Abstract
We studied the prognostic significance of plasmablastic (PB) multiple myeloma (MM) in Eastern Cooperative Oncology Group Phase III trial E9486. Two reviewers independently reviewed 453 cases. They agreed on 37 PB (8.2%) cases and 416 non-PB cases, achieving an 85% concordance (P < .0001). These PB cases had significantly lower hemoglobin and serum albumin levels, higher calcium and β 2-microglobuin levels, and higher percentage BM plasma cells (PC) by immunofluorescence. They had higher bone marrow PC labeling indices, higher serum soluble interleukin-6 receptor (sIL-6R) levels, and a higher probability of ras mutations. Three treatment regimens were used: vincristine, bis-chloro-ethyl nitrosourea (BCNU) melphalan, cyclophosphamide, and prednisone (VBMCP) alone; VBMCP with added cyclophosphamide (HiCy); or recombinant interferon α 2 (rIFNα2). Although the numbers are low, patients with PB had a significantly lower response rate versus non-PB MM when treated with VBMCP (treated, 47.1% v nontreated, 66.5% [P = .015]). Patients with nonresponding PB had a significantly higher progression rate than non-PB cases (30.6%v 11.8% [P < .0001]), especially with VBMCP alone (35.3% v 15.8% [P = .002]), and with added HiCy (37.5% v 9.8% [P < .0001]), but not with added rIFNα2. Event-free and overall survival of PB MM was shorter (median years, 1.1 v 2.7 and 1.9 v 3.7, respectively [P < .0001 for both]). In multivariate analysis, PB classification was also highly prognostic. There is no survival difference between the patients who were classified as PB by both reviewers versus patients classified as PB by only one reviewer. We conclude that PB MM is a discrete entity associated with more aggressive disease and shortened survival. Tumor cell rasmutations and increased sIL-6R may contribute to a higher proliferation rate and reduced survival. There were significant improvements in response and progression with the addition of HiCy and rIFNα2 to VBMCP, but the numbers were small and improved survival could not be shown.
MULTIPLE MYELOMA (MM) is a neoplastic disease of bone marrow (BM) plasma cells (PC) causing lytic bone lesions, anemia, renal insufficiency, and hypercalcemia. Survival varies from just a few months to 10 or more years. The plasma cell labeling index (PCLI) directly measures increased myeloma cell proliferation and has been shown to be a reliable prognostic factor.1 This increased PC may be accompanied by morphological differences. Earlier observations showed that morphological classification of MM identifies plasmablastic (PB) features, and can distinguish patients with a poor prognosis.1-9 PB features have been associated with other aspects suggesting aggressive disease, including high PCLI,3 high serum lactic dehydrogenase level,10,11 VLA-5 negativity among immature PCs, proliferation of myeloma cells in response to interleukin-6 (IL-6),12 and establishment of in vitro myeloma cell lines.13 We investigated morphological and biological features of patients with MM who were entering a large clinical trial to improve understanding of the prognostic impact of PB morphological changes in MM.
Definitions of PB MM differ, making it difficult to know if one can compare studies and apply PB classification in practice or in clinical trials. We described a simplified classification system to identify PB MM from BM aspirates.3 There have been three subsequent studies by other groups using this classification system. One study confirmed that this classification can identify a poor prognosis MM subset.10 A second study by the same investigators showed no long-term survivors in patients with PB MM.14 A third study showed that PB morphology did not represent an unfavorable category unless patients with the immature morphology were also included.15 To further develop this classification system, we tested it in a cooperative group clinical trial, with slides submitted at entry for study by clinical investigators and examination by two independent pathology reviewers.
We report the results of marrow aspirate slide review in 453 newly diagnosed MM cases entering Eastern Cooperative Oncology Group (ECOG) trial E9486 who also had studies on companion Myeloma Laboratory Group study E9487. We focused on survival analysis of PB cases and identification of clinical and biological correlates, including PCLI,ras mutations, and soluble IL-6 receptor (sIL-6R) level.16 We addressed the following questions: What are the clinical and biological correlates of PB MM? Is PB MM an adverse prognostic factor and does it add to prognostic information provided by existing prognostic factors? We also explored whether adverse effects of PB morphology might be offset by high-dose cyclophosphamide (HiCy) or recombinant human interferon α 2 (rIFNα2) added to the VBMCP regimen used in this trial.
MATERIALS AND METHODS
Patient selection.
We analyzed morphological data from untreated symptomatic patients with MM entered and eligible for ECOG clinical trial E9486 and companion laboratory study E9487 over a 5-year period ending in May 1992 and followed through July 1996. Of 653 patients entered on E9486, 561 were also entered on E9487. Of 538 eligible for both studies, 453 had morphological data. Of the 85 cases without morphological data, 31 were coded as not readable, 53 were coded as pending at the time of analysis, and 1 case was missing.
Patient demographics are described in Table1. Staging was performed according to a modification of the Durie Salmon criteria.17 Patients were randomly assigned to receive either regimen A, VBMCP; regimen B, VBMCP + HiCy; or regimen C, VBMCP + rIFNα2. Patients entered on the HiCy arm had to be less than 70 years of age. The study required submission of Wright-stained BM aspirate slides at the time of study entry. The 453 eligible patients had BM aspirates classified by two morphologists (J.M.B. and P.R.G.). Ancillary laboratory sample submission was initiated and was made mandatory after the first 18 months of accrual. Studies performed on samples sent to the Mayo Clinic Myeloma Tumor Biology Laboratory (Rochester, MN) included the PCLI, percentage PCs by immunofluorescence, serum C-reactive protein (CRP), and β 2-microglobulin levels (β2M). sIL-6R levels were performed at the laboratory of B.K. Other standard laboratory variables included BM examination; metastatic bone survey; serum and urine protein electrophoresis and immunoelectrophoresis; and measurement of serum calcium, creatinine, albumin, and hemoglobin levels at the member institutions. Patients fulfilled ECOG diagnostic criteria for MM and had to have symptomatic, progressive disease before protocol entry. Patients with monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM were excluded from this study.
Baseline Patient Characteistics by PB Status
| Patient Characteristic . | Non-PB . | PB . | P Value . | ||
|---|---|---|---|---|---|
| N . | % . | N . | % . | ||
| Sex | |||||
| Male | 250 | 60.1 | 26 | 70.3 | .292 |
| Female | 166 | 39.9 | 11 | 29.7 | |
| Age (y) | |||||
| Range | 23-84 | 45-83 | |||
| Median | 63 | 65 | |||
| Stage | |||||
| I, II | 191 | 45.9 | 11 | 29.7 | .060 |
| III | 225 | 54.1 | 26 | 70.3 | |
| Creatinine (mg/dL) | |||||
| <2 | 349 | 83.9 | 24 | 64.9 | .007 |
| 2-5 | 67 | 16.1 | 13 | 35.1 | |
| Calcium (mg/dL) | |||||
| <12 | 393 | 94.9 | 27 | 75.0 | .0002 |
| ≥12 | 21 | 5.1 | 9 | 25.0 | |
| Hemoglobin (g/dL) | |||||
| <10 | 142 | 34.1 | 22 | 59.5 | .004 |
| ≥10 | 274 | 65.9 | 15 | 40.5 | |
| PCLI (%) | |||||
| <1 | 287 | 73.4 | 18 | 51.4 | .0099 |
| ≥1 | 104 | 26.6 | 17 | 48.6 | |
| Heavy and light chain | |||||
| Light chain only | 45 | 11.1 | 3 | 8.1 | .784 |
| Heavy chain | 348 | 86.7 | 31 | 91.2 | |
| Monoclonal isotypes | |||||
| IgG | 238 | 58.9 | 24 | 64.9 | .891 |
| IgA | 106 | 26.1 | 9 | 24.3 | |
| IgD | 4 | 15.0 | 0 | 0 | |
| IgM | 1 | ||||
| Monoclonal light chain (serum) | |||||
| κ | 228 | 64.4 | 22 | 64.7 | 1.0 |
| λ | 126 | 35.6 | 12 | 35.3 | |
| Monoclonal light chain (urine) | |||||
| κ | 194 | 64.1 | 18 | 69.2 | .674 |
| λ | 108 | 35.9 | 8 | 30.8 | |
| Bence Jones protein (g/24h) | |||||
| ≤1 | 114 | 49.3 | 14 | 66.7 | .173 |
| >1 | 115 | 50.7 | 7 | 33.3 | |
| Patient Characteristic . | Non-PB . | PB . | P Value . | ||
|---|---|---|---|---|---|
| N . | % . | N . | % . | ||
| Sex | |||||
| Male | 250 | 60.1 | 26 | 70.3 | .292 |
| Female | 166 | 39.9 | 11 | 29.7 | |
| Age (y) | |||||
| Range | 23-84 | 45-83 | |||
| Median | 63 | 65 | |||
| Stage | |||||
| I, II | 191 | 45.9 | 11 | 29.7 | .060 |
| III | 225 | 54.1 | 26 | 70.3 | |
| Creatinine (mg/dL) | |||||
| <2 | 349 | 83.9 | 24 | 64.9 | .007 |
| 2-5 | 67 | 16.1 | 13 | 35.1 | |
| Calcium (mg/dL) | |||||
| <12 | 393 | 94.9 | 27 | 75.0 | .0002 |
| ≥12 | 21 | 5.1 | 9 | 25.0 | |
| Hemoglobin (g/dL) | |||||
| <10 | 142 | 34.1 | 22 | 59.5 | .004 |
| ≥10 | 274 | 65.9 | 15 | 40.5 | |
| PCLI (%) | |||||
| <1 | 287 | 73.4 | 18 | 51.4 | .0099 |
| ≥1 | 104 | 26.6 | 17 | 48.6 | |
| Heavy and light chain | |||||
| Light chain only | 45 | 11.1 | 3 | 8.1 | .784 |
| Heavy chain | 348 | 86.7 | 31 | 91.2 | |
| Monoclonal isotypes | |||||
| IgG | 238 | 58.9 | 24 | 64.9 | .891 |
| IgA | 106 | 26.1 | 9 | 24.3 | |
| IgD | 4 | 15.0 | 0 | 0 | |
| IgM | 1 | ||||
| Monoclonal light chain (serum) | |||||
| κ | 228 | 64.4 | 22 | 64.7 | 1.0 |
| λ | 126 | 35.6 | 12 | 35.3 | |
| Monoclonal light chain (urine) | |||||
| κ | 194 | 64.1 | 18 | 69.2 | .674 |
| λ | 108 | 35.9 | 8 | 30.8 | |
| Bence Jones protein (g/24h) | |||||
| ≤1 | 114 | 49.3 | 14 | 66.7 | .173 |
| >1 | 115 | 50.7 | 7 | 33.3 | |
Morphological classification system.
The two independent reviewers examined BM air-dried smears prepared with the Wright-Giemsa stain or Wright stain. Before the study began, an example slide set and classification criteria were sent to J.M.B. for study. The two reviewers then met after 6 months to compare interpretation of a test set of 50 cases entering the study. We did not change the original classification of either reviewer on those or subsequent cases.
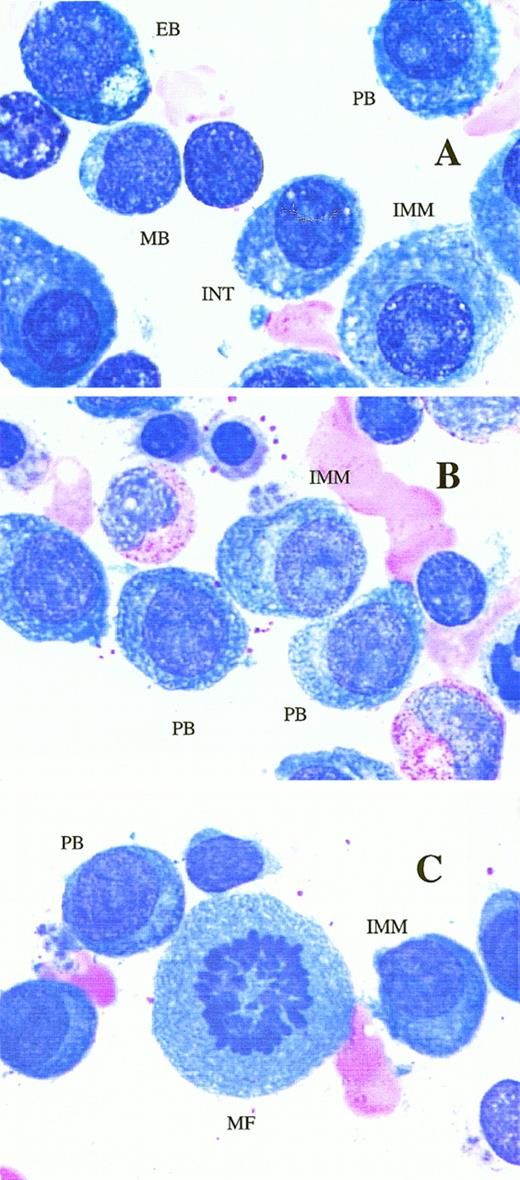
PB MM is a morphologic subset of myeloma defined on the Wright-stained BM aspirate slide as containing ≥2% PB.3 Briefly, areas of the aspirate slide are identified for evaluation. There must be a good spread of cells and high quality of staining, sufficient to identify critical features of the plasma cells, including nuclear chromatin pattern, nuclear size, nucleolar size, and cytoplasmic distribution. Each reviewer identifies 500 plasma cells and enumerates the percent PB. When the percent PB is very close to 2%, the reviewer counts another 500 plasma cells. PB are characterized as follows: the nucleus must have a fine reticular chromatin pattern (no or minimal chromatin clumping); the nucleus must be large (estimated to be greater than 10 μm), or it must have a large nucleolus (estimated to be greater than 2 μm); and the cytoplasm must have no or very little hof region, and less abundant cytoplasm (less than one-half of the nuclear area). In our previous study, 2% or more plasmablasts among the plasma cell population constituted a significant predictor of survival. Because of morphological heterogeneity, the reviewers thoroughly reviewed the entire area of a well-spread slide before concluding whether the case did or did not exhibit PB morphology in any area of the slide. Figure1 shows a representative example of PB MM. Several examples of typical plasmablasts can be seen.
Shown in panels A, B and C, are PB and immature (IMM) plasma cells. PB have fine reticular chromatin, scant cytoplasm (less than half the area of the nucleus), little or no hof, and either a large nucleus (>10 μm), or a large nucleolus (>2 μm). IMM plasma cells have the same nuclear features, but more abundant cytoplasm and usually a more prominent hof region.
Shown in panels A, B and C, are PB and immature (IMM) plasma cells. PB have fine reticular chromatin, scant cytoplasm (less than half the area of the nucleus), little or no hof, and either a large nucleus (>10 μm), or a large nucleolus (>2 μm). IMM plasma cells have the same nuclear features, but more abundant cytoplasm and usually a more prominent hof region.
After independent examination of the stained aspirate slides by each reviewer, and without knowledge of the other's interpretation, results were coded on submission forms and sent to the ECOG statistical office for data entry, storage, and analysis. Data were compared for agreement between the reviewers. To avoid bias or dominant influence by one investigator and to ensure the group of patients reported here represented PB MM, we classified the myeloma as PB only if both reviewers agreed.
PCLI.
PCLI were determined by immunofluorescence microscopy using a previously described technique.18 We used fluorescein-conjugated antiserum to κ and λ light chain on alcohol-fixed cytospin slides of BM mononuclear cells to identify clonal PCs and an antibody to 2-bromodeoxyuridine (BrdU) followed by a rhodamine-conjugated anti-mouse immunoglobulin (Ig) antisera to identify cells in S-phase of the cell cycle. A κ:λ ratio greater than 4:1 or less than 0.5:1 was considered abnormal. Cells stained with the light chain of the nondominant isotype were considered to be nonclonal and were not included in the labeling index determination. The labeling index was measured by counting the percentage of BrdU-labeled cells among 500 PCs on a slide stained with the anti-κ or anti-λ reagent identifying the dominant PC isotype.
Other assays.
CRP measurement was performed using immunoephelometry (Beckman Instruments, Inc, Brea, CA). The normal value is <0.8 mg/dL. The β2M measurement was performed using a microparticle enzyme immunoassay (MEIA Technology) on Abbott Diagnostics Instrumentation (Abbott Laboratories, Abbott Park, IL). The normal value is ≤2.7 μg/mL. These analyses were performed on fresh serum stored no longer than 2 weeks at 4°C. sIL-6R levels were performed by radioimmunoassay in the laboratory of B.K. in Nantes, France.16 Normal value is ≤300 ng/mL. Rasmutations were determined by polymerase chain reaction amplification of codons 12, 13, and 61 of the N- and K-ras genes followed by single-stranded conformation polymorphism,19oligonucleotide dot blot hybridization,20 direct DNA sequencing, or restriction fragment polymorphisms.21
Response to treatment.
Response to treatment was defined according to ECOG criteria. Objective response required a reduction in serum and urine M-protein to ≤50% of pretreatment level. If no measurable serum M-protein was present (<1.0 g/dL), a reduction in urine M-protein to ≤10% of pretreatment level was required. Protein requirements for response had to be verified on two consecutive determinations separated by at least 2 weeks. Patients with measurable soft tissue plasmacytomas had to have a 50% reduction in the sum of the products of the cross diameters. Serum and urine M-protein levels were measured by electrophoresis and not quantitative Ig levels, except where the serum M-spike was considered unreliable by the study chair. For objective response criteria to be met, there had to be no new bone lesions, no increase in lytic lesions, and no recurrence or persistence of hypercalcemia. Patients with nonsecretory MM and no soft tissue plasmacytomas were considered to have an objective response if the BM PC percentage decreased to less than 50% of pretreatment values. No response constituted the group who failed to meet objective response criteria outlined above. Patients who met two or more of the following criteria were considered to have relapse or progression: (1) an increase in serum M-protein to ≥50% above the lowest remission level or a rise of 2.0 g/dL; (2) an increase in 24-hour urine M-protein to 50% above the lowest remission value or an increase of 2 g/24 hours of M-protein and an M-protein of greater than 250 mg/24 hours; (3) an increase in soft tissue plasmacytomas by 50% as measured by the sum of the products of the cross diameters of each measurable lesion; (4) the definitive appearance of a new lytic bone lesion or an increase in size of existing lesions by 50%. Progression by skeletal disease alone required discussion with the study chair before removing the patient from study; and (5) a ≥50% increase in serum or urine M-protein plus one of the following: hypercalcemia ≥12 mg/dL, decrease in hemoglobin ≥2.0 g/dL to <11 g/dL in men or <10 g/dL in women in absence of evidence of myelodysplasia or other cause of anemia, increase in BM PCs by 50% or generalized bone pain. Patients who progressed without previous objective response were considered separately.
Statistical analysis.
Concordance was analyzed using the κ statistic. Distribution of patient characteristics among the PB and non-PB groups was tested for balance using Fisher's Exact test.22 Distribution of laboratory characteristics across the PB and non-PB groups were tested using the Wilcoxon test.23 Event free survival (EFS) was computed from the time of study registration to progression, relapse, or death. Overall survival (OS) was computed from the time of study registration to date of death or date last known to be alive. Survival curves were estimated by using the method of Kaplan and Meier24 with differences assessed by the log rank test.25 All P values were two sided, unless otherwise noted. The Cox model was used for multivariate analyses.26 To assess the joint effect of individual prognostic factors with PB morphology, all the significant factors were considered simultaneously to develop a multiple proportional hazards regression model. To arrive at a parsimonious model, a backward elimination procedure was adopted. At each step of the model fitting, the Wald test was used to eliminate variables until all the remaining variables had a P value of ≤.05.
RESULTS
Of 453 BM aspirates reviewed, 37 (8.2%) were classified as PB by both reviewers, and 416 were classified as non-PB by at least one of the reviewers. In 386 of 453 cases there was agreement between J.M.B. and P.R.G. as to whether cases were PB or not for an overall concordance of 85%. We used the κ statistic to evaluate the agreement between the reviewers. This statistic corrects for agreement caused by chance. If the agreement is high, then there is the possibility that there is usefulness in the morphological classification. The range of κ is from −1 (high disagreement) to 1 (high agreement). The κ statistic is .440 and the one-sided P value testing whether the value of κ = 0 (random agreement) versus κ > 0 is significant at P< .0001.
Clinical and laboratory characteristics.
A total of 627 patients were registered and eligible from ECOG Trial E9486/E9487. Of the 627, 453 had morphological and laboratory data. Table 1 shows the patient characteristics at on-study grouped by PB status. The 37 PB cases had similar distribution of age, sex, and Ig characteristics as did non-PB cases. PB cases had a higher incidence of serum creatinine (≥2 mg/dL), calcium (≥12 mg/dL), BM PCLI (≥1%), and hemoglobin (<10 g/dL; P ≤ .001). A higher proportion of PB myeloma cases were stage III (70.3% v54.1%; P = .06). Table 2presents median values of laboratory characteristics for PB cases at on-study. High values of β2M, PCLI, serum calcium, and creatinine and low values of albumin and hemoglobin are associated with PB morphology. As seen in Table 2, PB cases had higher serum levels of the sIL-6R than non-PB cases. PB cases also had a higher percentage of PCs measured during the performance of the PCLI by immunofluorescence microscopy (PCP-Imm, P = .004). Among 21 cases with >70% plasma cells, 23% were called PB compared with 7.4% for 311 cases with ≤40% plasma cells. The %PC estimated from the bone marrow aspirate from the referring institution failed to correlate with the showing of PB myeloma.
Associations With PB Morphology
| Characteristic . | Non-PB . | PB . | P Value . |
|---|---|---|---|
| β2M (mg/mL) | 3.5 | 4.2 | .0476 |
| PCLI (%) | 0.4 | .8 | .0019 |
| sIL-6R (ng/mL) | 187 | 227 | .0338 |
| CRP (mg/dL) | 3.3 | 2.4 | .1519 |
| PCP-Imm (%) | 25.0 | 34.0 | .0043 |
| Creatinine (mg/dL) | 1.2 | 1.4 | .1158 |
| Albumin (g/dL) | 3.6 | 3.3 | .0357 |
| Hemoglobin (g/dL) | 10.8 | 9.8 | .0015 |
| Calcium (mg/dL) | 9.4 | 10.2 | <.0001 |
| ras mutations | .303* | .582* | .013 |
| Characteristic . | Non-PB . | PB . | P Value . |
|---|---|---|---|
| β2M (mg/mL) | 3.5 | 4.2 | .0476 |
| PCLI (%) | 0.4 | .8 | .0019 |
| sIL-6R (ng/mL) | 187 | 227 | .0338 |
| CRP (mg/dL) | 3.3 | 2.4 | .1519 |
| PCP-Imm (%) | 25.0 | 34.0 | .0043 |
| Creatinine (mg/dL) | 1.2 | 1.4 | .1158 |
| Albumin (g/dL) | 3.6 | 3.3 | .0357 |
| Hemoglobin (g/dL) | 10.8 | 9.8 | .0015 |
| Calcium (mg/dL) | 9.4 | 10.2 | <.0001 |
| ras mutations | .303* | .582* | .013 |
*Conditional probability for ras mutations among patients with or without PB morphology; all other values are medians for the respective characteristics.
Response to treatment and progression.
Two-thirds (66.7%) of 627 patients who were eligible E9486 cases achieved an objective response. Similarly, 302 of the 442 patients (68.3%) evaluable for response and with morphological data who were registered and eligible to both E9486 and E9487 achieved an objective response. Twenty-one of the 36 PB cases (58.3%) evaluable for response had objective responses compared with 281 of 406 (69.2%) for non-PB cases (Table 3). Although the response rates for PB cases were lower, they were not significantly different. For nonresponders, there was a significant difference in the incidence of progression—30.6% for the PB cases compared with 11.8% for the non-PB cases (P < .0001). Of nonresponders who progressed, most (51%) did so by the first year and almost all (80%) progressed before the second year.
Response by Morphology
| Response . | Non-PB N (%) . | PB N (%) . | Total . |
|---|---|---|---|
| Objective | 281 (69.2) | 21 (58.3) | 302 |
| No change | 77 (19.0) | 4 (11.1) | 81 |
| Progression | 48 (11.8)† | 11 (30.6)† | 59 |
| Total* | 406 (100) | 36 (100) | 442 |
| Response . | Non-PB N (%) . | PB N (%) . | Total . |
|---|---|---|---|
| Objective | 281 (69.2) | 21 (58.3) | 302 |
| No change | 77 (19.0) | 4 (11.1) | 81 |
| Progression | 48 (11.8)† | 11 (30.6)† | 59 |
| Total* | 406 (100) | 36 (100) | 442 |
*Eleven patients were unevaluable for response (10 from the non-PB and 1 from the PB group).
Reflect a statistically significant difference, P < .0001.
Response to treatment regimens.
Table 4 presents the categories of response by each treatment: (A) VBMCP, (B) VBMCP + HiCy, and (C) VBMCP + rIFNα2. When the three treatment arms were analyzed for response separately, there were significant differences in objective response for PB versus non-PB cases for VBMCP, but not for VBMCP + HiCy or for VBMCP + rIFNα2. The difference in response rate for PB cases treated with VBMCP alone versus non-PB cases was statistically significant (47.1% v66.5%, P = .015). The objective response rate for PB MM for each of the regimens was incrementally higher: VBMCP alone, 47.1%; VBMCP + HiCy, 62.5%; and VBMCP + rIFNα2, 72.7%. However, because the number of patients with PB MM is small, the differences in response rates for PB cases across the three arms did not reach statistical significance.
Response Status for Different Morphologies by Treatment Regimen
| Response . | Treatment Regimen . | |||||
|---|---|---|---|---|---|---|
| Regimen A (VBMCP) . | Regimen B (VBMCP + HiCy) . | Regimen C (VBMCP + rIFNα2) . | ||||
| Non-PB N (%) . | PB N (%) . | Non-PB N (%) . | PB N (%) . | Non-PB N (%) . | PB N (%) . | |
| Objective | 105 (66.5) | 8 (47.1) | 71 (69.6) | 5 (62.5) | 105 (71.9) | 8 (72.7) |
| No change | 28 (17.7) | 3 (17.6) | 21 (20.6) | 0 (0.0) | 28 (19.2) | 1 (9.1) |
| Progression* | 25 (15.8) | 6 (35.3)3-152 | 10 (9.8) | 3 (37.5) | 13 (8.9) | 2 (18.2) |
| Total3-151 | 158 (100) | 17 | 102 (100) | 8 | 146 (100) | 11 (100) |
| Response . | Treatment Regimen . | |||||
|---|---|---|---|---|---|---|
| Regimen A (VBMCP) . | Regimen B (VBMCP + HiCy) . | Regimen C (VBMCP + rIFNα2) . | ||||
| Non-PB N (%) . | PB N (%) . | Non-PB N (%) . | PB N (%) . | Non-PB N (%) . | PB N (%) . | |
| Objective | 105 (66.5) | 8 (47.1) | 71 (69.6) | 5 (62.5) | 105 (71.9) | 8 (72.7) |
| No change | 28 (17.7) | 3 (17.6) | 21 (20.6) | 0 (0.0) | 28 (19.2) | 1 (9.1) |
| Progression* | 25 (15.8) | 6 (35.3)3-152 | 10 (9.8) | 3 (37.5) | 13 (8.9) | 2 (18.2) |
| Total3-151 | 158 (100) | 17 | 102 (100) | 8 | 146 (100) | 11 (100) |
*Progression without response.
Eleven patients were unevaluable for response: 3 in treatment A, 4 in treatment B, and 4 in treatment C. All were in the non-PB group except 1 in treatment C in the PB group.
Represent statistically significant response status differences between PB and non-PB (P < .05). Among the PB cases there were no significant differences in response status using the different regimens.
Disease progression after treatment regimens.
With VBMCP alone there was a higher incidence of progression in non-responders, 35.3% in PB MM compared with 15.8% in non-PB MM (P = .002). Similarly, in cases treated with VBMCP + HiCy there was a higher incidence of progression in nonresponders in PB MM of 37.5% compared with 9.8% in non-PB MM (P < .0001). In cases treated with VBMCP + rIFNα2 there was no difference in the incidence of progression in nonresponders. Although, in patients with nonresponding PB the incidence of progression was lower using the VBMCP + rIFNα2 regimen (18.2%) than with VBMCP alone (35.3%) or VBMCP + HiCy (37.5%) (Table 4). This difference was not statistically significant.
EFS.
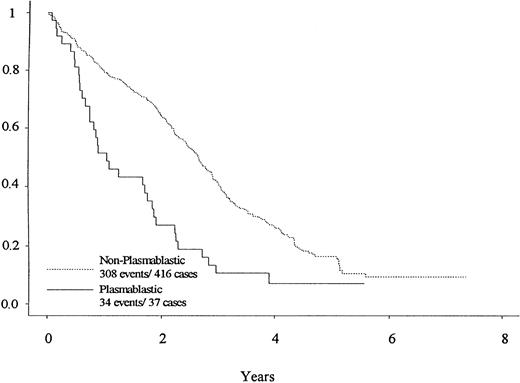
As shown in Fig 2, the PB cases have a significantly shorter EFS than the non-PB cases, with medians of 1.1 and 2.7 years (P < .0001). Comparing the EFS by treatment regimen, there were no significant differences in survival, perhaps because of the small numbers of PB cases in each arm.
EFS differences for patients with PB morphology (—) and non-PB morphology (---) are depicted. Note the greater number of events (relapse and progression) in the PB group, particularly in the first year. Median EFS of patients with PB morphology and non-PB morphology was 1.1 versus 2.7 years (P < .0001).
EFS differences for patients with PB morphology (—) and non-PB morphology (---) are depicted. Note the greater number of events (relapse and progression) in the PB group, particularly in the first year. Median EFS of patients with PB morphology and non-PB morphology was 1.1 versus 2.7 years (P < .0001).
Survival.
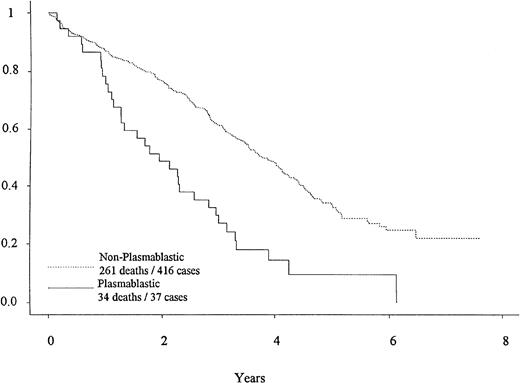
Overall survival by PB morphology is shown in Fig 3. PB cases had significantly shorter survival (P < .0001), with a median of 1.9 years, compared with 3.7 years for non-PB cases. The survival of the PB cases was not different when divided by treatment regimen. There is no survival difference between the patients who were classified as PB by both reviewers versus patients who were classified as PB by only one reviewer.
OS differences for patients with PB morphology (—) and non-PB morphology (---) are depicted. Note the greater number of deaths in the PB group, particularly in the first 2 years. Median survival of patients with PB morphology and non-PB morphology was 1.9 versus 3.7 years (P < .0001).
OS differences for patients with PB morphology (—) and non-PB morphology (---) are depicted. Note the greater number of deaths in the PB group, particularly in the first 2 years. Median survival of patients with PB morphology and non-PB morphology was 1.9 versus 3.7 years (P < .0001).
Univariate and multivariate analysis for prognostic factors.
PB morphology, in univariate analysis, imparts a poor prognosis compared with non-PB cases (P < .0001), as seen in Fig 3. Table 5 presents the univariate survival analysis of selected patient characteristics. The β2M, PCLI, sIL-6R, creatinine, albumin, hemoglobin, and calcium are all significant at P ≤ .001. CRP, level of urine Bence Jones protein, stage, and sex are significant at P < .05. In multivariate analysis (Table6), the following variables are reported in order of risk ratio, as jointly and independently associated with poor survival: PB morphology, high PCLI, serum sIL-6R, β2M, CRP, creatinine, and PC percentage by immunofluorescence.
Univariate Survival Analysis
| Characteristic . | N . | Median (y) . | P Value . |
|---|---|---|---|
| β2M (mg/mL) | |||
| <2.7 | 130 | 5.1 | <.0001 |
| ≥2.7 | 318 | 3.3 | |
| PCLI | |||
| <1% | 305 | 4.1 | <.0001 |
| ≥1% | 121 | 2.4 | |
| sIL-6R (ng/mL) | |||
| >270 | 332 | 3.9 | .0005 |
| ≤270 | 115 | 2.6 | |
| CRP (mg/dL) | |||
| <2 | 153 | 3.9 | .0254 |
| ≥2 | 293 | 3.5 | |
| Creatinine (mg/dL) | |||
| <2 | 373 | 3.9 | <.0001 |
| 2-5 | 80 | 2.8 | |
| Albumin (g/dL) | |||
| ≤3 | 98 | 2.8 | .0008 |
| >3 | 350 | 3.9 | |
| Hemoglobin (g/dL) | |||
| ≤10 | 164 | 2.9 | <.0001 |
| >10 | 289 | 4.1 | |
| Calcium (mg/dL) | |||
| <12 | 420 | 3.7 | .0002 |
| ≥12 | 30 | 2.2 | |
| Age (yr) | |||
| <65 | 257 | 3.7 | .0852 |
| ≥65 | 196 | 3.3 | |
| Stage | |||
| I, II | 202 | 4.1 | .0016 |
| III | 251 | 3.1 | |
| Sex | |||
| Male | 276 | 3.5 | .0414 |
| Female | 177 | 4.0 | |
| Bence Jones protein | |||
| <1 g/24 h | 128 | 4.1 | .0434 |
| >1 g/24 h | 122 | 3.5 | |
| Urine light chain | |||
| κ | 212 | 3.7 | .1560 |
| λ | 116 | 3.3 |
| Characteristic . | N . | Median (y) . | P Value . |
|---|---|---|---|
| β2M (mg/mL) | |||
| <2.7 | 130 | 5.1 | <.0001 |
| ≥2.7 | 318 | 3.3 | |
| PCLI | |||
| <1% | 305 | 4.1 | <.0001 |
| ≥1% | 121 | 2.4 | |
| sIL-6R (ng/mL) | |||
| >270 | 332 | 3.9 | .0005 |
| ≤270 | 115 | 2.6 | |
| CRP (mg/dL) | |||
| <2 | 153 | 3.9 | .0254 |
| ≥2 | 293 | 3.5 | |
| Creatinine (mg/dL) | |||
| <2 | 373 | 3.9 | <.0001 |
| 2-5 | 80 | 2.8 | |
| Albumin (g/dL) | |||
| ≤3 | 98 | 2.8 | .0008 |
| >3 | 350 | 3.9 | |
| Hemoglobin (g/dL) | |||
| ≤10 | 164 | 2.9 | <.0001 |
| >10 | 289 | 4.1 | |
| Calcium (mg/dL) | |||
| <12 | 420 | 3.7 | .0002 |
| ≥12 | 30 | 2.2 | |
| Age (yr) | |||
| <65 | 257 | 3.7 | .0852 |
| ≥65 | 196 | 3.3 | |
| Stage | |||
| I, II | 202 | 4.1 | .0016 |
| III | 251 | 3.1 | |
| Sex | |||
| Male | 276 | 3.5 | .0414 |
| Female | 177 | 4.0 | |
| Bence Jones protein | |||
| <1 g/24 h | 128 | 4.1 | .0434 |
| >1 g/24 h | 122 | 3.5 | |
| Urine light chain | |||
| κ | 212 | 3.7 | .1560 |
| λ | 116 | 3.3 |
Multivariate Model of Survival
| Variable . | Estimate . | P Value . | Risk Ratio . |
|---|---|---|---|
| PB | .655 | .0009 | 1.93 |
| PCLI | .497 | .0002 | 1.64 |
| sIL-6R | .404 | .0028 | 1.50 |
| β2M | .407 | .0083 | 1.50 |
| CRP | .257 | .0535 | 1.48 |
| Creatinine | .319 | .0395 | 1.38 |
| PCP-Imm | .279 | .0236 | 1.32 |
| Variable . | Estimate . | P Value . | Risk Ratio . |
|---|---|---|---|
| PB | .655 | .0009 | 1.93 |
| PCLI | .497 | .0002 | 1.64 |
| sIL-6R | .404 | .0028 | 1.50 |
| β2M | .407 | .0083 | 1.50 |
| CRP | .257 | .0535 | 1.48 |
| Creatinine | .319 | .0395 | 1.38 |
| PCP-Imm | .279 | .0236 | 1.32 |
DISCUSSION
Despite numerous publications on PB myeloma, there is a lack of clear understanding of the definition and significance of this entity. In a previous single-institution study of 100 patients with newly diagnosed MM seen at the Mayo Clinic, we showed the prognostic significance of PB morphology.3 Verifications by another group10,14 and positive studies at our institution1 29 justify looking at PB MM in a cooperative group. Advantages include uniform treatment and standard follow-up; uniformly collected laboratory data for correlative study; accurate data showing response, time of progression, and relapse afforded by a rigorous review and consensus by the principal investigator and data management team in the trial; and the large number of cases afforded by a cooperative group study. An added advantage is the integrated design of laboratory studies performed by investigators from different institutions working collaboratively and collectively as the ECOG Myeloma Laboratory Group. Out of this planned collaboration and prospective banking of sera and cells came the study of ras mutations and soluble IL-6 receptor analyses, correlated with PB and its outcome—studies not possible in a single-institution study.
In this series, we again confirmed that patients with PB features had poorer survival than those with simply immature morphology by using the classification method described in our previous publication.3 The 85% concordance and the κ statistic of .440 between two independent reviewers at separate institutions represents good reproducibility. We believe this to be an important step toward the development and acceptance of criteria for recognizing PB MM as a separate morphologic entity.
The identification of PB MM is not an artifact, but it is a discrete identifiable entity with distinguishing clinical and biological characteristics. There is no apparent relationship of PB morphology to ordinary clinical and laboratory features such as age or sex, presence or absence of bone lesions, or type of M-protein in the serum or urine. However, the fact that ras mutation carries with it a higher frequency of PB morphology suggests that PB MM may be, in part, a manifestation of an activating ras mutation that potentiates the growth of myeloma cells. A full analysis of ras mutations in E9486 including its relationship to PB morphology has been reported separately.27
PB cases also have higher serum levels of soluble IL-6R, a soluble cytokine receptor which can amplify myeloma cell proliferation in response to IL-6. Other studies show that sIL-6R is an important step in the pathogenesis of MM. There are increased levels detected in the serum in MM, compared with MGUS,16 and sIL-6R may amplify plasmablast response to cytokines as suggested by Klein and Bataille.30
PB cases tend to have more advanced and aggressive disease manifested by more frequent anemia, renal insufficiency, hypercalcemia, a higher PC percent by immunofluorescence, a higher PCLI and β2M, and a lower albumin level. The prognostic significance of PB MM can be shown even after one considers the status of these and other independent risk factors such as sIL-6R and CRP. This observation of independence from known prognostic factors shows that these factors do not adequately explain the poor prognostic impact of PB morphology. Although not a proven feature of PB MM, one can hypothesize that the aggressive behavior of plasmablasts and PB MM may in part reflect an increased IL-6 responsiveness associated with ras mutation. It will be important to further study and understand the biological basis for the poor prognosis associated with PB MM.
Treatment implications of PB MM suggested by this study are intriguing. The higher incidence of progression in nonresponders has been suggested previously.31 Although the numbers are small, there appeared to be a higher response rate with added HiCy or rIFNα2 and a lower rate of progression in nonresponders treated with added rIFNα2. There was not a survival advantage for either regimen. Although in this trial, combination chemotherapy using VBMCP with added rIFNα2 or HiCy did not improve survival, there may be subsets of patients who benefit from this treatment. In trial E9486, the addition of rIFNα2 during induction seemed to prolong survival in patients over the age of 70 and there was a trend toward longer survival in those with IgA myeloma. The potential of benefit for additional subgroups including those with PB MM should be considered in the design and analysis of future trials of rIFNα2 in MM.
An intergroup cooperative group MM clinical trial including ECOG, the Southwest Oncology Group, and Cancer and Leukemia Group B addresses the role of peripheral stem cell transplant in MM. We are particularly interested to see if peripheral stem cell transplant can improve survival in the PB MM subset. Four reviewers at different institutions will study BM aspirates to see whether patients with PB morphology are more likely to benefit from early intensive therapy with peripheral stem cell transplant.
In conclusion, PB MM represents a discrete entity in phase III trial E9486/E9487. It is an independent prognostic factor predicting shorter EFS and OS. A more frequently elevated level of PCLI, β2M, CRP, and creatinine, and a lower albumin level characterize PB MM as a more advanced and aggressive form of disease. Higher incidence ofras mutations and of elevations of sIL-6R suggest potential underlying mechanisms of aggressive behavior. Currently limited in use, PB classification of MM is potentially useful in practice and in clinical trials. Until more intensive therapy is definitively shown to be beneficial, we cannot conclude that it is a better approach for patients with PB MM.
ACKNOWLEDGMENT
We express our gratitude to Fran Silva for superb data management assistance, Roxanne Tabery for excellent technical support, and Kathleen Payne and Terrie Plank for fine secretarial support.
Supported in part by Public Service Grants No. CA 13650, CA 23318, CA 21076, CA 15947, CA 20365, CA 11083, CA 21115, and CA 62242 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. This study was conducted by The Eastern Cooperative Oncology Group. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Presented in part at the Meeting of the American Society of Hematology, December 4-8, 1992, Anaheim, CA, and at the Meeting of the American Society of Clinical Oncology, May 20-23, 1995, Los Angeles, CA.
Address reprint requests to Philip R. Greipp, MD, Division of Hematology, Mayo Clinic, Hilton Room 920, 200 1st Ave SW, Rochester, MN 55905.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal