To the Editor:
It has been generally believed that the central nervous system (CNS) is an immunologic sanctuary site and privileged from an attack of graft-versus-host disease (GVHD). However, several investigators have recently reported that the parenchymal cells, especially activated microglias, strongly expressed host class II major histocompatibility complex in rat GVHD models1,2 or in clinical cases with GVHD following allogeneic bone marrow transplantation (allo-BMT).3-5 We report a case with abrupt seizures, presented with partially reversible pathes of blight signal on T2-weighted magnetic resonance imaging (MRI), during the course of chronic GVHD following allo-BMT.
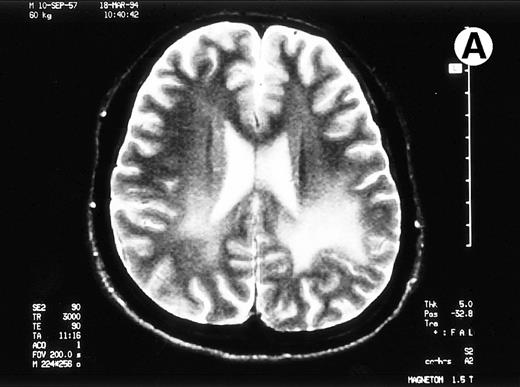
A 35-year-old man with a chronic myelocytic leukemia in a first chronic phase received an allo-BMT prepared for cyclophosphamide 120 mg/kg and total body irradiation 12 Gy from an HLA-identical sibling in October 1992. Acute GVHD developed in grade III and progressed to an extensive chronic GVHD (cGVHD), which included lichenoid lesions of buccal mucosa, oral dryness, keratoconjunctivitis sicca, and hypolacrimation in addition to skin lesion and liver dysfunction. We treated his cGVHD with 150 mg/d of cyclosporine (CsA) and 10 mg/d of prednisolone (PSL). With dose reduction of CsA from 12 months after allo-BMT, his cGVHD gradually deteriorated. Abrupt seizures, lasting a few minutes, developed on day 491 and day 496 when the patient received 100 mg/d of CsA and 10 mg/d of PSL. Neurological examination revealed no abnormalities. MRI detected the pathes of blight signal on T2-weighted method in white matter of brain adjacent bilateral posterior lateral ventricles (Fig 1A). The eloctroencephalogram revealed epileptic abnormal δ and θ waves on left frontal to central lobe. An examination of cerebrospinal fluid (CSF) revealed as follows: an initial pressure 120 mm H2O, protein 98 mg/dL, sugar 55 mg/dL, chrolide 123 mmol/L, cell count 38/μL with all lymphocytes and negative results in antibodies for cytomegalovirus, herpes simplex virus type-1, and toxoplasma. The concentration of interferon-γ was 0.3 U/L in serum and 1.0 U/L in CSF, which measured by radioimmunoassay kit (Medgenics, Belgium).
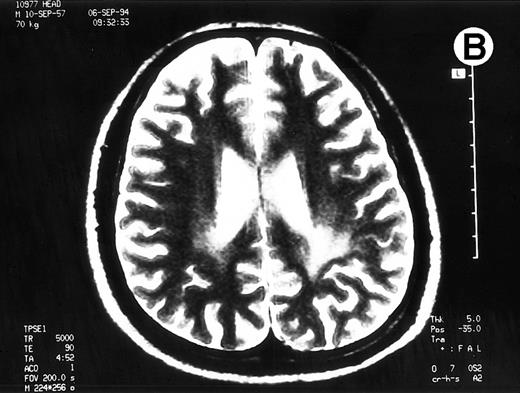
Paths of blight signal on T2-weighted MRI in white matter of brain adjacent bilateral posterior lateral ventricles. (A) On day 526 when we just withheld cyclosporine. (B) On day 698 when chronic GVHD resolved by readministration of cyclosporine.
Paths of blight signal on T2-weighted MRI in white matter of brain adjacent bilateral posterior lateral ventricles. (A) On day 526 when we just withheld cyclosporine. (B) On day 698 when chronic GVHD resolved by readministration of cyclosporine.
To rule out the possibility of CsA neurotoxicity, although the trough levels of CsA ranged from 130 to 170 ng/mL, we withheld CsA at day 526. Despite this attempt, the CNS lesions on MRI did not change, and we subsequently restarted 150 mg/d of CsA at day 537. Thereafter, the CNS lesions gradually resolved with 150 mg/d of CsA and 10 to 20 mg/d of PSL (Fig 1B), coinsident with resolution on the other signs of cGVHD. The seizures never recurred with 600 mg/d of sodium valproate. The patient has now minor CNS lesion and mild signs of cGVHD with 100 mg/d of CsA and with 90% of Karnovsky score at 62 months following allo-BMT.
The BMT patients developing leukoencephalopathy (LEC) have essentially all received the combination of intrathecal MTX and some form of cranial irradiation.6 The neurological disabilities of LEC are progressive, permanent, and often fatal. The present patient had received neither cranial irradiation before BMT nor intrathecal MTX before and after BMT. The CNS lesion on MRI has substantially reduced in size. Neurotoxicity of CsA has generally been seen when the trough serum level is above the therapeutic range, and the CNS lesion usually resolves within 1 to 2 weeks with discontinuation of CsA.7Our attempt with the abrupt discontinuation of CsA yielded no resolution in CNS lesion on MRI in this patient. Because of the absence in the episode of fever and no laboratory evidences of infections, progressive multifocal LEC or the other infectious meningoencephalitis were unlikely in this patient. Because his blood pressure was normotensive throughout the clinical course, the possibility of hypertensive encephalopathy was also unlikely. Furthermore, high levels of interferon-γ in CSF and serum in this patient strongly suggest the presence of immunological mechanism as in multiple sclerosis. Despite the lack of direct evidence such as a cerebral biopsy, we would like to propose that GVHD in CNS is truely present.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal