Abstract
Hypoxia underlies a number of biologic processes in which cellular migration and invasion occur. Because earlier studies have shown that the receptor for urokinase-type plasminogen activator (uPAR) may facilitate such events, we studied the effect of hypoxia on the expression of uPAR by first trimester human trophoblasts (HTR-8/SVneo) and human umbilical vein endothelial cells (HUVEC). Compared with control cells cultured under standard conditions (20% O2), HTR-8/SVneo cells and HUVEC cultured in 1% O2 expressed more uPAR, as determined by flow cytometric and [125I]-prourokinase ligand binding analyses. Increased uPAR expression paralleled increases in uPAR mRNA. The involvement of a heme protein in the hypoxia-induced expression of uPAR was suggested by the observations that culture of cells with cobalt chloride, or sodium 4,5-dihydroxybenzene-1,3-disulfonate (Tiron), an iron-chelating agent, also stimulated uPAR expression, and that the hypoxia-induced uPAR expression was inhibited by adding carbon monoxide to the hypoxic atmosphere. Culture of HTR-8/SVneo cells with vascular endothelial growth factor (VEGF) did not increase uPAR mRNA levels, suggesting that the hypoxia-mediated effect on uPAR expression by these cells did not occur through a VEGF-dependent mechanism. The functional importance of these findings is suggested by the fact that HTR-8/SVneo cells cultured under hypoxia displayed higher levels of cell surface plasminogen activator activity and greater invasion through a reconstituted basement membrane. These results suggest that hypoxia may promote cellular invasion by stimulating the expression of uPAR through a heme protein-dependent pathway.
HYPOXIC STRESS UNDERLIES a number of biologically-important processes in which cellular migration and invasion occur. For example, hypoxia within an expanding tumor leads to the release of vascular endothelial growth factor (VEGF) and stimulation of angiogenesis,1 the success of which depends on endothelial cell migration and invasion. Hypoxia may also play an important role in promoting tumor metastasis and invasion of the extracellular matrix.2-5 It is likely that specific responses to hypoxia also promote cellular migration during processes such as atherosclerotic plaque development, as suggested by the expression of hypoxia-inducible proteins by plaque macrophages,6 as well as during early human pregnancy, where blastocyst implantation and uterine invasion by trophoblast cells occur under conditions of relative hypoxia.7 8
The expression of pericellular plasminogen activator activity may facilitate the cellular migration and invasion that occur in the above settings.9 Generation of such activity is dependent on the binding of urokinase-type plasminogen activator (uPA) to the urokinase receptor (uPAR), a 55-kD glycoprotein anchored to the plasma membrane by a glycosyl-phosphatidylinositol moiety.10 The urokinase receptor promotes cellular migration through several mechanisms, one of which involves its ability to amplify and focus the expression of plasminogen activator activity to discrete sites on the cell surface.11,12 In addition, the uPAR mediates cellular adhesion to vitronectin,13promotes integrin-dependent migration,14 and initiates intracellular signaling events.15
In light of the above considerations, we have investigated whether hypoxia plays a role in regulating the expression of uPAR by invasive first trimester human trophoblasts and human umbilical vein endothelial cells (HUVEC). Our studies suggest that hypoxia stimulates uPAR expression and cellular invasion through a reconstituted extracellular matrix and that these effects are mediated through an oxygen-binding heme protein.
MATERIALS AND METHODS
Materials.
Tissue culture medium was obtained from GIBCO-BRL (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT) or from GIBCO-BRL. Cobalt chloride and 4,5-dihydroxybenzene-1,3-disulfonate (Tiron) were purchased from Sigma Chemical Co (St Louis, MO). Control mouse IgG2a and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were obtained from Dako Corp (Carpinteria, CA). The fluorogenic plasmin peptide substrate, HDVALL-AMC, was purchased from Bachem (King of Prussia, PA). Urokinase enzyme-linked immunosorbent assay (ELISA) kits, plasminogen, and the monoclonal anti-uPAR antibody used to measure uPAR expression by flow cytometry (monoclonal antibody [MoAb] 3937), were obtained from American Diagnostica, Inc (Greenwich, CT). Prourokinase was a generous gift from Drs Jack Henkin and Andrew Mazar (Abbott Laboratories, Abbott Park, IL). Matrigel was obtained from Collaborative Biomedical Products (Bedford, MA), and invasion chambers (8.0 μmol/L pore size) from Costar Corp (Toronto, Canada). Endothelial cell growth supplement was purified as described by Maciag et al.16 Recombinant VEGF was purchased from R & D Systems (Minneapolis, MN). Radiochemicals and Reflection NEF autoradiographic film were from Dupont/New England Nuclear (Mississauga, Canada), and Pharmacia Oligolabelling kits were obtained from Pharmacia Biotech (Piscataway, NJ). Iodobeads were purchased from Pierce (Rockford, IL). Nylon membranes were from Micron Separations, Inc (Westboro, MA). Airtight chambers used for culture of cells under hypoxic conditions were obtained from BellCo Biotechnology (Vineland, NJ).
Cells.
HTR-8/SVneo cells were obtained from explant cultures of human first trimester placenta and immortalized by transfection with a cDNA construct containing the SV40 large T antigen.17 These cells have been previously characterized17 and have been maintained in culture for more than 120 passages in RPMI 1640 supplemented with 5% FBS. They exhibit a high proliferation index and share various phenotypic similarities with the nontransfected parent HTR-8 cells such as in vitro invasive ability and lack of tumorigenicity in nude mice.17 HUVEC were isolated from pooled umbilical cords as previously described18 and cultured in Medium 199 containing 75 μg/mL endothelial cell growth supplement, 2 mmol/L glutamine, and 5% FBS.
Culture conditions to assess the cellular response to hypoxia.
For culture under hypoxic conditions, cells were placed in airtight chambers, which were flushed with a gas mixture containing 5% CO2 and 95% N2 until the oxygen concentration within the chamber, measured using a Miniox 1 oxygen analyzer (Catalyst Research Corp, Owings Mills, MD), was less than 0.5%. Cells were then incubated within the sealed chambers for up to 24 hours, at 37°C. Under these conditions, the O2 concentration equilibrates within 1 to 2 hours and remains at or below 1% throughout the incubation period.
Binding of molecular oxygen to the ferrous iron atom within the porphyrin ring of a heme protein induces a change in its conformation from the deoxy, or tense, to the oxy, or relaxed state, as described for hemoglobin.19 The conformation of a putative oxygen-sensing heme protein may be regulated in a similar manner, and such a protein may acquire signaling activity after assuming the deoxy state, as described for the FixL heme protein of Rhizobium meliloti.20 To determine whether the pathway underlying the regulation of uPAR by hypoxia involves such a protein, we cultured cells for 24 hours in the presence or absence of either 100 μmol/L cobalt chloride or 30 mmol/L Tiron,21 an iron-chelating agent. Cobalt displaces iron from the porphyrin ring, but binds oxygen with lower affinity than iron,22 while Tiron chelates intracellular iron in a manner similar to that described for desferrioxamine.23 Hence, culture of cells in the presence of either of these agents induces the conformation of heme proteins into the deoxy state, thereby initiating cellular hypoxic responses. Furthermore, like oxygen, carbon monoxide also binds to heme proteins, maintaining them in the oxy or relaxed state, even under hypoxic conditions. Therefore, another strategy used to assess the involvement of such a protein in regulation of uPAR expression by hypoxia was to culture HTR-8/SVneo cells for 24 hours under hypoxic conditions in the presence of 20% carbon monoxide, with the expectation that inclusion of the latter would block heme protein-mediated hypoxic responses.
Determination of uPAR expression by flow cytometry and urokinase binding analysis.
For flow cytometry, cells were released from flasks by incubation in cold phosphate-buffered saline (PBS) containing 5 mmol/L EDTA. One million cells were then incubated for 1 hour at 4°C, with either 10 μg/mL of MoAb 3937, or control mouse IgG2a, used at the same concentration. Bound antibody was detected using FITC-conjugated goat anti-mouse immunoglobulin. Cells were then fixed with 2% paraformaldehyde in PBS before analysis using a Coulter Elite Flow Cytometer (Beckman Coulter Instruments, (Miami, FL).
Measurement of uPAR expression by ligand binding analysis was performed using [125I]-prourokinase as the ligand, as we have described previously.24 Prourokinase was radiolabeled with125I, using Iodobeads, to a specific radioactivity of at least 106 cpm/μg. Cells were then chilled to 4°C, washed with cold PBS containing 1% bovine serum albumin (BSA), and incubated for 2 hours with increasing concentrations of [125I]-prourokinase in the absence (to determine total binding) or presence (to determine nonspecific binding) of a 100-fold molar excess of unlabeled prourokinase. Specific binding was defined as the difference between total and nonspecific binding and analyzed by nonlinear curve fitting methods (least squares method) using the Kaliedograph software program (Synergy Software, Reading, PA).
Northern blot analysis.
Total cellular RNA was isolated, separated by electrophoresis, and transferred to charged nylon membranes. After prehybridization at 42°C for 2 hours in 50% formamide, 5× Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), 6× SSC (1× SSC = 0.15 mol/L NaCl, 15 mmol/L sodium citrate, pH 7.0) and 100 μg/mL denatured salmon sperm DNA, membranes were hybridized overnight at 42°C with a full-length uPAR cDNA probe12 cloned in a Bluescript vector and labeled with [α-32P] deoxycytidine 5′-triphosphate (dCTP) using a Pharmacia Oligolabelling kit. The hybridization solution consisted of 6× SSC, 0.5% SDS, 100 μg/mL denatured salmon sperm DNA and 50% formamide. After serial washes, the membrane was developed by autoradiography using Dupont Reflection NEF film. To determine whether VEGF plays a role in uPAR upregulation in HTR-8/SVneo cells, as shown previously for endothelial cells,25 relative levels of uPAR mRNA in cells cultured for 24 hours under either 20% O2 alone, 1% O2, or 20% O2 + 10 ng/mL VEGF were also assessed by Northern blot analysis.
In vitro invasion assay.
A previously-described invasion assay was used to determine the effect of hypoxia on the invasion of HTR-8/SVneo cells through a reconstituted basement membrane.17 Briefly, Transwell invasion chambers were coated with 100μL of a 1.3 mg/mL solution of Matrigel diluted in cold RPMI 1640 medium. The Matrigel was then air-dried for 12 hours in a laminar flow cabinet. HTR-8/SVneo cells were labeled by incubation for 24 hours in the presence of 10 μCi/mL [3H]-thymidine. The cells were then harvested, adjusted to a concentration of 5.0 × 105/mL, and 100-μL aliquots added in triplicate to the upper wells of the invasion chambers. After a 24-hour incubation, cells in the upper and lower compartments of the chambers were harvested.17 The invasion index, reflecting the percentage of added cells that had invaded the Matrigel was determined by measurement of the radioactivity in the upper and lower compartments, as well as in the membrane.17
Determination of plasminogen activator (PA) and gelatinase levels in the culture medium.
Concentrations of gelatinase and plasminogen activators in the conditioned medium of HTR-8/SVneo cells cultured under standard or hypoxic conditions were compared using gel zymography, as previously described.17 The concentrations of uPA antigen in these samples were more accurately measured using a specific uPA ELISA.
Determination of cell surface PA activity.
Cell surface PA activity was determined using a modification of the fluorometric assay of Ellis et al.26 Briefly, HTR-8/SVneo cells were plated in quadruplicate wells of a 96-well tissue culture plate and allowed to grow to 95% confluency. After a 24-hour incubation under an atmosphere of either 20% O2 or 1% O2, the cells were washed twice and further incubated with fresh medium containing plasminogen and the plasmin peptide substrate, HDVLL-AMC, used at concentrations of 0.2 μmol/L and 0.5 mmol/L, respectively. Plasmin generation was assessed by determining the fluorescence within individual microplate wells 30 minutes later, using a Perkin-Elmer LS50B Luminescence Spectrophotometer (Perkin-Elmer Corp, Norwalk, CT) (excitation wavelength 360 nm, emission wavelength 460 nm).
RESULTS
Urokinase receptor expression is stimulated by hypoxia.
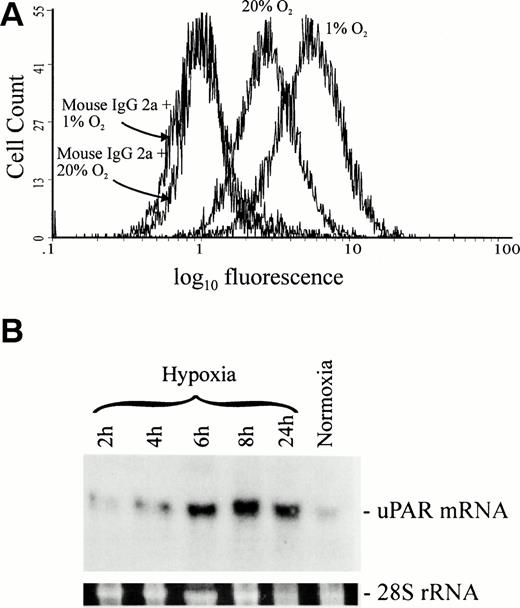
We first determined the effect of hypoxia on the expression of uPAR by HTR-8/SVneo cells (Fig 1A). In comparison to cells cultured under standard conditions (20% O2), the expression of uPAR by cells cultured in 1% O2 for 24 hours was 68% higher (n = 7, P = .007), as determined by flow cytometry. Equal amounts of nonimmune mouse IgG2a bound to cells cultured under both standard and hypoxic conditions, demonstrating that increased binding of anti-uPAR MoAb 3937 was not due to nonspecific interactions or increased Fc receptor expression. Similarly, assessment of uPAR expression by HTR-8/SVneo cells through measurement of [125I]-prourokinase binding showed that hypoxia stimulated the expression of uPAR by 88% (n = 9, P = .003), an increment similar to that detected using flow cytometry. This was accompanied by a parallel increase in the kd (Table 1), as well as by an increase in the cellular content of uPAR mRNA (Fig 1B). Increased uPAR mRNA expression was first apparent after 4 hours of exposure to hypoxia and reached a maximum level after 6 hours.
Effect of hypoxia on uPAR protein and mRNA levels in HTR-8/SVneo cells. (A) Analysis of uPAR expression by HTR-8/SVneo cells using flow cytometry showed an average increase of 68% in the mean fluorescence intensity when cells were cultured under hypoxic conditions and labeled with MoAb 3937. This figure is representative of seven independent experiments. (B) Northern blot analysis showed a 2.5-fold increase in uPAR mRNA levels after only 4 hours of hypoxic culture when compared with uPAR mRNA levels in cells cultured under standard conditions. The levels of uPAR transcript increased by fivefold at 6 hours of culture under hypoxia and remained high at 8 and 24 hours. Relative levels of uPAR mRNA were determined with a SigmaGel gel analysis program using 28S rRNA to correct for differences in the amount of RNA loaded onto the gel.
Effect of hypoxia on uPAR protein and mRNA levels in HTR-8/SVneo cells. (A) Analysis of uPAR expression by HTR-8/SVneo cells using flow cytometry showed an average increase of 68% in the mean fluorescence intensity when cells were cultured under hypoxic conditions and labeled with MoAb 3937. This figure is representative of seven independent experiments. (B) Northern blot analysis showed a 2.5-fold increase in uPAR mRNA levels after only 4 hours of hypoxic culture when compared with uPAR mRNA levels in cells cultured under standard conditions. The levels of uPAR transcript increased by fivefold at 6 hours of culture under hypoxia and remained high at 8 and 24 hours. Relative levels of uPAR mRNA were determined with a SigmaGel gel analysis program using 28S rRNA to correct for differences in the amount of RNA loaded onto the gel.
Effects of Hypoxia, Tiron, and Cobalt Chloride on the Binding of [125I]-Prourokinase to HTR-8/SVneo Cells
| Condition . | Bmax (sites/cell) ± SEM . | kd (nmol/L) ± SEM . | P (Bmax) . | P (kd) . | No. . |
|---|---|---|---|---|---|
| Standard versus hypoxia: | |||||
| Standard | 189,028 ± 39,222 | 1.26 ± 0.16 | 9 | ||
| Hypoxia | 356,728 ± 64,488 | 2.18 ± 0.42 | .003 | .025 | 9 |
| Standard versus Tiron: | |||||
| Standard | 208,980 ± 26,825 | 2.93 ± 0.59 | 6 | ||
| Tiron | 623,213 ± 102,360 | 8.08 ± 1.11 | .004 | .001 | 6 |
| Standard versus cobalt chloride: | |||||
| Standard | 223,026 ± 37,035 | 2.49 ± 0.47 | 8 | ||
| Cobalt chloride | 285,711 ± 38,324 | 3.58 ± 0.69 | .014 | .004 | 8 |
| Condition . | Bmax (sites/cell) ± SEM . | kd (nmol/L) ± SEM . | P (Bmax) . | P (kd) . | No. . |
|---|---|---|---|---|---|
| Standard versus hypoxia: | |||||
| Standard | 189,028 ± 39,222 | 1.26 ± 0.16 | 9 | ||
| Hypoxia | 356,728 ± 64,488 | 2.18 ± 0.42 | .003 | .025 | 9 |
| Standard versus Tiron: | |||||
| Standard | 208,980 ± 26,825 | 2.93 ± 0.59 | 6 | ||
| Tiron | 623,213 ± 102,360 | 8.08 ± 1.11 | .004 | .001 | 6 |
| Standard versus cobalt chloride: | |||||
| Standard | 223,026 ± 37,035 | 2.49 ± 0.47 | 8 | ||
| Cobalt chloride | 285,711 ± 38,324 | 3.58 ± 0.69 | .014 | .004 | 8 |
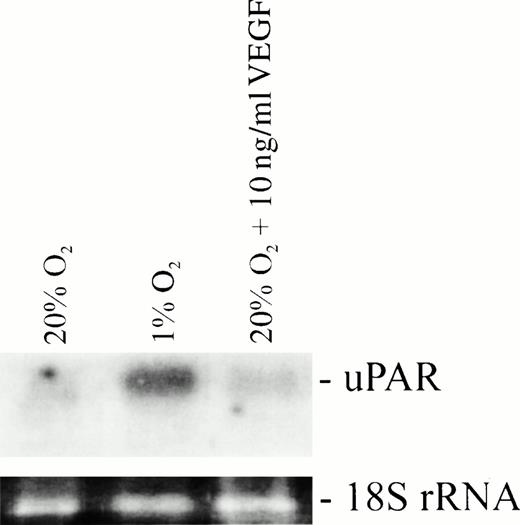
It has been previously reported that VEGF increases the expression of uPAR by vascular endothelial cells.25 Furthermore, recent studies have shown that hypoxia stimulates VEGF release by increasing transcriptional activation of the VEGF gene via hypoxia-inducible factor-1 (HIF-1), as well as through mRNA stabilization.27-30 In the present study, however, culture of HTR-8/SVneo cells in the presence of 10 ng/mL of VEGF for 24 hours under normoxic conditions did not result in increased levels of uPAR transcript (Fig 2), suggesting that the hypoxia-mediated upregulation of uPAR occurs through a VEGF-independent mechanism.
Effects of hypoxia and VEGF on uPAR mRNA levels in HTR-8/SVneo cells assessed by Northern blot analysis. VEGF did not increase the expression of uPAR mRNA when incubated with cells under normoxic conditions.
Effects of hypoxia and VEGF on uPAR mRNA levels in HTR-8/SVneo cells assessed by Northern blot analysis. VEGF did not increase the expression of uPAR mRNA when incubated with cells under normoxic conditions.
We were also interested in determining whether the effects of hypoxia on uPAR expression extended to other cell types such as endothelial cells. Figure 3 shows results in which similar effects of hypoxia on upregulation of uPAR expression were detected by radioligand binding studies using HUVEC. These results were also confirmed by flow cytometry (not shown).
Binding of [125I]-prourokinase to HUVEC, cultured under standard (solid squares) and hypoxic (open triangles) conditions. This figure is representative of three independent experiments in which a mean increase of 46% in the binding of [125I]-prourokinase to cells cultured under hypoxic conditions was observed. Error bars indicate standard error of triplicate samples for each point.
Binding of [125I]-prourokinase to HUVEC, cultured under standard (solid squares) and hypoxic (open triangles) conditions. This figure is representative of three independent experiments in which a mean increase of 46% in the binding of [125I]-prourokinase to cells cultured under hypoxic conditions was observed. Error bars indicate standard error of triplicate samples for each point.
Role of a heme protein in the regulation of uPAR expression.
To examine the potential involvement of a heme protein in the hypoxia-mediated stimulation of uPAR expression, we first examined the expression of uPAR by HTR-8/SVneo cells in response to incubation in the presence of cobalt chloride or Tiron. Both flow cytometric and radioligand binding studies showed a threefold increase in the expression of uPAR in response to Tiron (n = 6, P = .004), with a more modest 28% increase observed in response to cobalt chloride (n = 8, P = .014; Table 1). In each case, the kd increased as well, as observed following exposure of cells to hypoxia. Stimulation of uPAR expression by either cobalt or Tiron was associated with increased levels of uPAR mRNA, comparable to those observed in response to hypoxia (Fig 4).
Effect of hypoxia, carbon monoxide, cobalt chloride, and Tiron on the levels of uPAR mRNA in HTR-8/SVneo cells. Cells were cultured for 24 hours under the conditions listed over each lane of the figure. Levels of uPAR mRNA were increased 2.8, 1.8, 3.5, and >10-fold, respectively, within cells cultured under hypoxic (1% O2) conditions, hypoxic conditions in the presence of 20% carbon monoxide, and standard (20% O2) conditions in the presence of either cobalt chloride or Tiron, in comparison to cells cultured under standard conditions alone (lane 1). Carbon monoxide reduced the hypoxia-mediated increase in uPAR mRNA by 35%.
Effect of hypoxia, carbon monoxide, cobalt chloride, and Tiron on the levels of uPAR mRNA in HTR-8/SVneo cells. Cells were cultured for 24 hours under the conditions listed over each lane of the figure. Levels of uPAR mRNA were increased 2.8, 1.8, 3.5, and >10-fold, respectively, within cells cultured under hypoxic (1% O2) conditions, hypoxic conditions in the presence of 20% carbon monoxide, and standard (20% O2) conditions in the presence of either cobalt chloride or Tiron, in comparison to cells cultured under standard conditions alone (lane 1). Carbon monoxide reduced the hypoxia-mediated increase in uPAR mRNA by 35%.
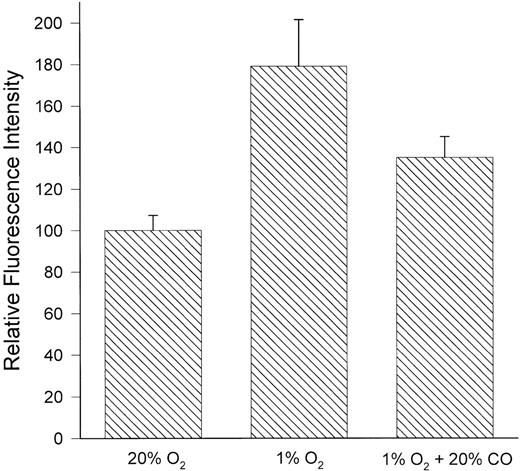
To further assess the potential role of a heme protein in the increased expression of uPAR in response to hypoxia, we examined the effects of carbon monoxide on this response. As expected, increased uPAR expression after culture of HTR-8/SVneo cells under hypoxic (1% oxygen) conditions was inhibited (56%) by inclusion of 20% carbon monoxide in the gas mixture (Fig 5); a parallel decrease was observed in the content of cellular uPAR mRNA (Fig 4). Furthermore, in two independent experiments, the inhibitory effects of carbon monoxide on the hypoxia-induced expression of uPAR were completely prevented by inclusion of cobalt chloride or Tiron in the medium (not shown), indicating that the inhibition of uPAR expression by carbon monoxide under hypoxic conditions was not due to nonspecific toxicity.
Inhibition of hypoxia-induced uPAR expression by carbon monoxide. HTR-8/SVneo cells were incubated for 24 hours under standard (20% O2) or hypoxic (1% O2) conditions, or hypoxic conditions in the presence of 20% carbon monoxide. The expression of uPAR was then assessed by flow cytometry using MoAb 3937. Carbon monoxide inhibited the hypoxia-induced expression of uPAR by 56%. Data represent the mean ± standard error of four independent experiments.
Inhibition of hypoxia-induced uPAR expression by carbon monoxide. HTR-8/SVneo cells were incubated for 24 hours under standard (20% O2) or hypoxic (1% O2) conditions, or hypoxic conditions in the presence of 20% carbon monoxide. The expression of uPAR was then assessed by flow cytometry using MoAb 3937. Carbon monoxide inhibited the hypoxia-induced expression of uPAR by 56%. Data represent the mean ± standard error of four independent experiments.
Hypoxia stimulates in vitro invasiveness via a heme protein.
To determine the functional correlates of hypoxia-induced uPAR expression, we compared the invasion of HTR-8/SVneo cells through a reconstituted basement membrane (Matrigel) under both standard (20% oxygen) and hypoxic (1% oxygen) conditions, observing an increase of 41.4% ± 7.4% (P = .003) under the latter (Table 2). Similar increases were observed when invasion assays were performed under 20% oxygen in the presence of either 100 μmol/L cobalt chloride (24.6 ± 8.4%;P = .028) or 30 mmol/L Tiron (29.3 ± 12.7%;P = .035) (Table 2). The role of a heme protein in the regulation of cellular invasiveness was confirmed by experiments in which hypoxia-stimulated invasion was inhibited by 87% in the presence of 20% carbon monoxide and was not significantly different from that which occurred in the presence of 20% oxygen (Table 2).
Effects of Hypoxia, Carbon Monoxide, Tiron, and Cobalt Chloride on Invasion of HTR-8/SVneo Cells Through Matrigel
| Condition . | Invasion Index* (%) ± SE . | P (v standard) . | No. . |
|---|---|---|---|
| Standard (20% O2) | 100 | 11 | |
| Hypoxia (1% O2) | 141.4 ± 7.4 | .003 | 11 |
| Standard + cobalt chloride | 124.6 ± 8.4 | .028 | 9 |
| Standard + Tiron | 129.3 ± 12.7 | .035 | 6 |
| Hypoxia + 20% CO | 104.0 ± 8.5 | .684 | 10 |
| Condition . | Invasion Index* (%) ± SE . | P (v standard) . | No. . |
|---|---|---|---|
| Standard (20% O2) | 100 | 11 | |
| Hypoxia (1% O2) | 141.4 ± 7.4 | .003 | 11 |
| Standard + cobalt chloride | 124.6 ± 8.4 | .028 | 9 |
| Standard + Tiron | 129.3 ± 12.7 | .035 | 6 |
| Hypoxia + 20% CO | 104.0 ± 8.5 | .684 | 10 |
*The invasion index was calculated as described in Graham et al.17
Effect of hypoxia on PA and gelatinase levels in culture medium and on cell surface PA activity.
Gel zymography showed a time-dependent reduction in the levels of PAs in medium conditioned by HTR-8/SVneo cells cultured for up to 24 hours under 1% O2 (Fig 6). These observations were supported by additional studies in which direct measurement of urokinase antigen levels in the conditioned medium of HTR-8/SVneo cells cultured under hypoxic conditions for 24 hours were reduced by a mean of 52%. In contrast, the expression of cell surface PA activity by HTR-8/SVneo cells cultured for 24 hours under hypoxic conditions was 20% higher than that expressed by cells cultured under standard conditions (P = .000007). These findings are consistent with binding of secreted urokinase to increased numbers of cellular uPAR.
Zymographic analysis of PAs present in the medium of HTR-8/SVneo cell cultures incubated for 8, 12, and 24 hours under normoxic or hypoxic conditions. Casein and plasminogen were incorporated into the acrylamide before polymerization as detailed elsewhere.17 Samples of serum-free medium containing 200 ng of protein were loaded onto each lane and separated by electrophoresis. After an overnight incubation in 5 mmol/L CaCl2 in TRIS buffer, gels were stained with Coomassie R-250 in 10% acetic acid/40% methanol and destained in 10% acetic acid/40% methanol. Clear areas represent caseinolytic activity. Note caseinolytic bands at 50 to 55 kD corresponding in size to uPA, and that at 24 hours of hypoxic culture, the caseinolytic bands were not discernible.
Zymographic analysis of PAs present in the medium of HTR-8/SVneo cell cultures incubated for 8, 12, and 24 hours under normoxic or hypoxic conditions. Casein and plasminogen were incorporated into the acrylamide before polymerization as detailed elsewhere.17 Samples of serum-free medium containing 200 ng of protein were loaded onto each lane and separated by electrophoresis. After an overnight incubation in 5 mmol/L CaCl2 in TRIS buffer, gels were stained with Coomassie R-250 in 10% acetic acid/40% methanol and destained in 10% acetic acid/40% methanol. Clear areas represent caseinolytic activity. Note caseinolytic bands at 50 to 55 kD corresponding in size to uPA, and that at 24 hours of hypoxic culture, the caseinolytic bands were not discernible.
Additional studies in which the levels of gelatinases in the conditioned medium of HTR-8/SVneo cells cultured under standard or hypoxic conditions were assessed by gelatin zymography showed that hypoxia did not affect the amounts of these proteins released in response to hypoxia (not shown). These observations suggest that the increased invasiveness of HTR-8/SVneo cells observed under hypoxic conditions is not attributable to the increased production or secretion of these proteinases, and instead results from another mechanism, potentially involving increased expression of uPAR.
DISCUSSION
Our results show that uPAR expression by human trophoblasts and endothelial cells is regulated by hypoxia. Furthermore, they suggest that these responses occur via a heme protein-dependent pathway. This conclusion is based on the results of experiments in which the ability of such a protein to regulate uPAR expression was assessed through the use of cobalt chloride and Tiron, each of which causes the functional displacement of ferrous iron from the heme moiety of the putative oxygen sensing protein. We also showed that the effects of hypoxia on uPAR expression were blocked by carbon monoxide. Although these results are consistent with the hypothesis that the hypoxia-induced stimulation of uPAR expression occurs through a heme protein, their absolute dependence on such a protein remains unproven until it is identified and cloned.
A similar pathway regulating the production of erythropoietin (EPO) has been extensively characterized31 and shown to involve the interactions of specific transcription factors, such as HIF-1,32 with discrete enhancer regions in the 3′-flanking sequences of the EPO gene.33Despite restricted cellular production of EPO, hypoxic induction of reporter genes containing the EPO enhancer has been observed in a wide variety of cell types, suggesting that HIF-1 may also mediate other adaptive responses to hypoxia.34 Inspection of the 5′-flanking region of the uPAR gene35 shows three potential HIF-1 binding sequences, each of which differs by only one nucleotide from the consensus sequence 5′-XACGTGCX-3′, originally identified in the genes encoding EPO and several glycolytic enzymes.36 These sequences, 5′-TTCGTGCT-3′, 5′-TAGGTGCT-3′ and 5′-TACGGGCC-3′, are located in the same orientation as the uPAR transcriptional unit, between nucleotides -501 and -494, -578 and -573, and -1204 and -1197, respectively. We did not identify any potential HIF-1 binding sites in the 3′-flanking regions of the uPAR gene.37
In addition to HIF-1,33 hypoxia may induce the activation of NF-κB, c-fos, c-jun, c-jun B, andjun D.38,39 While one group has also reported that hypoxia induces the activation of c-Src,40 a recent study failed to confirm these observations, despite culture of cells under hypoxic conditions that led to the induction of HIF-1, EPO, VEGF, and the glucose transporter-1, Glut-1.41 Furthermore, perturbation of c-Src activity in Hep3B cells did not affect the normoxic or hypoxic expression of the latter proteins.41 Whether other factors, in addition to HIF-1, contribute to the hypoxia-mediated stimulation of uPAR expression is unknown.
Although we propose that the increased expression of uPAR in response to hypoxia occurs primarily through HIF-1–mediated stimulation of uPAR gene expression, we also considered the possibility that these observations may reflect an autocrine effect of endogenously-released growth factors in response to hypoxia. For example, VEGF, the expression of which is markedly increased in response to hypoxia,1 stimulates the expression of uPAR by bovine and human endothelial cells.25 In our studies, however, culture of HTR-8/SVneo cells with exogenous VEGF did not result in increased levels of uPAR mRNA. These results suggest that, at least under the conditions used in these studies, the effect of hypoxia on uPAR expression is not mediated through VEGF. This conclusion is supported by the results of studies in which we observed that blocking anti-VEGF antibodies did not inhibit the increased expression of uPAR protein or mRNA induced by hypoxia (T. Fitzpatrick, K.R. McCrae, and C.H. Graham, unpublished observations, April 1997). However, it is possible that in other cell types, which produce greater amounts of VEGF, increased expression of uPAR in response to hypoxia may occur through additional pathways.
We observed that the levels of urokinase were progressively reduced in the conditioned medium of HTR-8/SVneo cells cultured for increasing intervals under hypoxic conditions. Although we have not determined precisely the mechanisms accountable for this observation, we believe it is likely that these decreased levels reflect increased binding of secreted uPA to cellular uPAR. This hypothesis is supported by the observation that cells cultured under hypoxic conditions expressed increased cell surface plasminogen activator activity. Nevertheless, we believe that the increased expression of uPAR induced by hypoxia is likely to be of importance in cellular migration and invasion in vivo, as it has been shown, for example, that invasive neoplastic cells may use uPA produced by neighboring stromal cells to facilitate invasion through extracellular barriers.42 Similar considerations apply to the increased kd for binding of uPA to cellular uPAR after exposure of cells to hypoxia. This modest increase, which is similar to that which accompanies the increased expression of uPAR in response to phorbol 12-myristate, 13-acetate (PMA),43 appears to result from additional glycosylation or other posttranslational modifications of the receptor. However, an increase in the kd of the magnitude observed here is not likely to significantly affect the degree of uPAR saturation with uPA in the presence of either increased endogenous uPA production or the production of significant amounts of uPA by neighboring cells. Finally, even in our system, the net effect of exposure of HTR-8/SVneo cells to hypoxia was an increase in the expression of cell surface PA activity, which we believe underlies the increased cellular invasiveness that occurred in parallel. Whether the increased in vitro invasiveness and in vivo metastatic capability of murine sarcoma cells under hypoxic conditions involve similar mechanisms has not yet been determined.5
Our findings may be relevant to several biological processes in which cell migration and invasion occur. For example, increased expression of uPAR by extravillous trophoblast cells may facilitate their invasion through hypoxic regions of the superficial endometrial stroma.44 In support of this hypothesis are studies in which trophoblast invasion of the uterine wall has been shown to be increased in pregnant rhesus monkeys in which uteroplacental blood flow was impaired following constriction of the abdominal aorta.45 It should also be noted that the human placenta is relatively hypoxic during the first 10 weeks of gestation,8a period when trophoblast cells are maximally invasive. In addition, increased expression of uPAR is enhanced in migrating endothelial cells in vitro,46 and the fact that neovascularization of transplanted tumors in vivo is inhibited by uPAR antagonists47,48 suggests a role for hypoxia-stimulated uPAR expression in tumor angiogenesis. Additional studies have shown that uPAR antagonists inhibit tumor metastasis,49,50 as well as local invasion,50,51 perhaps explaining, in part, the association of elevated uPAR levels in extracts of breast and other types of carcinomas with a poor clinical outcome.51Finally, the expression of hypoxia-inducible proteins by macrophages within atherosclerotic plaques shows that hypoxia occurs within this setting as well,6 and suggests that the migration of these and other cell types within the plaque may be facilitated by hypoxia-stimulated increases in uPAR.52
In conclusion, we have shown that the expression of uPAR is stimulated by hypoxia and that this response may involve an oxygen-sensing heme protein. Hence, we believe that the uPAR should be added to the expanding list of hypoxia-inducible proteins induced through such a pathway. Although it is likely that HIF-1 is involved in the hypoxia-mediated increase in uPAR expression, additional studies will be required to address this issue in different cell types and determine the physiologic importance of these observations.
ACKNOWLEDGMENT
We are grateful for the technical assistance of Shannyn Macdonald-Goodfellow and for the photographic work of Bob Temkin. We also thank Dr Jim Johnson for providing assistance with the flow cytometric analysis.
Supported by Grant No. T-3361 from the Heart and Stroke Foundation of Ontario (to C.H.G.), Grant No. HL 50827 from the National Institutes of Health, and Grant No. 95-0220 from the American Heart Association (to K.R.M.). C.H.G. is a Research Scholar from the Heart and Stroke Foundation of Canada.
Address reprint requests to Charles H. Graham, PhD, Department of Anatomy and Cell Biology, Botterell Hall, 9th Floor, Queen's University, Kingston, Ontario, Canada K7L 3N6.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Binding of [125I]-prourokinase to HUVEC, cultured under standard (solid squares) and hypoxic (open triangles) conditions. This figure is representative of three independent experiments in which a mean increase of 46% in the binding of [125I]-prourokinase to cells cultured under hypoxic conditions was observed. Error bars indicate standard error of triplicate samples for each point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3300/3/m_blod40935003x.jpeg?Expires=1769313993&Signature=VcxdQUzQcYvK0trinosJBH9aLCKNi5DPDAb6gsari48hNbkqaSiJF6HmyB8HOJQPdH08~I3cl76PRSCoBkjMAAOS9yGJm-IiCWTCeDOyIV3juybX9nZQtDpglfQOpEBJUXE3w0ZNeSapG1QKLR56R-86iVI8JHga90szUCYQFUR4elzsbNm6GB4F0Xl-pxurSM11v32JIw-B6mkacP6bBsTjb6~HSuQO2AlU9lqRHUn6xrVlgLNmpLQuXgKcqjeft5WGjOw-TsAEjlT6XSxMMe4qfTn7tBHxUMEyyVRfG0tF0B-PFurzp4VUJrVz-~gvTkPj00wHh3CzZ-7Yb12Q5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal