To the Editor:
Tissue factor pathway inhibitor (TFPI) is an important regulator in the extrinsic blood coagulation pathway.1-3 Although the regulatory biochemical role of TFPI is evident, the clinical significance of this proteinase inhibitor remains to be elucidated. The definition of a clinical TFPI deficiency seems to be more complex than that of other coagulation inhibitors, because the activity and concentration of circulating TFPI cannot be considered a true measure of in vivo levels. Its determination in plasma samples by immunological methods or functional assays has been shown to be inadequate in the detection of a clinical deficiency.4
Therefore, we investigated whether genetic variations in the TFPI gene contribute to the occurrence of hitherto unexplained cases of thrombophilia by analyzing DNA samples of patients with thromboembolic diseases and blood donors for mutations in the coding region of the TFPI gene by polymerase chain reaction followed by single-strand conformation polymorphism (PCR-SSCP).
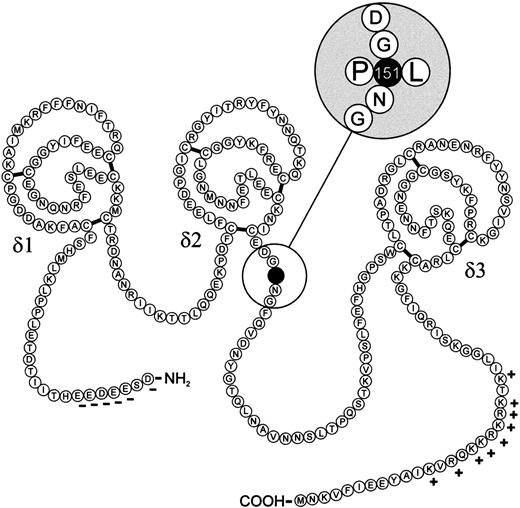
We selected 50 unrelated individuals with a thrombotic history for the determination of the genetic basis of their thrombosis (27 of them were shown to be carriers of the factor V Leiden mutation). While scanning all coding exons of the TFPI gene and the adjacent 5′ and 3′ intronic regions by PCR-SSCP analysis, an abnormal pattern suggesting the presence of a genetic variation was observed in a PCR fragment from exon 7 in one patient. DNA sequencing of this fragment showed a single heterozygous C to T mutation at nucleotide position 1 of exon 7, changing the codon CCG151 to CTG151, resulting in a Pro151 to Leu151 exchange in the amino acid sequence of the mature protein (Fig1).
Proposed secondary structure of the TFPI and the position of the amino acid exchange. The mature protein consists of 276 amino acids, forming three tandem Kunitz-type proteinase inhibitory domains, two connecting chains, an acidic N-terminus with negatively charged amino acids (−) and a basic carboxy-terminal end, containing a cluster of positively charged amino acids (+).5 6 The Pro151 to Leu151 change is located near the Kunitz-type inhibitor domain δ2 within the second connecting chain (magnification).
Proposed secondary structure of the TFPI and the position of the amino acid exchange. The mature protein consists of 276 amino acids, forming three tandem Kunitz-type proteinase inhibitory domains, two connecting chains, an acidic N-terminus with negatively charged amino acids (−) and a basic carboxy-terminal end, containing a cluster of positively charged amino acids (+).5 6 The Pro151 to Leu151 change is located near the Kunitz-type inhibitor domain δ2 within the second connecting chain (magnification).
The C→T transition at nucleotide position 536 is associated with the creation of a new recognition site for the restriction enzymeBseNI, providing a rapid means of screening further individuals for this mutation by PCR and restriction analysis. TFPI-exon 7 and its 5′ and 3′ flanking intronic regions were specifically amplified from genomic DNA by PCR using the primers TFPI-Ex7F, 5′-TCTATTTTAATTGGCTGTAT-3′, and TFPI-Ex7R, 5′-GCATGATAATAGTTTCCTGG-3′, for the amplification reaction. If the nucleotide C is present at position 536, the resulting 170-bp DNA fragment is not digestible withBseNI. However, if the nucleotide T is present at this position, a 27-bp and a 143-bp restriction fragment is generated.
To estimate the prevalence of the 536C→T exchange in the general population, 2,480 randomly chosen unrelated blood donors (18 to 60 years of age) were investigated by PCR and restriction analysis. Four persons heterozygous for this mutation were found. According to these results, the allelic frequency of the 536C→T mutation in the German population of the Westphalian area is approximately 0.08%.
To detect a greater number of subjects with the trait, which would allow a more precise estimation of the relative risk of those individuals to develop venous thrombosis, we investigated family members of the heterozygote blood donors and patients. In total, we found 13 heterozygote individuals within 6 different families. Two of them suffered from deep vein thrombosis. Homozygous carriers of this mutation were not found.
To determine a possible effect of the mutation on circulating TFPI, we measured the TFPI activity by a functional assay (Actichrome TFPI Activity Assay; American Diagnostica Inc, Greenwich, CT) and the protein concentration by an immunological assay (Imubind Total TFPI ELISA Kit; American Diagnostica Inc) in plasma of all individuals showing the TFPI mutation. Compared with the control group (blood donors without TFPI mutation), no statistically significant differences were detected. The mean values of the TFPI activity and TFPI concentration were 101% (SD, 15.5%; n = 12) and 40 ng/mL (SD, 10.4 ng/mL; n = 12) in individuals with the mutation and 100% (SD, 7.9%; n = 22) and 46 ng/mL (SD, 10.7 ng/mL; n = 22) in the control group. However, existing small differences might not have been recognized due to the low number of cases investigated so far.
Although the number of subjects with the TFPI mutation is somewhat too small to evaluate the risk of thrombophilia, we compared the prevalence of venous thromboembolism in this group with the prevalence in 466 blood donors who were thoroughly questioned about a possible history of venous thrombosis. Ten cases were found in this group. Our present results of the statistical analysis show a probability of 94.5% for the hypothesis that the presence of the TFPI trait is linked to thrombophilia (P = .055; odds ratio, 5.9; 95% confidence interval, 1.0 to 36.5). It is not yet possible to prove the thrombotic risk of subjects with this trait, because the mutation is found in both thrombotic and nonthrombotic subjects and the number of probands who are available for statistical analysis is relatively small. The low prevalence of the trait in the population makes an investigation of a large number of appropriate patients (n > 1,000) inevitable. Therefore, multicentric epidemiologic studies are warranted to elucidate the clinical significance of this mutation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal