suggested that monocytes were productively infected by KSHV in Kaposi's sarcoma lesions and KSHV was detected by PCR in the adherent monocytic cell population obtained after 8 days of culture of peripheral blood mononuclear cells of acquired immunodeficiency syndrome-associated Kaposi's sarcoma patients.40
We do not use the culture conditions described by Rettig et al1 because nobody has yet shown that such a method is successful to obtain DC. Moreover, Rettig et al1 presented no evidence that their infected cells were really DC in terms of endocytosis, expression of costimulatory molecules, antigen presentation, and activation of T lymphocytes. Thus, the present study may ascertain that fully characterized DC from most MM patients are not infected with KSHV. In addition, when we cultured stromal cells according to the protocol described by Rettig et al,1 we obtained a mixed population of fibroblastic and monocytic cells that were not infected with KSHV (unpublished results). However, one cannot exclude the possibility that a rare bone marrow cell that can be expanded under very specific conditions could be infected by KSHV in MM patients. We may only note that two groups failed to find KSHV seropositivity in patients with MM41,42in agreement with the results of Dr Chang (unpublished results). Our data, along with the lack of KSHV detection in tumoral samples 43, do not favor a causal role of KSHV in MM. In conclusion, this study shows that clinical-grade DC from most patients with MM are not infected with KSHV.
ACKNOWLEDGMENT
The authors appreciate the generous gift of rhIL-13 from A. Minty (Sanofi, Labège, France). We thank G. Cathala for expert technical advice, thank G. Fiol and M-C. Delteil for their help in generating DC samples, and acknowledge L. Milligan for her assistance in the preparation of the manuscript.
Supported by grants from ARC (Paris, France), LFNC (Paris, France), and AFS (Paris, France).
Address reprint requests to Bernard Klein, PhD, Institute for Molecular Genetics, CNRS, 1919 Route de Mende, 34033 Montpellier, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal