Previously, we have shown a mutant mouse DDD/1 with T-cell–specific homing defect that is regulated by an autosomal recessive gene,plt (paucity of lymph node T cells), and seems to be caused by lymph node (LN) stromal cells. In the present study, immunohistochemical analysis showed unusual distribution of T cells in LN, Peyer's patches (PP), and spleen from plt/plt, probably due to the failure of T cells to migrate from blood into the T-cell zone in LN or PP, or into the spleen white pulp across high endothelial venule or marginal zone, respectively, based on the experiments in which labelled T cells were injected intravenously and detected in the tissues. Analysis of surface L-selectin and CD44 suggested that T cells with memory phenotype, probably from afferent lymphatics, recruit intoplt/plt LN. Linkage mapping by simple-sequence length polymorphism of genomic DNA from 190 backcross progenies produced by intercrossing with MSM/Ms, linked plt most closely with D4Mit237, and localized at 24.7 cM from cetromere on chromosome 4. We discuss the possibility that a wild-type gene on plt locus encodes a chemokine inducing T-cell–specific homing into peripheral lymphoid tissues.
LYMPHOCYTES THAT have matured and differentiated in the primary lymphoid organs, thymus and bone marrow, are released into peripheral blood and circulate to secondary lymphoid organs such as lymph nodes (LN), Peyer's patches (PP), and spleen, in which they respond to antigens.1-4 They recirculate from lymph to blood and the recirculation seems to be essential for the immune surveillance. The lymphocyte homing into secondary lymphoid organs is initiated by the interaction of the homing receptors on the lymphocyte surface, such as molecules of the selectin and integrin families, and their ligand on the surface of the high endothelial venule (HEV).1-7 L-selectin and α4β7 integrin induce rolling of lymphocytes on HEV,5,6 followed by their arrest and firm adhesion through the interaction of αLβ2integrin/LFA-1 on lymphocytes with ICAM-1 on HEV.3,4 7Then, the lymphocytes transmigrate and home into the lymphoid tissues.
Recently, we have shown a mutant mouse in which the T-cell number in LN, but not B-cell number, is extremely small. The mutant mouse bears a T-cell specific homing deficiency into LN, which is regulated by a single autosomal recessive gene, plt (paucity of lymph node T cells).8 The mutation seems to be expressed on LN stromal cells, not on T cells themselves.8 T cells can bind to HEV in peripheral LN (PLN) from plt/plt, as well as from+/+, in Stamper-Woodruff assay, and the binding is inhibited by a monoclonal antibody (MoAb) specific for L-selectin or its ligand peripheral node addressin (PNAd), suggesting that these molecules are functional in plt/plt.8 Thus, some other factor(s) might be required for homing of T cells into PLN. Based on these findings, it is quite possible that plt is a mutant of the gene that encodes a factor to allow T cells to home into PLN.
In contrast with PLN, PP and spleen in plt/plt contain as many or more T cells than those in +/+.8 In this report we examined T-cell–homing deficiency on the functional and histological basis in PLN, mesenteric LN (MLN), PP, and spleen inplt/plt. In addition, as an approach to identify theplt gene, we mapped the gene on chromosome by the analysis with simple-sequence length polymorphism (SSLP) of genomic DNA9,10 in the intercrossing progeny between DDD/1 mouse (Mus musculus domesticus) [plt/plt]11 and Japanese wild-mouse–derived inbred strain MSM/Ms (Mus musculus molossinus) [+/+].12
MATERIALS AND METHODS
Mice.
DDD/1 (DDD/1-plt/plt) and DDD/1–Mtv-2/Mtv-2(DDD/1-+/+) were maintained in the specific pathogen-free conditions in animal facilities in the Institute of Medical Science, University of Tokyo (Tokyo, Japan).8,11,13MSM/Ms (MSM)12 were kindly provided by Dr Toshihiko Shiroishi (National Institute of Genetics, Mishima, Shizuoka, Japan). BALB/c mice were purchased from SLC (Hamamatsu, Shizuoka, Japan). BALB/c-plt/plt were produced by backcrossing of mice expressing plt phenotype that was detected with low content of T cells in biopsied inguinal LN by flow cytometry.8 14Sixth-backcrossed mice were used for experiments.
Antibodies.
Anti-TCR Cβ (H57-597), -CD3 (145-2C11), -Thy1.2 (30H12), -B220 (RA3-3A1/6.1), -CD4 (GK1.5), -CD8 (53-6-7.2), –L-selectin (MEL-14), -CD44 (KM201), and -FcRγII (2.4G2) MoAbs were used. Purified H57-597, 145-2C11, RA3-3A1/6.1, MEL-14, and KM201 were conjugated with fluorescein isothiocyanate (FITC) (Sigma, St Louis, MO) or with biotin-NHS (Vector, Burlingame, CA). FITC-conjugated anti-B220 MoAb (RA3-6B2) was purchased from Pharmingen (San Diego, CA). Antimetallophilic macrophage MoAb (MOMA-1)15 and Cy5-conjugated goat antirat IgG antibodies (Ab) were purchased from BMA Biomedicals Ltd (Augst, Swizerland) and Amersham (Buckinghamshire, England), respectively.
In vivo homing assay.
LN cells were collected from PLN and MLN of DDD/1-+/+. T cells were enriched from DDD/1-+/+ or DDD/1-plt/plt spleen cells through nylon wool column, followed by panning with goat antimouse IgG (Cappel, Durham, NC)-coated dish, and B cells by panning with anti-CD4 and -CD8 MoAb-coated dish after removal of adherent cells. T-cell– or B-cell–enriched preparation always contained more than 90% CD3+ or B220+ cells, respectively. These cells (4 × 107/mL) were labeled with 3 μmol/L 3'-acetyl-2'-carboxyethyl-6',7'-(dihydropyran-2”-one)-5 or 6-carboxyfluorescein diacethoxymethyl ester (BCECF-AM) (Dojindo, Kumamoto, Japan) at 37°C for 1 minute in Hank's balanced salt solution (HBSS) containing 5% fetal calf serum (FCS).8After washing, 1 × 107 or 5 × 107labeled cells were injected intravenously into mice. Fluorecence-positive cells in PLN, MLN, PP, and spleen were detected with flow cytometry or histology.
Assessment of leukocyte accumulation into peritoneum.
Mice were intraperitonealy injected with 1 mL of 2% thioglycollate (Nissui, Tokyo, Japan) to induce peritonitis.16 They were killed 24 or 48 hours later, intraperitonealy injected with 5 mL of phosphate-buffered saline (PBS) containing 2% FCS, 0.5 mmol/L EDTA, and 10 U/mL heparin (Takeda, Osaka, Japan), and massaged extensively. PBS was recovered from the peritoneum, and the cells were washed once, pelleted by centrifugation, resuspended in the buffer described above, and counted under a microscope. After washing, 5 × 105 cells in 5 μL FCS were smeared onto a glass slide. The cells stained with Giemsa's solution (Merck Japan, Tokyo, Japan) were examined morphologically under a microscope. The percentages of neutrophils and macrophages were calculated from more than 200 cells.
Immunohistochemical staining.
Cryostat sections (8 μm) from the lymphoid organs were dried and fixed with acetone. After washing with PBS, the section was incubated with 2% normal rabbit serum in PBS, containing 1% bovine serum albumin (BSA) to prevent nonspecific reactions, and then sequentially treated with 30H12 or RA3-3A1/6.1 MoAb with biotinylated rabbit antirat IgG polyclonal Ab (mouse serum adsorbed, Vector), and with horseradish peroxidase-conjugated streptavidin (Zymed, SanFrancisco, CA). Peroxidase activity was visualized with 3, 3'-diaminobenzidine tetrahydrochloride (Dojindo). The sections were counterstained with Gill's hematoxylin (Polysciences, Warrington, PA) and examined under a microscope.8 To study homing ability of BCECF-labeled lymphocytes injected into mice, a cryostat section from the spleen was stained with MOMA-1 MoAb that detects marginal zone macrophage (MZM)15 and Cy5-conjugated antirat IgG, then examined with a confocal laser microscope system (Bio-Rad Laboratories, Tokyo, Japan).
Flow cytometry.
Single-cell suspensions were prepared from PLN, PP, and spleen and were depleted of red blood cells by hemolysis. One million cells in each sample were stained with FITC-conjugated MoAb in PBS containing 3% FCS and 0.1% NaN3, or biotinylated MoAb followed by phycoerythrin-conjugated streptavidin (SA-PE) (Becton Dickinson, Mountain View, CA).17 Nonspecific reaction was blocked by preincubation with mouse IgG and 2.4G2 MoAb if necessary. The stained cells were analyzed on a FACScan (Becton Dickinson). Lymphocytes recognized with the forward and sideward scatter were analyzed. Dead cells positively stained with 7-aminoactinomycin D (Sigma) were gated out.14
Phenotype assessement.
(DDD/1-plt/plt × MSM) F1 hybrid and F2 progeny were produced. Backcross (BC) progeny were produced by mating male F1 to female DDD/1-plt/pltor female F1 to male DDD/1-plt/plt. Mice were phenotyped at 5 to 6 weeks of age. Lymphocytes of PLN (axillary, brachial, and inguinal) were counted, dual-stained with FITC-conjugated anti-TCR and biotinylated anti–L-selectin MoAb, followed by SA-PE, and analyzed in flow cytometry. Phenotype was determined based on the PLN cell count and the percentage of L-selectin+TCR+ cells. A section of spleen was immunohistochemically analyzed with anti-Thy1.2 or anti-B220 MoAb, andplt/plt showed the characteristic localization of T cells (Fig 1).
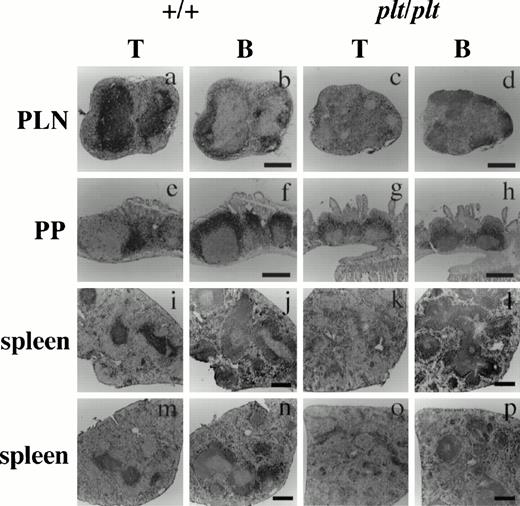
Histological analysis of T and B cells in PLN, PP, and spleen in +/+ and plt/plt. PLN (a-d), PP (e-h) and spleens (i-l) were from DDD/1-+/+ and DDD/1-plt/plt. Spleens from BALB/c-+/+ (m,n) and BALB/c-plt/plt (o, p) were also examined. Cryostat section was treated sequentially with 30H12 (anti-Thy1.2) or RA3-3A1/6.1 (anti-B220) MoAb, biotinylated rabbit antirat IgG (Vector) polyclonal Ab, and horse radish peroxidase-conjugated streptavidin (Zymed), and visualized with 3, 3'-diaminobenzidine tetrahydrochloride (Dojindo). The sections were counterstained with Gill's hematoxylin (Polysciences) and photographed under a microscope. Bar indicates 400 μm.
Histological analysis of T and B cells in PLN, PP, and spleen in +/+ and plt/plt. PLN (a-d), PP (e-h) and spleens (i-l) were from DDD/1-+/+ and DDD/1-plt/plt. Spleens from BALB/c-+/+ (m,n) and BALB/c-plt/plt (o, p) were also examined. Cryostat section was treated sequentially with 30H12 (anti-Thy1.2) or RA3-3A1/6.1 (anti-B220) MoAb, biotinylated rabbit antirat IgG (Vector) polyclonal Ab, and horse radish peroxidase-conjugated streptavidin (Zymed), and visualized with 3, 3'-diaminobenzidine tetrahydrochloride (Dojindo). The sections were counterstained with Gill's hematoxylin (Polysciences) and photographed under a microscope. Bar indicates 400 μm.
Genotype analysis.
For assessment of SSLP, 100 ng of genomic DNA from liver of DDD/1-plt/plt, MSM, and BC progenies were amplified in a 10 μL polymerase chain reaction (PCR) with MapPairs primer (Genetic Research Inc, Huntsville, AL).9,10 Information for microsatellite markers was obtained from the database of the Genetic and Physical Map of the Mouse Genome published by Massachusetts Institute of Technology (http://www.genome.wi.mit.edu./). The conditions for PCR were 30 seconds at 96°C (5 minutes for the first cycle), 1 minute at 55°C, and 1.5 minutes at 72°C for 35 cycles in 10 mmol/L Tris-HCl (pH9.0) containing 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L deoxynucleotide triphosphates, 0.1% Triton X-100, 0.2 μmol/L each primer, and 0.05 U rTaq DNA polymerase (Toyobo, Tokyo, Japan).18 In amplification for D4Mit237, concentration of MgCl2 was 2 mmol/L. To detect polymorphism in the CD72 gene, the following primers were used for PCR amplification of a simple sequence repeat found in genomic CD72 DNA sequence19: left, 5'-ATGGGAGATGCTGGATGGAGAT-3'; and right, 5'-CAGACCTAATTCCAACACTCAG-3'. The amplified products were subjected to electrophoresis on a 3% NuSieve 3:1 agarose gel (FMC Bioproducts, Rockland, ME), visualized with ethidium bromide staining, and photographed. The size of each PCR product from BC progeny was compared with that from parental strains, DDD/1-plt/plt and MSM. Individuals with wild-type phenotype (plt/+) and homogenous DDD/1/DDD/1 genotype for each marker, or with a mutant phenotype (plt/plt) and heterogenous DDD/1/MSM genotype, were assessed as a recombinant. Recombination distance between pltand each marker or between markers was analyzed by computer by using Map Manager v2.6.4 software provided by Drs Kenneth F. Manly and Robert Cudmore (Roswell Park Cancer Institute, State University of New York, Buffalo, NY). Information for chromosomal mapping was obtained from the database of the Genetic and Physical Map of the Mouse Genome by Massachusetts Institute of Technology or from National Center for Biothechnology Information by the National Institutes of Health (http://www.ncbi.nlm.nih.gov./).
RESULTS
Unusual distribution of T cells in PLN, PP, and spleen in plt/plt.
To analyze the effect of the plt mutation on peripheral lymphoid organs, the distribution of T and B cells in PLN, PP, and spleen from plt/plt was immunohistochemically compared with that from +/+. In PLN of +/+, T cells were densely distributed in the subcortical T-cell zones (Fig 1a). In PLN ofplt/plt, however, the cellularity of T cells was extremely low, and the stroma was much more dominant in the subcortical zone than in+/+ (Fig 1c). In PP of +/+, T cells were densely observed in the T-cell zones between B-cell zones filled with B220high and B220low B cells (Fig 1e, f), and few T cells were found in the B-cell zones. In plt/plt, the number and the size of PP were varied from mouse to mouse and from PP to PP, and most of PP were smaller than those in +/+. T cells were hardly observed in the distinct T-cell zones in PP fromplt/plt, but a lot of T cells were observed in the area occupied by B cells (Fig 1g, h). Also in spleen, the T-cell distribution was quite different between plt/plt and+/+. In +/+, spleen T cells colonized densely at the periarterial lymphatic sheath in the white pulp and spread sparsely in the red pulp (Fig 1i). In plt/plt spleen, however, few T cells were found in the white pulp, whereas numerous T cells distributed around the vascular sinusoid in the red pulp (Fig 1k). The unusual T-cell distribution in the spleen was observed not only in DDD/1-plt/plt but also in BALB/c-plt/plt (Fig 1o). In contrast, the B-cell distribution in PLN (Fig 1b, d), PP (Fig 1f, h), and spleen (Fig 1j, l, n, p), in plt/plt was not different from that in +/+. These results strongly suggest that theplt gene is involved in T-cell homing not only in PLN but also in PP and spleen.
Defect in T-cell migration into PLN, PP, and spleen white pulps in plt/plt.
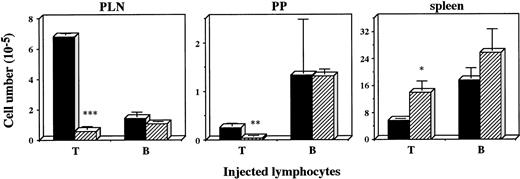
Defective recruitment of T cells into PLN was previously found inplt/plt.8 To examine whether the defect was also observed in other peripheral lymphoid organs, lymphocyte homing into PLN, PP, and spleen was assessed in plt/plt. Lymphocytes from+/+ PLN were labelled with fluorescent BCECF, intravenously injected into plt/plt and +/+, and the labelled lymphocytes were detected at 2 and 48 hours, and 6 days after the injection. The number of fluorescence-positive cells in PLN, MLN, or PP in plt/plt was always significantly smaller than that in+/+. In spleen, however, fluorescence-positive cells inplt/plt were more than those in +/+ throughout the observation period (data not shown). To compare T-cell homing with B-cell homing, fluorescence-labeled T or B cells enriched from +/+ spleen cells were injected into mice, and detected in PLN, PP, and spleen 48 hours after the injection. The labelled T cells in PLN and PP in plt/plt were fewer than those in +/+, but labelled B cells were detected inplt/plt as well as in +/+(Fig 2). In spleen, in contrast, the labelled T cells in plt/plt were more than in +/+ (Fig2). These results suggest a possibility that the homing deficiency inplt/plt results from some defect(s) in secondary lymphoid organs.
Homing of T and B cells into PLN, PP, and spleen in vivo. T and B cells were enriched from spleen of DDD/1-+/+, labeled with BCECF-AM (Dojindo) and 1 × 107 of the cells were intravenously injected. Fluorescence-positive cells in PLN, PP, and spleen of DDD/1-+/+ (filled column) or DDD/1-plt/plt (crosshatched column) were detected with flow cytometry at 48 hours after the injection. Cell number was calculated from the total cell number in each tissue and percentage of fluorescence-positive cells. Mean and SD from two mice is indicated. *P < .072, ** P < .05, *** P < .001.
Homing of T and B cells into PLN, PP, and spleen in vivo. T and B cells were enriched from spleen of DDD/1-+/+, labeled with BCECF-AM (Dojindo) and 1 × 107 of the cells were intravenously injected. Fluorescence-positive cells in PLN, PP, and spleen of DDD/1-+/+ (filled column) or DDD/1-plt/plt (crosshatched column) were detected with flow cytometry at 48 hours after the injection. Cell number was calculated from the total cell number in each tissue and percentage of fluorescence-positive cells. Mean and SD from two mice is indicated. *P < .072, ** P < .05, *** P < .001.
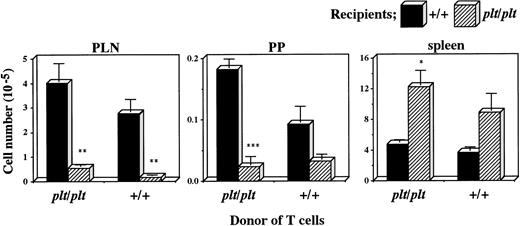
There was another possibility, however, that plt/plt T cells themselves had a homing deficiency into secondary lymphoid organs. To examine this possibility, plt/plt T cells were compared with those from +/+ for the migration into secondary lymphoid organs. T cells were prepared from +/+ or plt/pltspleen, labelled, intravenously injected into +/+ andplt/plt mice, and detected in PLN, PP, and spleen 40 hours after the injection. Labelled T cells from +/+ migrated into PLN, PP, and spleen in +/+ recipients (Fig 3), as described above. When those from plt/plt were injected into +/+ recipients, they migrated into these organs as well as, or rather better than those from+/+, whereas their homing into these organs was deficient when injected into plt/plt recipients (Fig 3). The deficiency was similar to that observed when +/+ T cells were intravenously injected into plt/plt recipients (Figs 2 and 3). Thus, the defect in the migration ability was not detected in T cells fromplt/plt. These results strongly support our previous suggestion that homing deficiency in plt/plt is caused by the defect in the stromal cells in secondary lymphoid organs, but not in T cells themselves.
Comparison of plt/plt T cells with those from+/+ for the migration into PLN, PP, and spleen. T cells were enriched from DDD/1-plt/plt or DDD/1-+/+spleen cells, and labelled with BCECF-AM. Ten million cells were intravenously injected into DDD/1-+/+ and DDD/1-plt/plt recipient mice. Forty hours after the injection, fluorescence-positive cells were detected in PLN, PP, and spleen in DDD/1-+/+ (filled columns) and in DDD/1-plt/plt(crosshatched columns) recipients. Cell number was calculated from the total cell number in each tissue and percentage of fluorescence-positive cells. Mean and SD from two recipient mice is indicated. *P < .053, ** P < .05, *** P < .005.
Comparison of plt/plt T cells with those from+/+ for the migration into PLN, PP, and spleen. T cells were enriched from DDD/1-plt/plt or DDD/1-+/+spleen cells, and labelled with BCECF-AM. Ten million cells were intravenously injected into DDD/1-+/+ and DDD/1-plt/plt recipient mice. Forty hours after the injection, fluorescence-positive cells were detected in PLN, PP, and spleen in DDD/1-+/+ (filled columns) and in DDD/1-plt/plt(crosshatched columns) recipients. Cell number was calculated from the total cell number in each tissue and percentage of fluorescence-positive cells. Mean and SD from two recipient mice is indicated. *P < .053, ** P < .05, *** P < .005.
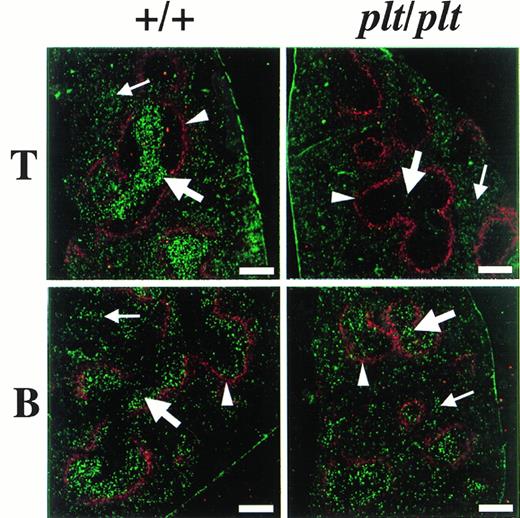
The distribution of fluorescence-labelled T and B cells in spleen 48 hours after the injection was also examined in plt/plt and in+/+. To identify the white pulp, the marginal zone was stained with MoAb specific for metalofilic macrophages. In plt/plt, few T cells labelled with fluorescence were detected in white pulp, whereas many labelled T cells were observed outside the marginal zone, ie, the red pulp (Fig 4). In +/+ spleen, the accumulation of labelled T cells was observed at the periarterial lymphatic sheath in the white pulp, as expected. Labelled B cells in the white pulp were detected in plt/plt as well as in+/+ (Fig 4). These results indicate that T cells migrate into the white pulp in +/+, but do not in plt/plt, although B cells are able to migrate into the white pulp in plt/plt as well as in +/+.
Localization of T and B cells homing into spleen. T and B cells were enriched from spleen of DDD/1-+/+, labeled with BCECF-AM (Dojindo), and 5 × 107 of the cells were intravenously injected into DDD/1-+/+ and DDD/1-plt/plt. Spleens from recipients were frozen at 48 hours after the injection. Cryostat section of the spleen was stained with MOMA-1 MoAb (BMA Biomedicals Ltd) and Cy5-conjugated antirat IgG (Amersham) to identify MZM, then examined under a confocal laser microscope system (Bio-Rad). The region sorrounded by MZM (red, indicated by arrowheads) is white pulp. Injected T or B cells (green) were identified in white pulp (thick arrow) and in red pulp (thin arrow). Bar at the lower right corner indicates 250 μm.
Localization of T and B cells homing into spleen. T and B cells were enriched from spleen of DDD/1-+/+, labeled with BCECF-AM (Dojindo), and 5 × 107 of the cells were intravenously injected into DDD/1-+/+ and DDD/1-plt/plt. Spleens from recipients were frozen at 48 hours after the injection. Cryostat section of the spleen was stained with MOMA-1 MoAb (BMA Biomedicals Ltd) and Cy5-conjugated antirat IgG (Amersham) to identify MZM, then examined under a confocal laser microscope system (Bio-Rad). The region sorrounded by MZM (red, indicated by arrowheads) is white pulp. Injected T or B cells (green) were identified in white pulp (thick arrow) and in red pulp (thin arrow). Bar at the lower right corner indicates 250 μm.
Increase in the proportion of PLN T cells with memory phenotype in plt/plt.
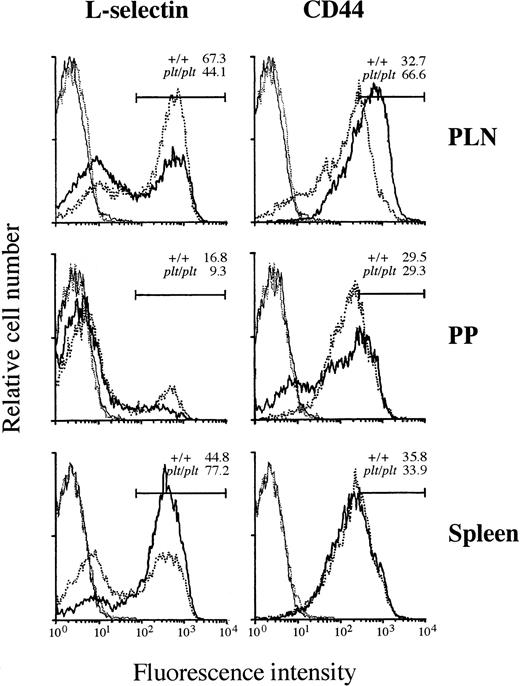
Although the T-cell–specific defect in plt/plt was found in the migration into PLN, PP, or spleen white pulp (Figs 2-4), some T cells were still detected in these lymphoid organs. To characterize these T cells, the expression of αL and α4integrins, L-selectin, and CD44 was examined. αL and α4 integrins were similary expressed on T cells from PLN, PP, and spleen in plt/plt and +/+ (data not shown). In PLN, the percentage of L-selectin+ T cells inplt/plt was smaller than in +/+, but that of T cells highly expressing CD44 (CD44high) in plt/plt was larger than in +/+ (Fig 5), suggesting that the frequency of memory T cells in plt/plt PLN was higher than in +/+, whereas predominant T cells expressed naive phenotype in +/+ PLN, as reported previously.20-22 In PP, although majority of T cells were L-selectin− both in plt/plt and in +/+, L-selectin+ T cells were less frequent in plt/pltthan in +/+. In plt/plt, the percentage of CD44high T cells was similar to, and that of those slightly expressing CD44 (CD44low) was larger than that in+/+ (Fig 5). The smaller percentage of L-selectin+T cells might suggest that the proportion of memory T cells inplt/plt was larger also in PP than in +/+. In spleen, L-selectin+ T cells were more frequent in plt/pltthan in +/+, but the obvious difference in the expression of CD44 was not observed among them. These L-selectin+ T cells might represent naive T cells. The expression of these molecules on B cells in PLN, PP, and spleen from plt/plt was not different from that from +/+ (data not shown).
Expression of L-selectin and CD44 on T-cell surface. Lymphocytes from LN, PP, and spleen of DDD/1-+/+ (dotted line) or DDD/1-plt/plt (solid line) were stained with FITC-conjugated H57-597 (anti-Cβ TCR) and biotinylated MEL-14 (anti–L-selectin) or KM201 (anti-CD44) MoAb followed by phycoerythrin-conjugated streptavidin (Becton Dickinson). The stained cells were analyzed in a FACScan (Becton Dickinson). T cells expressing TCR were gated and their expression of L-selectin or CD44 molecules (bold line) were analyzed. Cells stained only with FITC-conjugated H57-597 and phycoerythrin-conjugated streptavidin as a negative control (fine line) were also shown. Each line indicates the result as follows: bold dotted line: L-selectin or CD44 expression in +/+; bold solid line: L-selectin or CD44 expression in plt/plt; fine dotted line: negative control in +/+; fine solid line: negative control in plt/plt. The percentage of T cells expressing L-selectin or highly expressing CD44 in +/+ orplt/plt was indicated in the each panel. Reproducible results were obtained from three independent similar analyses.
Expression of L-selectin and CD44 on T-cell surface. Lymphocytes from LN, PP, and spleen of DDD/1-+/+ (dotted line) or DDD/1-plt/plt (solid line) were stained with FITC-conjugated H57-597 (anti-Cβ TCR) and biotinylated MEL-14 (anti–L-selectin) or KM201 (anti-CD44) MoAb followed by phycoerythrin-conjugated streptavidin (Becton Dickinson). The stained cells were analyzed in a FACScan (Becton Dickinson). T cells expressing TCR were gated and their expression of L-selectin or CD44 molecules (bold line) were analyzed. Cells stained only with FITC-conjugated H57-597 and phycoerythrin-conjugated streptavidin as a negative control (fine line) were also shown. Each line indicates the result as follows: bold dotted line: L-selectin or CD44 expression in +/+; bold solid line: L-selectin or CD44 expression in plt/plt; fine dotted line: negative control in +/+; fine solid line: negative control in plt/plt. The percentage of T cells expressing L-selectin or highly expressing CD44 in +/+ orplt/plt was indicated in the each panel. Reproducible results were obtained from three independent similar analyses.
Accumulation of neutrophils and macrophages into inflammatory site in plt/plt.
To examine the ability of plt/plt to recruit neutrophils and macrophages into inflammatory site, thioglycollate was intraperitonealy injected and the number of neutrophils and macrophages in the peritoneum was determined 12, 24, and 48 hours after injection. The number of neutrophils and macrophages in plt/plt peritoneum increased rapidly as in +/+ (data not shown), suggesting that the ability of plt/plt to recruit these cells was not affected.
plt gene locates on chromosome 4.
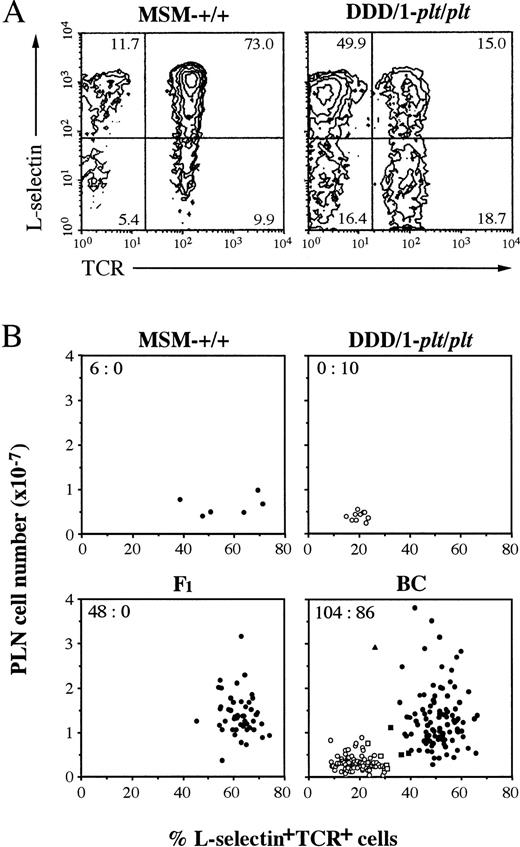
In our previous report, plt has been shown to be a autosomal recessive gene.8 For further analysis, we intercrossed DDD/1-plt/plt with MSM, an inbred strain derived from a Japanese wild mouse,12 to produce F1, BC, and F2 progeny. The phenotype of the progeny was assessed by PLN cell count and flow cytometric analysis of PLN cells, andplt/plt phenotype was distingushed from wild-type by paucity of T cells and by the low percentage of L-selectin+TCR+ cells (Fig 6A). The phenotype of individual mouse in MSM-+/+, DDD/1-plt/plt, F1, and BC was plotted against PLN cell number and percentage of L-selectin+TCR+ cells, as shown in Fig 6B. Inplt/plt phenotype, PLN cell number was less than 1 × 107, and the frequency of L-selectin+TCR+ cells was less than 31%. Mice with plt/plt phenotype, determined on this criteria, showed the characteristic distribution of T cells in spleen as shown in Fig 1k, o. Although the percentage of L-selectin+TCR+cells in PLN from MSM-+/+ was clearly higher than that from DDD/1-plt/plt, the number of PLN cells in MSM-+/+ was as small as DDD/1-plt/plt (Fig 6B), which was probably due to the small body weight of the former (11.82 ± 0.65 g) as compared with the latter (22.87 ± 1.48 g). One exceptional mouse in BC had only 26% L-selectin+TCR+ cells in PLN, but this mouse was identified as a heterozygote based on the large number of PLN cells and the quite similar distribution of T cells in spleen to that in +/+ (data not shown). Forty-eight F1 mice examined exclusively expressed wild-type phenotype. Wild-type:plt/plt ratio was 104:86 in BC. In the similar assessment, wild-type:plt/plt ratio was 72:28 in F2. Similar ratios were obtained in male and female progenies. These results confirmed that plt is an autosomal recessive gene, as previously reported.8
Phenotypic analysis of lymphocytes in PLN. Lymphocytes from PLN were counted and stained with FITC-conjugated H57-597 (anti-Cβ TCR) and biotinylated MEL-14 (anti–L-selectin) MoAb followed by phycoerythrin-conjugated streptavidin and analyzed in a FACScan (Becton Dickinson). (A) Total 10,000 cells of PLN from MSM-+/+ or DDD/1-plt/plt were analyzed and percentage was indicated at the corner of each quadrant.plt/plt was characteristically distinguished by small content of T cells, especially of L-selectin+TCR+cells (15.0%) compared with those of +/+ (73.0%). (B) PLN cell number and the percentage of L-selectin+TCR+ cells in PLN lymphocytes of individual mouse in MSM-+/+, DDD-plt/plt, F1, and BC progeny were assessed and represented with a dot. BC was pool of 101 (DDD/1-plt/plt × F1)BC and 89 (F1 × DDD/1-plt/plt)BC. plt/pltwas distinguished by paucity of PLN cell number (less than 1 × 107) and small content of L-selectin+TCR+ cells (less than 31%). The percentage of L-selectin+TCR+ cells of wild-type (+/+ or plt/+) were more than 32%. Some mice (□ or ▧) in BC were decided their phenotype by the T-cell distribution in the spleen in immunohistochemical analysis as shown in Fig 1i, k. Wild-type or plt/plt were indicated with black or white, respectively. One exceptional mouse (▴) in BC showed 26% L-selectin+TCR+ cells in PLN, but this was classified as a heterozygote based on large number of PLN cells and the quite similar distribution of T cells in spleen to that in+/+. The number of wild-type versus that of plt/pltis indicated at the upper corner of each group.
Phenotypic analysis of lymphocytes in PLN. Lymphocytes from PLN were counted and stained with FITC-conjugated H57-597 (anti-Cβ TCR) and biotinylated MEL-14 (anti–L-selectin) MoAb followed by phycoerythrin-conjugated streptavidin and analyzed in a FACScan (Becton Dickinson). (A) Total 10,000 cells of PLN from MSM-+/+ or DDD/1-plt/plt were analyzed and percentage was indicated at the corner of each quadrant.plt/plt was characteristically distinguished by small content of T cells, especially of L-selectin+TCR+cells (15.0%) compared with those of +/+ (73.0%). (B) PLN cell number and the percentage of L-selectin+TCR+ cells in PLN lymphocytes of individual mouse in MSM-+/+, DDD-plt/plt, F1, and BC progeny were assessed and represented with a dot. BC was pool of 101 (DDD/1-plt/plt × F1)BC and 89 (F1 × DDD/1-plt/plt)BC. plt/pltwas distinguished by paucity of PLN cell number (less than 1 × 107) and small content of L-selectin+TCR+ cells (less than 31%). The percentage of L-selectin+TCR+ cells of wild-type (+/+ or plt/+) were more than 32%. Some mice (□ or ▧) in BC were decided their phenotype by the T-cell distribution in the spleen in immunohistochemical analysis as shown in Fig 1i, k. Wild-type or plt/plt were indicated with black or white, respectively. One exceptional mouse (▴) in BC showed 26% L-selectin+TCR+ cells in PLN, but this was classified as a heterozygote based on large number of PLN cells and the quite similar distribution of T cells in spleen to that in+/+. The number of wild-type versus that of plt/pltis indicated at the upper corner of each group.
We mapped plt gene on chromosome by analyzing 190 BC progenies, which was a pool of 101 (DDD/1-plt/plt × F1)BC and 89 (F1 × DDD/1-plt/plt)BC mice. Genotype was determined with SSLP in genomic DNA.9,10 When three markers on each chromosome were analyzed in 22 BC progenies expressing plt/plt phenotype, markers on only chromosome 4 showed high linkage with the phenotype (D4Mit4: 100%; D4Mit9: 77.3%; and D4Mit16: 54.5%). To obtain precise linkage map of plt locus, further markers around D4Mit4 (Dietrich et al10, and Genetic and Physical Map of the Mouse Genome) were used for the analysis of 190 BC progenies.plt showed high linkage with D4Mit4, D4Mit286, and CD72, and was not segregated from D4Mit237 (Table 1).plt was mapped on 24.7 cM from the centromere when D4Mit149 was used as a centromere marker (Fig 7). CD72, which is the ligand for CD5 on T cells23 and expressed on B cells, linked with plt. CD72, however, locates on a distinct locus from plt, because a recombinant was obtained in BC (Table1, Fig 7).
Linkage Between plt and the Loci on Chromosome 4
| Locus Symbol . | Recombinant In . | Recombinant Frequency ± SE (%) . | ||
|---|---|---|---|---|
| 101 (DDD/1-plt/plt × F1)BC . | 89 (F1 × DDDplt/plt)BC . | 190 Total BC . | ||
| D4Mit4 | 4 | 1 | 5 | 2.6 ± 1.2 |
| D4Mit9 | 18 | 18 | 36 | 18.9 ± 2.8 |
| D4Mit108 | 4 | 2 | 6 | 3.2 ± 1.3 |
| D4Mit138 | 5 | 5 | 10 | 5.3 ± 1.6 |
| D4Mit149 | 29 | 18 | 47 | 24.7 ± 3.1 |
| D4Mit237 | 0 | 0 | 0 | 0.0 |
| D4Mit286 | 0 | 1 | 1 | 0.5 ± 0.5 |
| CD72 | 0 | 1 | 1 | 0.5 ± 0.5 |
| Locus Symbol . | Recombinant In . | Recombinant Frequency ± SE (%) . | ||
|---|---|---|---|---|
| 101 (DDD/1-plt/plt × F1)BC . | 89 (F1 × DDDplt/plt)BC . | 190 Total BC . | ||
| D4Mit4 | 4 | 1 | 5 | 2.6 ± 1.2 |
| D4Mit9 | 18 | 18 | 36 | 18.9 ± 2.8 |
| D4Mit108 | 4 | 2 | 6 | 3.2 ± 1.3 |
| D4Mit138 | 5 | 5 | 10 | 5.3 ± 1.6 |
| D4Mit149 | 29 | 18 | 47 | 24.7 ± 3.1 |
| D4Mit237 | 0 | 0 | 0 | 0.0 |
| D4Mit286 | 0 | 1 | 1 | 0.5 ± 0.5 |
| CD72 | 0 | 1 | 1 | 0.5 ± 0.5 |
Recombination between plt and 8 locus markers on chromosome 4 were examined in 101 {DDD/1-plt/plt × (DDD/1-plt/plt × MSM-+/+)F1}BC and 89 {(DDD/1-plt/plt × MSM-+/+)F1 × DDD/1-plt/plt}BC progenies. Phenotypes were assessed with cell count and flow cytometry of PLN, and immunohistochemistry of spleen.plt/plt was distinguished with paucity of PLN cell number (less than 1 × 107) and small content of L-selectin+ TCR+ cells (less than 31%) (Fig6B). Genomic DNA from BC progeny were amplified by PCR with MapPairs primer (Genetic Research Inc). To detect polymorphism in CD72 gene, the following primers were used, left: 5′-ATGGGAGATGCTGGATGGAGAT-3′ and right: 5′-CAGACCTAATTCCAACACTCAG-3′. The amplified products were subjected to electrophoresis, visualized with ethidium bromide staining, and photographed. The size of the PCR product was compared with that from parental strains to determine its genotype. Recombinant had wild-type phenotype (plt/+) and DDD/1/DDD/1 homozygote genotype, or mutant phenotype (plt/plt) and DDD/1/MSM heterozygote genotype for each locus. Recombination frequency was calculated with Map Manager software created by Drs Kenneth F. Manly and Robert Cudmore (Roswell Park Cancer Institute, State University of New York, Buffalo, NY).
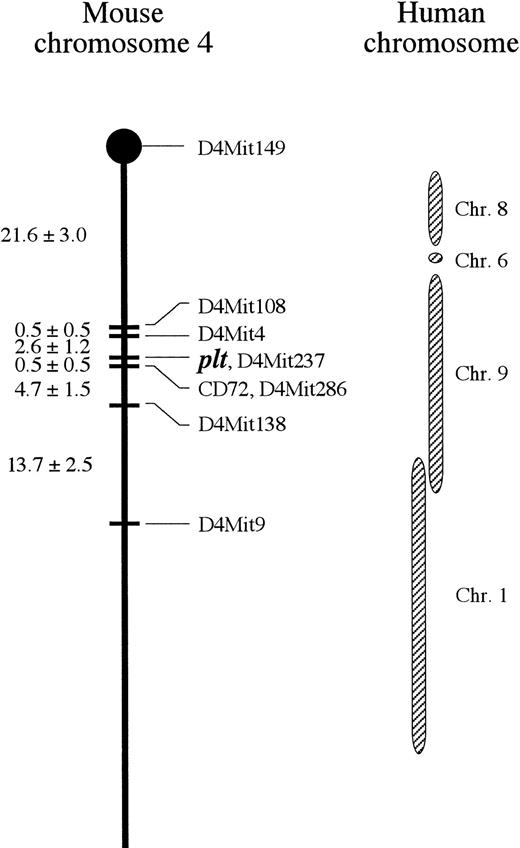
Linkage map of mouse chomosome 4 including pltlocus. Distance ± SE (cM) between each locus is expressed at left side. plt is located on 24.7 cM from cetromere and most closely linked with D4Mit237. Corresponding human chromosomes with mouse chromosome 4 are indicated.38
Linkage map of mouse chomosome 4 including pltlocus. Distance ± SE (cM) between each locus is expressed at left side. plt is located on 24.7 cM from cetromere and most closely linked with D4Mit237. Corresponding human chromosomes with mouse chromosome 4 are indicated.38
DISCUSSION
Previously, we have shown the T-cell–specific paucity in PLN inplt/plt mutant mice,8,17 and the paucity seems to result from the inability of PLN stroma to allow T cells to migrate from blood into PLN.8 In the present study, we investigated whether the plt mutation affected other peripheral lymphoid organs as well as PLN. Although the T-cell frequency in plt/pltPP is comparable with that in +/+ PP in our previous study,8 the distribution of T cells in plt/plt PP is different from that in +/+ PP. In plt/plt PP, T cells were unusually detected in the area occupied by B cells rather than in the distinct T-cell zone, and the T-cell zone and B-cell zones were unable to be distinguished (Fig 1). The unusual distribution of T cells in plt/plt PP might be due to the failure for T cells to migrate from blood into PP, because the labelled T cells injected intravenously were almost undetectable in plt/plt PP, whereas labelled and IV injected B cells were detected in plt/plt pp as well as in +/+ PP (Figs 2 and 3). Similar failure was observed in the T-cell migration into PLN inplt/plt. Thus, the T-cell migration from blood into PP is severely affected in plt/plt, as well as into PLN, which suggests that most of T cells in plt/plt PP might migrate from the surrounding submucosal tissue. The distribution of B cells inplt/plt PP was similar to that in +/+, except for the colocalization with T cells in plt/plt PP. Thus, pltmutation specifically affects the distribution of T cells not only in PLN, but also in PP.
It has been shown that T cells migrating from blood into PLN through HEV predominantly express a naive phenotype, whereas those from the afferent lymphatics express a memory phenotype.20 In PLN from plt/plt, the proportion of T cells with L-selectin- and CD44high, which are phenotypically memory T cells,22 is larger than in that from +/+, suggesting that most of T cells in plt/plt PLN migrated from the afferent lymphatics, whereas those in +/+ PLN migrated either from blood or afferent lymphatics, as described.24 Consistently, few labelled T cells intravenously injected were detected in plt/plt PLN, but many in +/+ PLN. In plt/plt PP, however, the proportion of T cells with this phenotype is not larger than that in +/+ PP. If most of T cells in PP were memory cells, as suggested,25their phenotype might be different from that in PLN.
The distribution of T cells in plt/plt spleen is quite different from that in +/+, although the T-cell content is never smaller than in +/+. In plt/plt spleen, few T cells were found in the white pulp but a lot of T cells were found in the red pulp (Fig 1). The number of the labelled T cells that were injected intravenously and migrated into plt/plt spleen was greater than that into +/+ spleen (Figs 2 and 3), but most of them were localized in the red pulp in plt/plt, whereas the major part of them in +/+ were detected in the periarterial lymphatic sheath in the white pulp (Fig 4). Characteristically, the labelled T cells inplt/plt spleen hardly migrated into the white pulp across the marginal zone (Fig 4). The labelled B cells injected intravenously, however, localized mainly in the white pulp even in plt/pltspleen. Thus, plt mutation selectively affects T-cell migration from blood into the white pulp in spleen, which might result in the unusual distribution of T cells in plt/plt spleen (Fig 1). The subpopulations of T cells in plt/plt spleen might be different from those of +/+ because the majority of T cells inplt/plt spleen expressed L-selectin, whereas about a half of T cells in +/+ spleen did not (Fig 5). This finding might suggest that naive T cells were dominant in plt/plt spleen. The expression of CD44, however, was quite similar on T cells in spleens from plt/plt and from +/+.
The defect of T-cell homing and the relative increase in memory-type T cells has been shown also in L-selectin–deficient mice.16,26 Although the T-cell–specific paucity inplt/plt seems to be due to the failure of PLN or spleen stromal cells to allow T-cell homing across the HEV or the marginal zone8 (Figs 3 and 4), respectively, low frequency of L-selectin+ T cells in plt/plt PLN and PP (Fig 5) raises a possibility that L-selectin+ T cells might be fewer in plt/plt than in +/+, resulting in a smaller number of L-selectin+ T cells that migrate into these lymphoid tissues in plt/plt than in +/+. It might be also possible that the paucity was due to the deficient expression of or dysfunction of L-selectin in plt/plt. These possibilities are unlikely, however, based on the following reasons: (1) The propotion of L-selectin+ T cells in spleen (Fig 5) and in peripheral blood (data not shown) is higher in plt/plt than in+/+, suggesting that the T-cell–specific paucity is not caused by a smaller number of L-selectin+ T cells; (2) T cells from plt/plt spleen are able to migrate into +/+ PLN or PP (Fig 3), as well as those from +/+; (3) B-cell distribution inplt/plt is quite similar in plt/plt and in +/+, but is impaired in L-selectin–deficient mice26; (4) The accumulation of neutrophiles and macrophages into the thioglycollate-stimulated peritoneum is not affected in plt/plt(data not shown), whereas that has been reported not to accumulate in L-selectin–deficient mice16; (5) In addition, the expression or function of L-selectin ligand is not affected inplt/plt.8 Thus, our findings suggest a factor(s) distinct from L-selectin, which is selectively required for T-cell homing into T-cell zone in PLN or PP, or spleen white pulp.
As described above, T-cell homing is strongly suggested to be regulated distinctly from B-cell homing. Consistently, Förster et al27 have reported a GTP-binding protein (G-protein)–coupled chemokine receptor, BLR1, which is selectively expressed on B cells and mediates B-cell homing into the B-cell zone of PP or into the white pulp in spleen. Although the mechanisms for the selective regulation of the B-cell homing is presently unknown, a signal(s) delivered through G-proteins might be included, because the treatment with pertussis toxin has been shown to induce the homing defect of T and B cells into PLN, PP, and spleen white pulp.28-33 It is well known that the activation of αL β2 integrin/LFA-1 on lymphocytes is required for binding to ICAM-1 on endothelial cells, which results in the adhesion of the lymphocytes to and their transmigration through the endothelial cells.7,34,35 A cytoplasmic protein, Cytohesin-1, might be involved for the activation of αLβ2 integrin/LFA-1, because Cytohesin-1 has been shown to bind to the intracellular domain of β2 integrin and to induce its adhesiveness to ICAM-1.36 Taken together, a certain chemokine(s) might bind to BLR1 and transduces signals to activate integrins on B cells through the BLR1-coupled G-protein and Cytohesin-1.
In this context, it might be also possible that the T-cell homing is mediated by a distinct chemokine(s) from that for the B-cell homing, which binds to the specific receptor on T cells and triggers a signal(s) through a G-protein(s) for the T-cell homing. The involvement of a signal(s) through G-protein in the homing defect of T cells inplt/plt might be supported by the findings that pertussis toxin-treated T cells retain the ability to bind to LN HEV in vitro,28 but are unable to home into PLN, PP, and spleen white pulp in vivo.28-33 This is quite similar to our findings that plt/plt LN HEV keep the ability to bind to T cells in vitro, but the stromal cells are unable to allow them to home into the lymphoid organs in vivo (Nakano et al8 and this report). Wild-type gene on plt locus might encode the chemokine for the T-cell homing in soluble or membrane-bound form, or regulate its function. The possibility is now under investigation in our laboratory.
Recently, CD43 on T cells has been shown to mediate their homing into PLN, PP, and spleen.37 Although it is possible that CD43 or its ligand(s) is affected in plt/plt, the possibility seems unlikely because of some differences among these homing defects. For example, anti-CD43 antibody inhibits the lymphocytes binding to PLN HEV in in vitro binding assay and the migration into spleen,37whereas those in plt/plt are not affected (Nakano et al8 and this report).
We have performed chromosomal mapping of plt gene with SSLP. Recombination between plt and each microsatellite as a locus marker showed the linkage of plt with the microsatellites on chromosome 4 (Table 1). A detailed analysis on chromosome 4 mappedplt on 24.7 cM from the centromere and linked it most closely with D4Mit237 (Fig 7). No recombination was observed betweenplt and D4Mit237 in 190 BC progenies examined. The locus occupied by D4Mit237 on mouse chromosome 4 corresponds to that on human chromosome 9p (Lyon et al,38 Genetic and Physical Map of the Mouse Genome, and National Center for Biotechnology Information). Around plt locus, there is no gene reported that encodes a molecule selectively affecting T cells, as far as we searched. Thus,plt seems to be a mutant of a novel gene whose product participates selectively in T-cell homing into peripheral lymphoid organs. Very recently, a novel chemokine with CX3C motif was reported.39 This chemokine is expressed as a membrane-bound form on TNF- or IL-1–activated, but not unstimulated, human umbilical vein endothelial cells, and serves as a chemoattractant for T cells and monocytes, suggesting that this chemokine is expressed on the endothelial cells at an inflammatory site. The wild-type gene ofplt locus is unlikely to be the CX3C chemokine, because the plt product functions in uninflammatory lymphoid organs in a T-cell–specific manner. In addition, the CX3C chemokine locates on human chromosome 16,39 whereasplt gene was mapped on mouse chromosome 4 corresponding to human chromosome 9.
Only lymphocytes among leukocytes can migrate from blood into lymph, but the mechanisms for the selective regulation remain to be elucidated. The investigation of the mechanisms for the function of theplt gene product will help us to elucidate the selective migration of lymphocytes and their recirculation.
ACKNOWLEDGMENT
We thank Dr T. Shiroishi (National Institute of Genetics, Mishima, Shizuoka, Japan) for providing MSM; Drs Y. Ishii, K. Yamaguchi, H. Hemmi (Toho University), and T. Yoshimoto (University of Tokyo) for their encouragement; and K. Nakano for her technical asistance.
Supported by Grant-in-Aids for Scientific Research (No. 08770344) from the Ministry of Education, Science, Sports, and Culture, Japan; and by the Uchida grant from the Japan Foundation of Cardiovascular Research (1996).
Address correspondence to H. Nakano, Department of Immunology, Toho University School of Medicine, Omori-nishi 5-21-16, Ota-ku, Tokyo 143, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal