To determine the relative in vivo importance of endothelial expressed adhesion molecules to eosinophil rolling, adhesion, and transmigration, we have induced eosinophilic peritonitis using ragweed allergen in P-selectin–deficient, intracellular adhesion molecule-1 (ICAM-1)–deficient and control wild-type mice. Circulating leukocytes visualized by intravital microscopy exhibited reduced rolling and firm adhesion in P-selectin–deficient mice and reduced firm adhesion in ICAM-1–deficient mice. Eosinophils exhibited reduced rolling and firm adhesion to endothelium in P-selectin–deficient mice. Eosinophil recruitment in P-selectin–deficient mice (∼75% inhibition of eosinophil recruitment) and ICAM-1–deficient mice (∼67% inhibition of eosinophil recruitment) was significantly reduced compared with wild-type mice. Eosinophil recruitment was not completely inhibited in P-selectin/ICAM-1 double-mutant mice (eosinophil recruitment inhibited ∼62%). However, pretreatment of P-selectin/ICAM-1–deficient mice with an anti-vascular cell adhesion molecule (VCAM) antibody induced near complete inhibition of eosinophil recruitment. Overall, these studies show that eosinophil rolling and firm adhesion is significantly reduced in P-selectin–deficient mice and that P-selectin, ICAM-1, and VCAM are important to eosinophil peritoneal recruitment after ragweed challenge.
THE RECRUITMENT OF eosinophils to sites of allergic inflammation in the peritoneal microcirculation in vivo is a multistep process characterized by initial eosinophil intravascular rolling and firm adhesion to endothelium, followed by sequential eosinophil diapedesis between endothelial cells and chemotaxis into tissues.1,2 In vitro studies of eosinophils using a rotational assay to simulate shear stress associated with blood flow have demonstrated a role for L-selectin in eosinophil adhesion to human umbilical vein endothelial cells placed on a horizontal rotator.3 Using intravital videomicroscopy, we have identified that the adhesion molecules used by eosinophils in vivo in the initial steps of rolling on endothelium (L-selectin– and VLA-4–dependent)2 differ from that used by other circulating leukocytes such as neutrophils (L-selectin–dependent and VLA-4–independent)2,4 under conditions of blood flow in vivo. These in vivo experiments with eosinophils identified that the paradigm of the strict separation of leukocyte selectins as rolling receptors, and leukocyte integrins as firm adhesion receptors under conditions of blood flow did not hold for all leukocyte integrins. The observation that an eosinophil expressed integrin, namely VLA-4, can function as a rolling receptor in vivo2 has subsequently been confirmed by other investigators studying the rolling function of VLA-4 expressed by T lymphocytes in vitro and in vivo.5 6
Whereas the above-noted studies have attempted to address the role of eosinophil expressed adhesion molecules (ie, L-selectin and VLA-4), this study focuses on the relative importance of adhesion molecules (P-selectin, intracellular adhesion molecule-1 [ICAM-1]) expressed by endothelial cells to eosinophil rolling, adhesion, and transmigration in vivo. Endothelial cells store P-selectin preformed in Weibel-Palade bodies.7 Upon in vitro stimulation with histamine or thrombin, Weibel-Palade bodies fuse with the endothelial cell surface membrane and expose P-selectin rapidly (within a few minutes of stimulation) and transiently (peak expression 20 to 30 minutes) to the luminal cell surface.7 In contrast to histamine and thrombin stimulation, cytokines such as tumor necrosis factor induce sustained P-selectin expression by cultured endothelial cells.8 The important in vivo functional role of P-selectin as a neutrophil adhesion counter-receptor has previously been shown in P-selectin–deficient mice.9-11 Additional studies demonstrate that neutrophils use P-selectin glycoprotein ligand-1 (PSGL-1) to roll on P-selectin12 and that neutralizing antibodies to P-selectin significantly attenuate neutrophil influx in animal models of inflammation.13 In limited studies of eosinophils and P-selectin, eosinophils have been shown to bind to both purified P-selectin14 and to P-selectin expressed by nasal polyp endothelium using static in vitro adhesion assays.15 Eosinophils also roll on P-selectin in vitro, as demonstrated in a flow chamber assay.16
Murine ICAM-1 has been molecularly cloned17 and, like human ICAM-1, has five extracellular Ig-like domains. Amino acid substitutions in the ICAM-1 extracellular domains have indicated that the primary binding site for the eosinophil counter receptor CD11a/CD18 (LFA-1) is located in the NH2-terminal first domain of ICAM-1.17 In vitro studies have shown that eosinophils bind to ICAM-118 and that levels of ICAM-1 expression are increased in the nasal mucosa of patients with perennial allergic rhinitis.19 In vivo studies using neutralizing antibodies to ICAM-1 in animal models of eosinophilic inflammation have produced conflicting results concerning the role of ICAM-1 in eosinophil recruitment, with some studies demonstrating inhibition of eosinophil recruitment,20 whereas other studies show no inhibition of eosinophil recruitment.21,22 To obviate methodologic concerns about studies using neutralizing antibodies to ICAM-1 (dose, route, and timing of administration of antibody, affinity of antibody, nonspecific binding of antibody to Fc receptors on inflammatory cells and not to ICAM-1 on endothelial cells), we have used ICAM-1–deficient mice to study the role of ICAM-1 in eosinophil adhesion and recruitment. Studies of neutrophil recruitment in P-selectin– and ICAM-1–deficient mice infected in the peritoneal cavity with streptococcus pneumoniae demonstrate an approximately 60% to 70% reduction in acute neutrophil migration into the peritoneal cavity in mice with either mutation alone and complete inhibition of neutrophil recruitment in P-selectin/ICAM-1 double-mutant mice.10 We have used the same P-selectin– and ICAM-1–deficient mice,10 as well as P-selectin/ICAM-1 double-mutant mice,10 to determine the relative importance of P-selectin and ICAM-1 to an eosinophilic inflammatory response in the peritoneum in response to ragweed allergen challenge (as opposed to streptococcus pneumoniae infection as the inflammatory stimulus for neutrophils in the above-mentioned studies).10
MATERIALS AND METHODS
Adhesion molecule knockout mice.
P-selectin–deficient, ICAM-1–deficient, P-selectin/ICAM-1 double-mutant, as well as C57BL/6 background control wild-type female mice aged 8 to 10 weeks were purchased from Jackson Laboratories (Bar Harbor, ME). The above-noted mice were developed by Dr Arthur Beaudet (Department of Molecular Genetics, Baylor College of Medicine, Houston, TX) and used previously in studies of neutrophil adhesion in a model of bacterial infection in mice.10
Mouse model of peritoneal eosinophilic inflammation: Ragweed allergen immunization and peritoneal allergen challenge.
The techniques used for ragweed immunization and challenge are similar to those previously described by other investigators.23-25Previous studies of neutrophil recruitment into the peritoneal cavity in these adhesion molecule-deficient mice were performed 2 to 4 hours after bacterial infection by Bullard et al,10 whereas the studies of eosinophil peritoneal recruitment we describe were performed at a later time point optimal for evaluation of eosinophil recruitment (24 to 48 hours after allergen challenge). Mice are immunized by a series of five injections of a 1:1,000 dilution of a ragweed pollen extract (Miles Inc, Spokane, WA): 0.1 mL is injected subcutaneously on days 0 and 1, and 0.2 mL is injected subcutaneously on days 6, 8, and 14. A control group of ragweed immunized mice (challenged with phosphate-buffered saline [PBS] diluent) and nonimmunized mice (prepared by subcutaneous injections of isotonic saline instead of the ragweed pollen extract) follow the same immunization schedule. Three to five mice are included in each group of mice studied. The mice are challenged on day 20 by the intraperitoneal injection of 0.2 mL of the ragweed allergen (or control PBS diluent).
To determine whether wild-type and adhesion molecule-deficient mice were equivalently sensitized to allergen, immediate hypersensitivity skin tests were performed. Wild-type and adhesion molecule-deficient mice were sensitized to ragweed as described. On day 20, 50 μL of ragweed antigen or diluent control was injected into the shaved backs of the different groups of mice. Immediately after antigen administration, 200 μL of 1% Evans blue dye was injected into the tail vein of the mice. Blueing of the skin at the antigen challenged (but not diluent challenged) sites occurred within 10 minutes of antigen challenge. The size of the blueing reaction (15 to 20 mm diameter) was not significantly different in wild-type, P-selectin–deficient, or ICAM-1–deficient mice.
In selected experiments to evaluate the contribution of vascular cell adhesion molecule (VCAM) expression to eosinophil recruitment, two groups of P-selectin/ICAM-1 double-mutant mice were immunized over a 14-day period with ragweed allergen as described above. On day 20 of the ragweed sensitization protocol, either a rat IgG1 antimouse VCAM monoclonal antibody (MoAb) mk 2.7 (1 mg/kg body weight; kindly provided by Dr E. Butcher, Stanford, CA)26or a species- and isotype-matched control MoAb was injected intravenously. Two hours after the antibody was administered, the mice were challenged by the intraperitoneal injection of allergen and the number of peritoneal eosinophils was enumerated 48 hours later.
Assessment of cells in the peritoneal cavity.
At time points before (day 0) and after immunization, as well as before and 48 hours after intraperitoneal allergen challenge (day 22), the mice were killed by cervical dislocation. Two milliliters of PBS containing 6 U/mL of heparin was injected intraperitoneally, the abdomen was massaged, and the peritoneal infusion was collected after the peritoneum was opened. An appropriate PBS dilution of the recovered peritoneal fluid was added to trypan blue, and the viability and total number of white blood cells were counted with a hemocytometer. Differential leukocyte counts were performed after brief acetone fixation and staining of the peritoneal cells with May-Grünwald-Giemsa stains. The percentage of eosinophils present on each slide was assessed by counting a minimum of 300 cells in random high-power fields using a light microscope (40× magnification) to display the slide image on a TV monitor (Videometric 150 image analysis program; American Innovision, San Diego, CA). In addition, because mast cells participate in allergen-induced inflammation, the number of resident peritoneal mast cells was compared in wild-type and adhesion molecule-deficient mice.
Assay for eosinophil peroxidase (EPO).
In addition to enumerating the number of eosinophils in the peritoneal cavity, EPO, an eosinophil cytoplasmic granule protein, was assayed using the substrate solution O-phenylenediamine dihydrochloride (OPD) and a calorimetric assay.27 Peritoneal cell pellets (105 cells) were lysed in 0.02% CTAB, 0.05% triton and added to 2 mL of assay buffer (0.1 mol/L phosphate buffer, pH 6.8, 8 mmol/L OPD, 0.01% H2O2). Reaction volumes were incubated in duplicate for 10 minutes at room temperature and read in a spectrophotometer at 492 nm wavelength (Shimadza UV160U, Tokyo, Japan).
Preparation of mice for detection of leukocyte rolling in the peritoneal microcirculation.
Adhesion molecule-deficient or control wild-type mice (25 to 35 g body weight) were anesthetized with a subcutaneous injection of saline solution containing a cocktail of ketamine hydrochloride and Xylazine (7.5 mg and 2.5 mg, respectively, per 100 mg body weight). The mice were then placed on a heating pad maintained at 37°C. A midline incision was made and the mesentery was gently exteriorized and spread on a heated glass window (37.5°C) of the stage of a Leitz intravital microscope (Wetzlar, Germany). The exteriorized portion of mouse mesentery was kept continuously moist with endotoxin-free isotonic saline solution (pH 7.4). Other parts of the intestine that were exposed but not microscopically observed were kept moist with isotonic saline-soaked cotton pads and the mesentery was covered with Saran Wrap. To minimize endotoxin contamination, Saran Wrap (Dow Brands LP, Indianapolis, IN) was presoaked with 1% E-Toxa-Clean (Sigma Chemical Co, St Louis, MO) overnight, followed by rinsing in 70% ethanol and endotoxin-free distilled water and a final wash with sterile isotonic saline solution.
Intravital microscopy and image analysis.
The passage of circulating leukocytes in the peritoneal microcirculation was made visible by transillumination using a Nikon 10× (numerical aperture [NA], 0.30), 20× (NA, 0.40), or 40× (NA, 0.55) water immersion objective (Melville, NY), as previously described.2,28 All microscopic images were recorded through a silicon-intensified tube camera (SIT68; Dage-MTI, Michigan City, IN) attached to the microscope and connected to a Sony monitor (Tokyo, Japan) and an SVHS video recorder (JVC HR-S66004) for off-line analysis of eosinophil rolling. Video recordings of different postcapillary and collecting venules (range, 20 to 65 μm) were analyzed for assessment of rolling and adhesion of leukocytes as previously described.2 28 Rolling leukocytes were quantitated by counting the number of cells interacting with the vessel wall in 1 minute in a plane perpendicular to a vessel axis, whereas those cells that were found to be stationary for at least 1 minute were considered as adherent or sticking cells. All studies were conducted between 0 to 1 hour after exteriorization of the mouse mesentery.
Isolation of murine eosinophils from interleukin-5 (IL-5) transgenic mice.
In selected experiments, mouse eosinophils of greater than 90% purity and greater than 98% viability were purified from IL-5 transgenic mice, kindly provided by Dr Colin Sanderson (Perth, Australia).29 IL-5 transgenic mice (age, 10 weeks old) have peripheral blood leukocyte differential cell counts exhibiting 42% ± 12% peripheral blood eosinophilia (n = 3). The contaminating white blood cells comprise 44% T lymphocytes, 2% mononuclear cells, and 10% neutrophils. To purify the eosinophils, we diluted blood drawn from six IL-5 transgenic mice 1:1 in PBS, 0.1 mmol/L EDTA and layered it onto a discontinuous density gradient of percoll (1.075, 1.080, 1.085, 1.090, and 1.095). Eosinophils (85% to 95% pure; 98% viable) banded between the 1.085 and 1.090 layers. Fluorescence-activated cell sorting analysis showed that the purified eosinophils expressed L-selectin, as well as α4 and β2 integrin cell surface receptors (data not shown).
Eosinophils with at least 98% viability and greater than 90% purity were selected and labeled with carboxy fluorescein diacetate (CFDA; Molecular Probes, Eugene, OR) as previously described for human eosinophils and murine mast cells.2 28 CFDA-labeled eosinophils were resuspended at a concentration of 0.5 × 107 cells/200 μL of PBS containing 0.01% glucose and kept at room temperature in the dark until used. Eosinophils were then injected into the tail vein of mice previously sensitized with ragweed allergen and challenged 24 hours before surgery with either ragweed or saline and observed by intravital fluorescence microscopy. All studies were conducted between 0 and 1 hour after exteriorization of the mouse mesentery.
Visualization of eosinophils in mouse mesentery.
The rolling of mouse eosinophils in mesenteric venules was made visible by stroboscopic epi-illumination using a video-triggered Xenon lamp (Chadwick Helmuth, El Monte, CA) and Leitz Ploemopak epi-illuminator employing an I2 filter block (Wetzlar, Germany). All images were recorded through a silicon-intensified tube camera (SIT68; Dage MTI, Michigan City, IN) using a 10× or 20× water immersion objective (Nikon), as described previously.2 The rolling fraction (Rf) and the rolling velocity of CFDA-labeled mouse eosinophils in ragweed and diluent PBS challenged mice (wild-type control and P-selectin–deficient mice) was determined by frame-by-frame analysis, as previously described.2 28
Statistics.
The number of eosinophils and mast cells, as well as EPO levels in peritoneal fluid, were compared by multiple comparisons of paired data by Student's t-test using a statistical software package (In Stat, San Diego, CA). P values of less than .05 were considered to be statistically significant. All results are given as the mean ± standard error of the mean (SEM). Rolling fractions of murine leukocytes and injected eosinophils were compared by multiple comparisons of paired data by Student's t-test using a statistical software package (SigmaStat, Jandel Scientific). Pvalues of less than .05 were considered statistically significant. All results are given as the mean ± standard error (SE).
RESULTS
Mouse model of eosinophilic peritonitis: P-selectin–deficient mice.
Control wild-type mice (n = 9 mice; 3 separate experiments), when immunized and challenged with ragweed allergen, developed a significant peritoneal cavity eosinophilia (17.3% ± 3.5% eosinophils; Fig 1) compared with wild-type mice that were not challenged with ragweed (0.9% ± 0.4% eosinophils;P = .03) or compared with wild-type mice that were immunized with ragweed and challenged with PBS diluent (2.5% ± 0.8% eosinophils; P = .04). In contrast to control wild-type mice, P-selectin–deficient mice immunized with ragweed developed less peritoneal eosinophilia when challenged with an intraperitoneal injection of ragweed allergen (P-selectin–deficient mice 4.3% ± 2.0% peritoneal eosinophils, ∼75% inhibition compared with ragweed-challenged wild-type mice, P = .005; Fig 1).
Comparison of eosinophil recruitment and EPO levels in P-selectin–deficient and wild-type mice. Ragweed-sensitized mice (P-selectin–deficient or control wild-type mice; n = 9 mice; 3 separate experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils (A) and EPO levels (B) were assessed. P-selectin–deficient mice developed significantly less peritoneal eosinophilia (P = .005) and lower EPO levels (P = .05) compared with control wild-type mice after allergen.
Comparison of eosinophil recruitment and EPO levels in P-selectin–deficient and wild-type mice. Ragweed-sensitized mice (P-selectin–deficient or control wild-type mice; n = 9 mice; 3 separate experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils (A) and EPO levels (B) were assessed. P-selectin–deficient mice developed significantly less peritoneal eosinophilia (P = .005) and lower EPO levels (P = .05) compared with control wild-type mice after allergen.
There was a trend to an increase in the total leukocyte count in the peritoneal cavity of wild-type mice after, compared with before, allergen challenge (89.4 ± 53.6 × 105 peritoneal cells after allergen; 44.0 ± 14.8 × 105peritoneal cells before allergen; n = 3 experiments; P = .19) and P-selectin–deficient mice (94.8 ± 41.4 × 105peritoneal cells after allergen; 76.6 ± 13.6 × 105 peritoneal cells before allergen; n = 3 experiments;P = .48), but this did not reach statistical significance.
Mouse model of eosinophilic peritonitis: ICAM-1–deficient mice.
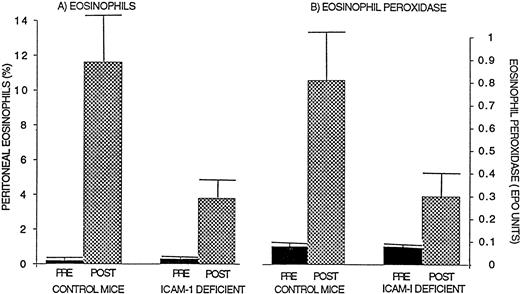
Control wild-type mice (n = 10 mice; 3 separate experiments), when immunized and challenged with ragweed allergen, developed a significant peritoneal cavity eosinophilia (11.6% ± 2.9% eosinophils) compared with wild-type mice that were not challenged with ragweed (0.2% ± 0.1% eosinophils; P = .05) or compared with wild-type mice that were immunized with ragweed and challenged with PBS diluent (1.4% ± 0.6% eosinophils). In contrast to control wild-type mice, ICAM-1–deficient mice immunized with ragweed developed less peritoneal eosinophilia when challenged with an intraperitoneal injection of ragweed allergen (ICAM-1–deficient mice 3.8% ± 1.1% peritoneal eosinophils, ∼67% inhibition compared with ragweed challenged wild-type mice; P = .03; Fig 2).
Comparison of eosinophil recruitment and EPO levels in ICAM-1–deficient and wild-type mice. Ragweed-sensitized mice (ICAM-1–deficient or control wild-type mice; n = 10 mice; 3 separate experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils (A) and EPO levels (B) were assessed. ICAM-1–deficient mice developed significantly less peritoneal eosinophilia (P = .03) and lower EPO levels (P = .05) compared with control wild-type mice after allergen.
Comparison of eosinophil recruitment and EPO levels in ICAM-1–deficient and wild-type mice. Ragweed-sensitized mice (ICAM-1–deficient or control wild-type mice; n = 10 mice; 3 separate experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils (A) and EPO levels (B) were assessed. ICAM-1–deficient mice developed significantly less peritoneal eosinophilia (P = .03) and lower EPO levels (P = .05) compared with control wild-type mice after allergen.
As with studies of P-selectin–deficient mice, there was a trend to an increase in the total leukocyte count in the peritoneal cavity of wild-type mice after versus before allergen challenge (73.3 ± 50.5 × 105 peritoneal cells after allergen; 34.2 ± 10.2 × 105 peritoneal cells before allergen; n = 3 experiments; P = .22) and ICAM-1–deficient mice (48.9 ± 20.6 × 105 peritoneal cells after allergen; 37.1 ± 7.1 × 105 peritoneal cells before allergen; n = 3 experiments; P = .37), but this did not reach statistical significance.
Kinetic studies of eosinophilic peritonitis.
Because peak eosinophil recruitment in this model of eosinophilic peritonitis occurs from 24 to 72 hours after allergen challenge, our initial studies (reported above) focused on the 48-hour post-allergen challenge time point. We also performed studies of eosinophil recruitment at earlier time points. Control wild-type mice, when immunized and challenged with ragweed allergen, developed a significant peritoneal eosinophilia (18.3% ± 1.7 % eosinophils) 24 hours after allergen challenge compared with ICAM-1–deficient mice (6.3% ± 1.8% eosinophils; P = .01) or compared with P-selectin–deficient mice (5.3% ± 0.6% eosinophils; P = .001). These studies show that there was no significant difference in the degree of inhibition of eosinophil recruitment in ICAM-1–deficient mice at 24 hours after allergen challenge (66% inhibition vwild-type mice) compared with 48 hours after allergen challenge (67% inhibition v wild-type mice). Similarly, our studies of P-selectin–deficient mice showed that there was no significant difference in the degree of inhibition of eosinophil recruitment in P-selectin–deficient mice at 24 hours after allergen challenge (72% inhibition v wild-type mice) compared with 48 hours after allergen challenge (75% inhibition v wild-type mice).
We also performed studies at 12 hours after allergen challenge that are more difficult to interpret because of the very low level of allergen-induced eosinophil recruitment in wild-type mice at 12 hours (3% eosinophils) compared with 24 hours (18% eosinophils) or 48 hours (17% eosinophils) after allergen challenge. Nevertheless, there is a similar degree of inhibition of eosinophil recruitment 12 hours after allergen challenge in ICAM-1–deficient mice (62% inhibition compared with wild-type mice) and P-selectin–deficient mice (63% inhibition compared with wild-type mice). These studies clearly demonstrate the importance of P-selectin and ICAM-1 at the 24-hour and 48-hour post-allergen challenge time points and also suggest a role for P-selectin and ICAM-1 as early as the 12-hour time point (the caveat being the low numbers of eosinophils recruited in wild-type mice).
Peritoneal EPO levels.
Control wild-type mice (n = 9 mice; 3 separate experiments), when immunized and challenged with ragweed allergen, developed a significant increase in peritoneal cavity EPO levels (1.35 ± 0.35 EPO units) compared with wild-type mice that were not challenged with ragweed (0.08 ± 0.05 EPO units; P = .01) or compared with wild-type mice that were immunized with ragweed and challenged with PBS diluent (0.14 ± 0.06 EPO units; P = .01). In contrast, P-selectin–deficient and ICAM-1–deficient mice immunized with ragweed developed lower levels of peritoneal EPO when challenged with an intraperitoneal injection of ragweed allergen (P-selectin–deficient mice 0.54 ± 0.16 EPO units, ∼60% inhibition compared with ragweed-challenged wild-type mice 1.35 ± 0.35 EPO units; P= .05; Fig 1; ICAM-1–deficient mice 0.30 ± 0.07 EPO units, ∼63% inhibition compared with ragweed challenged wild-type mice 0.81 ± 0.24 EPO units; P = .05; Fig 2).
Peritoneal mast cells and mononuclear cells.
In contrast to the changes noted in the number of recruited eosinophils in control wild-type and adhesion molecule-deficient mice after allergen challenge, there were no significant differences in the number of resident peritoneal mast cells in wild-type and ICAM-1–deficient mice before (ICAM-1 wild-type 4.1% ± 1.3% vICAM-1–deficient 4.7% ± 0.9%) or after allergen challenge (ICAM-1 wild-type 2.8% ± 0.4% v ICAM-1–deficient 3.2% ± 0.3%).
There was a slight, but statistically insignificant, reduction in peritoneal mast cells in P-selectin–deficient compared with control wild-type mice before (P-selectin wild-type 3.2% ± 0.5% vP-selectin–deficient 2.0% ± 0.3%) and after allergen challenge (P-selectin wild-type 1.5% ± 0.7% v P-selectin–deficient 1.1% ± 0.5%). There was also a trend for antigen-challenged P-selectin–deficient, ICAM-1–deficient, as well as wild-type mice to have a lower percentage of peritoneal mast cells compared with their respective pre-antigen control. This is probably due to the fact that antigen challenge induces mast cell degranulation, rendering some post-antigen degranulated peritoneal mast cells invisible with the granule-based stain we have used to enumerate mast cells.
Mononuclear cells comprised the majority of peritoneal cells in wild-type (96.1 ± 0.1% before allergen v 83.3% ± 3.7% after allergen), P-selectin–deficient (97.6% ± 0.3% before allergen v 91.5% ± 2.1% after allergen), and ICAM-1–deficient mice (94.9% ± 0.8% before allergen v93.3% ± 1.2% after allergen). Rare neutrophils that comprised less than 1% to 2% of peritoneal cells were occasionally noted in wild-type mice challenged with allergen.
Peripheral blood leukocytes.
There was no significant difference in the number of circulating eosinophils in wild-type (2.1%) compared with P-selectin–deficient (2.0%) or ICAM-1–deficient mice (2.2%). P-selectin/ICAM-1–deficient mice had a slight increase in circulating eosinophils (3.1%) compared with wild-type mice (2.1%). As previously noted,10 there was a mild increase in the total peripheral blood leukocyte cell count as well as the percentage of neutrophils in P-selectin/ICAM-1–deficient, ICAM-1–deficient, and P-selectin–deficient mice compared with wild-type mice (wild-type mice 6.4 ± 0.8 × 103 total leukocytes/μL; 2.1% ± 0.2% eosinophils, 14.8% ± 6.3% neutrophils, 83.1% ± 7.4% mononuclear cells; P-selectin–deficient mice 8.1 ± 0.7 × 103 total leukocytes/μL; 2.0% ± 0.3% eosinophils, 22.4% ± 10.6% neutrophils, 75.6% ± 8.4% mononuclear cells; ICAM-1–deficient mice 11.5 ± 0.6 × 103 total leukocytes/μL; 2.2% ± 0.1% eosinophils, 27.1% ± 10.3% neutrophils, 70.7% ± 12.6% mononuclear cells; and P-selectin/ICAM-1–deficient mice 11.8 ± 0.6 × 103 total leukocytes/μL; 3.1% ± 0.2% eosinophils, 29.6% ± 9.8% neutrophils, 67.3% ± 12.7% mononuclear cells).
Intravital microscopy and leukocyte rolling.
The effect of ragweed challenge on leukocyte rolling and firm adhesion in mesenteric venules of wild-type (n = 11 mice, 34 venules) as well as P-selectin (n = 6 mice, 19 venules) and ICAM-1–deficient mice (n = 5 mice, 18 venules) was examined by intravital microscopy (Table 1). There was no significant difference in hemodynamic parameters measured (venular diameter, mean blood flow velocity, shear rate, and shear stress; Table 2) in wild-type compared with P-selectin–deficient or ICAM-1–deficient mice. Leukocyte rolling was observed predominantly in postcapillary and collecting venules and was rarely observed in arterioles. In wild-type mice, challenge with ragweed allergen resulted in a fourfold increase in leukocyte rolling compared with challenge with PBS diluent (ragweed challenge 68.1 ± 11.1 rolling leukocytes/minute v PBS diluent challenge 16.4 ± 5.3 rolling leukocytes/minute; P = .004; Table 1). However, leukocyte rolling in ragweed-challenged P-selectin–deficient mice (4.2 ± 0.3 rolling leukocytes/minute) was significantly less than that observed in control ragweed-challenged wild-type mice (68.1 ± 11.1 rolling leukocytes/minute; P = .0001). PBS diluent challenge failed to induce detectable leukocyte rolling in P-selectin–deficient mice during the 1-hour observation period.
Effect of Ragweed Challenge on Leukocyte Rolling and Adhesion in Mesenteric Venules
| Leukocyte Rolling per Minute (mean ± SE) . | Leukocyte Adhesion (adherent leukocytes/100 μm venule length; mean ± SE) . | ||||||
|---|---|---|---|---|---|---|---|
| Mice . | Ragweed Challenged . | Diluent Challenged . | P . | Mice . | Ragweed Challenged . | Diluent Challenged . | P . |
| Wild-type | 68.1 ± 11.1 | 9.1 ± 1.5 | .004 | Wild-type | 10.6 ± 2.2 | 2.3 ± 0.9 | .02 |
| P-selectin–deficient | 4.2 ± 0.3 | 0-150 | NS | P-selectin–deficient | 2.8 ± 1.2 | 0.2 ± 0.4 | .04 |
| ICAM-1–deficient | 45.1 ± 4.5 | 13.9 ± 2.2 | .002 | ICAM-1–deficient | 1.1 ± 0.4 | 0.6 ± 0.3 | NS |
| Leukocyte Rolling per Minute (mean ± SE) . | Leukocyte Adhesion (adherent leukocytes/100 μm venule length; mean ± SE) . | ||||||
|---|---|---|---|---|---|---|---|
| Mice . | Ragweed Challenged . | Diluent Challenged . | P . | Mice . | Ragweed Challenged . | Diluent Challenged . | P . |
| Wild-type | 68.1 ± 11.1 | 9.1 ± 1.5 | .004 | Wild-type | 10.6 ± 2.2 | 2.3 ± 0.9 | .02 |
| P-selectin–deficient | 4.2 ± 0.3 | 0-150 | NS | P-selectin–deficient | 2.8 ± 1.2 | 0.2 ± 0.4 | .04 |
| ICAM-1–deficient | 45.1 ± 4.5 | 13.9 ± 2.2 | .002 | ICAM-1–deficient | 1.1 ± 0.4 | 0.6 ± 0.3 | NS |
Values are the mean ± SE of 5 to 6 mice per experimental group. In each mouse, a total of 3 to 10 postcapillary venules were analyzed. The number of rolling leukocytes was quantitated by counting the leukocytes passing through a perpendicular plane during a 1-minute interval. Firm adhesion of leukocytes is expressed as the number of adherent cells/100 μm of venular length.
Abbreviation: NS, not significant.
No leukocyte rolling was detected during the 1-hour observation period.
Hemodynamic Parameters of the Postcapillary Venules of Allergen- and Saline-Challenged Mice
| Hemodynamic Parameters . | Wild-Type . | P-Selectin–Deficient . | ICAM-1–Deficient . | |||
|---|---|---|---|---|---|---|
| Ragweed . | Saline . | Ragweed . | Saline . | Ragweed . | Saline . | |
| Venular diameter (μm) | 48 ± 4 | 47 ± 9 | 38 ± 5 | 40 ± 3 | 46 ± 3 | 44 ± 5 |
| Vblood* (mm/s) | 2.6 ± 0.6 | 2.1 ± 0.5 | 1.5 ± 0.3 | 1.8 ± 0.2 | 1.2 ± 0.1 | 1.7 ± 0.3 |
| Shear rate (s−1) | 248 ± 23 | 196 ± 11.4 | 170 ± 31 | 260 ± 34 | 173 ± 14 | 330 ± 47 |
| Shear stress (dyn/cm1) | 6.2 ± 0.6 | 4.9 ± 0.3 | 4.2 ± 0.8 | 6.5 ± 0.9 | 4.2 ± 0.3 | 8.3 ± 1.2 |
| Hemodynamic Parameters . | Wild-Type . | P-Selectin–Deficient . | ICAM-1–Deficient . | |||
|---|---|---|---|---|---|---|
| Ragweed . | Saline . | Ragweed . | Saline . | Ragweed . | Saline . | |
| Venular diameter (μm) | 48 ± 4 | 47 ± 9 | 38 ± 5 | 40 ± 3 | 46 ± 3 | 44 ± 5 |
| Vblood* (mm/s) | 2.6 ± 0.6 | 2.1 ± 0.5 | 1.5 ± 0.3 | 1.8 ± 0.2 | 1.2 ± 0.1 | 1.7 ± 0.3 |
| Shear rate (s−1) | 248 ± 23 | 196 ± 11.4 | 170 ± 31 | 260 ± 34 | 173 ± 14 | 330 ± 47 |
| Shear stress (dyn/cm1) | 6.2 ± 0.6 | 4.9 ± 0.3 | 4.2 ± 0.8 | 6.5 ± 0.9 | 4.2 ± 0.3 | 8.3 ± 1.2 |
There was no statistically significant difference in hemodynamic parameters obtained between the various groups (ragweed vsaline; P-selectin or ICAM-1 v wild-type).
Mean blood flow velocity (millimeters per second) was calculated from the highest eosinophil velocity in each of the different venules assuming a parabolic flow. The wall shear stress was determined from the shear rate assuming blood viscosity of 0.025 poise.
Allergen challenge of ICAM-1–deficient mice with ragweed resulted in a threefold increase in leukocyte rolling (45.1 ± 4.5 rolling leukocytes/minute) compared with PBS diluent-challenged ICAM-1–deficient mice (13.9 ± 2.2 rolling leukocytes/minute;P = .002). Furthermore, no significant differences in leukocyte rolling was observed between ragweed-challenged wild-type mice and ragweed challenged ICAM-1–deficient mice.
Intravital microscopy and leukocyte firm adhesion.
We next examined the effect of allergen challenge on leukocyte firm adhesion (adherent leukocytes/100 μm venule length) in the mesenteric venules (Table 1). In wild-type mice, intraperitoneal challenge with ragweed resulted in a fourfold increase in leukocyte adhesion compared with PBS diluent challenge (ragweed challenge 10.6 ± 2.2 adherent leukocytes v PBS diluent challenge 2.3 ± 0.9 adherent leukocytes; P = .02). Leukocyte adhesion in ragweed-challenged P-selectin–deficient mice was found to be significantly reduced compared with ragweed-challenged wild-type mice (P-selectin–deficient 2.8 ± 1.2 adherent leukocytes v wild-type mice 10.6 ± 2.2 adherent leukocytes; P = .0001). Minimal adhesion of murine leukocytes was observed in P-selectin–deficient mice that were challenged with PBS diluent (0.2 ± 0.4 adherent leukocytes). Although allergen challenge had induced significant leukocyte rolling in ICAM-1–deficient mice, it failed to induce significant leukocyte adhesion in the ICAM-1–deficient mice (1.1 ± 0.4 adherent leukocytes in ragweed-challenged ICAM-1–deficient mice; v 10.6 ± 2.2 adherent leukocytes in ragweed-challenged wild-type mice;P = .007). As observed with P-selectin–deficient mice, PBS diluent administration failed to induce significant firm adhesion of rolling leukocytes in ICAM-1–deficient mice (0.6 ± 0.3 adherent leukocytes).
Intravital microscopy and eosinophil rolling and adhesion in P-selectin–deficient mice.
Because the above-noted studies characterized total leukocyte, but not eosinophil, rolling and adhesion, we examined the ability of purified murine eosinophils to roll and adhere to vascular endothelium in vivo (Table 3). Eosinophils were purified from IL-5 transgenic mice and labeled with CFDA before injection into the tail vein. The passage of the fluorescently labeled eosinophils in the mesenteric circulation was made visible by stroboscopic epi-illumination (Fig 3). Similar to studies of total leukocyte rolling, allergen challenge with ragweed induced a 1.8-fold increase in eosinophil rolling in the mesenteric venules of wild-type mice challenged with ragweed (eosinophil Rf: 26.7% ± 2.6%) compared with wild-type mice challenged with PBS diluent (eosinophil Rf: 9.1% ± 1.5%; P = .002; Table 3). The rolling of eosinophils in venules of ragweed challenged P-selectin–deficient mice was found to be dramatically reduced (eosinophil Rf: 1.2% ± 0.8%; P = .0002 vragweed-challenged wild-type mice). PBS diluent challenge failed to induce detectable eosinophil rolling in P-selectin–deficient mice.
Effect of Ragweed Challenge on Eosinophil Rolling and Adhesion to Endothelium in Mesenteric Venules of Wild-Type and P-Selectin–Deficient Mice
| Eosinophil Rolling Fraction (%; mean ± SE) . | Eosinophil Adhesion (adherent eosinophils/100 μm venule length; mean ± SE) . | ||||||
|---|---|---|---|---|---|---|---|
| Mice . | Ragweed Challenged . | Diluent Challenged . | P . | Mice . | Ragweed Challenged . | Diluent Challenged . | P . |
| Wild-type | 26.7 ± 2.6 | 9.1 ± 1.5 | .002 | Wild-type | 3.3 ± 1.1 | 0.4 ± 0.3 | .001 |
| P-selectin–deficient | 1.2 ± 0.8 | 0* | NS | P-selectin–deficient | 0.8 ± 0.5 | 0* | .03 |
| Eosinophil Rolling Fraction (%; mean ± SE) . | Eosinophil Adhesion (adherent eosinophils/100 μm venule length; mean ± SE) . | ||||||
|---|---|---|---|---|---|---|---|
| Mice . | Ragweed Challenged . | Diluent Challenged . | P . | Mice . | Ragweed Challenged . | Diluent Challenged . | P . |
| Wild-type | 26.7 ± 2.6 | 9.1 ± 1.5 | .002 | Wild-type | 3.3 ± 1.1 | 0.4 ± 0.3 | .001 |
| P-selectin–deficient | 1.2 ± 0.8 | 0* | NS | P-selectin–deficient | 0.8 ± 0.5 | 0* | .03 |
Values are mean ± SE of 5 mice per experimental group. In each group, 2 to 5 postcapillary venules were investigated. The rolling of injected eosinophils (fluorescently labeled) in mesenteric venules are expressed as a fraction of total injected cells (Rf, %). Firm adhesion of injected eosinophils (fluorescently labeled) are expressed as the number of adherent eosinophils/100 μm of venule length.
Abbreviation: NS, not significant.
No eosinophil rolling or adhesion was detected during the 1-hour observation period.
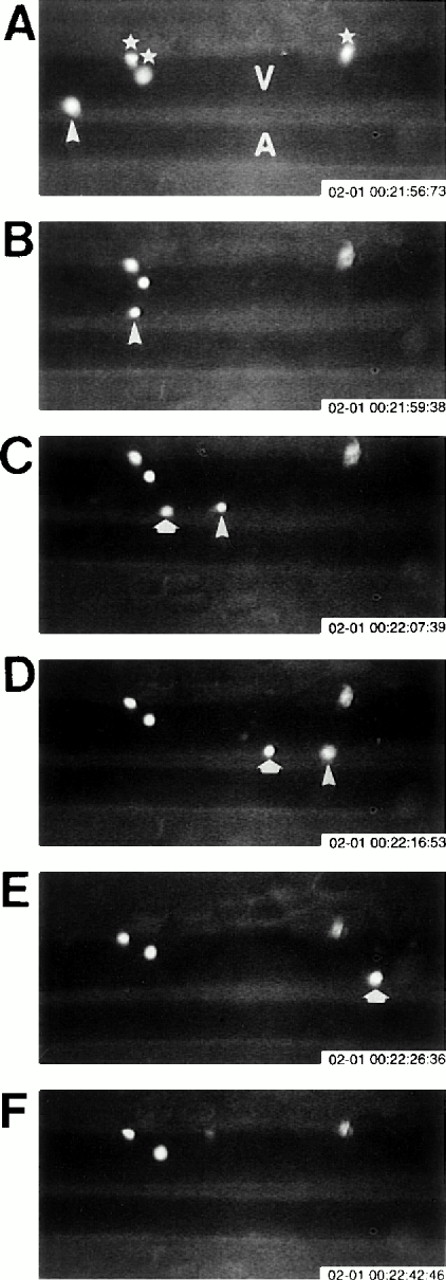
Eosinophil rolling and firm adhesion visualized by intravital videomicroscopy. Ragweed-sensitized wild-type mice were challenged with an intraperitoneal injection of ragweed. Twenty-four hours after intraperitoneal ragweed allergen challenge, fluorescently labeled eosinophils in the peritoneal microcirculation were visualized in vivo using intravital videomicroscopy. (A) through (F) are sequential videotape images of the same venule (V) and arteriole (A). Blood flow in the venule is from left to right. Three firmly adherent eosinophils (⋆; A) are visualized in the same relative position in (A) through (F), whereas two different eosinophils (eosinophil #1 = ▵; and eosinophil #2 = ⇧) rolling along the venular endothelium are noted in different positions in (A) through (D) (rolling eosinophil #1 = ▵) and (C) through (E) (rolling eosinophil #2 = ⇧).
Eosinophil rolling and firm adhesion visualized by intravital videomicroscopy. Ragweed-sensitized wild-type mice were challenged with an intraperitoneal injection of ragweed. Twenty-four hours after intraperitoneal ragweed allergen challenge, fluorescently labeled eosinophils in the peritoneal microcirculation were visualized in vivo using intravital videomicroscopy. (A) through (F) are sequential videotape images of the same venule (V) and arteriole (A). Blood flow in the venule is from left to right. Three firmly adherent eosinophils (⋆; A) are visualized in the same relative position in (A) through (F), whereas two different eosinophils (eosinophil #1 = ▵; and eosinophil #2 = ⇧) rolling along the venular endothelium are noted in different positions in (A) through (D) (rolling eosinophil #1 = ▵) and (C) through (E) (rolling eosinophil #2 = ⇧).
The ability of eosinophils to firmly adhere to mesenteric endothelium in P-selectin–deficient and wild-type mice after allergen challenge was also determined. In wild-type mice ragweed challenge resulted in an eightfold increase in eosinophil adhesion (ragweed challenge 3.3 ± 1.1 adherent eosinophils v 0.4 ± 0.3 adherent eosinophils after PBS diluent challenge; P = .001; Table 3). As observed with murine leukocytes, reduced levels of eosinophil adhesion was observed in P-selectin–deficient compared with wild-type mice that were challenged with ragweed (0.8 ± 0.5 adherent eosinophils in ragweed challenged P-selectin–deficient mice v 3.3 ± 0.5 adherent eosinophils in ragweed challenged wild-type mice,P = .001), whereas no detectable adhesion of eosinophils was observed in PBS diluent-challenged P-selectin–deficient mice. A comparison of velocity distribution profiles of rolling eosinophils showed that the eosinophil rolling velocity in ragweed-challenged P-selectin–deficient mice (216.3 ± 31.9 μm/s) was significantly greater than the eosinophil rolling velocity in ragweed-challenged wild-type mice (35.7 ± 8.3 μm/s;P < .001).
P-selectin/ICAM-1 double-mutant mice.
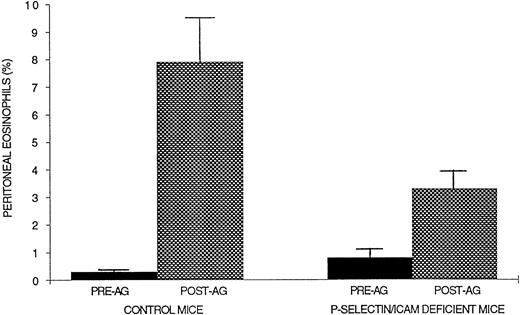
Because studies with neutrophil emigration into the peritoneum during streptococcus pneumoniae inducing peritonitis demonstrated complete inhibition of neutrophil emigration into the peritoneum of P-selectin/ICAM-1 double-mutant mice,10 we performed similar experiments evaluating eosinophil recruitment after ragweed allergen challenge. In contrast to studies with neutrophils in which there was complete inhibition of neutrophil emigration,10eosinophil recruitment in P-selectin/ICAM-1 double-mutant mice challenged with allergen was not completely inhibited (eosinophil recruitment inhibited ∼62%; P = .01; Fig 4) and was similar to that noted with either P-selectin–deficient (eosinophil recruitment inhibited ∼75%) or ICAM-1–deficient mice (eosinophil recruitment inhibited ∼67%) challenged with allergen.
Evaluation of eosinophil recruitment in P-selectin/ICAM-1–deficient double-mutant mice. Ragweed-sensitized mice (P-selectin/ICAM-1–deficient or control wild-type mice) were challenged with an intraperitoneal injection of ragweed. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils was assessed by light microscopy. P-selectin/ICAM-1–deficient double-mutant mice developed significantly less peritoneal eosinophilia compared with control wild-type mice after allergen (P = .01).
Evaluation of eosinophil recruitment in P-selectin/ICAM-1–deficient double-mutant mice. Ragweed-sensitized mice (P-selectin/ICAM-1–deficient or control wild-type mice) were challenged with an intraperitoneal injection of ragweed. Forty-eight hours later, the percentage of transmigrated peritoneal eosinophils was assessed by light microscopy. P-selectin/ICAM-1–deficient double-mutant mice developed significantly less peritoneal eosinophilia compared with control wild-type mice after allergen (P = .01).
Experiments were performed with an anti-VCAM antibody to determine the contribution of VCAM to eosinophil recruitment in P-selectin/ICAM-1–deficient mice. Pretreatment of P-selectin/ICAM-1 double-mutant mice with either an anti-VCAM or control antibody before the day-20 peritoneal allergen challenge resulted in near complete inhibition of eosinophil recruitment into the peritoneal cavity 48 hours later only in the mice receiving the anti-VCAM antibody (∼93% inhibition of eosinophil recruitment). In contrast, the species- and isotype-matched antibody had no additional effect on eosinophil recruitment in the control group of P-selectin/ICAM double-mutant mice (∼53% inhibition of eosinophil recruitment; wild-type mice 16.6% ± 8.4% peritoneal eosinophils; P-selectin/ICAM-1–deficient mice pretreated with control antibody 7.8% ± 1.2% peritoneal eosinophils; P-selectin/ICAM-1–deficient mice pretreated with an anti-VCAM antibody 1.1% ± 0.2% peritoneal eosinophils; Fig 5).
Inhibition of eosinophil recruitment in P-selectin/ICAM-1–deficient double-mutant mice treated with an anti-VCAM antibody. Ragweed-sensitized P-selectin/ICAM-1–deficient mice were pretreated with either a rat IgG1 antimouse VCAM MoAb or a species- and isotype-matched control antibody. Two hours after the antibody was administered intravenously, the mice were challenged by the intraperitoneal injection of allergen and the number of peritoneal eosinophils enumerated 48 hours later.
Inhibition of eosinophil recruitment in P-selectin/ICAM-1–deficient double-mutant mice treated with an anti-VCAM antibody. Ragweed-sensitized P-selectin/ICAM-1–deficient mice were pretreated with either a rat IgG1 antimouse VCAM MoAb or a species- and isotype-matched control antibody. Two hours after the antibody was administered intravenously, the mice were challenged by the intraperitoneal injection of allergen and the number of peritoneal eosinophils enumerated 48 hours later.
DISCUSSION
In this study, we demonstrate that endothelial-expressed P-selectin and ICAM-1 subserve important functions in the recruitment of eosinophils to sites of allergic inflammation. In addition, we show that eosinophil rolling is significantly reduced in P-selectin–deficient mice at sites of allergen challenge 24 hours after challenge. Because significant peritoneal eosinophilia (but not neutrophilia) is present 48 hours after intraperitoneal allergen challenge,23-25 our recruitment studies focused on analyzing the influx of eosinophils at 48 hours.23-25 Our study confirmed previous studies in several laboratories that have shown that intraperitoneal allergen challenge induces a peritoneal eosinophilia that is present at 8 hours and peaks between 24 and 72 hours.23-25 A small influx of neutrophils that peaks at 8 hours and is no longer present after 24 hours has also previously been noted in this mouse model.23-25 Our studies using intravital microscopy have demonstrated that total leukocyte rolling in mesenteric venules is significantly induced in ragweed-challenged wild-type and ICAM-1–deficient mice. In contrast, total leukocyte rolling is significantly inhibited in ragweed-challenged P-selectin–deficient mice. Studies with fluorescently labeled eosinophils confirm that not only total leukocyte, but also eosinophil rolling is significantly reduced in P-selectin–deficient mice. In addition, eosinophils roll appreciably faster in P-selectin–deficient compared with wild-type mice, suggesting that the absence of P-selectin weakens the strength of the initial eosinophil endothelial cell interaction, resulting in eosinophils rolling at higher velocities. Intravital microscopy studies also demonstrated that total leukocyte firm adhesion was significantly reduced in ragweed-challenged ICAM-1–deficient and P-selectin–deficient mice as compared with ragweed-challenged wild-type mice. These observations underscore the importance of ICAM-1 as a firm adhesion receptor in vivo. The reduced firm adhesion of leukocytes in P-selectin–deficient mice challenged with allergen also suggests that leukocyte rolling and adhesion are sequential at sites of allergen challenge. A model of sequential leukocyte adhesion would require initial leukocyte rolling before firm adhesion. Thus, the absence of significant leukocyte rolling in the mesenteric venules of P-selectin–deficient mice would not allow for the subsequent firm adhesion of leukocytes to firm adhesion receptors such as ICAM-1. Indeed, studies with fluorescently labeled eosinophils confirmed that not only total leukocytes, but also eosinophils, exhibited reduced rolling and firm adhesion in P-selectin–deficient as compared with wild-type mice challenged with allergen.
Our studies with ICAM-1–deficient mice (∼67% inhibition of eosinophil recruitment) also demonstrated an important role for this endothelial-expressed adhesion molecule in recruitment of eosinophils after allergen challenge. Studies with antibodies to ICAM-1 in animal models of asthma have previously shown conflicting results regarding the role of ICAM-1 in eosinophil recruitment into the allergen-challenged lung.20-22 In a primate model of asthma, antibodies against ICAM-1 decreased BAL eosinophil infiltration (∼65%) and attenuated bronchial hyperresponsiveness,20whereas in a mouse model antibodies to ICAM-1 had no effect on eosinophil recruitment.21,22 The conflicting results in these studies may relate to methodologic differences in the studies in which different animal models (mouse v primate), different antibodies (rat α mouse ICAM-1 MoAb YN1/1.7 v MoAb R6.5), different route of antibody administration (intraperitoneal vintravenous), and different antigens (ascaris v ovalbumin) were used.20-22 Our study using ICAM-1–deficient mice would suggest that ICAM-1 is important to eosinophil recruitment to the peritoneal cavity in mice, as has previously been demonstrated for eosinophil recruitment to the lung in primates.20 A potential confounding variable in the studies of the ICAM-1–deficient mice we have used in this study is the recent demonstration that these mice are not completely deficient in ICAM and do express small amounts of alternatively spliced ICAM (as assessed by immunohistochemistry).30 At present, the in vivo biologic and functional significance of these alternative spliced forms of ICAM are not known. The demonstration in this study that eosinophil recruitment was inhibited approximately 67% in ICAM-1–deficient mice suggests that ICAM-1 plays a significant role in eosinophil recruitment. Although it is possible that alternative isoforms of ICAM-1 could contribute to the approximately 33% of eosinophil recruitment not inhibited in ICAM-1 mutant mice, our studies using neutralizing antibodies to VCAM in P-selectin/ICAM-1 double-mutant mice suggest that VCAM is more likely than these alternate isoforms of ICAM to participate in eosinophil recruitment into the peritoneal cavity.
Previous studies by Gonzalo et al31 and our group32 have shown that eosinophil recruitment into the lungs of ovalbumin-challenged P-selectin–deficient mice is inhibited 70% to 80% compared with wild-type mice 3 hours after allergen challenge. In contrast, Gonzalo et al31 showed that eosinophil recruitment into the lungs of ovalbumin-challenged mice was increased 2.5-fold 7 hours after allergen inhalation challenge. The difference in eosinophil requirements for P-selectin at 7 hours compared with other time points after allergen challenge may depend on the vascular bed studied (pulmonary v peritoneal), the antigen used (ovalbumin by Gonzalo et al and ragweed in our model), or the method of sensitization and challenge (single v multiple repetitive challenge). It is difficult to directly compare kinetic results in the two studies (pulmonary versus peritoneal), because the time 0 hours in the Gonzalo et al pulmonary study was preceded by 7 consecutive days of daily inhaled allergen, whereas in our peritoneal study the single day-20 intraperitoneal injection of allergen was not preceded by daily allergen challenge for 7 days. Further studies will need to define whether repetitive allergen challenge compared with single allergen challenge alters the kinetic requirements of eosinophils for P-selectin.
Overall, these studies demonstrate that P-selectin and ICAM-1 play an important role in eosinophil recruitment to sites of allergic inflammation. However, neither of these adhesion molecules is completely able to inhibit eosinophil recruitment, and neither adhesion molecule is specific for eosinophils as P-selectin and ICAM-1 are also important to neutrophil10 and T lymphocyte11,33,34 adhesion. Studies with P-selectin/ICAM-1 double-mutant mice suggest that approximately 25% to 40% of eosinophil recruitment at sites of allergen challenge in the peritoneal cavity occurs through a P-selectin/ICAM-1–independent pathway. In terms of P-selectin–independent endothelial mechanisms of eosinophil rolling along vascular endothelium, E-selectin and VCAM-1 are important candidate endothelial expressed rolling receptors. In addition, VCAM-1 can function as an ICAM-1–independent eosinophil firm adhesion pathway. Our studies in which we were able to nearly completely inhibit eosinophil recruitment in P-selectin/ICAM-1 double-mutant mice pretreated with neutralizing antibodies to VCAM, suggest that VCAM contributes most significantly to P-selectin/ICAM-1–independent eosinophil recruitment. VCAM-1 expressed by endothelial cells can function both as a firm adhesion and a rolling receptor.6Studies using either T cells or α4 integrin transfected cells have shown that cells expressing an α4 integrin can roll on purified VCAM-1, but not on ICAM-1 or fibronectin.6 Studies using eosinophils have also demonstrated that neutralizing antibodies to α4, but not to β2 integrins can inhibit eosinophil rolling in vivo,2 presumably through an interaction between eosinophil expressed α4 and endothelial expressed VCAM-1. However, at present, in vitro flow chamber or in vivo studies with eosinophils and neutralizing antibodies to VCAM-1 have not been published. The importance of P-selectin and VCAM-1, but not E-selectin to eosinophil rolling is also suggested from in vitro flow chamber studies and in vivo studies of the rabbit microcirculation in which E-selectin was shown to preferentially support neutrophil35,36 but not eosinophil rolling.37However, in this study, we have not directly evaluated the role of E-selectin in supporting eosinophil rolling. Nevertheless, overall, these studies suggest that P-selectin supports both eosinophil and neutrophil rolling,10,12 whereas VCAM-1 supports eosinophil but not neutrophil rolling and adhesion and E-selectin predominantly supports neutrophil but not eosinophil rolling.35-38
In summary, we report the first demonstration of murine eosinophil rolling and firm adhesion in mouse mesenteric venules at sites of allergen challenge using intravital microscopy. These studies also provide evidence for the importance of vascular P-selectin, ICAM-1, and VCAM-1 in the rolling, adhesion, and recruitment of murine eosinophils and leukocytes in a model of allergic eosinophilic peritonitis.
ACKNOWLEDGMENT
The authors thank Lauri Doval for expert secretarial support during the preparation of the manuscript, Dr Colin Sanderson for providing IL-5 transgenic mice, and Gregory K. Hughes for technical support.
Supported by National Institutes of Health Grants No. AI 33977 and AI 38425 (to D.H.B.) and AI 35796 (to P.S.).
Address reprint requests to David H. Broide, MBCHB, University of California, San Diego, 9500 Gilman Dr, La Jolla, CA 92093-0635.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal