Abstract
Transcription factors of the NFAT (nuclear factor of activated T cells) family regulate the expression of many genes encoding immunoregulatory cytokines and cell surface proteins during the immune response. The NFAT protein NFAT1 (NFATp) is expressed and functional in T cells, B cells, mast cells, and natural killer cells. Here we report a detailed analysis of the enhanced eosinophil responses of NFAT1-deficient mice, observed in an in vivo model of allergic inflammation. In addition to the pleural eosinophilia described previously, NFAT1−/− mice that have been sensitized with antigen display a significant increase, relative to wild-type mice, in the numbers of eosinophils in bone marrow and peripheral blood. After restimulation with antigen in vitro, antigen-responsive T cells from the draining lymph nodes of NFAT1−/− mice show increased expression of mRNA encoding the Th2 cytokines interleukin-4 (IL-4), IL-5, and IL-13. Consistent with this finding, there is a pronounced increase in the levels of IL-5 and IL-13 in the pleural cavities of sensitized NFAT1−/− mice after allergen challenge in vivo. Furthermore, development of eosinophilia depends on overexpression of IL-4 and IL-5, because it is strongly inhibited by administration of neutralizing antibodies to either of these cytokines. These results indicate that NFAT1-deficient mice are prone to develop a classically allergic phenotype characterized by eosinophilia and increased production of Th2 cytokines. Thus, the presence of NFAT1 might inhibit the allergic response, perhaps by interfering with the development of Th2 immune responses, and the lack or dysfunction of NFAT1 could potentially underlie certain cases of atopic disease.
ATOPY OR ALLERGIC disease is a complex familial disorder with multiple manifestations, including allergic asthma, rhinitis, conjunctivitis, and dermatitis.1-3 Both eosinophils and mast cells have been implicated in the pathogenesis of allergic disease. The hallmark of these diseases is a pronounced increase in the level of serum IgE, reflecting the actions of the cytokines interleukin-4 (IL-4) and IL-13 to promote B-cell isotype switching to IgE.4 IgE produced by allergen-reactive B cells binds to Fcε receptors present on the surface of mast cells and basophils; when challenged with allergen, these cells release vasoactive mediators that directly damage the surrounding tissue, as well as chemotactic factors and cytokines that promote leukocyte infiltration and exacerbate the inflammatory response.2,4Likewise, eosinophils accumulate at sites of allergic inflammation, produce leukotrienes and other inflammatory mediators, and contribute significantly to tissue damage at sites of allergic inflammation.5 The magnitude of asthmatic responses is related to the number of eosinophils present in the lung,6and suppression of eosinophil accumulation at the site of inflammation impairs the development of airway hyperreactivity.7 8
By promoting IgE production and eosinophil recruitment, respectively, the cytokines IL-4 and IL-5 play a critical role in the development of atopic disease. The genes encoding these cytokines are clustered on human chromosome 5 and mouse chromosome 11, and the gene cluster is closely linked to atopy in certain families.1 IL-4, IL-5, and IL-13 are coordinately produced by Th2 cells, which are recognized as key regulators of allergy and asthma.1-3,9 Th2 cells are the predominant CD4+ T cells in the inflamed tissues of patients with atopic dermatitis and in the bronchoalveolar lavage fluids of patients with allergic asthma.10,11 In a murine model of allergic airway inflammation, severe combined immunodeficiency disease (SCID) mice lacking T and B cells showed no antigen-induced airway hyperreactivity,12 and similarly depletion of CD4+ T cells prevented antigen-induced airway hyperreactivity and pulmonary eosinophilia.13 In contrast, neither IgE-deficient nor mast-cell–deficient mice showed significant diminution of eosinophilic inflammation and airway hyperresponsiveness,14-16 confirming the importance of Th2 cells in these processes. The differentiation of uncommitted T-cell precursors into Th2 cells is largely driven by IL-4.17,18In murine models of airway hyperreactivity, BALB/c mice that had been treated with anti–IL-4 during antigen sensitization showed a significant decrease of airway hyperreactivity in response to antigen challenge,12 whereas IL-4–deficient C57BL/6 mice showed almost no eosinophilia and much less peribronchial inflammation.14
Proteins belonging to the NFAT (nuclear factor of activated T cells) family of transcription factors control the transcription of many genes involved in allergy and eosinophil function.19 NFAT proteins are expressed in T cells, B cells, mast cells, and natural killer (NK) cells, where they are activated by stimulation of calcium-mobilizing antigen and Fc receptors and regulate the expression of appropriate inducible genes.20-24 Binding sites for NFAT proteins have been described in the promoter/enhancer regions of diverse inducible genes encoding cytokines and cell-surface receptors, including interferon γ (IFN-γ), IL-2, IL-3, IL-4, IL-5, IL-13, tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), CD40L, FasL, and CTLA-4.19 Given these observations, it was surprising that mice lacking the family member NFAT1 (NFATp) showed increased pleural accumulation of eosinophils and increased serum IgE levels in an in vivo model of allergic inflammation25 and displayed an unexpected pattern of IL-4 gene expression.26 Here we show that the pleural eosinophilia and inflammation observed in NFAT1-deficient mice depends on overexpression of both IL-4 and IL-5 and is characterized by the development of a Th2 cytokine profile. Thus, NFAT1 deficiency in mice leads to the development of a classically allergic phenotype, unlike that seen in nonallergic or intrinsic asthma where IL-5 is important but there is no involvement of IgE or IL-4.11 Given the known genetic predisposition of allergic disease in humans, it is possible that certain forms of human atopy could involve a deficiency in NFAT1.
MATERIALS AND METHODS
Animals.
NFAT1-deficient and control wild-type+/+ mice were generated on a mixed genetic 129/Sv × C57BL/6 background.25 Eight- to 12-week-old wild-type and NFAT1−/− mice, bred and maintained independently, were used in all experiments. C57BL/6 and 129/Sv mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Sensitization and allergic pleurisy.
Animal sensitization and allergic pleurisy were assessed as described with minor modifications.27 Mice were immunized in the hind footpad by subcutaneous injection of 0.1 mL of ovalbumin (OVA; 200 μg) emulsified in complete Freund's adjuvant. Allergic pleurisy was induced in ovalbumin-sensitized mice by intrathoracic injection of 0.1 mL OVA (12 μg) in phosphate-buffered saline (PBS) 14 days after sensitization. Control animals were injected intrathoracically with PBS alone. Animals were killed 24 hours later, and the thoracic cavity was rinsed with 0.3 mL PBS containing heparin (10 U/mL). The pleural washes were collected, their volume was measured, and the cells were evaluated as described below.
Designated groups of mice were injected intraperitoneally with 1 mg neutralizing anti–IL-4 monoclonal antibody (MoAb; 11B11; gift from Biological Response Modifiers Program, National Cancer Institute, Frederick, MD) weekly during the sensitization protocol as well as 30 minutes before the intrathoracic injection. Neutralizing anti–IL-5 MoAb (TRFK-5; PharMingen, San Diego, CA) was administered intraperitoneally 30 minutes before the intrathoracic injection at two different concentrations (1 mg/kg and 5 mg/kg). There was no difference in OVA-induced eosinophil accumulation in sensitized animals when nontreated animals were compared with animals treated with an isotype control antibody (rat IgG1; PharMingen). Values for wild-type mice (mean ± SEM eosinophils × 105/cavity) were 5.2 ± 1.4 in nontreated mice, 5.8 ± 1.6 in mice treated with 1 mg/kg control IgG1, and 7.9 ± 2.9 in mice treated with 5 mg/kg IgG1, whereas the corresponding values for NFAT1−/− mice were 17.8 ± 5.0 for nontreated mice, 18.1 ± 6.5 for mice treated with 1 mg/kg control IgG1, and 17.4 ± 5.8 for mice treated with 5 mg/kg control IgG1.
Collection of bone marrow and peripheral blood cells.
Bone marrow cells were isolated from the left femur. The femoral head and condyles were removed, and the displaceable cells were recovered by flushing the marrow cavity of the femur shaft with 3 mL PBS containing heparin (10 U/mL). Blood samples were collected from the mice tail vein. Total and differential nucleated bone marrow and peripheral blood cells were evaluated as described below.
Cell counts.
Total cell counts were obtained after dilution of the pleural fluid, bone marrow cell suspension, and peripheral blood in Turk solution (2% acetic acid). Differential analysis of leukocytes was done under oil immersion objective in cytocentrifuged smears stained with Diff-Quik (Baxter, FL). Eosinophils, macrophages/monocytes, lymphocytes, and neutrophils were enumerated based on morphology and staining characteristics.
Cytokine analysis.
Pleural fluid obtained as described above was centrifuged (1,000g, 10 minutes), and the cell-free supernatant was assessed for cytokine content. IL-4 (Genzyme, Cambridge, MA), IL-5 (Endogen, Woburn, MA), and IL-13 (R&D Systems, Minneapolis, MN) protein levels were determined by enzyme-linked immunosorbent assay (ELISA).
For cytokine mRNA analysis, wild-type and NFAT1−/− mice were sensitized with OVA as described above. Ten days later, draining lymph nodes were removed, and cells were incubated without or with 1 mg/mL OVA for 6 hours. Cytokine mRNA expression in total cellular RNA from each sample was analyzed with a multiple cytokine RNase protection assay (Ribo-Quant; PharMingen).
IgE measurement.
Blood serum for IgE analysis was obtained by cardiac puncture 15 days after sensitization with ovalbumin as described above. Serum IgE was measured by sandwich ELISA using anti-mouse IgE MoAb R35-72 as capture antibody and biotinylated anti-mouse IgE R32-92 as detection antibody (PharMingen).
Statistical analysis.
Statistical analysis of values from wild-type and NFAT1−/− and between control and treated groups was determined using an unpaired Student's t-test for single comparison or an analysis of variance followed by the Student-Newman-Keuls for multiple comparisons. P < .05 was considered to be statistically significant as indicated in the figure legends.
RESULTS
Allergic response to OVA sensitization and challenge in NFAT1-deficient mice.
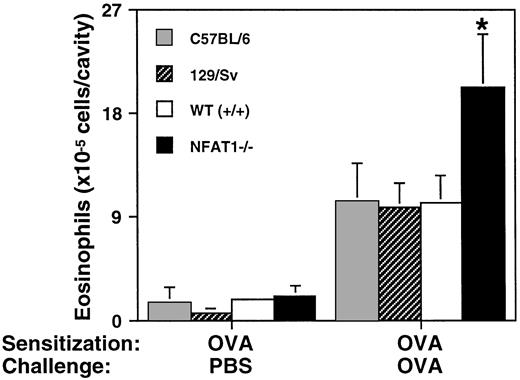
Eosinophil tissue infiltration at the site of inflammation is one of the major events in the allergic response. We previously showed that NFAT1-deficient mice display increased eosinophil accumulation in a model of allergic pleurisy.25 To investigate if this phenotype was related to the mixed genetic background of these mice, we compared them with mice of the 129/Sv and C57BL/6 parental strains. As shown in Fig 1, eosinophil accumulation in the pleural cavity in response to antigen challenge was indistinguishable in the parent strains and wild-type mice, whereas eosinophil accumulation in the pleural cavity of NFAT1−/− mice was significantly greater under the same conditions. These results suggested that the increased eosinophil infiltration in NFAT1−/− mice is not related to their mixed genetic background, but rather to their lack of expression of NFAT1 protein.
Allergic pleurisy in wild-type, NFAT1−/−, and parental strain mice. Shown are the total number of eosinophils in the pleural cavity of C57BL/6, 129/Sv, wild-type (+/+), and NFAT1−/− mice sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Data are expressed as mean ± SEM of values from 5 mice. (*) Indicates significantly greater response relative to wild-type mice (P < .05).
Allergic pleurisy in wild-type, NFAT1−/−, and parental strain mice. Shown are the total number of eosinophils in the pleural cavity of C57BL/6, 129/Sv, wild-type (+/+), and NFAT1−/− mice sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Data are expressed as mean ± SEM of values from 5 mice. (*) Indicates significantly greater response relative to wild-type mice (P < .05).
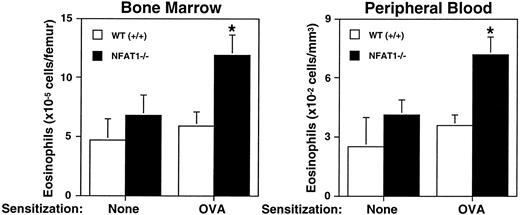
Several studies have indicated an association of atopic disease with bone-marrow response to allergen. Myeloid progenitor cells (granulocyte-macrophage colony-forming units [GM-CFU]) are increased in response to Ascaris suum antigen in canines,28 and in atopic human blood there are higher numbers of eosinophil-basophil colony-forming units (Eo/B-CFU).29,30 Indeed, it has been suggested that there is a mobilizable pool of bone marrow eosinophils that can be rapidly recruited to blood and migrate into tissues in response to chemoattractant.31 To evaluate eosinophil differentiation in response to allergen, wild-type and NFAT1−/− mice were sensitized with OVA, and eosinophil counts in bone marrow and peripheral blood were assessed. As shown in Fig2, NFAT1−/− mice that had been sensitized with ovalbumin displayed increased eosinophil numbers in bone marrow and peripheral blood when compared with wild-type mice. Moreover, the number of bone marrow and blood eosinophils in sensitized mice showed a positive correlation with eosinophil numbers in the pleural cavity in response to allergen challenge (data not shown). These data indicated that the enhanced eosinophil accumulation at the site of allergic inflammation was due not merely to a local reaction, but rather reflected a general upregulation of the allergic response.
Analysis of bone marrow response to OVA sensitization. Shown are the total number of eosinophils in bone marrow and peripheral blood of wild-type (+/+) and NFAT1−/− mice that were either not sensitized (none) or were sensitized with OVA. Data are expressed as mean ± SEM of values from 5 to 6 mice. (*) Indicates significantly greater response relative to wild-type mice (P < .05).
Analysis of bone marrow response to OVA sensitization. Shown are the total number of eosinophils in bone marrow and peripheral blood of wild-type (+/+) and NFAT1−/− mice that were either not sensitized (none) or were sensitized with OVA. Data are expressed as mean ± SEM of values from 5 to 6 mice. (*) Indicates significantly greater response relative to wild-type mice (P < .05).
OVA-sensitized cells in lymph nodes of NFAT1−/− mice display a Th2 cytokine profile.
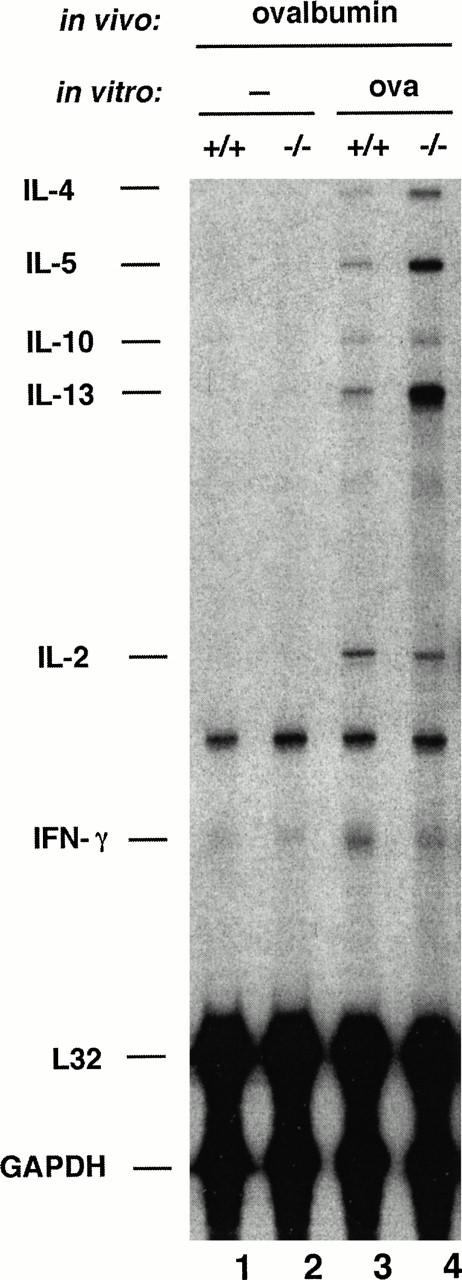
Eosinophil migration into inflamed tissue is coordinated by an interacting network of cytokines, chemokines, adhesion molecules, and inflammatory mediators, and chemoattractant production at the site of inflammation is essential for eosinophil recruitment.5 The cytokine IL-5, which is produced by T cells and mast cells as well as by eosinophils themselves, is recognized as a primary regulator of eosinophil growth, differentiation, chemotaxis, and activation.32-34 Furthermore, several studies have shown higher expression of different cytokines, such as IL-4, IL-5, and IL-13 at sites of allergic inflammation.35-38 To determine whether the eosinophilia seen in NFAT1−/− mice was related to differential cytokine expression, we used an RNase protection assay where multiple cytokines can be assessed in a sample of very few cells. Wild-type and NFAT1−/− mice were immunized in the footpads with OVA, using the same protocol as for antigen sensitization for later intrapleural challenge, cells from the draining lymph node were restimulated in vitro with OVA, and the expression of cytokines was assessed. As shown in Fig3, no cytokines were detected in unstimulated cells (lanes 1 and 2). However, although only a small fraction of lymph node cells were expected to be OVA-responsive, NFAT1−/− mice showed a striking increase in production of the Th2 cytokines IL-4, IL-5, and IL-13, relative to wild-type mice, after restimulation with OVA (Fig 3, compare lanes 3 and 4).
Analysis of cytokine expression by OVA-sensitized wild-type (+/+) and NFAT1−/− cells. Mice were sensitized in vivo with OVA, the draining lymph node cells were left unstimulated (−) or restimulated in vitro with 1 mg/mL of OVA for 6 hours and the total cellular RNA was analyzed by RNase protection assay for cytokine mRNA levels. The detected cytokines (IL-4, IL-5, IL-10, IL-13, IL-2, and IFN-γ) and the housekeeping genes (L32 and GAPDH) are indicated. These results are representative of experiments using three different pairs of wild-type and NFAT1-deficient mice.
Analysis of cytokine expression by OVA-sensitized wild-type (+/+) and NFAT1−/− cells. Mice were sensitized in vivo with OVA, the draining lymph node cells were left unstimulated (−) or restimulated in vitro with 1 mg/mL of OVA for 6 hours and the total cellular RNA was analyzed by RNase protection assay for cytokine mRNA levels. The detected cytokines (IL-4, IL-5, IL-10, IL-13, IL-2, and IFN-γ) and the housekeeping genes (L32 and GAPDH) are indicated. These results are representative of experiments using three different pairs of wild-type and NFAT1-deficient mice.
Analysis of cytokine production in the in vivo allergic response.
Because lymph node cells from NFAT1−/− mice overexpressed IL-4, IL-5, and IL-13 after OVA stimulation in vitro, we investigated the production of these cytokines in vivo in the model of allergic pleurisy. Pleural washes from OVA-sensitized wild-type and NFAT1−/− mice were assessed by ELISA for IL-4, IL-5, and IL-13 protein content. The levels of IL-5 and IL-13 in the wild-type mice challenged with OVA increased 6- and 15-fold, respectively, when compared with mice challenged with PBS. However, in our experimental conditions only the increase in IL-13 (P = .005), but not in IL-5 (P = .065), reached significant values (P < 0.05). In NFAT1-deficient animals, the levels of both cytokines IL-5 (P = .018) and IL-13 (P = .018) were significantly different when OVA-challenged and PBS-injected mice were compared (Fig4). Interestingly, NFAT1−/−mice presented increased levels of IL-5 and IL-13 in the pleural cavity after OVA challenge when compared with wild-type mice (Fig 4), whereas IL-4 was not detected in either mouse group (data not shown). The lack of detection of IL-4 in the pleural washes, although this cytokine is overexpressed in vitro (Fig 3), could be due to IL-4 consumption in the in vivo response or an insufficient sensitivity of the ELISA for IL-4. The finding of IL-5 overproduction in the pleural cavity of NFAT1-deficient animals is consistent with the enhanced eosinophil infiltration observed in these mice. IL-5 is a selective chemoattractant for eosinophils33 and has the ability to prime and activate these cells.32-34 In addition, IL-5–deficient mice do not show eosinophilia39 and fail to develop airway hyperresponsiveness and eosinophil infiltration in an experimental model of asthma.8
Analysis of in vivo cytokine production during allergic pleurisy. Measurement of IL-5 and IL-13 production in pleural cavity of wild-type (+/+) and NFAT1−/− mice sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Pleural washes were obtained 24 hours after OVA challenge and assessed for cytokine levels by ELISA. Data are expressed as mean ± SEM of values from 5 mice. (*) Indicates significantly greater values relative to wild-type mice (P < .05).
Analysis of in vivo cytokine production during allergic pleurisy. Measurement of IL-5 and IL-13 production in pleural cavity of wild-type (+/+) and NFAT1−/− mice sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Pleural washes were obtained 24 hours after OVA challenge and assessed for cytokine levels by ELISA. Data are expressed as mean ± SEM of values from 5 mice. (*) Indicates significantly greater values relative to wild-type mice (P < .05).
Role of IL-5 and IL-4 in the enhanced in vivo allergic response.
Both IL-4 and IL-5 genes are known targets for NFAT proteins.19 To investigate the involvement of these cytokines in mediating eosinophil recruitment in NFAT1-deficient mice, we used an in vivo treatment with neutralizing anti–IL-4 and anti–IL-5 MoAbs in the model of allergic pleurisy. Wild-type and NFAT1−/− mice were sensitized with OVA and treated with anti–IL-5 MoAb at two different concentrations before allergen challenge. As shown in Fig 5, a low concentration of anti–IL-5 (1 mg/kg) completely abrogated OVA-induced eosinophil recruitment in the wild-type mice but only slightly inhibited the eosinophil accumulation observed in NFAT1−/− mice under the same conditions. Interestingly, treatment with a fivefold higher dose of anti–IL-5 (5 mg/kg) completely inhibited eosinophil accumulation in the NFAT1−/− mice (Fig 5), consistent with the observed IL-5 overproduction in the pleural cavity of these mice (Fig 4). Thus, IL-5 is likely to be the major mediator contributing to the high numbers of eosinophils present in NFAT1-deficient mice in the in vivo allergic response (Fig 1). Although various chemokines and lipid mediators including eotaxin, RANTES, platelet-activating factor, and leukotriene B4 have been implicated in eosinophil recruitment, our results indicate that these other chemoattractants do not act independently of IL-5 in NFAT1−/− or wild-type mice. However, they could synergize promoting high levels of eosinophil recruitment. In fact, it has been shown that IL-5 and eotaxin synergize as chemoattractants for eosinophils in an in vivo model of eosinophil migration.31 40
Analysis of allergic eosinophil accumulation into the pleural cavity after treatment with anti–IL-5 and anti–IL-4 neutralizing MoAb. Total numbers of eosinophils in the pleural cavity of wild-type (+/+) and NFAT1−/− mice were assessed in animals sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Mice were left untreated (none) or treated with anti–IL-5 or anti–IL-4 neutralizing MoAb as described in Materials and Methods. Different concentrations of anti-IL–5 (1 mg/kg and 5 mg/kg) were used for treatment as indicated. Data are expressed as mean ± SEM of values from 5 to 12 mice. (*) Indicates significantly greater values relative to the PBS-challenged controls (P < .05), and (+) indicates significantly smaller values than the groups challenged with OVA but not treated with antibody (P < .05).
Analysis of allergic eosinophil accumulation into the pleural cavity after treatment with anti–IL-5 and anti–IL-4 neutralizing MoAb. Total numbers of eosinophils in the pleural cavity of wild-type (+/+) and NFAT1−/− mice were assessed in animals sensitized with OVA and challenged in the pleural cavity with PBS or OVA. Mice were left untreated (none) or treated with anti–IL-5 or anti–IL-4 neutralizing MoAb as described in Materials and Methods. Different concentrations of anti-IL–5 (1 mg/kg and 5 mg/kg) were used for treatment as indicated. Data are expressed as mean ± SEM of values from 5 to 12 mice. (*) Indicates significantly greater values relative to the PBS-challenged controls (P < .05), and (+) indicates significantly smaller values than the groups challenged with OVA but not treated with antibody (P < .05).
The cytokine IL-4 has been implicated as a major cytokine in Th2 differentiation leading to IL-5 production, and it also promotes IgE class switching in B lymphocytes.17 18 To investigate the importance of IL-4 in eosinophil tissue infiltration, wild-type and NFAT1−/− mice were treated with neutralizing anti–IL-4 MoAb during the sensitization period, and the allergic pleurisy was evaluated. As shown in Fig 5, anti–IL-4 treatment significantly inhibited eosinophil accumulation in both wild-type and NFAT1−/− mice, although the inhibition was not complete. Furthermore, anti–IL-4 treatment also inhibited the antigen-induced increase of serum IgE levels in the wild-type and NFAT1−/− mice (data not shown). It is important to note that anti–IL-5 treatment specifically inhibited eosinophil recruitment in the allergic pleurisy, whereas anti–IL-4 treatment also had an effect on migration of other leukocytes (data not shown). These results suggest that the overexpression of IL-4 plays a key role during the immunization process, which drives the immune response of NFAT1-deficient mice toward Th2 differentiation and an increased allergic response.
DISCUSSION
Our results indicate that the pleural eosinophilia observed in NFAT1−/− mice correlates with a classically Th2 pattern of cytokine expression, involving the cytokines IL-4, IL-5, and IL-13. The eosinophilia is abrogated or considerably inhibited by treatment of the mice with anti–IL-5 and anti–IL-4 neutralizing MoAbs, respectively (Fig 5). Moreover, antigen-responsive cells from the pleural cavity and from draining lymph nodes of NFAT1−/− mice show a striking upregulation of these three cytokines relative to wild-type mice (Figs 3 and 4). Furthermore, NFAT1−/− mice show increased numbers of eosinophils in bone marrow and peripheral blood after allergen sensitization (Fig 2). The differentiation, maturation, and proliferation of bone marrow eosinophils is promoted by GM-CSF, IL-3, and IL-5.41-45 The role of these cytokines in increased bone marrow eosinophil levels in NFAT1−/− mice remains to be determined. Although IL-3 and GM-CSF might play a role in eosinophilia following allergen challenge, our data on IL-5 mRNA (Fig 3) and protein (Fig 4) overexpression, and more importantly the ability of anti–IL-5 MoAb to completely inhibit the enhanced eosinophil accumulation in NFAT1−/− mice (Fig 5), indicate that IL-5 is a major effector molecule for eosinophil accumulation in NFAT1-deficient mice. However, we cannot rule out a parallel or synergistic effect for IL-3, GM-CSF, or other effector molecules.
Earlier controversy about the relative importance of IL-4 and IL-5 in mediating allergic responses8,12 was resolved by proposing at least two different cellular mechanisms for the allergic response, one dependent on IgE and IL-4 and mediated by mast cells, and another dependent on IL-5 and mediated by eosinophils.2 These different mechanisms were linked with the genetic background of the experimental animal strain, where BALB/c mice are high IgE responders and C57BL/6 are naturally hyporesponsive.2 Our results indicate that NFAT1−/− mice, genetically of mixed 129/Sv × C57BL/6 background, combine both the proposed mechanisms that they show a characteristic allergic phenotype with high eosinophilia that is associated with increased serum IgE levels25 and is dependent on overexpression of both Th2 cytokines IL-4 and IL-5. In effect, the loss of NFAT1 protein leads a hyporesponsive mouse strain to develop a high IgE- and IL-4–dependent allergic response.
Extending this line of thinking to asthma and atopy, two different types of human asthma have been described, one allergic and another unrelated form known as intrinsic or nonallergic.9 Whereas allergic asthma usually manifests at a young age and shows a strong familial occurrence and correlation with IgE levels, intrinsic asthma usually begins in adulthood, is characteristically not familial, and is not related to high levels of serum IgE.9,11 Nevertheless, patients with intrinsic asthma resemble patients with allergic asthma in that they show increased numbers of eosinophils in the peripheral blood and bronchoalveolar fluid and similar bronchial histopathologies.11 Allergic asthmatics display a characteristic Th2 cytokine profile, with increased levels of both IL-4 and IL-5 in peripheral blood and bronchoalveolar fluid, whereas the corresponding fluids from patients with intrinsic asthma show increases of IL-2 and IL-5 but no increase in IL-4.11 Our results suggest that NFAT1-deficient mice display a phenotype resembling that of allergic rather than that of nonallergic asthma in being characterized by a Th2 cytokine profile. The genetic basis for atopic disease and the propensity to develop a type 2 cytokine response is currently under intense investigation in several laboratories,1,46 47 and it is possible that certain forms of human atopy could also involve a selective deficiency in the transcription factor NFAT1.
The immune phenotype of NFAT1-deficient mice illustrates three important points. First, these mice are immunocompetent rather than immunodeficient and do not show any gross impairment in the production of NFAT-dependent cytokines, indicating that the lack of NFAT1 is compensated for by the presence of other NFAT proteins. Second, the increased secondary immune responses and increased cell proliferation observed in NFAT1-deficient mice suggests that NFAT1 may actually have an overall negative effect on immune responsiveness in normal mice. This behavior is not unprecedented. For example, in signal transduction pathways, kinases that are activated early during a response often activate feedback processes that contribute to the late downregulation of the same response. Finally, the unusual hypereosinophilia of NFAT1-deficient mice in a model of allergy, and their tendency toward the late production of Th2-type cytokines suggests that NFAT1 critically influences Th differentiation during the normal immune response. NFAT1 could act to promote the transcription of genes encoding cytokines or effector proteins that skew T-cell differentiation toward the Th1 pathway or cytokines that suppress differentiation toward the Th2 pathway. Alternatively, NFAT1 could inhibit the production of effector proteins having the opposite effect. These possibilities are not mutually exclusive. Given the importance of Th1-Th2 cytokine production in asthma, allergy, and other clinical situations, it is of considerable interest to understand the mechanisms by which NFAT1 exerts its profound effects on T-cell differentiation and function. Moreover, NFAT1-deficient mice represent a good model in which to study the effect of background genes and NFAT1 itself on the process of T-cell differentiation toward Th1 and Th2 phenotypes.
ACKNOWLEDGMENT
The authors thank Drs A. Lichtman and A. Abbas for helpful discussions.
Supported by National Institutes of Health Grant Nos. RO1 CA42471 and PO1 AI35297 to A.R.; A.K. was supported by a fellowship of the Deutsche Forschungsgemeinschaft (Germany); P.T.B. is a Research Fellow of the Pew Foundation (USA) and CNPq (Brazil); and A.R. is Scholar of the Leukemia Society of America (USA).
Address reprint requests to Anjana Rao, PhD, The Center for Blood Research, Harvard Medical School, 200 Longwood Ave, Boston, MA, 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal