Abstract
We have previously shown that intravenously injected peripheral blood (PB) or bone marrow (BM) cells from newly diagnosed chronic myeloid leukemia (CML) patients can engraft the BM of sublethally irradiated severe combined immunodeficient (SCID) mice. We now report engraftment results for chronic phase CML cells in nonobese diabetic (NOD)/SCID recipients which show the superiority of this latter model. Transplantation of NOD/SCID mice with 7 to 10 × 107 patient PB or BM cells resulted in the continuing presence of human cells in the BM of the mice for up to 7 months, and primitive human CD34+ cells, including those detectable as colony-forming cells (CFC), as long-term culture-initiating cells, or by their coexpression of Thy-1, were found in a higher proportion of the NOD/SCID recipients analyzed, and at higher levels than were seen previously in SCID recipients. The human CFC and total human cells present in the BM of the NOD/SCID mice transplanted with CML cells also contained higher proportions of leukemic cells than were obtained in the SCID model, and NOD/SCID mice could be repopulated with transplants of enriched CD34+ cells from patients with CML. These results suggest that the NOD/SCID mouse may allow greater engraftment and amplification of both normal and leukemic (Ph+) cells sufficient for the quantitation and characterization of the normal and leukemic stem cells present in patients with CML. In addition, this model should make practical the investigation of mechanisms underlying progression of the disease and the development of more effective in vivo therapies.
CHRONIC MYELOID LEUKEMIA (CML) is a clonal multilineage myeloproliferative disorder. It is now defined by the presence in the clonal cells of a unique genetic abnormality. In most cases, this is caused by a characteristic reciprocal exchange of genetic material, manifested cytogenetically as the Philadelphia chromosome (Ph),1 and involving the translocation of the c-abl proto-oncogene from chromosome 9 to a new position on chromosome 222 within the BCR-1 gene.3This BCR-ABL fusion gene encodes a 210-kD chimeric protein product which has increased tyrosine kinase activity compared with the normal c-abl protein,4 and its presence in the cytoplasm5 is also believed to be key to its transforming activity. CML follows a biphasic or triphasic clinical course: an initial chronic phase characterized by the presence of a dominant clone of BCR-ABL+/Ph+ cells which differentiate normally, followed inevitably a few years later by a blastic phase resembling acute leukemia.6 In some patients, the emergence of the blastic phase may be preceded by clinical signs of disease progression, referred to as an accelerated phase.
Much of our understanding of the biological changes underlying the pathophysiology of chronic phase CML has come from in vitro studies of the properties and behavior of lineage-restricted and multipotent colony-forming cells (CFC),7 and more recently, long-term culture-initiating cells (LTC-IC) present in the peripheral blood (PB) and bone marrow (BM) of patients with CML.8 Early studies of long-term marrow cultures of cells from patients with CML together with clinical experience demonstrating the ability of patients to achieve cytogenetic remissions after treatment with high-dose chemotherapy9,10 or interferon-α11 showed the common persistence of a suppressed but functionally intact reservoir of normal (Ph−) stem cells despite dominance of the Ph+ clone in the CFC and more mature compartments of PB and BM cells. Ph+ LTC-IC have also been shown, but conditions that would allow the sustained growth of leukemic cells from either the PB or BM of patients with CML have not yet been identified.12-14 It has thus been difficult to investigate mechanisms underlying the in vivo expansion of the most primitive neoplastic (Ph+) cells, or the role that p210BCR-ABL plays in initiating the disease.
Early attempts by other groups to establish an in vivo transplantation model of chronic phase CML by using severe combined immunodeficient (SCID) mice as recipients of human cells were not successful.15,16 Even blast crisis CML cells, when injected into SCID mice intraperitoneally, under the renal capsule, or into subcutaneously implanted human fetal bone fragments, were found to disseminate poorly to the BM of the mice.15-17 More recently, we reported that reproducible and sustained engraftment of SCID mice with intravenously transplanted chronic phase cells could be obtained if large enough numbers of cells from patients with high white blood cell (WBC) counts were injected and the mice were conditioned by a near-lethal dose of irradiation,18 based on our prior success with this approach using transplants of normal human adult BM or umbilical cord blood cells.19,20 However, we also found that the level of human hematopoiesis obtained in SCID mice was generally low, regardless of the source of the injected cells, and even with very large transplants of CML cells, only a minority of the cells later found to be present in the mice were leukemic. Subsequently we discovered that nonobese diabetic (NOD)/SCID mice, which have additional defects in natural killer (NK) cell activity as well as defective macrophage and complement function,21 allow superior engraftment of normal22-24 and leukemic25 human hematopoietic cells. It therefore seemed likely that these mice might also prove to be better recipients of CML cells. We now describe an improved in vivo model for CML, using the NOD/SCID mouse as a recipient. By comparison with the SCID model, engraftment of NOD/SCID mice with multiple types of normal and leukemic human cells was higher and could be achieved with lower numbers of CD34+ cell–enriched populations. These experiments provide a foundation for the future characterization of the phenotype and properties of normal and Ph+ cells that have long-term in vivo repopulating activity, as well as for the development of strategies to selectively manipulate normal and Ph+stem cell populations in vivo.
MATERIALS AND METHODS
Patient cells.
BM and PB samples were obtained from patients with informed consent according to procedures approved by the Human Experimentation Committee at the Princess Margaret Hospital, Toronto; the Toronto Hospital; and the Vancouver Health Sciences Hospital. All patients had Ph+ CML and were in chronic phase at the time the sample was taken (Table 1). Fresh PB or BM was diluted 1:2 with Iscove's modified Dulbecco's medium (IMDM; GIBCO-BRL, Burlington, Ontario, Canada) containing 10% fetal calf serum (FCS) and enriched for mononuclear cells by Ficoll density gradient centrifugation. CD34+ cells were then selected from some of these samples by using QBEnd10, an antibody that detects a class II CD34 epitope (generously provided by Dr Dinesh Jacob, Quantum Biosystems, Cambridge, UK), and anti-mouse Ig-coated magnetic beads (Dynabeads M-450, Dynal, Great Neck, NY) as described previously.26-28 CD34+ cells were detached from the beads with Pasteurella haemolytica glycoprotease (Cedarlane, Hornby, Ontario, Canada) under conditions that efficiently removed class I and class II CD34 epitopes (as detected by using QBEnd10-phycoerythrin) from 106 KGIa cells suspended in 50 μl of RPMI within 20 minutes at 37°C. The percentage of CD34+ cells in the glycoprotease-selected fraction was determined by flow cytometry using antibodies to glycoprotease-resistant epitopes of CD34 and CD45, and light scatter, as described.29 In some experiments, unseparated or CD34-enriched cells were suspended in 10% dimethyl sulfoxide, frozen at −70°C, stored in liquid nitrogen until required, and then thawed before transplantation into mice.
Clinical Data for Patients' Samples Used in This Study
| Patient No.-150 . | Age . | Sex . | PB WBC Count (×109/L) . | Prior Treatment . |
|---|---|---|---|---|
| 2 | 26 | M | 395 | None |
| 3 | 26 | M | 45 | Hydroxyurea × 2 mos |
| 4 | 63 | M | 58 | None |
| 5 | 50 | F | 160 | None |
| 6 | 48 | M | 135 | None |
| 7 | 54 | M | 132 | None |
| 10 | 51 | F | 135 | None |
| 12 | 67 | F | 189 | None |
| 13 | 72 | M | 218 | None |
| 14 | 63 | M | 123 | None |
| 15 | 37 | M | 82 | Hydroxyurea × 1 wk |
| 16 | 34 | F | 120 | None |
| 17 | 63 | M | 87 | One 4-wk course of busulphan 30 mos previously |
| Patient No.-150 . | Age . | Sex . | PB WBC Count (×109/L) . | Prior Treatment . |
|---|---|---|---|---|
| 2 | 26 | M | 395 | None |
| 3 | 26 | M | 45 | Hydroxyurea × 2 mos |
| 4 | 63 | M | 58 | None |
| 5 | 50 | F | 160 | None |
| 6 | 48 | M | 135 | None |
| 7 | 54 | M | 132 | None |
| 10 | 51 | F | 135 | None |
| 12 | 67 | F | 189 | None |
| 13 | 72 | M | 218 | None |
| 14 | 63 | M | 123 | None |
| 15 | 37 | M | 82 | Hydroxyurea × 1 wk |
| 16 | 34 | F | 120 | None |
| 17 | 63 | M | 87 | One 4-wk course of busulphan 30 mos previously |
Abbreviations: M, male; F, female.
All patients had Ph+ CML and were in chronic phase at the time the sample was taken.
Analysis of mice.
NOD/SCID mice were bred and maintained in the defined flora animal colony at the Ontario Cancer Institute, Toronto. Unless otherwise stated, 7.5 to 10 × 107 light density cells or various numbers of enriched CD34+ cells were injected into the tail vein of sublethally irradiated (400 cGy using a 137Cs γ-irradiator) 8-week-old NOD/SCID mice. Some mice also received various combinations of intraperitoneally injected human cytokines on alternate days as indicated. Cytokines used included mast cell growth factor (MGF, 10 μg), PIXY321 (7 μg), interleukin-3 (IL-3, 6 μg), granulocyte-macrophage colony-stimulating factor (GM-CSF, 6 μg), G-CSF (6 μg), and Flt-3 ligand (FL, 6 to 10 μg) (MGF, PIXY, and FL from Immunex; IL-3, GM-CSF, and G-CSF from Amgen; for the last two experiments, stem cell factor from Amgen was used instead of MGF). Mice were killed 1 to 7 months after transplantation, or as early as 2 weeks posttransplant if they appeared to be sick. The cells present in eight bones (ie, both femurs, tibiae, iliac crests, and humeri) were obtained in IMDM containing 10% FCS. The proportion of all human cells in the BM of transplanted mice was quantified by Southern blot analysis using a human-specific probe as previously described,19 or by flow cytometry on a FACScan analyzer (Becton Dickinson, San Jose, CA) after staining with human-specific monoclonal antibodies directed against CD45 and CD71.18,23 Cells obtained from the four leg bones of some mice were shipped on wet ice by overnight courier to Vancouver for additional phenotyping studies, isolation of CD34+ cells, plating in CFC and LTC-IC assays, and cytogenetic analyses as reported.18,23 In some instances CFC assays on unseparated cells were also performed by using selective conditions that do not allow coexisting mouse CFC to be detected.30
BCR Southern analyses.
DNA was extracted from cells obtained from the BM of transplanted mice, digested, and separated by gel electrophoresis. Southern blot analysis was then performed using a BglII/HindIII probe that contains the human BCR exon 1.31 The relative intensities of the germline and rearranged bands were determined by scanning the developed film on a Computing Densitometer (Model 300A; Molecular Dynamics, Sunnyvale, CA) followed by analysis using ImageQuant software (Version 3.3; Molecular Dynamics). The percentage of leukemic cells was calculated assuming that normal cells contribute two copies to the germline band and leukemic cells contribute one copy each to the germline and rearranged bands. The limit of detection for this assay is approximately 0.1 μg of DNA.
Statistical analysis.
Comparisons of the level of engraftment in NOD/SCID versus SCID mice were performed using the Mann-Whitney Rank Sum Test (SigmaStat version 1.0; Jandel Software; Labtronics, Inc, Guelph, Ontario, Canada). Results are expressed as mean ± SEM.
RESULTS
Engraftment of NOD/SCID mice by BM and PB cells from patients with chronic phase CML.
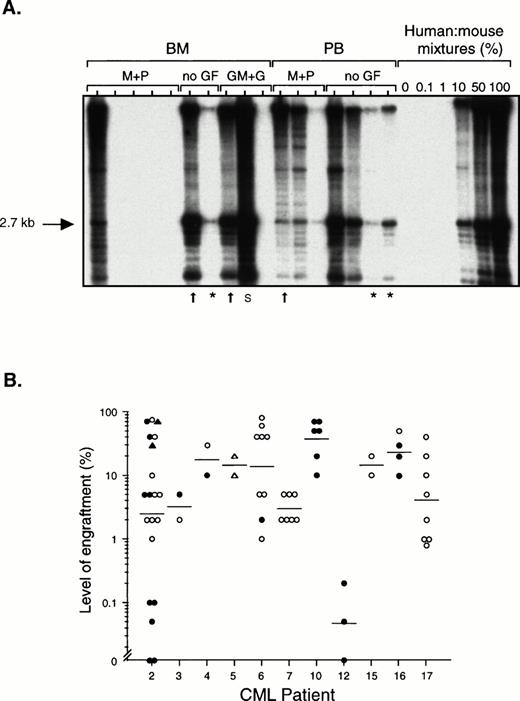
Fresh or previously frozen light density cells from the BM or PB of 11 patients with chronic phase CML (Table 1) were transplanted by tail vein injection into sublethally irradiated NOD/SCID mice. The extent of human cell engraftment in the BM of these mice was determined by Southern blotting using a human-specific DNA probe, or by flow cytometric detection of cells expressing the human-specific markers CD45 and CD71. DNA results from one experiment are shown in Fig1A. In several of the mice in this experiment, more than 50% of all the cells in the BM were human, although there was considerable variability in the level of engraftment, even among mice treated identically. The three lanes marked by an asterisk indicate mice that were killed more than 5 months after transplantation, showing the durability of the engraftment. Fig1B shows the proportion of human cells detected in the BM of all 65 mice transplanted with chronic phase cells that were analyzed. In 56 of these (86%), at least 1% of the cells in the BM were human, and in 31 (48%), at least 10% of the cells were human, including two mice transplanted with only 8 × 106 PB cells from patient 5. Very high levels of human cells (40% to 80%) could be detected in 16 of the 65 mice (25%) for up to 7 weeks posttransplant. All 9 of 9 mice that were analyzed between 3 and 6.5 months posttransplant were engrafted and 6 of these mice contained between 1% to 10% human cells, indicating that the graft persists for a long period of time. Human cell engraftment (>1% human cells) was observed in mice transplanted with cells from 10 of the 11 patients. The degree of engraftment tended to be higher after transplantation of the same number of PB as compared with BM cells. (The BM of all 39 mice transplanted with CML PB cells contained at least 1% human cells, whereas this was true for only 18 of 26 mice (69%) transplanted with CML BM cells.) Treatment of transplanted mice with various human cytokines on alternate days did not obviously enhance the level of human cell engraftment (data not shown).
Level of human cell engraftment in the BM of NOD/SCID mice transplanted with chronic phase CML cells. (A) Southern blot in which each lane contains DNA from a single mouse transplanted with 108 light density cells from the BM or PB from CML patient 2. Some of the mice subsequently received intraperitoneal injections of human MGF (M), PIXY321 (P), GM-CSF (GM), and G-CSF (G) on alternate days (no GF = no growth factors). Mice were killed between 3 and 29 weeks posttransplant, or earlier if they appeared sick (arrows). Mice killed after 5 months are indicated by an asterisk. DNA extracted from cells obtained from the BM or spleen (S) of the mice was digested withEcoRI and probed to detect a human-specific a-satellite sequence (p17H8).39 The level of human cell engraftment was determined by comparing the intensity of the 2.7-kb band with those of defined mixtures of human and mouse DNA as shown. (B) Level of engraftment in the BM of 65 mice transplanted with BM (• and ▴) or PB (○ and ▵) cells from the 11 patients with chronic phase CML studied, as determined by Southern blot analysis (• and ○) or flow cytometry (▴ and ▵) using antibodies to CD45 and CD71. The number of cells transplanted per mouse ranged from 7.5 × 107 to 108 except for two mice that received 2 × 107PB cells and two mice that received 8 × 106 PB cells. (—) Indicate geometric means.
Level of human cell engraftment in the BM of NOD/SCID mice transplanted with chronic phase CML cells. (A) Southern blot in which each lane contains DNA from a single mouse transplanted with 108 light density cells from the BM or PB from CML patient 2. Some of the mice subsequently received intraperitoneal injections of human MGF (M), PIXY321 (P), GM-CSF (GM), and G-CSF (G) on alternate days (no GF = no growth factors). Mice were killed between 3 and 29 weeks posttransplant, or earlier if they appeared sick (arrows). Mice killed after 5 months are indicated by an asterisk. DNA extracted from cells obtained from the BM or spleen (S) of the mice was digested withEcoRI and probed to detect a human-specific a-satellite sequence (p17H8).39 The level of human cell engraftment was determined by comparing the intensity of the 2.7-kb band with those of defined mixtures of human and mouse DNA as shown. (B) Level of engraftment in the BM of 65 mice transplanted with BM (• and ▴) or PB (○ and ▵) cells from the 11 patients with chronic phase CML studied, as determined by Southern blot analysis (• and ○) or flow cytometry (▴ and ▵) using antibodies to CD45 and CD71. The number of cells transplanted per mouse ranged from 7.5 × 107 to 108 except for two mice that received 2 × 107PB cells and two mice that received 8 × 106 PB cells. (—) Indicate geometric means.
Presence of primitive human hematopoietic cells in the BM of engrafted mice.
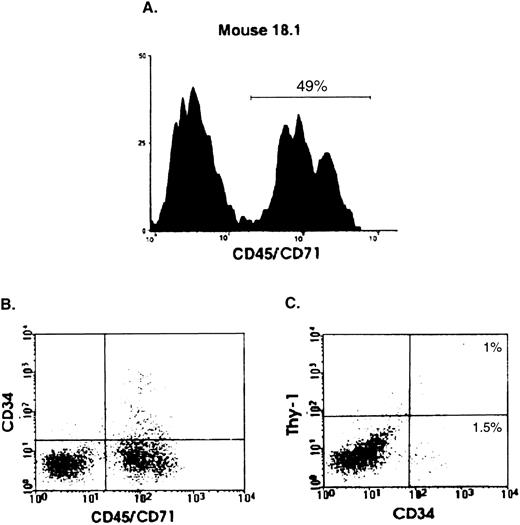
To determine whether primitive human hematopoietic cells could be detected in the transplanted mice, BM cells from 33 engrafted mice (1% to 80% human cells) were examined by flow cytometry for expression of human Thy-1 and/or CD34. All but one of the mice were analyzed between 2 and 7 weeks; one mouse was sacrificed after 6.5 months. The example shown in Fig 2 is from a mouse that was sacrificed 4 weeks posttransplant; 49% of the viable (PI−) cells in the BM of this mouse were human, as indicated by CD45/CD71 staining (panel A). Approximately 2.5% of the human cells were CD34+ and 1.0% (∼40% of the CD34+ human cells) were also positive for human Thy-1 (panels B and C). Of the 33 mice analyzed, all had detectable CD34+ cells in the BM (mean, 1.8 × 104; range, 7.1 × 102 to 5.4 × 105 per four leg bones), and 16 of 33 (48%) had detectable numbers of more primitive CD34+Thy-1+ cells (mean, 1.4 × 104; range, 8.7 × 102 to 4.2 × 104 per four leg bones).
Detection by flow cytometry of human CD34+Thy-1+ cells in the BM of transplanted mice. Analysis of a representative mouse (18.1) transplanted with 108 BM cells from patient 10 and killed 1 month later. (A) Histogram showing the proportion of viable (PI−) human (CD45/71)+ cells present in the BM of this mouse. (B) and (C) Percentage of CD34+ and CD34+Thy-1+ cells determined by staining with human-specific anti-CD34–FITC and anti-Thy-1–phycoerythrin antibodies. Gates defining positive cells were set to exclude greater than 99.9% of cells stained with monoclonal antibodies of the same isotype and labeled with the corresponding fluorochromes.18 23
Detection by flow cytometry of human CD34+Thy-1+ cells in the BM of transplanted mice. Analysis of a representative mouse (18.1) transplanted with 108 BM cells from patient 10 and killed 1 month later. (A) Histogram showing the proportion of viable (PI−) human (CD45/71)+ cells present in the BM of this mouse. (B) and (C) Percentage of CD34+ and CD34+Thy-1+ cells determined by staining with human-specific anti-CD34–FITC and anti-Thy-1–phycoerythrin antibodies. Gates defining positive cells were set to exclude greater than 99.9% of cells stained with monoclonal antibodies of the same isotype and labeled with the corresponding fluorochromes.18 23
To determine the numbers of human CFC present, unseparated BM cells or FACS-sorted human CD34+ cells were plated in methylcellulose under human-specific or standard conditions, respectively. CFC were detected in 37 of 45 (82%) mice analyzed. All mice that had detectable human cell engraftment (>0.1% human cells) also contained multiple types of lineage-restricted CFC, as well as CFC that generated multilineage colonies. Human CD34+ cells isolated from 31 mice were assayed for LTC-IC by using a 6-week CFC readout after maintenance of the cells on human growth factor (Steel factor, IL-3, and G-CSF)-producing murine fibroblasts.32Human LTC-IC were detected in 16 of these mice.
Both normal and leukemic cells engraft the BM of NOD/SCID mice transplanted with CML cells.
We previously found that the majority of human progenitors present in the BM of SCID mice that have been engrafted with PB or BM cells from patients with chronic phase CML were not leukemic.18 A similar analysis was therefore undertaken in the present study. From a total of 17 mice, 109 colonies were obtained and analyzed cytogenetically and 69 of these (71% ± 8%, Table2) were found to contain the Ph chromosome. However, there was significant inter-mouse variability in the proportion of human progenitors present that were leukemic. In the one mouse where metaphases could be obtained from human LTC-IC–derived colonies, 15 of 17 were normal but the other 2 were Ph+(Table 2).
Detection of Leukemic CFC in the BM of Mice Transplanted With Chronic Phase CML Cells
| Patient No. . | Cell Source . | Time of Analysis (d) . | No. of Mice . | Metaphase Analysis . | |
|---|---|---|---|---|---|
| No. of Ph+ Colonies/ No. of Colonies Analyzed (%) . | |||||
| 2 | BM | 14 | 1 | 1/1 (100) | |
| PB | 17 | 1 | 4/4 (100) | ||
| 4 | BM | 19 | 1 | 20/25 (80) | |
| PB | 10 | 1 | 3/6 (50) | ||
| 5 | PB* | 33 | 1 | 4/4 (100) | |
| 6 | BM | 16 | 1 | 10/16 (62.5) | |
| PB | 21 | 1 | 3/8 (37.5) | ||
| 7 | PB | 46-50 | 5 | 6/10 (60) | |
| 10 | BM | 29-51 | 5 | 18/35 (51.4) | |
| Mean ± SEM | 71 ± 8% | ||||
| Patient No. . | Cell Source . | Time of Analysis (d) . | No. of Mice . | Metaphase Analysis . | |
|---|---|---|---|---|---|
| No. of Ph+ Colonies/ No. of Colonies Analyzed (%) . | |||||
| 2 | BM | 14 | 1 | 1/1 (100) | |
| PB | 17 | 1 | 4/4 (100) | ||
| 4 | BM | 19 | 1 | 20/25 (80) | |
| PB | 10 | 1 | 3/6 (50) | ||
| 5 | PB* | 33 | 1 | 4/4 (100) | |
| 6 | BM | 16 | 1 | 10/16 (62.5) | |
| PB | 21 | 1 | 3/8 (37.5) | ||
| 7 | PB | 46-50 | 5 | 6/10 (60) | |
| 10 | BM | 29-51 | 5 | 18/35 (51.4) | |
| Mean ± SEM | 71 ± 8% | ||||
*Human LTC-IC from this mouse were also analyzed and found to be predominantly normal (15 of 17 CFC progeny generated cytogenetically normal colonies); however, 2 of 17 were Ph+.
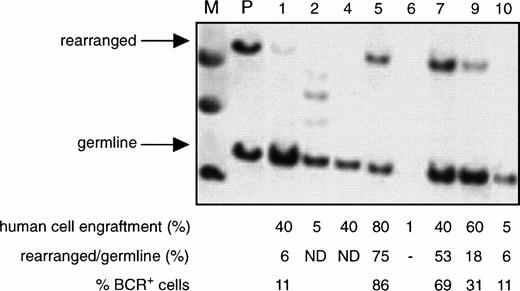
The proportion of leukemic (BCR+) cells among all the human cells present in the BM of the NOD/SCID mice transplanted with CML cells, as determined by Southern blotting using a human-specific 5′ BCR probe, was found to range from 8% to 100% (66% ± 7%, mean ± SEM, n = 24 mice). A representative blot is shown in Fig3. Interestingly, in the blot of the BM from one of the mice shown, extra bands were seen, suggesting the presence of additional chromosomal rearrangements. However, this could not be definitively established by digestion of the same sample with a different restriction enzyme because of the limited quantity of DNA available. The limit of sensitivity of this Southern blotting procedure seemed to be ∼5%, because the germline band was generally not detectable if the level of human cell engraftment was below this value. Overall, there was no apparent correlation between the level of Ph+ cells and the proportion of human cells in the BM of the mice, the source (PB v BM) of cells transplanted, or whether or not human growth factors were injected. Two mice transplanted with 108 PB or BM cells had high levels of engraftment (30% to 40%) of human cells in the spleen and Southern analysis of the DNA obtained from this site showed 4% and 10% BCR+ cells, respectively (data not shown).
Detection of normal and leukemic human cells in the BM of engrafted mice. Southern analysis of DNA extracted from the BM of NOD/SCID mice transplanted with 108 PB cells from patient 6 and assessed 3 to 6 weeks later. Each numbered lane contains the DNA from a separate mouse. The DNA was digested with Bgl II and then probed using a Bgl II/HindIII fragment containingBCR exon 131 to detect germline and rearrangedBCR genes. (M) Indicates the molecular weight marker. (P) Indicates DNA extracted from the PB of the patient. The level of human cell engraftment in the BM of each mouse was determined separately by Southern blot analysis using a human-specific probe as described in Fig1A. The percentage of BCR+ cells was determined as described in Materials and Methods. (ND), not detected.
Detection of normal and leukemic human cells in the BM of engrafted mice. Southern analysis of DNA extracted from the BM of NOD/SCID mice transplanted with 108 PB cells from patient 6 and assessed 3 to 6 weeks later. Each numbered lane contains the DNA from a separate mouse. The DNA was digested with Bgl II and then probed using a Bgl II/HindIII fragment containingBCR exon 131 to detect germline and rearrangedBCR genes. (M) Indicates the molecular weight marker. (P) Indicates DNA extracted from the PB of the patient. The level of human cell engraftment in the BM of each mouse was determined separately by Southern blot analysis using a human-specific probe as described in Fig1A. The percentage of BCR+ cells was determined as described in Materials and Methods. (ND), not detected.
Populations enriched in CD34+ cells from patients with chronic phase CML can engraft NOD/SCID mice.
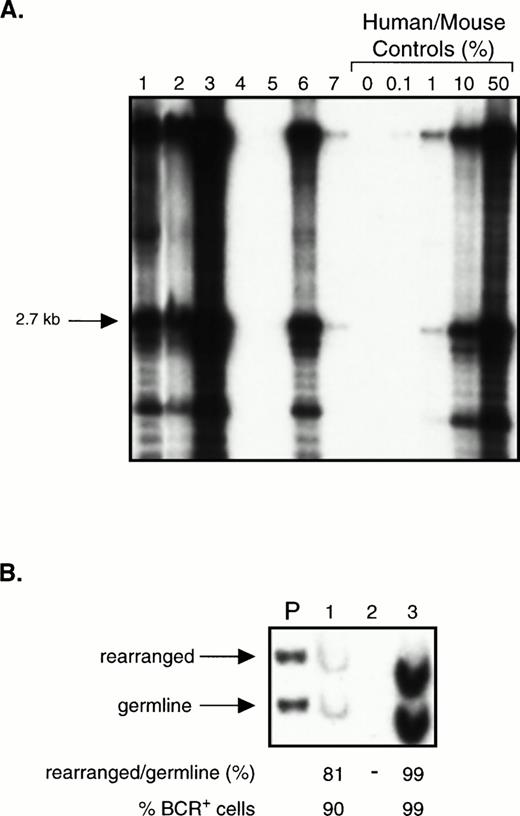
To assess whether CD34+ cells could engraft NOD/SCID mice, we transplanted mice with 105 to 2 × 106fresh or thawed populations of enriched CD34+ cells from three chronic phase patients (purity, 63% to 90%). Cells from 6 of these mice were analyzed between 2 and 3 weeks because of morbidity. The rest were analyzed after 5 weeks, including 2 mice that were killed after 19 weeks. Fig 4A shows a Southern blot analysis of 7 mice transplanted with enriched CD34+cells from one patient. In total, 13 of the 15 mice analyzed had detectable human cells in their BM by Southern blot, at levels ranging from less than 0.1% to 70%. The 2 mice with no detectable engraftment had received the lowest dose of enriched CD34+ cells (105). Two mice that received thawed cells had sufficiently high levels of engraftment with human cells to be assessed by BCR Southern analysis. In these, the percentage of leukemic cells was found to be 90% and 99% (Fig 4B).
Transplantation of CD34+ cell-enriched chronic phase CML cells into NOD/SCID mice. (A) Southern blot showing the level of human cell engraftment in the BM of mice transplanted with 2 × 105 (lanes 4 and 5) or 2 × 106 (lanes 1 through 3 and 6 and 7) CD34+ cells (purity 63%) isolated from the PB of a patient with chronic phase CML. Mice were analyzed after 5 to 7 weeks except for mouse 3 and mouse 6 which were analyzed after only 2 to 3 weeks because of morbidity. DNA analysis was performed as described in Fig 1A. (B) BCR Southern analysis of the BM of mice 1 through 3 from (A). For methods, refer to Fig 3.
Transplantation of CD34+ cell-enriched chronic phase CML cells into NOD/SCID mice. (A) Southern blot showing the level of human cell engraftment in the BM of mice transplanted with 2 × 105 (lanes 4 and 5) or 2 × 106 (lanes 1 through 3 and 6 and 7) CD34+ cells (purity 63%) isolated from the PB of a patient with chronic phase CML. Mice were analyzed after 5 to 7 weeks except for mouse 3 and mouse 6 which were analyzed after only 2 to 3 weeks because of morbidity. DNA analysis was performed as described in Fig 1A. (B) BCR Southern analysis of the BM of mice 1 through 3 from (A). For methods, refer to Fig 3.
Engraftment of CML cells is superior in NOD/SCID mice compared with SCID mice.
To compare the level of human cell engraftment obtained using NOD/SCID and SCID mice as recipients, the levels of human cell engraftment in the 65 NOD/SCID mice transplanted from 11 donors (data from Fig 1) were compared with those previously observed in 66 SCID mice transplanted from 10 donors using the same transplantation system.18 In each study, all patients were newly diagnosed and untreated (with the few exceptions noted). Engraftment in NOD/SCID mice was significantly higher than in SCID mice, both when data from all of the transplanted mice were pooled (5% ± 1% v 1% ± 1%,P < .0001) and when the mean levels of engraftment from individual patients were compared (12% ± 3% v2% ± 1%, P = .015; Fig 5).For a more direct comparison, cells from an additional patient with chronic phase CML were transplanted into mice of both strains. One NOD/SCID mouse transplanted with 10 × 107 unseparated BM cells was killed after 18 days because it appeared to be sick. Very high numbers of human myeloerythroid CFC were found in the murine BM, as well as a small number of multilineage CFC. Human CD34+cells and LTC-IC were also detected. A second NOD/SCID mouse transplanted with 1.5 × 106 enriched CD34+cells from the same donor and killed after 14 days had similarly high numbers of human CFC in the BM. Flow cytometric and LTC studies were not performed in this mouse. In contrast, two SCID mice transplanted with 10 × 107 unseparated cells remained healthy and had 30- to 60-fold fewer human CFC in the BM after 6 weeks. No human CD34+ cells were detectable. The vast majority (>90%) of human progenitors present in the BM of both NOD/SCID mice were Ph+ by cytogenetic analysis.
Comparison of engraftment of chronic phase CML cells in NOD/SCID versus SCID mice. Each (○) represents the mean level of human cell engraftment obtained after transplantation of PB or BM cells from individual patients with chronic phase CML into groups of NOD/SCID or SCID mice, as indicated on the horizontal axis. Engraftment in NOD/SCID mice was significantly higher than in SCID mice (P = .015). The raw data for the SCID mice can be found in the report of Sirard et al.18
Comparison of engraftment of chronic phase CML cells in NOD/SCID versus SCID mice. Each (○) represents the mean level of human cell engraftment obtained after transplantation of PB or BM cells from individual patients with chronic phase CML into groups of NOD/SCID or SCID mice, as indicated on the horizontal axis. Engraftment in NOD/SCID mice was significantly higher than in SCID mice (P = .015). The raw data for the SCID mice can be found in the report of Sirard et al.18
DISCUSSION
In this study we describe an experimental in vivo model of human CML which involves the intravenous injection of patient PB or BM cells into sublethally irradiated NOD/SCID mice. The BM of these mice was routinely found to contain at least 1% human cells for up to 6.5 months after transplantation of 108 light density cells from 10 of the 11 high WBC count chronic phase patients studied. Although the highest levels of human cells (up to 80%) were detected within 7 weeks posttransplant, the detection of human cells at the 1% to 10% levels for up to 6.5 months indicates the graft persists for long periods of time. Similar engraftment kinetics have recently been reported for normal human BM.23 CML PB cells engrafted as well as, or better than, BM cells, as was noted for similarly transplanted SCID mice.18 Although marked variability (>10-fold) in levels of engraftment were again seen, both between individual recipients of the same cells and between recipients of cells from different patients, the levels of engraftment seen here in NOD/SCID recipients were much higher than those noted previously using SCID hosts.18 For example, 25% of NOD/SCID recipients had 40% to 80% human cells, whereas only 3% of SCID recipients contained similarly high levels. Intermediate stages of human hematopoietic cell development detectable as in vitro CFC were found in a similar proportion of NOD/SCID and SCID recipients of CML cells (82% v77%), but more primitive cell types were found more frequently in the NOD/SCID mice (LTC-IC, 52% v 13%; CD34+ cells, 100% v 44%; CD34+Thy-1+ cells, 48%v 0%).
The NOD/SCID mice were repopulated with both normal and leukemic human cells after transplantation of either PB or BM from patients with chronic phase disease. This was shown by analysis of colonies derived from both unsorted and purified human CD34+ CFC obtained from the BM of engrafted mice (71% Ph+ CFC), as well as by Southern analysis of the total human cell population present (up to 99% bcr rearranged). The CML cells made up a much larger proportion of the human graft in NOD/SCID recipients compared with SCID mice, in which only 17% of CFC were Ph+ and the lower engraftment levels precluded Southern analysis. These findings both confirm that primitive normal and leukemic cells are present in the PB and BM of patients with chronic phase CML at diagnosis,33and that at least some of these have in vivo repopulating ability.18 The higher levels of engraftment that seem to be obtained in NOD/SCID mice suggest that this system may be further developed to provide an assay for these in vivo repopulating cells from patients with CML to allow their quantitation, as well as their further phenotypic and functional characterization. It is interesting to note that the predominance of genotypically normal human cells that was seen in similarly transplanted SCID mice18 was reversed in the NOD/SCID mice studied here. This suggests that the added immunodeficiency of the NOD/SCID strain, which includes deficient NK cell and macrophage function, may be more important for promoting the engraftment of the leukemic cells than of their normal counterparts, and there is some evidence that primitive Ph+ cells may be more sensitive to NK cells.34 35 However, these differences may also simply reflect interpatient variability in the relative and absolute prevalence of primitive normal and leukemic cells in their PB or BM. Thus, paired studies of cells from the same patients will be required to definitively resolve this question.
One of the hallmarks of CML is the inevitable progression of the disease to an acute leukemia. CML thus provides an important system in which to study the genetic changes that cause this to occur. As was seen in SCID mice,18 transplantation of blast crisis CML cells from one patient into NOD/SCID mice resulted in the rapid and extensive engraftment amplification of a leukemic population (data not shown). We previously reported that 100% of CFC derived from the BM of SCID mice transplanted with blast crisis cells were Ph+.18 Analysis of the total population of human cells present in the BM of the NOD/SCID recipients of blast crisis cells studied here also indicated that a high proportion were leukemic. It is also interesting that in one mouse transplanted with chronic phase cells (Fig 3, lane 2), extra bands were seen onBCR Southern analysis, suggesting either the outgrowth of a pre-existing subclone that was not detectable in the patient, or possibly that additional rearrangements had occurred in vivo posttransplant. Although such events would likely be rare, this model provides an in vivo system in which to analyze particular mutations that may contribute to leukemic progression, as the patterns of engraftment after transplantation of chronic phase or blast crisis cells seem to be distinct. For example, it might be possible to evaluate the role of various oncogenes in the progression of CML by transfecting them into chronic phase cells and then studying the engraftment potential of the transduced cells after transplantation into NOD/SCID mice.
The ability of NOD/SCID mice to be engrafted with leukemic cells after the transplantation of enriched populations of CD34+ cells from patients with chronic phase CML represents a first step towards determining the phenotype of CML stem cells. Previous studies of leukemic (Ph+/BCR+) LTC-IC have suggested that the CD34+HLA-DR− fraction of CML PB and BM is preferentially enriched for genotypically normal LTC-IC,36-38 whereas CD71, CD38, and Thy-1 are not useful markers for discriminating between Ph+ and Ph− LTC-IC.33 37 The ability to detect engrafting cells capable of initiating human CML in NOD/SCID mice after transplantation of purified cells now provides a means to test whether the phenotypic properties of leukemic LTC-IC extend to CML cells with engrafting potential. In addition, CML-engrafted NOD/SCID mice should offer new opportunities for devising and testing novel therapeutic strategies.
ACKNOWLEDGMENT
We thank I. McNiece (Amgen), D. Williams (Immunex), and Novartis for providing cytokines; StemCell Technologies (Vancouver, BC) for media; P. Lansdorp for antibodies; N. Jamal, H. Messner, M. Baker, D. Roy, and M. Barnett for providing patient samples; and members of the Dick lab for critically reviewing the manuscript.
J.C.Y.W., T.L., and J.D.C. contributed equally to this work.
Supported by grants from the Medical Research Council (MRC) of Canada, the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society and the Terry Fox Run, and Novartis; postdoctoral fellowships from the Leukemia Research Fund of Canada, the MRC (J.C.Y.W.), and the NCIC (T.L.); a Terry Fox Cancer Research Scientist award (C.J.E.); a Research Scientist award from the NCIC (J.E.D.); and an MRC Scientist Award (J.E.D.).
Address reprint requests to J.E. Dick, PhD, Dept of Genetics, Research Institute, Hospital for Sick Children, 555 University Ave, Toronto, Ontario, Canada M5G 1X8.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal