Abstract
Chronic myelogenous leukemia (CML) is characterized by the continuous proliferation and abnormal circulation of malignant hematopoietic progenitors. This may be related to the unresponsiveness of CML progenitors to β1 integrin adhesion receptor-mediated inhibition of progenitor proliferation by the marrow microenvironment. In hematopoietic cell lines, the BCR-ABL oncogene product, p210BCR-ABL, interacts with a variety of cytoskeletal elements important for normal integrin signaling. We studied the role of p210BCR-ABL in abnormal integrin function in CML by evaluating the effect of inhibition of BCR-ABL expression with antisense oligodeoxynucleotides (AS-ODNs) on integrin-mediated adhesion and proliferation inhibition of malignant primary progenitors from CML marrow. Preincubation of CML CD34+HLA-DR+(DR+) cells with breakpoint-specific AS-ODNs significantly increased adhesion of CML progenitors to stroma and fibronectin (FN). Pretreatment with breakpoint-specific ODNs also resulted in significant inhibition of CML progenitor proliferation after ligand or antibody-mediated β1 integrin engagement. Breakpoint-specific ODNs were significantly more effective in restoring CML progenitor adhesion and proliferation inhibition than control ODNs. BCR-ABL mRNA and p210BCR-ABL levels in CML CD34+ cells were significantly reduced after incubation with breakpoint-specific AS-ODN. These studies indicate a role for BCR-ABL in abnormal circulation and defective integrin-dependent microenvironmental regulation of proliferation of CML hematopoietic progenitors.
CHRONIC MYELOGENOUS leukemia (CML) results from the malignant transformation of a hematopoietic stem cell and is characterized by a massive expansion of hematopoietic progenitors, as well as more differentiated hematopoietic cells, originating from the malignant clone. In addition, large numbers of circulating progenitors are seen in CML, reflecting their enhanced release from the bone marrow cavity.1 Retention and homing of progenitors to the marrow microenvironment is in large part dependent on members of the β1 integrin family of adhesion receptors, in particular the α4β1 receptor.2-4 Signaling through β1 integrin receptors also plays an important role in the regulation of proliferation,5,6 differentiation,7 and survival8 of a variety of cell types. We have shown that signals transduced from the extracellular matrix by β1 integrins play an important role in microenvironmental regulation of normal hematopoietic progenitor proliferation.9,10 In contrast to normal hematopoietic progenitors, CML progenitors show deficient adhesion to the microenvironment.11-13 Although malignant CML CD34+ HLA-DR+ cells express normal levels of β1 integrins on their cell surface, CML progenitors adhere significantly less to α4β1 and α5β1 binding regions of fibronectin (FN), indicating that β1 integrin receptor function is abnormal in CML.13 We have shown that in addition to defective adhesion, CML progenitors are unresponsive to β1 integrin-mediated inhibition of proliferation.14 Therefore, defects in integrin function may not only be responsible for the abnormal circulation, but also the continuous proliferation of CML progenitors, leading to their selective expansion and growth advantage.
CML is characterized cytogenetically by a translocation between chromosomes 9 and 22 [t(9;22)(q34;q11)], which leads to the fusion of the c-ABL gene on chromosome 9 with the BCR gene on chromosome 22.15,16 The BCR-ABL oncogene plays a critical role in the pathogenesis of CML. Transfection of hematopoietic cell lines with BCR-ABL cDNA results in cellular transformation,17 and transduction of mouse stem cells with BCR-ABL is sufficient to induce a CML-like syndrome.18 Several studies have examined intracellular pathways leading to BCR-ABL–induced transformation. When expressed in cell lines, p210BCR-ABL may activate the RAS/MAPK pathway by binding to and/or phosphorylating proteins involved in regulation of RAS function, such as RAS-GAP, SHC, and GRB2.19,20 These observations may explain how BCR-ABL leads to growth factor–independent proliferation of factor-dependent cell lines. However, primary chronic phase CML hematopoietic progenitor cells do not show growth factor–independent proliferation and do not, for the most part, differ from normal progenitors in their growth factor responsiveness.21,22 On the other hand, recent studies suggest that p210BCR-ABL interacts with a number of cytoskeletal proteins that are also involved in “inside-out” and “outside-in” signaling through β1 integrins, such as F-actin, FAK, Crkl, and paxillin.23-26 These studies led us to hypothesize that interactions between p210BCR-ABL and cytoskeletal elements important for β1 integrin signaling are responsible for abnormal integrin function and disturbed microenvironmental regulation of progenitor proliferation in CML.
Antisense oligodeoxynucleotides (AS-ODNs) complementary to the BCR-ABL breakpoint can inhibit BCR-ABL expression, suppress CML-blast crisis progenitor proliferation in vitro,27 and suppress CML cell line growth in severe combined immunodeficient (SCID) mice.28 In the present study, we evaluated the effect of inhibition of BCR-ABL expression by AS-ODNs directed against the BCR-ABL breakpoint on β1 integrin-mediated adhesion and proliferation inhibition of CML progenitors. Our studies indicate that inhibition of BCR-ABL expression in primary malignant CML CD34+HLA-DR+ cells with breakpoint-specific AS-ODNs restores integrin-mediated adhesion of CML colony-forming cells (CFC) to stroma and FN substrates and restores inhibition of CML CFC proliferation after contact with stroma and FN substrates or after antibody-mediated integrin stimulation. These results indicate a role for p210BCR-ABL in abnormal β1 integrin function in CML.
MATERIALS AND METHODS
Seventeen patients with CML and eight normal healthy volunteers were evaluated after informed consent was obtained using guidelines approved by the Committee on the Use of Human Subjects at the University of Minnesota and the Institutional Review Board at the City of Hope National Medical Center. Fifteen patients were in the chronic phase of CML, whereas two were in accelerated phase. Nine patients were receiving treatment with hydroxyurea alone, which was discontinued at least 4 days before study. Eight patients had been previously treated with interferon (IFN)-α, which had been discontinued for at least 3 months before study. The b2a2 BCR-ABL breakpoint was present in six patients, whereas the b3a2 breakpoint was present in 11 patients. Heparinized bone marrow samples were obtained by aspiration from the posterior iliac crest. Bone marrow mononuclear cells (BMMNC) were isolated by Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient separation (specific gravity, 1.077) for 30 minutes at 37°C and 400g.
Selection of CD34+DR+ progenitor populations.
CD34+ cell enriched populations were selected from CML or normal BMMNC using sequential avidin-biotin immunoadsorbtion columns (CellPro Inc, Bothell, WA)29 or immunomagnetic column separation (Miltenyi Biotech Inc, Auburn, CA). CD34+HLA-DR+ (DR+) cells were then selected by fluorescence-activated cell sorting (FACS) as previously described.30 31
Adhesive substrates.
Bone marrow stromal layers were established from normal BMMNC in T-75 or T-150 flasks as previously described, irradiated at 1,250 cGy using a Cesium irradiator to eliminate hematopoietic cells, and subcultured in 24-well plates (Costar, Cambridge, MA).31 Human plasma fibronectin was a kind gift from Dr James B. McCarthy (University of Minnesota, Minneapolis, MN). FN was purified as a byproduct of factor VIII production by sequential ion exchange and gelatin chromatography32 and 75-kD tryptic fragments containing the cell binding sequence RGDS and 33- and 66-kD COOH-terminal heparin binding fragments were purified as previously described.32
AS-ODNs.
18 or 24 mer phosphodiester AS-ODNs to BCR-ABL junctional regions were added to CML or normal DR+ cells as indicated in the Results (Table 1). In addition to AS-ODNs specific to the breakpoint present in the cells being studied, cells were incubated with missense ODNs and ODNs to the alternate breakpoint, eg, b2a2 if the tested cells contained the b3a2 breakpoint, as controls (control ODNs).
AS-ODN Sequences Used
| 18-mer | |
| b2a2 Antisense | 5′-GAA-GGG-CTT-CTT-CCT-TAT-3′ |
| b2a2Missense | 5′-GAA-CGG-CAT-CTA-CGT-TAT-3′ |
| b3a2 Antisense | 5′-GAA-GGG-CTT-TTG-AAC-TCT-3′ |
| b3a2Missense | 5′-GAA-GTG-CTG-TTG-AAC-TCT-3′ |
| b3a2 Missense (TAT) | 5′-GAA-GTG-CTG-TTG-CAC-TAT-3′ |
| 24-mer | |
| b2a2 Antisense | 5′-GCT-GAA-GGG-CTT-CTT-CCT-TAT-TGA-3′ |
| b3a2 Antisense | 5′-GCT-GAA-GGG-CTT-TTG-AAC-TCT-GCT-3′ |
| 18-mer | |
| b2a2 Antisense | 5′-GAA-GGG-CTT-CTT-CCT-TAT-3′ |
| b2a2Missense | 5′-GAA-CGG-CAT-CTA-CGT-TAT-3′ |
| b3a2 Antisense | 5′-GAA-GGG-CTT-TTG-AAC-TCT-3′ |
| b3a2Missense | 5′-GAA-GTG-CTG-TTG-AAC-TCT-3′ |
| b3a2 Missense (TAT) | 5′-GAA-GTG-CTG-TTG-CAC-TAT-3′ |
| 24-mer | |
| b2a2 Antisense | 5′-GCT-GAA-GGG-CTT-CTT-CCT-TAT-TGA-3′ |
| b3a2 Antisense | 5′-GCT-GAA-GGG-CTT-TTG-AAC-TCT-GCT-3′ |
Incubation of DR+ cells with AS or control ODNs.
AS or control ODNs were added initially at a concentration of 40 μg/mL and a second time 18 hours later (30 μg/mL) for a total of 36 hours of incubation. To minimize AS-ODN degradation, cell incubation was performed in a serum-free medium.33 To induce proliferation of FACS-selected normal or CML DR+ cells, which are usually in G0,14 the serum-free medium was supplemented with low levels of cytokines (SFM-LC), similar to normal stroma-conditioned long-term bone marrow culture (LTBMC) medium (granulocyte colony-stimulating factor [25 pg/mL], granulocyte-macrophage colony-stimulating factor [10 pg/mL], leukemia inhibitory factor [50 pg/mL], stem cell factor [200 pg/mL], interleukin-6 [1 ng/mL], and macrophage inflammatory protein-1α [200 ng/mL]),34 which we have previously used to induce DR+ cell proliferation.14 Incubation of normal and CML progenitors in this serum-free medium maintains CFC viability, does not alter CFC adhesion, and induces CFC proliferation, as measured by the thymidine suicide assay.
Coculture of DR+ cells with stromal layers.
A total of 10,000 CML and normal DR+ cells cultured in SFM-LC were washed and resuspended in 0.5 mL LTBMC medium and either plated on irradiated normal stromal layers or kept in suspension for 4 hours, as previously described.14 After coculture, stromal layers were washed three times with warm Iscove's modified Dulbecco's medium (IMDM) and nonadherent cells collected.30 Adherent cells were obtained using Trypsin EDTA (Sigma). Both fractions were plated in short-term methylcellulose assay and the percent adherent CFC was calculated as: [stroma-adherent CFC divided by (stroma-adherent CFC + stroma-nonadherent CFC)] × 100. Thymidine suicide assays were performed to evaluate the percent of CFC in S-phase in the different populations.
Coculture of DR+ cells with fibronectin substrates.
75-kD and 33-66-kD (60 μg/mL) proteolytic fragments from FN were adsorbed to wells of a 48-well plate as previously described.14 Control wells were adsorbed with 5 mg/dL bovine serum albumin (BSA; >99% pure, fatty acid free; Sigma) alone or with poly-L-Lysine (1:50,000; Sigma). A total of 10,000 CML or normal DR+ cells cultured for 96 hours in SFM-LC were washed and resuspended in 0.5 mL IMDM with 0.3% BSA and cultured either in ligand-coated or control wells at 37°C and 5% CO2. After 4 hours, nonadherent cells were removed by standardized horizontal shaking of the plates followed by four to five consecutive washings with warm IMDM. Adherent cells were removed by trypsinization.14 Both fractions were plated in short-term methylcellulose assay and the percent adherent CFC was calculated as: [Adherent CFC divided by (adherent CFC + nonadherent CFC)] × 100. Thymidine suicide assays were performed on the different fractions to determine their proliferative status.
Cross-linking of integrin receptors with monoclonal antibodies.
A total of 104 CML or normal DR+ cells were cultured in SFM-LC for 96 hours, washed, and resuspended in 0.5 mL IMDM with 0.3% BSA, aliquoted in 5-mL tubes (Becton Dickinson Labware, Lincoln Park, NJ) and incubated with monoclonal antibody P4C10, directed against the β1 integrin receptor (1:2,500; mouse ascites; Life Technologies, GIBCO-BRL, Gaithersburg, MD), or control mouse IgG (2 mg/mL; 1:2,500; Sigma Chemical Co, St Louis, MO) for 30 minutes at room temperature. After washing, cells were incubated with goat antimouse antibodies (1:500; Biosource International, Camarillo, CA) for 30 minutes at room temperature. Cells were then incubated at 37°C in a humidified atmosphere with 5% CO2 for 4 hours in the continued presence of the secondary antibodies, after which they were washed and their proliferative status was assessed by thymidine suicide assays.14
Thymidine suicide assays.
The different cell fractions recovered after culture with stroma or ligand-coated plates, or kept in suspension, with or without addition of antibodies, were washed in serum-free IMDM (pH 7.3) and divided into two equal fractions. They were then suspended in 200 μL serum-free IMDM and incubated at 37°C with or without 5 mCi3H-thymidine (6.7 Ci/mmol; New England Nuclear, Boston, MA). After 20 minutes, 5 mL cold thymidine (500 mg/mL in IMDM with 20% fetal calf serum; Sigma) was added to all samples to competitively inhibit further 3H-thymidine uptake. Cells were washed in IMDM and plated in methylcellulose progenitor culture for 14 to 18 days. The percentage of CFC in S-phase was calculated as the percentage of CFC killed in the presence of 3H-thymidine {% CFC in S-phase = [(CFC present in absence of 3H-thymidine − CFC present in presence of 3H-thymidine) × 100] divided by CFC present in the absence of3H-thymidine}.35 The reproducibility and specificity of this assay have been previously shown.9,13 35
Methylcellulose progenitor culture.
DR+ cells recovered from adhesion and thymidine suicide assays were plated in methylcellulose culture as previously described.36 Cultures were incubated in a humidified atmosphere at 37°C and 5% CO2 for 14 to 18 days and assessed for the presence of colony-forming unit-granulocyte macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E), and CFU-MIX colonies.
Polymerase chain reaction (PCR) analysis.
After incubation with AS-ODNs, CML DR+ cells were counted and frozen at −70°C in PBS. Total cellular RNA was extracted from thawed samples using the method of Chomczynski and Saachi.37 After reverse transcription, amplification of BCR-ABL mRNA was performed with the primers shown in Table 2, using 30 to 40 cycles of single-step PCR. Amplified samples were size separated by gel electrophoresis, ethidium bromide stained, and photographed.38 β-Actin controls were run for each sample. The ratio of BCR-ABL to β-actin was determined by densitometry.
PCR Primers
| BCR-ABL |
| 5′-CTC-CAG-ACT-GTC-CAC-AGC-ATT-CCG-3′ |
| 5′-TCA-GAC-CCT-GAG-GCT-CAA-AGT-C-3′ |
| β-Actin |
| 5′-TAC-CTC-ATG-AAG-ATC-CTC-A-3′ |
| 5′-TTC-GTG-GAT-GCC-ACA-GGA-C-3′ |
| BCR-ABL |
| 5′-CTC-CAG-ACT-GTC-CAC-AGC-ATT-CCG-3′ |
| 5′-TCA-GAC-CCT-GAG-GCT-CAA-AGT-C-3′ |
| β-Actin |
| 5′-TAC-CTC-ATG-AAG-ATC-CTC-A-3′ |
| 5′-TTC-GTG-GAT-GCC-ACA-GGA-C-3′ |
Western blotting.
After incubation with AS-ODNs, CML DR+ cells were counted and protein extracts obtained by incubating with lysis buffer (1% Triton-X 100; 1 mmol/L EDTA; 150 mmol/L NaCl; 50 mmol/L Tris-HCl; 10 mmol/L sodium orthovanadate; 10 mmol/L sodium fluoride; 10 mmol/L phenylmethyl sulfonyl fluoride [PMSF]; 0.15 U/mL Aprotinin; 10 U/mL leupeptin, pH 7.5). Protein extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to a nitrocellulose filter. p145c-ABL and p210BCR-ABL were visualized with an anti–c-ABL monoclonal antibody (antibody 24-11; Santa Cruz Biotechnology Inc, Santa Cruz, CA or antibody Ab-3; Oncogene Science Inc, Manhasset, NY), peroxidase-conjugated goat antimouse IgG (Jackson Immunoresearch Laboratories Inc, West Grove, PA) and a Western blot chemiluminescence reagent (Amersham Life Sciences, Arlington Heights, IL). The ratio of BCR-ABL to c-ABL was determined by densitometry.
Statistical analysis.
Results of experimental points obtained from multiple experiments were reported as mean ± standard error of mean (SEM). Significance levels for differences between different samples was determined using two-tailed Student's t-test.
RESULTS
Anti–BCR-ABL AS-ODNs restore CML CFC adhesion to stroma or FN.
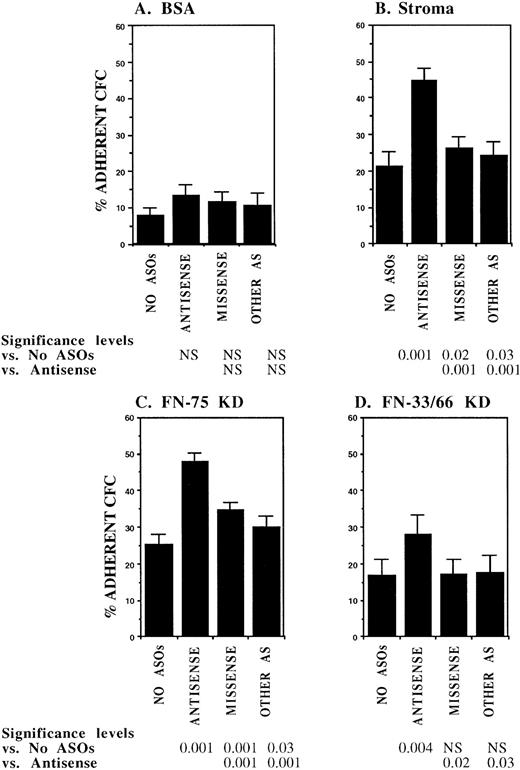
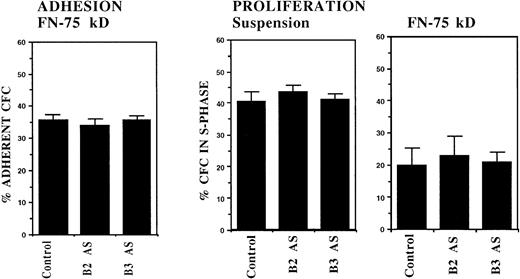
In initial experiments, CML DR+ cells were incubated with 18-mer AS-ODNs specific for the BCR-ABL breakpoint (b3a2 or b2a2) present in the cells that were being evaluated. Negative controls included missense AS-ODNs, eg, b2a2 missense if the b2a2 breakpoint was present, and AS-ODNs to the alternate breakpoint, eg, b3a2 if the tested cells contained the b2a2 breakpoint. Preincubation with breakpoint-specific AS-ODNs or control ODNs did not significantly affect subsequent colony growth (data not shown). Incubation with breakpoint-specific AS-ODNs significantly increased adhesion of CML CFC to stromal layers (Fig1). Control AS-ODNs had significantly less effect on CML CFC adhesion. Breakpoint-specific AS-ODNs also enhanced CML CFC adhesion to 75-kD and 33-66-kD fragments of FN recognized by α4β1 and α5β1 integrins, respectively,32 significantly more than control ODNs (Fig1). These observations indicate that breakpoint-specific BCR-ABL AS-ODNs can restore β1 integrin-mediated adhesion of CML CFC. The effects of specific AS-ODNs on adhesion of CML cells containing either the b2a2 or b3a2breakpoint were equivalent.
Anti–BCR-ABL AS-ODNs restore CML CFC adhesion to stroma or fibronectin. A total of 10,000 DR+ cells were incubated in serum-free medium with or without breakpoint-specific and control ODNs after which their adhesion to BSA (A); stromal-adherent layers (B); FN 75-kD (C); and FN 33-66-kD (D) coated wells was evaluated as described in the text. Results shown represent the mean ± SEM for six (A, C, and D) or nine (B) separate experiments.
Anti–BCR-ABL AS-ODNs restore CML CFC adhesion to stroma or fibronectin. A total of 10,000 DR+ cells were incubated in serum-free medium with or without breakpoint-specific and control ODNs after which their adhesion to BSA (A); stromal-adherent layers (B); FN 75-kD (C); and FN 33-66-kD (D) coated wells was evaluated as described in the text. Results shown represent the mean ± SEM for six (A, C, and D) or nine (B) separate experiments.
Anti–BCR-ABL AS-ODNs restore β1 integrin-dependent inhibition of CML CFC proliferation.
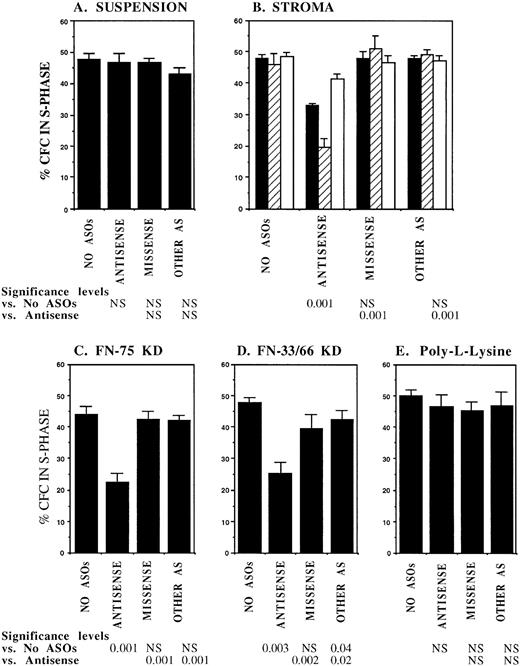
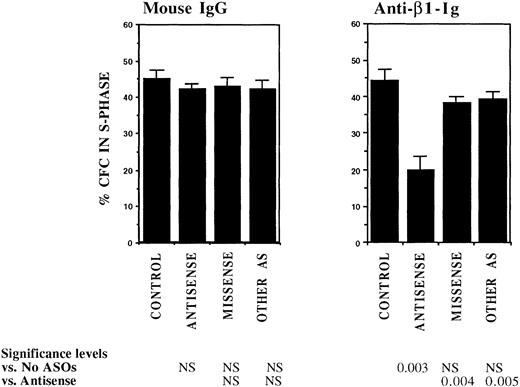
FACS selected CML DR+ cells, which are usually in G0,13 were induced to proliferate by culture for 4 days in serum-free medium supplemented with cytokines at concentrations similar to those present in normal stroma-conditioned LTBMC medium (SFM-LC).34 After 4 days, approximately 50% of CML DR+ CFC were in S-phase as evaluated by thymidine suicide assays. Proliferating CML DR+ cells were exposed to AS and control ODNs during the last 36 hours of culture and then cocultured with stroma and FN substrates for 4 hours, and the percentage of CFC in S-phase was evaluated using thymidine suicide assays. Consistent with previous studies, proliferation of untreated CML CFC was similar whether cells were kept in suspension or were cocultured with stroma or FN fragments.13 Preincubation with either breakpoint-specific AS-ODNs or control ODNs did not alter proliferation of CML CFC kept in suspension. However, significant reduction in proliferation of CML CFC preincubated with breakpoint-specific AS-ODNs was seen after coculture with stroma (Fig 2), primarily as a result of inhibition of proliferation of adherent CFC. CML CFC proliferation was also significantly reduced when DR+ cells pretreated with AS-ODNs were cocultured with FN 75-kD and 33-66-kD substrates (Fig 2). In contrast to breakpoint-specific AS-ODNs, control ODNs did not significantly reduce proliferation of CML CFC cocultured with either stroma or FN (Fig 2). Inhibition of CML CFC proliferation was not a nonspecific effect of adhesion because proliferation of CML CFC treated with AS-ODNs was not reduced after coculture with the adhesogen poly-L-lysine (Fig 2). In additional experiments, we examined the effect of antibody-mediated stimulation of β1 integrins on CML CFC proliferation. Proliferation of untreated CML CFC was not significantly altered by cross-linking of β1 integrin receptors with blocking anti-β1 integrin mouse monoclonal antibodies and secondary antimouse antibodies (Fig 3). However, proliferation of CML CFC pretreated with anti–BCR-ABL AS-ODNs, but not control ODNs, was significantly inhibited after antibody-mediated cross-linking of β1 integrin receptors (Fig 3). These observations indicate that breakpoint-specific BCR-ABL AS-ODNs can restore β1 integrin-mediated inhibition of CML CFC proliferation.
Anti–BCR-ABL AS-ODNs restore inhibition of CML CFC proliferation after coculture with stroma or FN. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. After incubation, cells were either kept in suspension (A) or cocultured with stroma (B); FN 75-kD (C); FN 33-66-kD (D); or poly-L-lysine (E) coated wells for 4 hours, after which proliferation was evaluated using thymidine suicide assays as described in the text. For stroma (B), results for adherent (hatched bars), nonadherent (open bars), and total (adherent + nonadherent) cells (solid bars) are shown separately. The significance values in (B) represent differences in proliferation of total (adherent + nonadherent) cells. Results represent the mean ± SEM for six separate experiments.
Anti–BCR-ABL AS-ODNs restore inhibition of CML CFC proliferation after coculture with stroma or FN. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. After incubation, cells were either kept in suspension (A) or cocultured with stroma (B); FN 75-kD (C); FN 33-66-kD (D); or poly-L-lysine (E) coated wells for 4 hours, after which proliferation was evaluated using thymidine suicide assays as described in the text. For stroma (B), results for adherent (hatched bars), nonadherent (open bars), and total (adherent + nonadherent) cells (solid bars) are shown separately. The significance values in (B) represent differences in proliferation of total (adherent + nonadherent) cells. Results represent the mean ± SEM for six separate experiments.
Anti–BCR-ABL AS-ODNs restore inhibition of CML CFC proliferation after cross-linking with anti-β1 integrin antibodies. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. Cells were then incubated with anti-β1 integrin antibodies or control mouse antibodies for 30 minutes followed by incubation with goat antimouse antibodies for 4 hours. Proliferation of CFC with or without antibody treatment was assessed using thymidine suicide assays. Results represent the mean ± SEM for four separate experiments.
Anti–BCR-ABL AS-ODNs restore inhibition of CML CFC proliferation after cross-linking with anti-β1 integrin antibodies. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. Cells were then incubated with anti-β1 integrin antibodies or control mouse antibodies for 30 minutes followed by incubation with goat antimouse antibodies for 4 hours. Proliferation of CFC with or without antibody treatment was assessed using thymidine suicide assays. Results represent the mean ± SEM for four separate experiments.
The effect of anti–BCR-ABL AS-ODNs on CML progenitor adhesion and proliferation is specific.
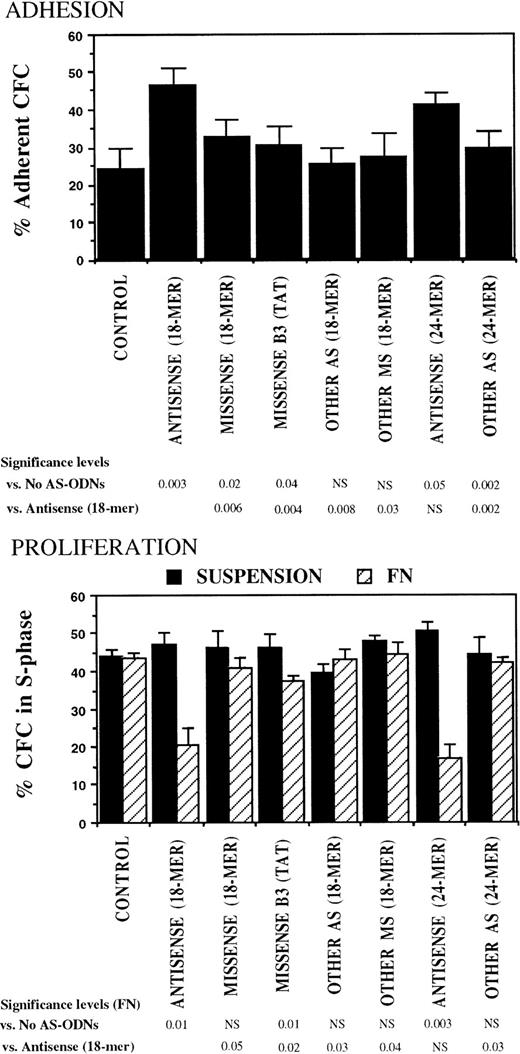
The specificity of the observed effects of breakpoint-specific AS-ODNs on CML CFC adhesion and proliferation was further evaluated in experiments in which CML DR+ cells were incubated with additional breakpoint-specific and control AS-ODNs. These included 24-mer b2a2 and b3a2breakpoint-specific AS-ODNs and a control 18-mer missense anti-b3a2-ODN containing a TAT sequence 5′ from the breakpoint, which is associated with nonspecific effects in BCR-ABL–containing cell lines.39 Both 18- and 24-mer breakpoint-specific AS-ODNs restored CML CFC adhesion to FN and proliferation inhibition after contact with FN, significantly more than any of the control ODNs (Fig 4).
Anti–BCR-ABL AS-ODNs restore CML CFC adhesion to and proliferation inhibition by fibronectin significantly more than control ODNs. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. After incubation, cells were either kept in suspension or were cocultured with FN substrates, and their adhesion and proliferation were evaluated as described in the text. Results represent the mean ± SEM for four separate experiments.
Anti–BCR-ABL AS-ODNs restore CML CFC adhesion to and proliferation inhibition by fibronectin significantly more than control ODNs. A total of 10,000 DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. After incubation, cells were either kept in suspension or were cocultured with FN substrates, and their adhesion and proliferation were evaluated as described in the text. Results represent the mean ± SEM for four separate experiments.
In another set of experiments, incubation of normal DR+cells with anti–BCR-ABL ODNs did not alter the adhesion of normal CFC to FN (Fig 5). In addition, inhibition of normal CFC proliferation usually seen after coculture with FN substrates was unaltered after preincubation with BCR-ABL AS-ODNs. These results indicate that the effects of BCR-ABL AS-ODNs on CML CFC adhesion and proliferation are not the result of nonspecific effects of ODNs on adhesion or proliferation.40
Anti–BCR-ABL AS-ODNs do not alter normal CFC adhesion or proliferation. A total of 10,000 normal DR+ cells were incubated in serum-free medium for 96 hours with or without addition of b2a2 and b3a2breakpoint-specific ODNs. After incubation, cells were either kept in suspension or were cocultured with FN- (75 kD) coated wells for 4 hours and their adhesion and proliferation were evaluated as described in the text. Results represent the mean ± SEM for eight separate experiments.
Anti–BCR-ABL AS-ODNs do not alter normal CFC adhesion or proliferation. A total of 10,000 normal DR+ cells were incubated in serum-free medium for 96 hours with or without addition of b2a2 and b3a2breakpoint-specific ODNs. After incubation, cells were either kept in suspension or were cocultured with FN- (75 kD) coated wells for 4 hours and their adhesion and proliferation were evaluated as described in the text. Results represent the mean ± SEM for eight separate experiments.
Anti–BCR-ABL AS-ODNs inhibit BCR-ABL expression in CML DR+ cells.
DR+ cells incubated with breakpoint-specific AS-ODNs or control ODNs were evaluated by reverse transcriptase (RT)-PCR for BCR-ABL mRNA levels and by Western blotting for p210BCR-ABL levels to determine the effect of BCR-ABL AS-ODNs on BCR-ABL expression. We show that exposure of CML DR+ cells to breakpoint-specific AS-ODNs, but not control ODNs, results in significant reduction of BCR-ABL mRNA levels (Table 3 and Fig 6). Similarly, significant reduction in p210BCR-ABL levels was observed after incubation with breakpoint-specific AS-ODNs resulted in CML DR+ cells, but not control AS-ODNs (Table 4 and Fig 7). These studies indicate that, under the conditions used, incubation with breakpoint-specific AS-ODNs can, at least partially, inhibit BCR-ABL expression.
Effect of AS-ODNs on BCR-ABL mRNA Expression
| Sample . | Control . | BreakpointSpecific AS-ODN . | Missense ODN . | AS-ODN to Other Breakpoint . |
|---|---|---|---|---|
| 1 | 1.0 | 0.29 | 0.83 | 1.83 |
| 2 | 1.0 | 0.23 | 0.31 | 0.46 |
| 3 | 1.0 | 0.07 | 0.7 | 0.64 |
| Mean ± SEM | 1.0 ± 0 | 0.2 ± 0.07 | 0.61 ± 0.16 | 0.97 ± 0.42 |
| Significance (compared with control) | P < .007 | NS | NS |
| Sample . | Control . | BreakpointSpecific AS-ODN . | Missense ODN . | AS-ODN to Other Breakpoint . |
|---|---|---|---|---|
| 1 | 1.0 | 0.29 | 0.83 | 1.83 |
| 2 | 1.0 | 0.23 | 0.31 | 0.46 |
| 3 | 1.0 | 0.07 | 0.7 | 0.64 |
| Mean ± SEM | 1.0 ± 0 | 0.2 ± 0.07 | 0.61 ± 0.16 | 0.97 ± 0.42 |
| Significance (compared with control) | P < .007 | NS | NS |
DR+ cells were incubated in serum-free medium with or without addition of breakpoint-specific and control ODNs. PCR amplification of BCR-ABL mRNA extracted from 25,000 DR+cells was performed as described in the text, amplified samples were size separated by gel-electrophoresis, ethidium bromide stained, and photographed. β-Actin controls were run for each sample. Densitometry was performed and the results were expressed as the ratio of BCR-ABL to β-actin, with controls normalized to 1.0.
Abbreviation: NS, not significant.
Anti–BCR-ABL AS-ODNs inhibit BCR-ABL mRNA expression. CML DR+ cells were incubated in serum-free medium with or without addition of breakpoint-specific and control ODNs. PCR amplification of BCR-ABL mRNA extracted from 25,000 DR+cells was performed as described in the text, amplified samples were size separated by gel-electrophoresis, ethidium bromide stained, and photographed. β-Actin controls were run for each sample. In the representative experiment shown here, the b3a2breakpoint was present in the cells studied. Cells were incubated either without any ODNs (CONTROL), or with b3a2antisense ODNs (B3A2AS), b3a2 missense ODNs (B3A2MS), or b2a2 antisense ODNs (B2A2 AS).
Anti–BCR-ABL AS-ODNs inhibit BCR-ABL mRNA expression. CML DR+ cells were incubated in serum-free medium with or without addition of breakpoint-specific and control ODNs. PCR amplification of BCR-ABL mRNA extracted from 25,000 DR+cells was performed as described in the text, amplified samples were size separated by gel-electrophoresis, ethidium bromide stained, and photographed. β-Actin controls were run for each sample. In the representative experiment shown here, the b3a2breakpoint was present in the cells studied. Cells were incubated either without any ODNs (CONTROL), or with b3a2antisense ODNs (B3A2AS), b3a2 missense ODNs (B3A2MS), or b2a2 antisense ODNs (B2A2 AS).
Effect of AS-ODNs on p210BCR-ABLExpression
| Sample . | Control . | BreakpointSpecific AS-ODN . | Missense ODN . | AS-ODN to Other Breakpoint . |
|---|---|---|---|---|
| 1 | 1.0 | 0.43 | 0.76 | 1.52 |
| 2 | 1.0 | 0.27 | 1.42 | 0.86 |
| 3 | 1.0 | 0.44 | 0.85 | 1.94 |
| 4 | 1.0 | 0.17 | 0.53 | 0.64 |
| 5 | 1.0 | 0.75 | 0.9 | 0.84 |
| Mean ± SEM | 1.0 ± 0 | 0.41 ± 0.1 | 0.9 ± 0.15 | 1.16 ± 0.25 |
| Significance (compared with control) | P < .004 | NS | NS |
| Sample . | Control . | BreakpointSpecific AS-ODN . | Missense ODN . | AS-ODN to Other Breakpoint . |
|---|---|---|---|---|
| 1 | 1.0 | 0.43 | 0.76 | 1.52 |
| 2 | 1.0 | 0.27 | 1.42 | 0.86 |
| 3 | 1.0 | 0.44 | 0.85 | 1.94 |
| 4 | 1.0 | 0.17 | 0.53 | 0.64 |
| 5 | 1.0 | 0.75 | 0.9 | 0.84 |
| Mean ± SEM | 1.0 ± 0 | 0.41 ± 0.1 | 0.9 ± 0.15 | 1.16 ± 0.25 |
| Significance (compared with control) | P < .004 | NS | NS |
DR+ cells were incubated in serum-free medium for 96 hours with or without addition of breakpoint-specific and control ODNs. Protein extracts obtained from 1 to 2 × 10e6 cells were subjected to gel electrophoresis and Western blotting with an anti–c-ABL antibody as described in Materials and Methods. Densitometry was performed and results expressed as the ratio of p210BCR-ABL to p140c-ABL, with controls normalized to 1.0.
Abbreviation: NS, not significant.
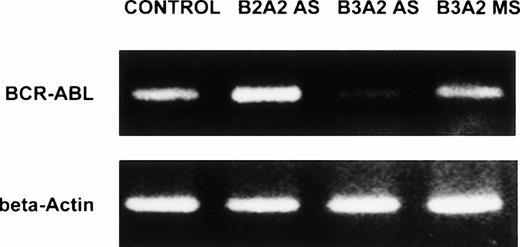
Anti–BCR-ABL AS-ODNs inhibit p210BCR-ABLexpression. CML CD34+ cells from a patient with a known b3a2 BCR-ABL splice site were incubated in serum-free medium with addition of either breakpoint-specific antisense ODNs (AS) or with b3a2 specific missense ODNs (MS) for 48 hours as described in the legend. mRNA obtained from 20 × 103 cells was used for RT-PCR for either the BCR-ABL mRNA or β-actin (top panel). Protein extracts obtained from 1 × 106 cells were subjected to SDS-PAGE and Western blotting with an anti–c-ABL antibody (Ab3) as described in Materials and Methods (bottom panel).
Anti–BCR-ABL AS-ODNs inhibit p210BCR-ABLexpression. CML CD34+ cells from a patient with a known b3a2 BCR-ABL splice site were incubated in serum-free medium with addition of either breakpoint-specific antisense ODNs (AS) or with b3a2 specific missense ODNs (MS) for 48 hours as described in the legend. mRNA obtained from 20 × 103 cells was used for RT-PCR for either the BCR-ABL mRNA or β-actin (top panel). Protein extracts obtained from 1 × 106 cells were subjected to SDS-PAGE and Western blotting with an anti–c-ABL antibody (Ab3) as described in Materials and Methods (bottom panel).
DISCUSSION
Adhesion receptor-mediated interactions with the extracellular matrix play an important role in the regulation of cellular proliferation, differentiation, and survival.5-8 Neoplastic transformation is often associated with altered cell-matrix interactions and perturbation of normal microenvironmental regulation of cell growth and function.41-43 The abnormal circulation and unregulated expansion of malignant hematopoietic cells in CML may be related to defective function of β1 integrin adhesion receptors. We hypothesized that the BCR-ABL oncogene may induce abnormal β1 integrin function in CML progenitors through its interactions with cytoskeletal proteins critical for normal integrin function. We show here that downregulation of BCR-ABL expression with breakpoint-specific AS-ODNs results in restoration of β1 integrin function in CML progenitors. These studies suggest a possible mechanism by which the BCR-ABL oncogene may contribute to abnormal unregulated proliferation and abnormal circulation of malignant hematopoietic progenitors in CML.
In previous studies, BCR-ABL AS-ODNs have inhibited clonogenic growth of CML-blast crisis progenitors but have failed to consistently inhibit growth of CML-chronic phase progenitors.27,44 In the present studies, the growth of chronic phase CML CFC was not altered after 48 hours of exposure to BCR-ABL AS-ODNs. In addition, BCR-ABL AS-ODNs did not by themselves alter the percentage of CML progenitors in S-phase. However, the present studies indicate that downregulation of BCR-ABL expression with BCR-ABL breakpoint-specific AS-ODNs is associated with restoration of progenitor-microenvironmental interactions in CML. Malignant CML progenitors show deficient adhesion to stroma and FN through α4β1 and α5β1 integrins. Treatment with breakpoint-specific AS-ODNs resulted in enhanced CML progenitor adhesion to stroma and α4β1 and α5β1 integrin binding fragments of FN. In normal progenitors, β1 integrin-mediated adhesion to stroma and FN, or engagement of the integrin receptors with anti-β1 antibodies, leads to inhibition of progenitor proliferation. CML hematopoietic progenitors, in contrast, are unresponsive to β1 integrin-mediated inhibition of proliferation.13Breakpoint-specific AS-ODNs, besides restoring CML CFC adhesion, also restore inhibition of CML progenitor proliferation after coculture with stroma, FN, or anti-β1 integrin antibodies. Reduced BCR-ABL expression does not seem to represent increased numbers of normal cells given the lack of reduction in CFC numbers after culture, the tiny fraction of BCR-ABL–negative cells in the starting CD34+population, and the lack of significant expansion in normal CFC number after 36 hours of culture in medium with low cytokine concentrations. These observations support the hypothesis that p210BCR-ABLis responsible for abnormal β1 integrin-mediated adhesion and inhibition of proliferation in CML progenitors.
When interpreting the results of experiments using AS-ODNs, it is important to recognize that cellular responses observed after exposure to AS-ODNs may not necessarily be the result of specific inhibition of the mRNA targeted by that ODN.45 In previous studies, missense ODNs, and AS-ODNs to the breakpoint not present in the cells being evaluated, have had nonspecific effects on BCR-ABL–transduced cell lines.46 In the present studies, however, significant inhibition of BCR-ABL expression at the protein and mRNA level in CML CD34+ cells was observed with breakpoint-specific BCR-ABL AS-ODNs, but not control ODNs. In addition, 18- and 24-mer breakpoint-specific AS-ODNs restored β1 integrin-mediated adhesion and proliferation inhibition of CML CFC significantly more than control missense ODNs, control AS-ODNs to the breakpoint not present in the cells being studied, and a control b3a2missense ODNs containing the TAT sequence present in anti-b2a2 AS-ODNs,39 which is particularly associated with nonspecific effects. Our studies indicate that even partial inhibition of BCR-ABL expression in CML primary progenitors is sufficient to allow restoration of integrin-dependent microenvironmental regulation. Although AS-ODNs can nonspecifically alter cell adhesion,40 this does not appear to be the case here because normal CFC adhesion or adhesion-mediated proliferation inhibition was not affected by BCR-ABL AS-ODNs.
BCR-ABL–expressing cell lines express several-fold more p210BCR-ABL than primary CML hematopoietic cells.47 As a result, in previous studies using CML cell lines, high concentrations of phosphorothioate AS-ODNs, which are more likely to have nonspecific effects, have been required to achieve inhibition of BCR-ABL expression. The selective effects of breakpoint-specific BCR-ABL AS-ODNs on BCR-ABL expression and CML CFC adhesion and proliferation in our studies may thus be related to the use of phosphodiester instead of phosphorothioate AS-ODNs, the use of serum-free medium to prevent phosphodiester AS-ODN degradation, and the use of relatively low concentrations of AS-ODNs, all of which reduce the likelihood of nonspecific effects.45 Higher concentrations or prolonged exposure to AS-ODNs, or the use of phosphorothioate AS-ODNs, may possibly have resulted in more complete inhibition of BCR-ABL expression in our studies, but would probably have caused increased nonspecific effects.
p210BCR-ABL may induce abnormal integrin function in CML by associating with and/or abnormally phosphorylating cytoskeletal proteins or cytoskeleton-associated signaling proteins important for normal integrin function. We have recently shown that β1 integrin receptor capping after antibody-mediated receptor engagement and cross-linking, which is an indicator of the ability of the integrin receptor to interact with the cytoskeleton,10 is significantly impaired in BCR-ABL–transformed cells, indicating abnormal receptor cytoskeletal interactions.48 Inhibition of BCR-ABL mRNA and protein expression by insertion of antisense gene constructs restores β1 integrin capping in these cells, supporting a role for BCR-ABL in defective capping.49 Integrin cytoplasmic domains do not have intrinsic enzymatic activity, but mediate “inside-out” and “outside-in” signaling through interactions with cytoskeletal elements such as α-actinin, talin, vinculin, paxillin, and tensin in focal adhesion complexes.50-52 These proteins, besides connecting integrin receptors to actin microfilaments, also serve as a framework for the association of other signaling proteins, including protein tyrosine kinases such as FAK and SRC and adapter proteins such as GRB-2 and CRK,52,53 thereby linking integrin receptor stimulation to various intracellular signaling pathways. Integrin stimulation is also associated with cytoskeletal translocation and activation of the phosphoinositide 3-kinase (PI-3 kinase),54 which may also play an important role in modulation of integrin function. The focal adhesion-associated proteins paxillin, FAK, vinculin, talin, and tensin are constitutively phosphorylated in BCR-ABL–transfected cell lines.24,26,55 Recent studies suggest that the adapter proteins CRKL and CBL are prominent p210BCR-ABL substrates in primary CML cells25 and may form a complex with p210BCR-ABL and link it with paxillin and the p85 regulatory subunit of PI-3 kinase.56-58 Finally, other studies have shown that the COOH terminus of c-ABL has F- and G-actin binding domains with actin bundling activity, that p210BCR-ABL shows enhanced actin binding through this COOH-terminal domain, and that this interaction may be important for the transforming ability of BCR-ABL.23 59 Some or all of these interactions of p210BCR-ABL with integrin-related proteins may contribute to abnormal integrin function in CML.
Some other recent observations also support the concept that BCR-ABL may alter integrin-mediated adhesion, as well as signaling from the extracellular matrix (ECM). Proliferation of normal fibroblasts requires signals generated both by cytokine stimulation, as well as cell attachment via integrins. BCR-ABL transfection has been reported to abrogate the anchorage requirement, but not the growth factor requirement, for fibroblast proliferation.60 Cytokine and serum starvation of hematopoietic cell lines or primary hematopoietic progenitors is associated with failure of integrin-mediated adhesion to FN, but is reversed after readdition of cytokines.61,62 In contrast, the same cell lines transfected with BCR-ABL and serum and cytokine starved continued to adhere to FN, but adhesion could not be upregulated by readdition of cytokines.62 The proadhesive effect of BCR-ABL therefore mimics the effect of cytokines in BCR-ABL–negative cell lines. These studies suggest that BCR-ABL can, in different cellular contexts, alter inside-out signaling pathways that regulate integrin-mediated adhesion and growth signaling. The differences between the effects of BCR-ABL in these studies and our own may be related to the differences in the cell types and the assay conditions used. In the former studies, fibroblasts and cell lines rather than primary hematopoietic progenitors were used. Further, the cell lines were serum and cytokine starved before adhesion studies, which may explain their relatively deficient adhesion. It is possible that BCR-ABL may prevent decrease in cell adhesion after cytokine withdrawal by preventing downregulation of Rho and Rac,63required for actin stress fiber formation, through constitutive activation of intracellular signal molecules such as p62DOKand pl20GAP.64,65 In addition, BCR-ABL protects cells that are cytokine starved from apoptotic cell death.21 The decrease in adhesion of BCR-ABL–negative cell lines, but not BCR-ABL–containing cell lines, after cytokine starvation may therefore be related to increased stress on these “normal” cells. Reduced adhesion of CML progenitors in our studies is not the result of reduced growth of adherent progenitors, as the total number of colonies, adherent + nonadherent, generated from CML DR+ cells after coculture with control BSA and FN fragments was similar, and proliferation of the CML progenitors that adhered to FN was not reduced when measured in thymidine suicide assays.
In conclusion, we show that inhibition of BCR-ABL expression with breakpoint-specific AS-ODNs restores β1 integrin-mediated adhesion and proliferation inhibition in CML CFC. Our studies link the BCR-ABL oncogene with altered integrin-dependent microenvironmental regulation and abnormal circulation of hematopoietic progenitor proliferation in CML. Future studies will be directed towards understanding the specific molecular mechanisms underlying p210BCR-ABL-induced disruption of integrin function in CML progenitors.
ACKNOWLEDGMENT
The authors would like to acknowledge the excellent technical assistance of Heidi Munthe, Denise Malone, and Brad Anderson.
Supported in part by National Institutes of Health (Bethesda, MD) Grants No. R29 CA74455, PO1 CA 45814, PO1 CA65493, and R01 HL 49930; and the University of Minnesota Hospitals and Clinics (Minneapolis). R.B. is a recipient of an American Society of Clinical Oncology Young Investigators Award (Alexandria, VA). C.M.V. is a Scholar of the Leukemia Society of America (New York, NY).
Address reprint requests to Ravi Bhatia, MD, Department of Hematology and Bone Marrow Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91006.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal