Abstract
Multiple myeloma (MM) is usually characterized by production of a single serum monoclonal protein of constant isotype and light-chain restriction. Multiple Ig isotypes and isotype switches, which are rare in untreated patients, are reported to be more common in patients undergoing myeloablative therapy. These additional protein bands, detected by immunofixation electrophoresis (IFE), could be due to altered paraprotein production by the malignant plasma cell clone or oligoclonal Ig production during recovery of B-cell function after myeloablative therapy. We analyzed abnormal protein bands (APB), distinct from the presenting paraprotein, in 550 patients receiving high-dose therapy with autologous hematopoietic cell transplantation at a single institution. Fifty-five patients (10%) had APB, 48 had oligoclonal bands (OB), and 23 had an apparent isotype switch (IS) on IFE (16 had both OB and IS). Morphologic and flow cytometric examination of bone marrow in 17 patients with IS showed no evidence of a clonal plasma cell isotype switch. Patients with APB had significantly higher complete response to therapy (67% v 37%,P = .001). To assess the independent prognostic relevance of APB, a multivariate analysis was performed among 471 patients surviving at least 12 months from first transplant (all patients developing APB had done so by 12 months from first transplant). APB (in 50 patients) was a favorable feature for both event-free (rank 3, P = .004) and overall survival (rank 3, P = .0005). We propose that OB and IS are likely to be due to recovery of Ig production rather than alterations in the biology of the malignant plasma cell clone.
THE MALIGNANT PLASMA cell clone in patients with multiple myeloma (MM) usually produces a single abnormal unique monoclonal antibody with a constant isotype and light-chain restriction (paraprotein). This characteristic is important in both diagnosis and management of patients with MM. Biclonal and triclonal paraproteins are detected rarely at the time of diagnosis1,2 and switching of paraprotein isotype has been reported following high-dose chemotherapy.3 The prevalence, etiology, and clinical significance of this finding is not known. In patients with MM, the appearance of abnormal proteins bands (APB) distinct from the presenting paraprotein on serum immunofixation electrophoresis (IFE), could be due to either a change in paraprotein production by the malignant plasma cell clone, emergence of a second malignant clone, or a temporary result of myeloablative therapy. Previous studies4-6 have shown that antibody production is impaired after both allogeneic and autologous bone marrow (BM) transplantation and that transient oligoclonal bands (OB) detectable by serum IFE are common during the recovery of Ig production. Because the analysis of monoclonal proteins is important in monitoring response to therapy in MM, the appearance of new single or OB may pose problems in patient management. We conducted a retrospective analysis of the clinical records, serial serum IFE, and BM studies of 550 myeloma patients enrolled in trials of tandem autotransplants to determine the frequency and clinical significance of APB. All 550 patients completed a first transplant in support of high-dose melphalan and 78% completed a second transplant. Ten percent of patients developed APB, which were usually transient and coincided with the recovery of normal Ig production associated with a marked tumor response. APB appear to be unrelated to the underlying B-cell malignancy.
MATERIALS AND METHODS
The records of all 550 successive myeloma patients enrolled in tandem autotransplant trials between January 1991 and November 1995 were reviewed with follow-up to July 1996 (median follow-up, 21 months; range, 0 to 73 months); all completed one autotransplant with high-dose melphalan and 78% completed a second transplant. Fifty-five patients (10%) had APB on serum IFE (median follow-up, 27 months; range, 5 to 64 months).
Patients.
All patients had MM staged by standard criteria.7 The nature of serum or urine monoclonal proteins on initial evaluation at UAMS was determined by IFE in the clinical immunology laboratory at the University Hospital. Table 1 summarizes the paraprotein analysis at diagnosis in the 55 patients studied.
Paraprotein Characterization at Initial Diagnosis in 55 Patients Who Subsequently Developed APB
| Ig . | No. of Patients . |
|---|---|
| IgGκ | 12 |
| IgGλ | 7 |
| IgGλ & free λ | 1 |
| IgAκ | 8 |
| IgAλ | 7 |
| IgDλ | 1 |
| IgDλ & free λ | 1 |
| Free κ | 9 |
| Free λ | 8 |
| Nonsecretory | 1 |
| Ig . | No. of Patients . |
|---|---|
| IgGκ | 12 |
| IgGλ | 7 |
| IgGλ & free λ | 1 |
| IgAκ | 8 |
| IgAλ | 7 |
| IgDλ | 1 |
| IgDλ & free λ | 1 |
| Free κ | 9 |
| Free λ | 8 |
| Nonsecretory | 1 |
Therapy.
Several high-dose regimens using combinations of high-dose alkylating agents and total body irradiation (TBI) were used as previously described.8 The high-dose regimens used in the 55 patients with APB are summarized in Table 2. Two patients were transplanted with autologous BM, 9 received both autologous BM and peripheral blood stem cells (PBSC), 43 were transplanted with PBSC, and 1 received syngeneic BM. Most patients were maintained on either interferon α-2b or glucocorticoid therapy.
High-Dose Regimens Used in 55 Patients With APB
| High-Dose Regimen . | Transplant × 2 . | Transplant × 1 . |
|---|---|---|
| Mel 200 | 33 | 9 |
| Mel 140/TBI | 0 | 1 |
| Mel 200 then Mel/TBI | 5 | — |
| Mel 200 then Mel/Cy | 6 | — |
| Mel 140 then Cy/carbo/etoposide | 1 | — |
| High-Dose Regimen . | Transplant × 2 . | Transplant × 1 . |
|---|---|---|
| Mel 200 | 33 | 9 |
| Mel 140/TBI | 0 | 1 |
| Mel 200 then Mel/TBI | 5 | — |
| Mel 200 then Mel/Cy | 6 | — |
| Mel 140 then Cy/carbo/etoposide | 1 | — |
Melphalan was used alone in a dose of 200 mg/m2 (Mel 200) or at a dose of 140 mg/m2 together with total body irradiation (Mel 140/TBI) (radiation dose of 850 to 1,125 cGy) or with cyclophosphamide (dose 100 to 120 mg/kg) (Mel/Cy). One patient had a first transplant after Mel 140 and a second high-dose therapy with cyclophosphamide, carboplatin (carbo), and etoposide.
Response.
Partial remission (PR) was defined as greater than or equal to 75% tumor mass reduction with a normal BM aspirate and biopsy on two occasions at least 2 months apart. Patients with complete response (CR) also had undetectable paraprotein in serum and urine by IFE.
Serum monoclonal antibody identification.
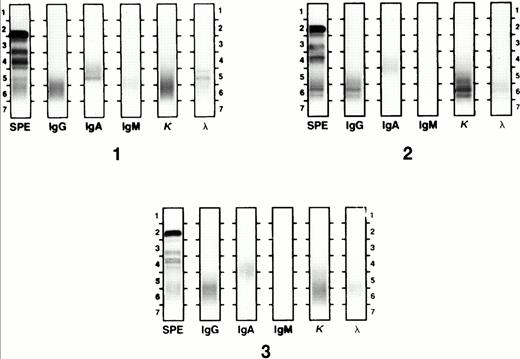
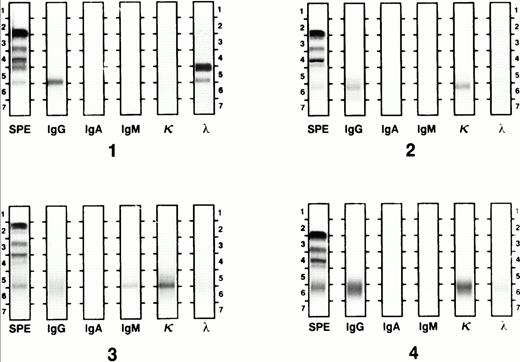
Ig class and light chain were determined using the Beckman Paragon Electrophoresis System (Beckman Instruments, Irvine, CA) according to the manufacturer's protocol. Briefly, serum proteins were separated in agarose gel media by electrophoresis at alkaline pH. Antiserum against specific Ig classes and light-chain types was applied and identification was made after staining of immunoprecipitated antigen-antibody complexes. Serum IFE was performed at presentation and as indicated when monoclonal protein bands were identified by serum protein electrophoresis or when disease relapse was evident in the BM. All IFE gels were retrospectively reviewed in a blinded fashion. Gels were reported as showing OB when two or more discrete Ig bands of the same isotype, distinct from the presenting myeloma associated paraprotein, were seen (Fig 1). OB were distinguished from paraprotein by isotype, light chain, and electrophoretic mobility. An apparent isotype switch (IS) was defined as a single distinct protein band with a different heavy chain class or light-chain type from the original paraprotein (Fig 2). This included the appearance of an intact serum Ig band in patients presenting with light-chain disease only. Patients with OB of one isotype and a single protein band of a different isotype (distinct from the paraprotein), or who had OB and IS at different times in their clinical course, were considered to have both OB and IS. Paraprotein and abnormal protein bands were quantitated by integration of densitometer scans of serum protein electrophoresis gels. The lower limit of detection of this method is 0.1 g/dL. To determine APB duration, the analysis was confined to APB episodes developing after the last transplant, so that their spontaneous course could be assessed, ie, without further transplant intervention, which terminated the presence of APB.
Oligoclonal bands by IFE. Gel 1 shows an IgA λ monoclonal band that resolved with pretransplant chemotherapy. Four months after Mel200 transplant, the patient's serum had IgG κ oligoclonal bands (gel 2), which resolved after the second transplant (gel 3).
Oligoclonal bands by IFE. Gel 1 shows an IgA λ monoclonal band that resolved with pretransplant chemotherapy. Four months after Mel200 transplant, the patient's serum had IgG κ oligoclonal bands (gel 2), which resolved after the second transplant (gel 3).
Apparent IS by IFE. Gel 1 shows a IgG λ monoclonal protein with free λ light chain. The IgG λ monoclonal protein band resolved with pretransplant chemotherapy and a single IgG κ band appeared (gel 2). After the second transplant, the IgG pattern was polyclonal and a new IgM κ monoclonal band appeared (gel 3). This band was replaced by a polyclonal IgG pattern 1 month later (gel 4).
Apparent IS by IFE. Gel 1 shows a IgG λ monoclonal protein with free λ light chain. The IgG λ monoclonal protein band resolved with pretransplant chemotherapy and a single IgG κ band appeared (gel 2). After the second transplant, the IgG pattern was polyclonal and a new IgM κ monoclonal band appeared (gel 3). This band was replaced by a polyclonal IgG pattern 1 month later (gel 4).
BM morphology and flow cytometric studies were reviewed for patients with an apparent isotype switch.
BM morphology.
BM aspirate smears and trephine biopsy sections were evaluated as follows: (1) no morphologic evidence of MM if less than 5% plasma cells with no atypical clusters or sheets of plasma cells in aspirate smears or biopsy sections, and no significant plasma cell atypia; (2) atypical plasmacytosis if 5% to 10% plasma cells in aspirate smears or biopsy sections, or less than 5% plasma cells with mild cytological atypia; (3) residual/recurrent MM if greater than 10% plasma cells in aspirate smears, large clusters or sheets of atypical plasma cells in biopsy sections, or less than 10% plasma cells with moderate-marked cytologic atypia.
Flow cytometric analysis.
Bivariate flow analysis of cytoplasmic Ig and DNA content was performed to evaluate for neoplastic plasma cells using a modification of the technique reported by Barlogie et al.9 Ficoll-Hypaque separated cells were exposed to anti–light chain reagents (fluorescein-conjugated F[ab']2 fragment, DAKO, Carpenteria, CA) and counterstained for DNA with propidium iodide (Sigma, St Louis, MO). Proportions of tumor cells were determined by applying an electronic gating procedure to the brightly light-chain stained cells with abnormal DNA content (aneuploid population) or to the relative excess of kappa or lambda light-chain stained cells with normal DNA content (diploid population).
RESULTS
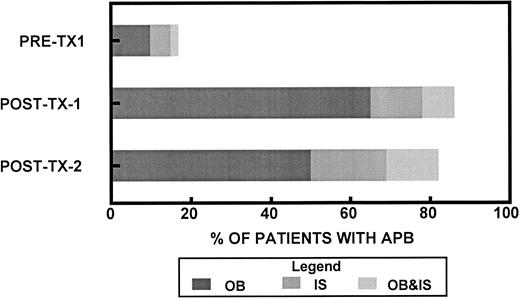
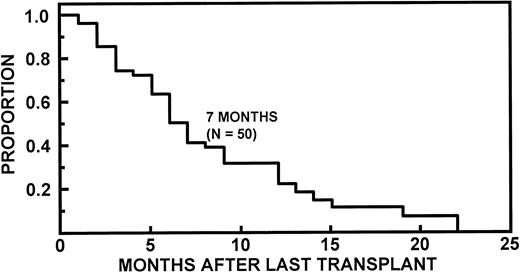
Fifty-five (10%) of the 550 study patients underwent high-dose therapy and had APB distinct from the paraprotein present at diagnosis. Forty-eight patients (87%) had OB, 23 patients (42%) had apparent IS, and 16 patients (29%) had both OB and IS. In 44 patients who developed APB after first transplant, the median interval from transplant was 2 months (range, 1 to 5 months). The percentage of patients with APB before transplantation and after transplants 1 and 2 is shown in Fig 3. APB were quantified by serum protein electrophoresis in 54 patients. Twenty-three of these 54 patients (43%) had APB, which were below the level of detection (< 0.1 g/dL) for all measurements. APB could be quantified at least once in 31 patients (57%) with a median maximum value of 0.5 mg/dL (range, 0.1 to 2.4 g/dL). Seven patients (13%) had a maximum APB level exceeding 1 g/dL. In five of these patients, there was progression to a polyclonal Ig pattern, and in two patients, the APB decreased to levels less than 0.1 g/dL. There was no association between the incidence of APB and the source of hematopoietic cells. Because high-dose therapy led to resolution of APB present before transplantation, the duration of APB after last transplant was used to determine the spontaneous duration of APB (Fig 4). The median duration of APB was 7 months (range, 1 to 22 months).
Time of detection of APB. Fifty-five of 550 patients had APB detected on serum IFE. The percentage of patients who had APB detected before the first transplant (PRE–Tx-1) (n = 55), after the first transplant (POST–Tx-1) (n = 55), and after the second transplant (POST–Tx-2) (n = 45) is shown. Twenty-eight patients had APB in more than one interval. Forty-eight patients had OB; of these, 24 (50%) had OB detected in more than one interval, 2 patients (4%) before and after the first transplant, 3 patients (6%) before and after the first transplant and after the second transplant, and 19 patients (40%) after both first and second transplants. Twenty-three patients had apparent IS; of these five (22%) had IS detected in more than one interval (one patient had both OB and IS in more than one interval). Two patients (9%) had IS before the first transplant and after the second transplant and three patients (13%) after both first and second transplant.
Time of detection of APB. Fifty-five of 550 patients had APB detected on serum IFE. The percentage of patients who had APB detected before the first transplant (PRE–Tx-1) (n = 55), after the first transplant (POST–Tx-1) (n = 55), and after the second transplant (POST–Tx-2) (n = 45) is shown. Twenty-eight patients had APB in more than one interval. Forty-eight patients had OB; of these, 24 (50%) had OB detected in more than one interval, 2 patients (4%) before and after the first transplant, 3 patients (6%) before and after the first transplant and after the second transplant, and 19 patients (40%) after both first and second transplants. Twenty-three patients had apparent IS; of these five (22%) had IS detected in more than one interval (one patient had both OB and IS in more than one interval). Two patients (9%) had IS before the first transplant and after the second transplant and three patients (13%) after both first and second transplant.
Kaplan-Meier curves showing duration of APB. APB were detected after last transplant in 50 patients.
Kaplan-Meier curves showing duration of APB. APB were detected after last transplant in 50 patients.
The clinical outcome of patients with APB was compared with that of patients without APB to determine if APB had prognostic significance. In 51 of 55 (93%) patients with APB, the myeloma paraprotein had disappeared or was resolving at the first detection of APB. CR was significantly higher in patients with APB (67% v 37%,P = .001) and significantly fewer patients had less than PR (32% v 9%, P = .001). Appreciating that APB were first documented in all patients within 12 months of first transplant, a landmark analysis was performed comparing the 50 patients with APB and 421 patients without APB who survived at least 12 months post transplant. Both event-free survival (EFS) (50 v 29 months;P = .006) and overall survival (OS) (71+ v 54 months;P = .0004) were superior in patients with APB. On multivariate analysis that included previously recognized pretreatment prognostic variables (especially cytogenetics, beta-2–microglobulin [β2M], duration of standard therapy before transplant, and C-reactive protein [CRP]),10 APB was an independent favorable parameter for both EFS (P = .004; after less than or equal to 12 months of prior therapy and favorable cytogenetics) and OS (P = .0005: after cytogenetics and duration of prior therapy).
The isotype change and characteristics of the 23 patients with IS is shown in Table3. Seven patients had only IS (Table 3, patients 5, 7, 8, 11, 13, 19, and 21), the other 16 had both IS and OB. The median IS peak value was 0.2 mg/dL (range, < 0.1 to 0.5 mg/ dL); 48% of patients had less than 0.1 mg/dL (detection limit) for all measurements.
Apparent IS in 23 Patients
| Patient No. . | Time . | Isotype Change . | Maximum IS (g/dL) . | Duration (mo) . |
|---|---|---|---|---|
| 1 | pre–Tx-1 | Aλto Gκ | <0.1 | 1 |
| 2 | pre–Tx-1 | λ-freeto Gκ | 0.3 | 1 |
| 3 | post–Tx-1 | Gκ to Gκ + Gλ | 0.4 | 3 |
| 4 | post–Tx-1 | Gκ to Gκ + Gλ | 0.4 | 2 |
| 5 | post–Tx-1 | Aλ to Aλ + Gλ | 0.2 | 3 |
| 6 | post–Tx-1 | Aλ to Gλ | 0.2 | 3 |
| 7 | post–Tx-1 | Gκ to Gκ × 2 + Gλ + Mλ | 0.3 | 2 |
| 8 | post–Tx-1 | Gλ to Gλ + Mλ + Mκ | <0.1 | 2 |
| 9 | post–Tx-1 | Gλ to Gλ + Gκ | <0.1 | 3 |
| 10 | post–Tx-2 | Gλ + λ-free to κ-free | <0.1 | 3 |
| 11 | post–Tx-2 | λ-free to Gκ to Mλ | <0.1 | 6 |
| 12 | post–Tx-2 | Gκ to nil to Gκ + Gλ | <0.1 | 5 |
| 13 | post–Tx-2 | λ-free to nil to Gλ | 0.3 | 7+ |
| 14 | post–Tx-2 | λ-free to nil to Aκ + Gκ | <0.1 | 6 |
| 15 | post–Tx-2 | κ-free to Gκ + Gλ oligo to Gλ | <0.1 | 1+ |
| 16 | post–Tx-2 | Aλ to Gλ | <0.1 | 2 |
| 17 | post–Tx-2 | Aκ to Gκ oligo to poly to Gλ to Mλ to poly | <0.1 | 4 |
| 18 | post–Tx-2 | Gκ to Gκ + Mλ oligo to Mλ + Gκ oligo to poly | 0.4 | 3 |
| 19 | post–Tx-1 & 2 | Gλ to Gκ to Mκ | <0.1 | 8 |
| 20 | post–Tx-1 & 2 | κ-free to Mλ to Gκ oligo to Gλ to poly | 0.3 | 7 |
| 21 | post–Tx-1 & 2 | Gκ to Gκ + Gλ to Gκ + Gλ + Mλ | 0.5 | 9 |
| 22 | pre–Tx-1 and post–Tx-2 | κ-free to Gλ × 2 to poly to Mλ × 2 to Mλ | 0.4 | 4+ |
| 23 | pre–Tx-1 and post–Tx-2 | λ-free to Gκ × 2 to nil to Gκ + Gλ oligo to Gκ | 0.2 | 6+ |
| Patient No. . | Time . | Isotype Change . | Maximum IS (g/dL) . | Duration (mo) . |
|---|---|---|---|---|
| 1 | pre–Tx-1 | Aλto Gκ | <0.1 | 1 |
| 2 | pre–Tx-1 | λ-freeto Gκ | 0.3 | 1 |
| 3 | post–Tx-1 | Gκ to Gκ + Gλ | 0.4 | 3 |
| 4 | post–Tx-1 | Gκ to Gκ + Gλ | 0.4 | 2 |
| 5 | post–Tx-1 | Aλ to Aλ + Gλ | 0.2 | 3 |
| 6 | post–Tx-1 | Aλ to Gλ | 0.2 | 3 |
| 7 | post–Tx-1 | Gκ to Gκ × 2 + Gλ + Mλ | 0.3 | 2 |
| 8 | post–Tx-1 | Gλ to Gλ + Mλ + Mκ | <0.1 | 2 |
| 9 | post–Tx-1 | Gλ to Gλ + Gκ | <0.1 | 3 |
| 10 | post–Tx-2 | Gλ + λ-free to κ-free | <0.1 | 3 |
| 11 | post–Tx-2 | λ-free to Gκ to Mλ | <0.1 | 6 |
| 12 | post–Tx-2 | Gκ to nil to Gκ + Gλ | <0.1 | 5 |
| 13 | post–Tx-2 | λ-free to nil to Gλ | 0.3 | 7+ |
| 14 | post–Tx-2 | λ-free to nil to Aκ + Gκ | <0.1 | 6 |
| 15 | post–Tx-2 | κ-free to Gκ + Gλ oligo to Gλ | <0.1 | 1+ |
| 16 | post–Tx-2 | Aλ to Gλ | <0.1 | 2 |
| 17 | post–Tx-2 | Aκ to Gκ oligo to poly to Gλ to Mλ to poly | <0.1 | 4 |
| 18 | post–Tx-2 | Gκ to Gκ + Mλ oligo to Mλ + Gκ oligo to poly | 0.4 | 3 |
| 19 | post–Tx-1 & 2 | Gλ to Gκ to Mκ | <0.1 | 8 |
| 20 | post–Tx-1 & 2 | κ-free to Mλ to Gκ oligo to Gλ to poly | 0.3 | 7 |
| 21 | post–Tx-1 & 2 | Gκ to Gκ + Gλ to Gκ + Gλ + Mλ | 0.5 | 9 |
| 22 | pre–Tx-1 and post–Tx-2 | κ-free to Gλ × 2 to poly to Mλ × 2 to Mλ | 0.4 | 4+ |
| 23 | pre–Tx-1 and post–Tx-2 | λ-free to Gκ × 2 to nil to Gκ + Gλ oligo to Gκ | 0.2 | 6+ |
The single band Ig isotype and light chain are specified for each observation. Nil denotes absence of the Ig region, oligo denotes presence of oligoclonal protein bands, and poly the presence of a normal Ig pattern on IFE. Peak IS is the highest value obtained by quantification of the apparent isotype switch protein band. + indicates that the IS was present on the last follow-up sample. The paraprotein associated with the patient's MM is underlined. Two patients had IS before transplantation (Tx), 7 after Tx-1, 9 only after Tx-2, 3 after Tx-1 and Tx-2, and 2 pre–Tx-1 and post–Tx-2.
The median duration of IS ocurring after the last transplant was 5 months (range, 1 to 7 months). IS was still present on IFE at last follow-up in four patients (Table 3, patients 13, 15, 22, and 23) at 1, 4, 6, and 7 months. In one of four patients exhibiting IS at last follow-up, the IS protein was less than 0.1 g/dL for all measurements, and in three patients it had decreased to less than 0.1 g/dL.
Because the monoclonal protein band of IS could be due to altered paraprotein production by the myeloma clone, the presence of IS was correlated with morphologic and flow cytometric examination of BM to determine if the IS was associated with relapse of disease or emergence of a clone of malignant plasma cells with a different light-chain restriction. Seventeen of the 23 patients with IS had concurrent IFE and BM studies on 26 relevant specimens (one to three specimens per patient) (Fig 5). Twenty-one of the 26 (81%) BM specimens from patients with IS (76% of patients) had normal BM morphology and no evidence of a neoplastic plasma cell population by flow cytometric analysis. Despite the normal BM studies, eight related serum specimens had persistent or recurrent myeloma paraprotein. Two patients (three specimens) had an atypical plasmacytosis with normal flow cytometry at the time of IS. One of these patients (two specimens) had residual disease detectable by IFE that was missed by flow cytometry. Two patients had abnormal flow cytometric studies at the time of IS. Patient 21 (Table 3), had a normal BM morphologic study, but an abnormal flow cytometric study suggestive of plasma cell aneuploidy, but without sufficient plasma cells to determine light-chain restriction. Patient 17 (Table 3) who developed treatment related acute myeloid leukemia (t-AML) with an associated IS (IgMλ), had no original IgAκ paraprotein on IFE and no evidence of MM on BM morphology study. Flow cytometry analysis, which had previously been reported as normal, showed an excess of 14% λ-diploid cells consistent with the IFE findings.
Results of IFE and BM examination for 26 specimens in 17 patients with apparent IS or IS and paraprotein (IS & PARA). BM was examined microscopically for morphologic abnormalities (ABN) and by flow cytometry (fluorescence-activated cell sorting [FACS]) for clonal Ig light chain expression.
Results of IFE and BM examination for 26 specimens in 17 patients with apparent IS or IS and paraprotein (IS & PARA). BM was examined microscopically for morphologic abnormalities (ABN) and by flow cytometry (fluorescence-activated cell sorting [FACS]) for clonal Ig light chain expression.
The 10 patients with IS who failed to achieve CR or relapsed after treatment had disease characterized by a paraprotein with the same IFE characteristics as that detected at diagnosis. To determine if the relapse was associated with the IS protein, flow cytometry studies were used to evaluate the light-chain restriction of the myeloma cells at diagnosis and relapse. Eight of the 10 patients had flow cytometry data at diagnosis, which showed abnormal cell populations expressing the same light chain as that of the serum paraprotein, but only 3 had clonal abnormalities on flow cytometry at relapse; all of these had the same light chain as the original paraprotein. In addition, one patient presenting with λ light-chain disease (Table 3, no. 14) had both κ and λ aneuploid BM cell populations on initial flow cytometric analysis and subsequently showed IgAκ and IgGκ monoclonal bands on IFE. This patient may have had biclonal myeloma.
DISCUSSION
We have reported a single institution's experience with APB in 550 adult patients with MM receiving autologous hematopoietic cell transplantation. The appearance of OB during recovery from myeloablation is well described in both experimental autologous and allogeneic BM transplantation, as well as during clinical trials of transplantation with myeloablative and nonmyeloablative therapy.4,5,6,11 Careful analysis of children recovering from myeloablative therapy has shown that most have transient OB by IFE at some stage during the initial recovery phase.5 The mechanism responsible for the restricted antibody response is not fully understood, but may be due to either abnormal T-cell control of B-cell proliferation and maturation, failure of normal B-cell affinity maturation, or failure of recall response to antigen. This limitation of antibody response is characteristic of B-cell ontogeny in infancy,12 and the IFE patterns seen in patients with OB and IS may reflect a recapitulation of B-cell ontogeny after myeloablation.
We have shown that APB occurred in 16 MM patients (3%) after nonmyeloablative therapy. This may be unique to MM patients or alternatively may be a feature of recovery from all myelosuppressive chemotherapy. The suppression of normal Ig production by myeloma cells may facilitate detection of OB above background level during the recovery period after myelosuppression. Alternatively, these postchemotherapy patients may have been specifically identified because of the repeated monitoring of serum Ig required in this population for evaluation of disease response.
Of the 23 patients with single bands on IFE suggesting an IS, 16 (70%) also had OB. In these patients, the single protein bands (IS) were usually transient and associated with the development of OB or the progression of OB to a normal polyclonal Ig pattern. In addition, the single protein band (IS) often had the same electrophoretic mobility as one of the subsequently developing OB. When these patients relapsed, the IFE paraprotein band was identical to that at presentation. IS did not resolve during the follow-up period of this study in 4 of 23 patients (17%) for between 1 and 7 months, but in all patients, was below the limit of detection by serum protein electrophoresis at last measurement. Analysis of BM by flow cytometry for neoplastic plasma cells showed that IS was not associated with detectable light-chain restriction or an aneuploid cell population in 24 of 26 (92%) specimens examined (91% of patients). One patient with IS had an aneuploid population (light-chain restriction of clone not determined) with evidence of residual disease on IFE and normal BM morphology. The only patient in the series who had an abnormal plasma cell population by flow cytometry showing the same switched light chain as the band seen on IS, concurrently developed t-AML. Monoclonal gammopathy has been described as a feature of both myelodysplastic syndromes and acute leukemia.2 13
Our data suggests that the OB and IS are most likely to be transient phenomena associated with recovery of B-cell function. The single bands identified on IFE are not due to changes in behavior or isotype switching by the malignant plasma cell clone. Instead, single Ig bands (IS) are, in most cases, a feature of the development or resolution of oligoclonal bands.
The detection of APB on IFE did not have adverse prognostic significance in this retrospective analysis, consistent with the proposed etiology of the APB phenomenon. The initial development of APB was associated with near or complete suppression of the myeloma clone as measured by paraprotein production in 93% of patients, suggesting that the appearance of APB may be a surrogate marker of normal immune reconstitution with alleviation of the immunosuppressive effects of the myeloma clone. In patients surviving at least 12 months after first transplant, those with APB had a significantly better survival. If the presence of APB indicate recovery of immune function, this suggests a significant role for immunosurveillance in long-term myeloma clone suppression.
Because IS may cause a monoclonal peak on serum protein electrophoresis, we believe that IFE is required to investigate all increases in monoclonal protein, especially after therapy. Although APB appear to have no adverse clinical significance, the detection of IS in patients with MM requires careful monitoring. New paraprotein bands may be due to lymphoproliferative disorders complicating immunosuppression, may represent a rare case of true clonal isotype switching, or may occur in association with other hematologic disorders complicating treatment of myeloma, such as the t-AML that occurred in one patient in this series.
Supported in part by Grant No. CA55819 from the National Cancer Institute, Bethesda, MD.
Address reprint requests to B. Barlogie, MD, PhD, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, 4301 West Markham St, Slot 776, Little Rock, AR 72205.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Results of IFE and BM examination for 26 specimens in 17 patients with apparent IS or IS and paraprotein (IS & PARA). BM was examined microscopically for morphologic abnormalities (ABN) and by flow cytometry (fluorescence-activated cell sorting [FACS]) for clonal Ig light chain expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3518/3/m_blod40934005x.jpeg?Expires=1767775221&Signature=xIsKHqwyZGFtul3NT8KobS6oB9Gd8ml3xv2o-EMdP7uzFVHhMXNWCgT5dGeVZGe2rAAxt0iTkpMgDqPsZQ4t8NR4DqO-U7uUVWU-V679IdfYTYG8iS3E6GtGzAISxWmDEiZESLBlCq1~0mGJV0Sw8ccwoPJ0SPEFkZ7-AXdOwxAO8JK0ZuJVD9uAnR0B28sN3gGJ5ZqSi5YEn38B5C~zdkwSayrTzD4Gesrak0vRwvBAqdWo0AIDEU4Rckm2YVfUhvYt8NgFTGI0GLJsU26SutJ2ljxXxeItcoq8sJsWKGeV0Kp4TF1hjpr7bH67~j8EA9dmoVBmzf0XD5wBZXvPEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal