Abstract
To characterize the effect of human immunodeficiency virus-1 (HIV-1)nef expression in human monocytes/macrophage (HMØ) and U937 on the levels of FcγRs, HLA antigens, and monokines, elutriated HMØs and U937 cells were transfected with an adenovirus-mediated Nef expression system. Nef-expressing cells downmodulated FcγRI, FcγRII, and upregulated HLA class I molecules. Nef-expressing HMØs, treated with lipopolysaccharide (LPS) or phorbol 12-myristate 13-acetate (PMA), overexpressed tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-10. However, IL-6 was induced by LPS and inhibited by PMA. Additionally, a subpopulation of Nef-expressing HMØs underwent apoptosis. Our data suggest that HIV-1 nefdownmodulated FcγRs in myeloid cells in a manner similar to that previously reported for its effect on CD4+ in T cells.
THE VIRAL GENE, nef, encodes a 27- to 34-kD protein and is present only in the human immunodeficiency virus (HIV) and in primate lentiviruses, simian immunodeficiency virus (SIV).1-3 Although the precise function(s) of this gene is not fully understood, it is known that Nef is required to maintain high viral loads during persistent SIV infection and disease in macaques.4 HIV-1 nef downregulates cell surface CD4 and blocks interleukin-2 (IL-2) gene induction in T cells as well as in monocytic cell lines.5-12 It has been shown that nefperturbs cell activation pathway(s), thereby influencing viral replication in the host.13,14 HIV-infected human monocytes/macrophages (HMØs) play an important role in the pathogenesis of disease by acting as a persistent virus reservoir.15,16 HMØs express both CD4 and Fcγ receptors (FcγRs) for IgG.17 It is not known how FcγR responds to HIV gene products in HMØs. Virus-infected HMØs secrete immunomodulators, inflammatory monokines, neurotoxins, and neuroexcitotoxins.18-21 Cytokines/monokines generated during the immune response regulate both immune function and viral gene expression, thereby affecting the progression to acquired immunodeficiency syndrome (AIDS).16,22 Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-10 can either enhance or suppress HIV replication during the virus life cycle.16,23-31 Overexpression of Nef during early HIV infection promotes active virus replication and has been suggested to increase monokine/cytokine production.4 18 However, the Nef gene by itself has not been shown to modulate monokines directly.
Our preliminary studies have suggested that Nef expression alters myeloid cells surface receptors.32 In the present study, we determined whether nef expression modulates expression of FcγRI, II, and III; HLA class I and II; CD4; IL-2R molecules; and the monokines TNF-α, IL-1β, IL-6, and IL-10 in dividing and differentiated primary HMØs and U937 cells. Because several studies have shown the potential involvement of apoptotic cell death during HIV infection,33,34 we also examined Nef-expressing HMØs for apoptosis. To study the expression of cell surface molecules, monokines and apoptosis, we selected adenovirus (Ad)-mediated transfection and retrovirus-mediated transduction,35-37 because transfection of DNA by chemical or electroporation methods were found inefficient in macrophages.38 To increase Nef expression, we used a replication-deficient adenovirus that had previously been shown to enhance the delivery of cointernalized macromolecules into the cells.37,39-41 Because HMØs express high numbers of transferrin receptors,42-44 we conjugated nefplasmid DNA with transferrin and used this conjugate together with an adenovirus mutant dl312 to transfect HMØs. The present results show that Nef expression in HMØs and U937 cells modulated cell-surface expression of FcγRI and II, CD4, and HLA class I molecules. Activation of Nef-expressing HMØs with phorbol 12-myristate 13-acetate (PMA) or lipopolysaccharide (LPS) altered the levels of TNF-α, IL-1β, IL-10, and IL-6. Furthermore, for the first time, we have shown that HIV Nef mediates apoptosis in a subpopulation of HMØs.
MATERIALS AND METHODS
Human monocytes/macrophages.
Monocytes (95% to 98% pure) were prepared from peripheral blood mononuclear cells (PBMCs) of HIV-1–seronegative donors by centrifugal elutriation45,46; purified cells were cultured.21
U937 cells.
The human histiocytic lymphoma cell line U937,47 expressing many monocytic characteristics including FcγRs obtained from ATCC (Rockville, MD), was propagated in RPMI 1640 containing 10% fetal bovine serum (FBS), penicillin, streptomycin, and glutamine (Inovar Biologicals, Gaithersburg, MD).
PA317 cells.
Mouse embryo fibroblasts, derived from NIH/3T3 cells,48were purchased from ATCC. Transfection of retrovirus vectors into these cells resulted in production of virions able to infect human cells. PA317 cells were used to prepare nef- or fen-expressing retrovirus stocks. Cell culture conditions were the same as for the U937 cells.
293 cells.
Primary human embryonic kidney cells transformed by human adenovirus type 5 DNA49 were obtained from ATCC and cultured in Eagle's minimum essential medium (MEM) with Earle's buffered saline solution (BSS), 10% FBS, and antibiotics to prepare an adenovirus stock of dl312.
Proliferation assay of HMØs.
HMØs were cultured and maintained in macrophage medium with L-glutamine (GIBCO-BRL, Life Technologies, Gaithersburg, MD) with 5% filtered FBS (Intergen, Purchase, NY) and gentamicin sulfate (GIBCO-BRL) at 37°C, 5% CO2. Twenty-six thousand cells were plated in each well of a 96-well flat-bottom culture plate (Costar, Cambridge, MA) in the presence or absence of 5 ng/mL IL-3 (R & D System, Minneapolis, MN) and different concentrations (1 to 20 ng/mL; Fig 1) of granulocyte-macrophage colony-stimulating factor (GM-CSF; R & D System). Cell proliferation assay was performed as previously described.35 In brief, at different days (1, 2, 4, and 6), 0.1 μmol/L or 2.5 μCi/mL of [3H] thymidine (ICN Pharmaceuticals Inc, Costa Mesa, CA) was added and the cells were harvested after 6 hours. The cells were dissociated in an enzyme-free EDTA solution (Speciality Media Inc, Lavallette, NJ) before harvesting on a filtermat paper (Skatron Inc, Sterling, VA). Filters were then counted in a Beckman LS 5800 liquid scintillation counter (Beckman Instrument Inc, Fullerton, CA.
Dose-response effect of IL-3 and GM-CSF on DNA synthesis in freshly isolated primary HMØs. Purified MØs were cultured in serum-free macrophage medium alone as a control or in the presence of 5 ng/mL IL-3 and at concentrations of 1, 10, or 20 ng/mL GM-CSF, as indicated. Cells (2.6 × 104) were seeded in each well of a 96-well plate. On different days, as shown in the figure, cells were pulsed with 0.1 μmol/L or 2.5 μCi/mL [3H] thymidine and harvested after 6 hours, and counts were taken. The data presented here are the average of counts from 6 individual wells ± standard error.
Dose-response effect of IL-3 and GM-CSF on DNA synthesis in freshly isolated primary HMØs. Purified MØs were cultured in serum-free macrophage medium alone as a control or in the presence of 5 ng/mL IL-3 and at concentrations of 1, 10, or 20 ng/mL GM-CSF, as indicated. Cells (2.6 × 104) were seeded in each well of a 96-well plate. On different days, as shown in the figure, cells were pulsed with 0.1 μmol/L or 2.5 μCi/mL [3H] thymidine and harvested after 6 hours, and counts were taken. The data presented here are the average of counts from 6 individual wells ± standard error.
Nef, fen, and β-gal retrovirus vectors.
A Moloney murine leukemia virus-based retrovirus vector encodingnef or fen (antisense) with neo-R gene, LnefSN, and LfenSN from HIV-1 SF-2 isolate, nucleotide position 8504 to 9573,36 was used to transduce HMØs and U937 cells. The fragment contained the entire nef coding region as well as 289 bp of 5′ and 140 bp of 3′ noncoding sequences. Virus made by PA317 amphotropic retrovirus packaging cells48 was stored at −20°C and used to transduce cells. Retrovirus vector containing the β-gal gene50 (kindly provided by M.V. Eiden, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) was used to transduce HMØs.
Retrovirus-mediated transduction of nef, fen, andβ-gal into HMØs.
Purified HMØs were transduced with a retroviral vector containingnef or fen, nef, and β-gal together using two separate retrovirus vectors. Briefly, HMØs, 1 to 2 × 107 cells/flask (Costar) were cultured in macrophage media plus 5% FBS and 5 ng/mL IL-3 in the presence or absence of 20 ng/mL GM-CSF for 4 days (Fig 1). On day 4, polybrene (5 μg/mL; Sigma Chemical Co, St Louis, MO) was added to the cells to assist gene transfer51 in the presence of 1 mL (1 × 105 PFU) of tissue culture supernatant containing thenef retroviral vector. Cells were cultured for 48 hours; transduced cells were used for β-gal staining, apoptosis, Nef immunoprecipitation, fluorescence-activated cell sorting (FACS), and cytokine analysis.
Transduction of nef and fen into U937.
U937 cells (1 × 105/well) were plated into each well of a 6-well flat-bottom plate (Costar) and cultured as described above. The cells were then transduced with nef or fencontaining 1 mL of tissue culture supernatant of retrovirus in the presence of polybrene.7 Cells and virus were incubated for 48 hours. Media containing G418 (2 mg/mL; GIBCO-BRL) were added to the culture. Nef transduction-positive cells were resistant to G418 and were selected after 10 days in culture7 for immunoprecipitation of Nef, turnover of cytoplasmic FcγRs, and analysis of FcγRs and other cell surface antigens.
Selection of nef-transduction–positive cells.
U937 cells and HMØs were treated with G418 after retrovirus transduction to select the nef-positive cells. U937 cells were selected under the above-described conditions; however, G418 was toxic to HMØs. Therefore, nef-transduced HMØs in the absence of G418 were used. To determine the transduction efficiency, HMØs were also transduced with both nef and β-gal genes; they were then stained49 for β-gal, and the blue cells were quantified.
Adenovirus vector system.
A replication-deficient mutant of AD, dl312 (kindly provided by T. Shenk, Princeton University, Princeton, NJ), was used.52AD, dl312 was propagated in 293 cells49 and purified by CsCl2 double-density centrifugation.39 Titrated virus stock was stored in Tris-Cl buffer, pH 7.5, containing 20% glycerol and stored at −70°C. AD, dl312 was used with HIV-1nef plasmid for efficient protein expression in HMØs.
Conjugation of human apotransferrin with poly(L-lysine).
Human apotransferrin (Sigma) was conjugated with poly(L-lysine) (Sigma) using a previously published procedure.53 54 In brief, 100 mg of apotransferrin was mixed with 10 mg sodium periodate (Sigma) at 4°C for 1 hour. Excess periodate was removed by Sephadex G-25; pH of the protein solution was adjusted to 7.5 by sodium bicarbonate. Apotransferrin was incubated with 10 mg poly(L-lysine) and 10 mg cyanocarbohydride (Sigma). Conjugated apotransferrin was then separated from unconjugated apotransferrin with a MonoS column (Pharmacia Biotech, Piscataway, NJ) using a salt gradient (0.7 to 2.5 mol/L NaCl). Conjugated protein was dialyzed against 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer and stored at −70°C until used.
Coupling of apotransferrin-poly(L-lysine) with nef plasmid DNA.
Apotransferrin (500 μg) was incubated with 5 mmol/L ferric citrate (Sigma) solution for 5 minutes at room temperature, followed by the addition of 100 μg of nef plasmid DNA for 4 hours.
Adenovirus and lipofectamine-mediated nef transfection into HMØs.
To express HIV-1 Nef protein, a plasmid containing a 1.0-kb nefgene fragment of HIV-1 SF2 clone (kindly provided by J. A. Levy, University of California, San Francisco, CA) was used. HMØs were transfected either with HIV-1 nef or HIV-1 fen plasmid alone. Adenovirus- (Ad-) and lipofectamine-mediated HIV-1 nefor HIV-1 fen were also transfected into the HMØs using a recently described transfection protocol.37 In brief, 5 × 106 cells were cultured in 8 mL of macrophage media with 5% FBS, IL-3 (5 ng/mL), and GM-CSF (20 ng/mL) in 25-cm2 tissue culture flasks (Costar) and incubated for 4 days at 37°C, 5% CO2 until transfection.Nef or fen plasmid DNA conjugated to transferrin-poly(L-lysine) at 20 μg DNA/flask was mixed with lipofectamine (20 μg/flask) in a total volume of 200 μL. The mixture was added to the HMØs. After 10 minutes, 100 μL of AD, dl312 (100 PFU/cell) were added to the experimental flasks. Cells were incubated at 37°C in 5% CO2 for 4 hours. Cells were washed; 5 mL of fresh macrophage media containing IL-3, GM-CSF, and 5% FBS with antibiotics was added. Flasks were incubated for an additional 24 hours. Cells from these flasks were used for the analysis of β-gal, Nef protein, FcγRs, and other cell surface antigens.
Assessment of nef-transduced/transfected HMØ survival.
HMØs and U937 cells, transduced with retrovirus vector containingnef and fen, were assessed for toxicity by phase-contrast microscopy and trypan blue exclusion.55Similarly, Ad-mediated nef-transfected HMØs were also examined for signs of toxicity.
Determination of apoptosis in nef-transduced/transfected HMØs.
Ad-mediated nef-transfected and retrovirus-transduced HMØs were examined for apoptosis by an in situ nick/end-labeling technique using an in situ apoptosis detection kit; cells on the slides were stained by peroxidase (ApopTag plus; Oncor, Gaithersburg, MD; catalogue no. S7101-kit).
Assesment of β-gal–expressing HMØs.
Nef-transduced/transfected HMØs with β-gal andnef were scraped and collected from the flasks. The cells were centrifuged at 1,100 rpm for 10 minutes, and the pellet was resuspended in phosphate-buffered saline (PBS). After two washes in PBS, the cells were resuspended at a concentration of 106 cells/mL. The cells were placed (100 μL/well) in a poly(L-lysine)–coated 96-well plate and kept at room temperature for 30 minutes. Cells were fixed for 2 minutes with a cold mixture containing 0.8% formalin (Mallinckrodt Chemicals Inc, Paris, KY) and 0.5% glutaraldehyde (Sigma) in PBS. Cells were then washed twice with 0.15 mol/L Tris-HCl buffer, pH 7.8, and dried for 2 to 3 minutes. A freshly prepared staining solution containing 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide (Aldrich Chemical Co, Milwaukee, WI), 2 mmol/L MgCl2, and 1 mg/mL X-gal (GIBCO-BRL) was filtered and added to the well and incubated at 37°C without CO2 for 2 hours; blue cells were quantified.
Analysis of steady-state Nef protein levels in retrovirus-/Ad-mediated nef-transduced/transfected HMØs.
To analyze Nef protein in the transduced or transfected cells, immunoprecipitation methods were performed as described.35In brief, the cells were starved for 1 hour in cysteine- and methionine-free RPMI 1640 and then labeled with 200 μCi/mL and 1,000 Ci/mmol of 35 S-cysteine and 35 S-methionine (ICN Pharmaceuticals), respectively, for 4 hours. Cells were lysed in buffer containing 50 mmol/L Tris-HCl, pH 7.5, 0.1% sodium dodecyl sulfate (SDS), 300 mmol/L NaCl, 0.5% deoxycholate, 1.0% Triton X-100, 1 mg/mL leupeptin, 1 mg/mL aprotinin, and 50 mg/mL phenylmethylsulfonyl fluoride (PMSF; Sigma). Cellular extracts were immunoprecipitated overnight with polyclonal rabbit anti-Nef antisera (NIH AIDS and Research Reagent Program, Rockville, MD) and fractionated on a reducing 10% SDS-polyacrylamide gel. The gels were dried and exposed to x-ray film.
Flow cytometric analysis of FcγRs, HLA, CD4, and IL-2R molecules in Ad-mediated nef-transfected HMØs and U937 cells.
Flow cytometric analysis was performed on a Becton Dickinson FACScan using Consort 30, and Lysis II software as previously described (Becton Dickinson, Mountain View, CA).10 Ten million Ad-mediated fen- or nef-transfected HMØ/U937 cells were washed twice with modified Hank's buffer (GIBCO-BRL) containing 0.1% bovine serum albumin, 0.1% sodium azide, and 25 mmol/L HEPES, pH 7.2, without phenol red, and placed on ice. Washed cells were then filtered through a nylon monofilament cloth (53 μ opening; Small Parts Inc, Miami, FL); filtered cells were counted. Cells (5 × 105) were taken in 0.1 mL of Hank's buffer for each cell surface antigen analysis. Ten microliters of a 10 mg/mL solution of nonfluorescent human IgG (Sigma Immunochemicals, St Louis, MO) was added to all the tubes except those containing FcγRs to minimize nonspecific binding. Human γ-globulin (Pierce, Rockford, IL; 20 μL of stock concentration [12 mg/mL]) was added to the FcγRs tubes to block Fc region-specific binding of monoclonal antibody according to the manufacturer's protocol (Medarex Inc, West Lebanon, NH). The following antihuman antibody-fluorescein conjugates were used: antihuman CD4, antihuman CD14, and antihuman HLA-ABC (Olympus Corporation Immunochemicals, Lake Success, NY); antihuman HLA-DR (Beckton Dickinson Immunocytometry System, San Jose, CA); antihuman IL-2R (T Cell Diagnostics, Cambridge, MA); and antihuman FcγRI, II, and III (Medarex, Inc). After the addition of IgG and γ-globulin, antibody-fluorescein conjugates were added according to the manufacturer's protocol and kept on ice for 30 minutes. The cells were then washed with 2 mL of Hank's buffer, centrifuged, and resuspended in 1 mL of the same buffer; 5 mg/mL of propidium iodide was added and the cells were analyzed for FcγRI, II, and III; HLA-ABC; HLA-DR; CD4; CD14; and IL-2R molecules. A total of 10,000 cells was analyzed; living cells were gated, and the relative fluorescence intensity was measured and plotted for each of the above-mentioned cell surface antigens. The effect of Nef expression on cell surface antigens in U937 cells and HMØs was compared.
Turnover of FcγRI and II in Nef-expressing U937 cells.
Turnover of cytoplasmic FcγRs was analyzed as previously described for CD4.36 In brief, 1 × 106Nef-expressing exponentially growing U937 cells were starved for 30 minutes in cysteine- and methioneine-free RPMI 1640 and then pulse-labeled for 30 minutes with Trans35S-label (ICN Pharmaceuticals) at 0.2 mCi/mL. Pulse-labeled cells were washed, aliquoted, and chased for 0, 4, 12, 20, and 36 hours, incubating them in complete growth medium. At designated times, aliqouts were washed in cold PBS, pH 7.4. Cytoplasmic proteins were extracted in RIPA buffer consisting of PBS containing 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1 mmol/L PMSF. Radiolabeled FcγRs were immunoprecipitated using protein A-Sepharose CL-4B (GIBCO-BRL). In brief, cell lysates were incubated with protein-A for 30 minutes and centrifuged, and the supernatants were incubated with 1 μg of antihuman FcγRI or FcγRII antibodies (Medarex, Inc) at 4°C for 2 hours. The immune complexes were washed in RIPA buffer and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. The radioactivity incorporated into protein is quantified by exposing the dried gel to a storage phosphor screen and scanning the screen with a phosphor imaging instrument (Molecular Dynamics, Sunnyvale, CA).
Analysis of TNF-α, IL-1β, IL-6, and IL-10 by enzyme-linked immunosorbent assay (ELISA) in Ad-mediated nef-transfected HMØs.
HMØs were cultured for 4 days and nef or fentransfection was performed in an Ad expression system as described above. Two days after transfection, cells were washed and media were changed to remove growth factors and transferrin. Fresh media containing 5% FBS were replaced and treated with LPS (100 ng/mL; Sigma) or PMA (1 or 20 ng/mL; Calbiochem, San Diego, CA) for 72 hours. The tissue culture fluid was centrifuged at 1,100 rpm for 10 minutes, and the supernatant was assayed for TNF-α, IL-1β, IL-6, and IL-10 by ELISA. An enzyme immunoassay kit (Endogen Inc, Cambridge, MA) to quantitatively determine cytokines was used according to the manufacturer's protocol.
RESULTS
Purified HMØs treated with IL-3 and GM-CSF proliferated on days 1, 2, 4, and 6 (Fig 1). This proliferation was dose-dependent and was maximal on day 4 of culture in the presence of 5 ng/mL IL-3 and 20 ng/mL GM-CSF.
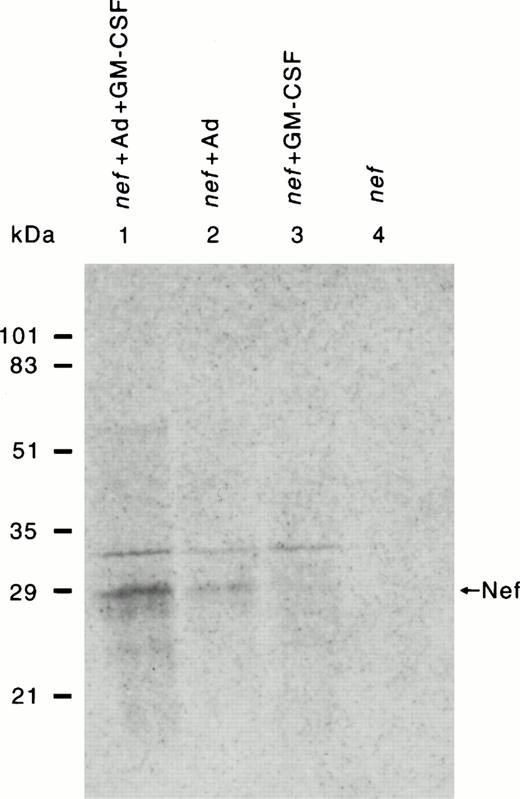
Retrovirus-mediated transduction produced very low levels of Nef expression. The adenovirus-enhanced delivery of the nef plasmid conjugated with transferrin resulted in expression of Nef protein (Fig 2), allowing us to investigate Nef activity in HMØs. Only 1% to 2% of the cells were positive for β-gal expression in retrovirus-mediated nef gene transfer in HMØs. However, Nef protein could not be immunoprecipitated from such low levels of transduction in HMØs. Retrovirus vector-mediated Nef-expressing U937 cells (Figs 3 and4) were selected with G418 treatment, and Nef protein was precipitable in the selected U937 cells (data not shown). Ad-mediated transfection of HMØs yielded better expression of the Nef and β-gal genes (Figs 2 and 5b) than retrovirus vector-mediated gene expression (data not shown). About 10% of Ad-mediated transfected HMØs were β-gal positive; these cells were identified as intense or light blue by histochemical analysis (Fig5b) Ad-mediated transfected cells were analyzed for Nef protein by immunoprecipitation (Fig 2). HMØs transfected with nef, in the presence of AD, dl312 and GM-CSF had the highest Nef expression (Fig 2, lane 1). The absence of GM-CSF markedly reduced Nef expression (Fig 2, lane 2). The absence of Ad-d1312 from the transfection resulted in minimum Nef expression in the presence or absence of GM-CSF (Fig 2, lane 3 and 4).
Detection of HIV-1 Nef expression in HMØs after Ad-d1312–mediated transfection of nef cDNA. HMØs were transfected either with nef cDNA alone or cDNA conjugated with transferrin-poly(L-lysine) in the presence of a replication-deficient AD, dl312. HMØs were cultured in macrophage media in the presence of IL-3 (5 ng/mL) and GM-CSF (20 ng/mL). After 4 days in culture, cells were transfected and cultured in macrophage media containing FBS, IL-3, with/without GM-CSF for 24 hours. The cells were then labeled with 200 μCi/mL of 35 S-labeled cysteine/methionine for 4 hours after starving them with cysteine/methionine-free media for 1 hour. Cells were then harvested, lysed, and immunoprecipitated with Nef antibody followed by SDS-PAGE and autoradiography. HMØs transfected with nef, AD, dl312, and lipofectamine in the presence of GM-CSF had the highest levels of Nef expression (lane 1). The absence of GM-CSF markedly reduced Nef expression (lane 2). The absence of Ad from the transfection resulted in minimal Nef expression in the presence of GM-CSF (lane 3) or in the absence of GM-CSF (lane 4).
Detection of HIV-1 Nef expression in HMØs after Ad-d1312–mediated transfection of nef cDNA. HMØs were transfected either with nef cDNA alone or cDNA conjugated with transferrin-poly(L-lysine) in the presence of a replication-deficient AD, dl312. HMØs were cultured in macrophage media in the presence of IL-3 (5 ng/mL) and GM-CSF (20 ng/mL). After 4 days in culture, cells were transfected and cultured in macrophage media containing FBS, IL-3, with/without GM-CSF for 24 hours. The cells were then labeled with 200 μCi/mL of 35 S-labeled cysteine/methionine for 4 hours after starving them with cysteine/methionine-free media for 1 hour. Cells were then harvested, lysed, and immunoprecipitated with Nef antibody followed by SDS-PAGE and autoradiography. HMØs transfected with nef, AD, dl312, and lipofectamine in the presence of GM-CSF had the highest levels of Nef expression (lane 1). The absence of GM-CSF markedly reduced Nef expression (lane 2). The absence of Ad from the transfection resulted in minimal Nef expression in the presence of GM-CSF (lane 3) or in the absence of GM-CSF (lane 4).
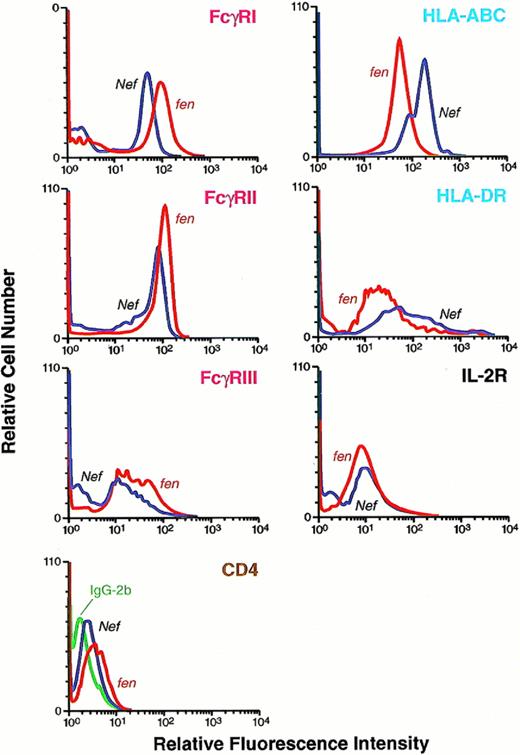
Cell surface expression of human FcγRI, II, and III; HLA class I and II; CD4; CD14; and IL-2R molecules in U937 cells expressing HIV-1 Nef or HIV-1 fen. U937 cells were transduced with retroviral vector containing nef or fenand selected after treatment with G418. Selected cells expressing Nef and Fen were cultured and analyzed for cell surface molecules as described in Fig 6. Downregulation of FcγRI and II and CD4 and upregulation of HLA class I molecules and no change in HLA class II, CD14, and IL-2R were observed in the Nef-expressing population of U937 cells.
Cell surface expression of human FcγRI, II, and III; HLA class I and II; CD4; CD14; and IL-2R molecules in U937 cells expressing HIV-1 Nef or HIV-1 fen. U937 cells were transduced with retroviral vector containing nef or fenand selected after treatment with G418. Selected cells expressing Nef and Fen were cultured and analyzed for cell surface molecules as described in Fig 6. Downregulation of FcγRI and II and CD4 and upregulation of HLA class I molecules and no change in HLA class II, CD14, and IL-2R were observed in the Nef-expressing population of U937 cells.
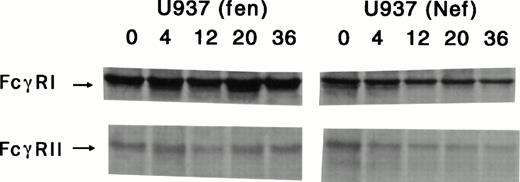
Determination of FcγRI and II turnover in retrovirus vector-mediated Nef and Fen-expressing U937 cells. Cells were starved for 1 hour and pulse-labeled with 35S-labeled cysteine/methionine for 30 minutes; cytoplasmic extracts were prepared at the indicated time points and analyzed by immunoprecipitation with anti-FcγRI and II antibodies. As shown, Nef-expressing U937 cells had significantly lower half-lives of FcγRI and II than those of thefen-transduced U937 cells.
Determination of FcγRI and II turnover in retrovirus vector-mediated Nef and Fen-expressing U937 cells. Cells were starved for 1 hour and pulse-labeled with 35S-labeled cysteine/methionine for 30 minutes; cytoplasmic extracts were prepared at the indicated time points and analyzed by immunoprecipitation with anti-FcγRI and II antibodies. As shown, Nef-expressing U937 cells had significantly lower half-lives of FcγRI and II than those of thefen-transduced U937 cells.
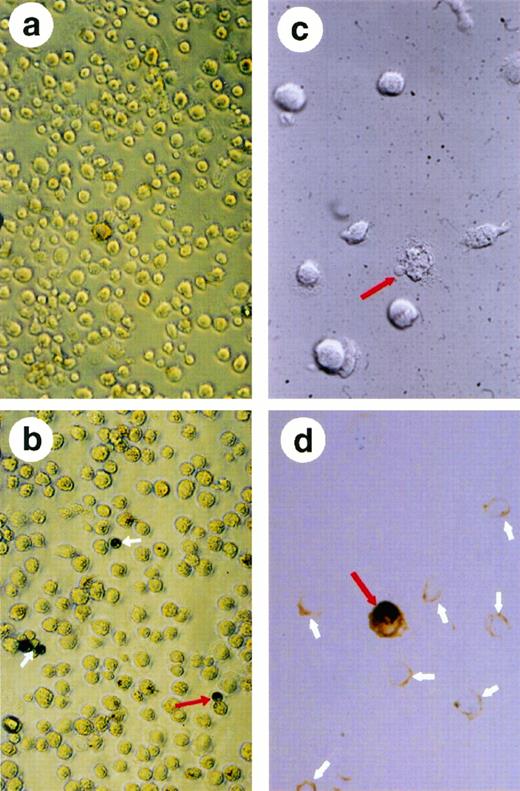
Analysis of the β-gal-positive cells after transfection of HMØs with β-gal and nef cDNA conjugated with transferrin-poly (L-lysine). A replication-deficient AD, dl312 was added to mediate HIV-1 nef and β-gal transfection. The transfected MØs were washed and resuspended in PBS; the resuspended cells were then placed in a poly(L-lysine)-coated 96-well plate, fixed, and stained for β-gal. The experiment was repeated four times and a representative field is shown. (a) shows the nontransfected HMØs that are negative in β-gal staining. (b) shows HMØs transfected with AD, dl312 and the β-gal plasmid. Fields were chosen to show distinct β-gal staining. Positive cells are blue and are shown with white arrows. A dividing MØ with positive β-gal staining is indicated with a red arrow. Microscopic examination of apoptotic cells identified by condensation of cytoplasm and chromatin in HMØs transfected with HIV-1 nef (c and d). MØs were cultured on slide chambers after transfection; cells were fixed in 10% neutral-buffered formalin and stained with the ApopTag peroxidase kit. A red arrow shows unstained apoptotic cells with blebbing (c). A single stained apoptotic cell (red arrow) and many unstained nonapoptotic cells (white arrows) are shown in (d).
Analysis of the β-gal-positive cells after transfection of HMØs with β-gal and nef cDNA conjugated with transferrin-poly (L-lysine). A replication-deficient AD, dl312 was added to mediate HIV-1 nef and β-gal transfection. The transfected MØs were washed and resuspended in PBS; the resuspended cells were then placed in a poly(L-lysine)-coated 96-well plate, fixed, and stained for β-gal. The experiment was repeated four times and a representative field is shown. (a) shows the nontransfected HMØs that are negative in β-gal staining. (b) shows HMØs transfected with AD, dl312 and the β-gal plasmid. Fields were chosen to show distinct β-gal staining. Positive cells are blue and are shown with white arrows. A dividing MØ with positive β-gal staining is indicated with a red arrow. Microscopic examination of apoptotic cells identified by condensation of cytoplasm and chromatin in HMØs transfected with HIV-1 nef (c and d). MØs were cultured on slide chambers after transfection; cells were fixed in 10% neutral-buffered formalin and stained with the ApopTag peroxidase kit. A red arrow shows unstained apoptotic cells with blebbing (c). A single stained apoptotic cell (red arrow) and many unstained nonapoptotic cells (white arrows) are shown in (d).
Initial examination of the HMØs by trypan blue exclusion showed 6% to 10% more cell death in nef-transfected/-transduced cells than in control cells. This observation led us to investigate the possibility that Nef expression results in apoptotic cell death. Our studies using in situ nick-end labeling show that the Nef-expressing population of HMØs contained more apoptotic cells (Fig 5d) than did their normal counterparts. We observed Nef-mediated apoptosis in about 2% to 5% of HMØs.
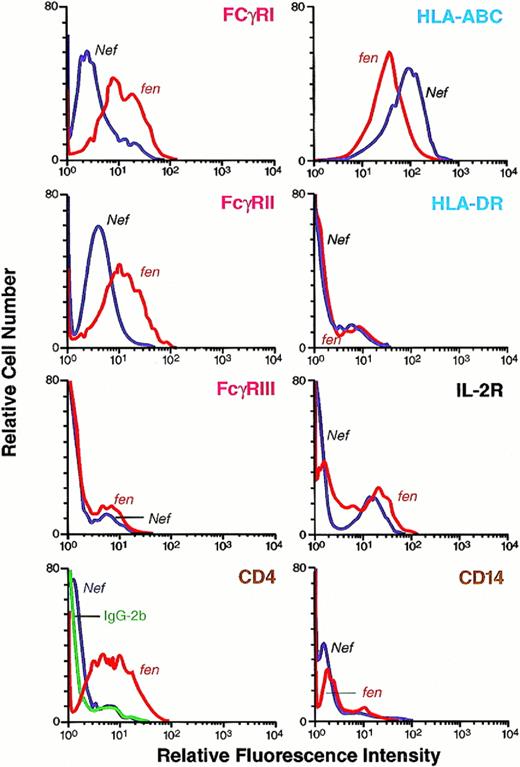
To investigate whether expression of cell surface molecules were modulated by Nef, Ad-d1312–mediated nef- andfen-transfected HMØs were analyzed by flow cytometry. We found that FcγRI and FcγRII were downmodulated in the Nef-expressing primary HMØs (Fig 6). Downmodulation of FcγRIII was minimal in HMØs (Fig 6). Retrovirus-mediated transduction downregulated FcγRI and II in U937 cells. We could not detect FcγRIII in the U937 cells (Fig 3); CD4 was also downregulated in U937 cells and minimally downregulated in HMØs. HLA-DR expression on the cell surface was unchanged in both HMØs and U937 cells (Figs 3and 6). Among the other cell surface markers examined, HLA class I molecules were upregulated in both Nef-expressing HMØs and U937 cells (Figs 3 and 6). Nef-mediated downmodulation of cell surface FcγRs and CD4 on HMØs is specific, because other molecules, such as IL-2R, HLA II, and CD14, had no effect on the nef-transfected HMØs and Nef-expressing U937 cells (Figs 3 and 6).
Cell surface expression of human FcγRI, II, and III; HLA class I and II; CD4; and IL-2R molecules in an HMØ population transfected with HIV-1 Nef or HIV-1 fen plasmid. After AD, dl312-mediated transfection of nef cDNA, HMØs were cultured and analyzed for expression of cell surface molecules. Cells in separate tubes were suspended in modified Hank's buffer. FITC-labeled specific monoclonal antibody was added; after incubation, cells were washed and analyzed by fluorescence histograms. FITC-labeled similar isotype antibody was added as control in each case. Downregulation of FcγRI, II, and III and CD4 and upregulation of HLA class I molecules and no change in IL-2R and HLA class II were observed in a Nef-expressing population of HMØs.
Cell surface expression of human FcγRI, II, and III; HLA class I and II; CD4; and IL-2R molecules in an HMØ population transfected with HIV-1 Nef or HIV-1 fen plasmid. After AD, dl312-mediated transfection of nef cDNA, HMØs were cultured and analyzed for expression of cell surface molecules. Cells in separate tubes were suspended in modified Hank's buffer. FITC-labeled specific monoclonal antibody was added; after incubation, cells were washed and analyzed by fluorescence histograms. FITC-labeled similar isotype antibody was added as control in each case. Downregulation of FcγRI, II, and III and CD4 and upregulation of HLA class I molecules and no change in IL-2R and HLA class II were observed in a Nef-expressing population of HMØs.
The action of nef in U937 cells and steady-state levels of total FcγRI and FcγRII were compared with retrovirus-mediated control fen- and Nef-expressing cells. FcγRI expression in both nef- and fen-tranduced cells was higher than FcγRII levels. Phosphor-imaging analysis of these gels confirmed that both fen- and nef-transduced cells synthesized equivalent levels of the FcγRI proteins (Fig 4, time 0); the FcγRI protein in Nef-expressing cells had a significantly abbreviated lifespan, going from the normal t1/2 of approximately 150 hours (Fig 4, U937fen) to approximately 36 hours in the U937 (Nef) cell. Similarly, FcγRII was also synthesized by cells expressing both Nef and Fen. As shown in Fig 4, FcγRII had a t1/2 of 185 hours in cells transduced with the control vector (fen). In contrast, FcγRII in Nef-expressing cells was rapidly degraded; the t1/2 was approximately 24 hours.
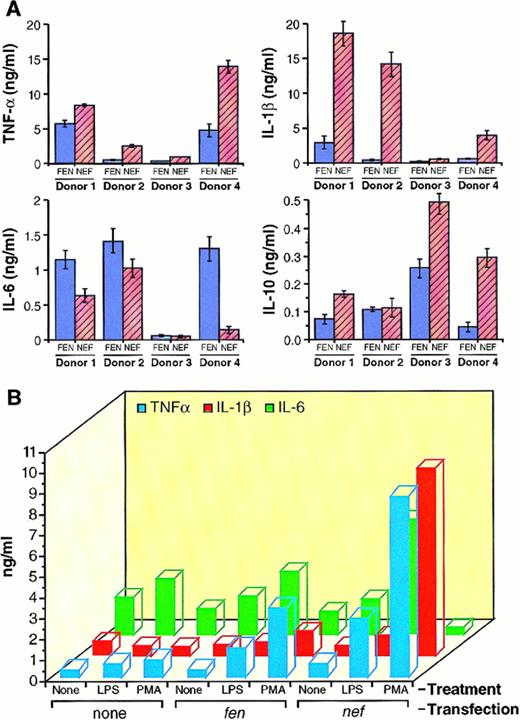
Because different HIV proteins have been shown to alter cytokine levels, we studied the effect of Nef expression on the proinflammatory cytokines. As shown in Fig 7A and B, both PMA and LPS induced basal levels of TNF-α, IL-1β, IL-6, and IL-10 in HMØs. PMA-treated Nef-expressing HMØs induced more TNF-α, IL-1β, and IL-10 (Fig 7Aa, b, and d), whereas HMØs express less IL-6 compared with control cells (Fig 7Ac). Four different donors' HMØs were studied and the amount of monokine levels varied from donor-to-donor (Fig 7A). After induction with PMA or LPS, Nef-expressing cells produced twofold to fourfold and twofold to 20-fold more TNF-α and IL-1β, respectively, than control cells (Fig7B). PMA and LPS induced twofold to eightfold more IL-10 than the control cells (data not shown). LPS induced twofold to fourfold more IL-6 secretion in the Nef-expressing HMØs when compared with control cells (Fig 7B).
(A) Analysis of TNF-α, IL-1β, IL-6, and IL-10 by ELISA from HIV-1 nef-transfected HMØs. Purified HMØs were cultured in macrophage media in the presence of IL-3 (5 ng/mL) and GM-CSF (20 ng/mL). After 4 days in culture, cells were transfected with HIV-1 nef cDNA conjugated with transferrin-poly(L-lysine) in the presence of AD, dl312 and incubated for 48 hours. Cells were washed to remove growth factors and transferrin. HMØs were cultured in macrophage media containing 5% FBS and treated with 20 ng/mL PMA for donors no. l, 2, and 4 and 1 ng/mL for donor no. 3 for 72 hours. Conditioned media were analyzed for TNF-α (a), IL-1β (b), IL-6 (c), and IL-10 (d) by ELISA. HMØs from 4 donors were analyzed and represented individually. (B) Comparative analysis of cytokines produced by Ad-d1312-mediated nef-transfected HMØ treated with LPS and PMA. Cells were transfected and maintained as described in (A). HMØs were treated with LPS (100 ng/mL) or PMA (20 ng/mL) for 72 hours; conditioned media were analyzed for the presence of cytokines by ELISA. Values presented are the average of four different experiments.
(A) Analysis of TNF-α, IL-1β, IL-6, and IL-10 by ELISA from HIV-1 nef-transfected HMØs. Purified HMØs were cultured in macrophage media in the presence of IL-3 (5 ng/mL) and GM-CSF (20 ng/mL). After 4 days in culture, cells were transfected with HIV-1 nef cDNA conjugated with transferrin-poly(L-lysine) in the presence of AD, dl312 and incubated for 48 hours. Cells were washed to remove growth factors and transferrin. HMØs were cultured in macrophage media containing 5% FBS and treated with 20 ng/mL PMA for donors no. l, 2, and 4 and 1 ng/mL for donor no. 3 for 72 hours. Conditioned media were analyzed for TNF-α (a), IL-1β (b), IL-6 (c), and IL-10 (d) by ELISA. HMØs from 4 donors were analyzed and represented individually. (B) Comparative analysis of cytokines produced by Ad-d1312-mediated nef-transfected HMØ treated with LPS and PMA. Cells were transfected and maintained as described in (A). HMØs were treated with LPS (100 ng/mL) or PMA (20 ng/mL) for 72 hours; conditioned media were analyzed for the presence of cytokines by ELISA. Values presented are the average of four different experiments.
DISCUSSION
Our results of proliferation assays of HMØs are comparable to those of previous findings.56 57 We selected optimally dividing and differentiated cells for transfection/transduction of nef and β-galactosidase (β-gal). As judged by β-gal staining, about 10% of the cell population was found positive using AD, d312-mediated transfection system. Only a subpopulation of HMØs proliferate when treated with IL-3 and GM-CSF. However, this method of transfection proved to be an effective system to study the function of nefgene in this heterogenous population of HMØs.
HIV proteins are toxic to cells,58-60 and Nef protein is toxic and impairs induced cell proliferation.61,62Therefore, both nef-transduced and nef-transfected HMØs were monitored for cell viability. HIV proteins have been shown to be toxic to different human cells and induce apoptosis in T cells and nonmyeloid cells.63-66 Our results indicate for the first time that Nef expression in an HMØ subpopulation in the presence of IL-3 and GM-CSF induce apoptosis. Apoptotic MØs have also been identified in PBMC populations in HIV patients (A.D. Bradley, Immunex Corp, Seattle, WA; D.R. Clark, University of Arizona, Tucson, AZ; personal communications, July, 1996). However, in other studies, there was no apoptosis in HIV-infected HMØs.67Because HMØs account for approximately 1% of the PBML population, the number of cells examined in the above-mentioned study67 may be insufficient to conclude whether HMØs are apoptotic. In our experiments, a small percentage of the diversified population of HMØs68 undergoes apoptosis, possibly due to their great susceptibility. It has been previously reported that Nef expression in HMØs induces the neurotoxic monokines, IL-6 and TNF-α.57,69 Furthermore, Nef is toxic to certain cells, impairs induced cell proliferation, shares some properties with scorpion peptides,61 70 and induces apoptosis in a subpopulation of HMØs. These facts suggest that Nef is directly toxic to certain cells and may indirectly contribute to neuropathology in AIDS patients.
FcγRI and FcγRII were downmodulated in the Nef-expressing HMØs and U937 cells (Figs 3 and 6). Downmodulation of FcγRIII was minimal in HMØs and FcγRIII was not detected in U937 cells. This was not surprising, because FcγRIII is not usually expressed in U937 cells.71 CD4 downmodulation was previously noted in Nef-expressing lymphoid and nonlymphoid cells of different species and is considered to be a posttranslational process.7,8,36,72Furthermore, Nef acts on CD4 molecules that are released from the endoplasmic reticulum and migrate to the cell surface, which triggers their endocytosis and degradation in lysosomes with a decreased t1/2.8,36 Downmodulation of FcγRs and CD4 may have several effects on the host immune function. It has been shown that downmodulation of CD4 in T cells results in resistance for new viral infection, thereby promoting virus persistence.8 In HMØs, downmodulation of CD4 by Nef might produce similar results. In macrophages, FcγRs are responsible for multiple functions such as (1) endocytosis of antigen-antibody complexes via clathrin-coated pits; (2) signalling exocytosis; (3) release of proteins and inflammatory monokines; (4) antigen-dependent cellular cytotoxicity, triggering lysis of tumor cells; and (5) generating and releasing superoxide radicals.71,73-78 FcγRs can bind to the Fc portion of the antibody molecule, facilitate cytoplasmic uptake of the virus-antibody complex, and mediate immunopathogenesis in many virus infections.79-81 Downregulation of FcγRI and II affects the above-mentioned functions of macrophages and may promote persistent viral infection.
HLA class I molecules were upregulated in both Nef-expressing HMØs and U937 cells (Figs 3 and 6). Macrophages use major histocompatibility complex (MHC) class I molecules to present endogenous peptides and exogenous antigens, whereas the class II pathway presents exogenous antigen only.82-84 This allows macrophages to monitor tissues for the presence of viral infections and tumor cells.85 In Nef-expressing macrophages, downregulation of FcγRI and II prevents efficient uptake, processing, and presentation of exogenous antigens. Thus, overexpression of HLA class I in Nef-expressing macrophages may partially compensate the antigen-processing and presentation functions that have been altered due to FcγRI and II downregulation. In U937 cells chronically infected with HIV-1, MHC class I molecules were downmodulated by the virus.86 Viral infection and monokines, including IL-10, interferon (IFN), and TNF-α upregulate MHC class I molecules.87-89 In our study, the combination of Nef, TNF-α, and IL-10 might upregulate HLA class I. Overexpression of HLA class I is also associated with autoimmune diseases.90Nef-expressing macrophages lack some of the important HMØ functions, but not antigen presentation, because they overexpress HLA class I. Late-stage HIV infection is associated with autoimmune diseases,90 which may be due in part to HLA class I overexpression by HIV-infected macrophages.
To examine the mechanism of FcγRs downmodulation, U937 cells were used, because cells that stably expressed Nef could be selected by culturing in the presence of cells with G418.7,91 Reduction of t1/2 has also been shown to downmodulate CD4.8 36 Here, for the first time, we have shown that Nef downmodulates surface FcγRI and FcγRII.
Monokines (TNF-α, IL-1β, IL-6, and IL-10) play a very important role in AIDS pathogenesis and can be induced by different stimuli including LPS,92-95 PMA,96 and UV light97 in myeloid cells. Therefore, we analyzed whether Nef-expressing HMØs in the presence of LPS or PMA can alter secretion of TNF-α, IL-1β, IL-6, and IL-10. Induction of IL-6 by LPS but not by PMA in Nef-expressing cells showed that LPS-mediated IL-6 induction differed from PMA. Cytokines/monokines regulate both immune function and viral replication, and contribute to immunopathogenesis during HIV infection.16 In HIV-infected individuals, secretion of the proinflammatory monokines TNF-α, IL-1β, and IL-6 is increased in HMØs, lymphoid tissues, plasma, and cerebrospinal fluid; these monokines can also enhance viral expression when added to acutely or chronically infected cell cultures.18 In contrast, IL-10 can either induce or suppress HIV expression, depending on the culture system used.16 IL-10 can inhibit HIV replication by blocking secretion of TNF-α and IL-6.30 TNF-α has been shown to be the most potent inducer of HIV replication.24,27 In Nef-expressing human T cells, signal transduction is altered.6,98,99 Nef can upregulate TNF-α, IL-4, IL-8, and IL-13 and downregulate IL-2 and IFN-γ after induction by a mitogenic combination of PMA and ionomycin.100 Furthermore, IL-10 mRNA increased during the acute phase of infection of Cynomolgus macaques with a pathogenic primary isolate of SIVmac consisting of a full-lengthnef.101 Among other regulatory proteins, HIV-1 Tat induces overexpression of TNF-α and IL-6.102,103Moreover, gp120 enhances expression of TNF-α, IL-1β, IL-10, IFN-β, complement proteins, nitric oxide, and endothelin-1 in many cell types, including macrophages.104 105 Our results suggest that HIV-1 Nef upregulates TNF-α, IL-1β, and IL-10 in LPS- or PMA- stimulated HMØs. Although the mechanism of induction of monokines by HIV-1 Nef is unknown, upregulation of these monokines in an activated state may regulate cell-mediated and humoral immune responses as well as control viral gene transcription and virus replication, thereby contributing to viral pathogenesis.
In summary, our present study shows that Nef downmodulates FcγRI and II and CD4; upregulates HLA class I molecules in HMØs; induces apoptosis in a subpopulation of HMØs; and modulates monokine expression in the presence of LPS and PMA. Moreover, the Ad vector system described here has potentially attractive therapeutic applications for gene delivery into HMØs.
ACKNOWLEDGMENT
The authors acknowledge the editorial guidance of Devera Schoenberg, MS. C.N.S.V. dedicates this paper to the memory of Dr Somnath Ghosh and Ashwatha Shetty. We are grateful to Charles Carter for providing the monocytes.
S.K.D and C.N.S.V. contributed equally to this paper.
Address reprint requests to Swapan K. De, PhD, Bldg 30, Room 232, Oral Infection and Immunity Branch, National Institute of Dental Research, NIH, Bethesda, MD 20892.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Dose-response effect of IL-3 and GM-CSF on DNA synthesis in freshly isolated primary HMØs. Purified MØs were cultured in serum-free macrophage medium alone as a control or in the presence of 5 ng/mL IL-3 and at concentrations of 1, 10, or 20 ng/mL GM-CSF, as indicated. Cells (2.6 × 104) were seeded in each well of a 96-well plate. On different days, as shown in the figure, cells were pulsed with 0.1 μmol/L or 2.5 μCi/mL [3H] thymidine and harvested after 6 hours, and counts were taken. The data presented here are the average of counts from 6 individual wells ± standard error.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/6/10.1182_blood.v91.6.2108/2/m_blod4061601.jpeg?Expires=1769151691&Signature=Xl3LgRv5sIhDRw~xVAP8bR3x-LKLT9ONxfbfl0EI1NKW6U51SkSZ9SrQDW9aptMe7WqwkgopgPrc-Szfqx9g1-7wR4I~~h2byVbDCzAmh9K-UJ3Dzfic0b90XG8v3WR6juloLcLsKMA3UEo3cqlgU5LnBr1-eHfeAuFCxA~jn0TemesaUAJiwcC9t~MiROuMP7lojPpbc1nkPrZChMsbYz5Wkf9gzv0jXuRECoTNWP-y7kLRkFLHSZXdLiVC09tFluyBBWks0VD0QdrNU3zhv2TF58qqlqmJqWIdyfkHgj3gKZJL9pxky9agAWJuJCj2TRwjLjMtaaMSO8X-ahHFAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal