Abstract
In a single institution, we have used recombinant interferon- (IFN-) to treat 116 newly diagnosed Philadelphia-positive (Ph+) chronic myeloid leukemia (CML) patients and analyzed the predictive factors for response and survival. The patients whose median age was 50.3 years (range, 9 to 70) were administered IFN- (5 million units/m2/d) subcutaneously. The IFN- dose was subsequently adjusted to maintain the white blood cell and platelet counts between 1.5 and 5 × 109/L, 50 and 100 × 109/L, respectively. At diagnosis, the Sokal score was used to classify the patients into three groups: low (n = 57), intermediate (n = 42), and high risk (n = 16). A complete hematological response (CHR) was achieved in 93 cases (80.2%). Of the 116 patients, 113 were available for cytogenetic evaluation. Fifty patients (43%) achieved a major cytogenetic response (MCR) (=65% marrow Ph− cells), 37 of them having a complete cytogenetic response (CCR). The estimated 5-year survival of the 116 patients was 68% ± 11% (95% confidence interval [CI]) with a median follow-up of 42 months (range, 3 to 114) and 85% ± 11% (95% CI) with a median follow-up of 30.9 (range, 3 to 111) when patients were censored at the time of transplantation. Event-free survival at 5 years (adding death and transplant as event) was 46% ± 11% (95% CI). Using proportional hazards regression to study time-dependent variables, we confirmed that the most significant factor associated with survival was the cytogenetic response (MCR or CCR) (P < .0001). This factor was independent compared with the Sokal score and baseline variables used to calculate the Sokal score. Moreover, using either univariate or multivariate analysis, the achievement of CHR within 3 months was strongly correlated with MCR (P < .0001). Minimum cytogenetic response (mCR, ie, at least 5% of Ph− metaphases) at 3 months was also a significant predictive factor for MCR (P < .0001). These results show that IFN- can induce a high rate of hematological and cytogenetic response when administered in doses leading to myelosuppression. Factors such as the achievement of CHR and mCR within 3 months could be useful to identify early those patients who will not respond to IFN- and who need alternative treatments such as stem cell transplantation.
CHRONIC MYELOGENOUS LEUKEMIA (CML) is a clonal myeloproliferative disorder characterized by acquisition of the Philadelphia chromosome (Ph) in leukemic stem cells and their progeny.1,2 The abnormal Ph chromosome is the result of a reciprocal translocation between chromosomes 9 and 22. The major consequence of this translocation is the fusion of the ABL gene to the BCR gene on chromosome 22.3 The BCR-ABL fusion gene, which is transcribed into an 8.6-kb chimeric mRNA, encodes a 210-kD hybrid protein that is probably responsible for the chronic phase of the disease, but its critical function has not yet been defined.4 The cells belonging to the malignant clone can be detected by either chromosome analysis or molecular techniques designed to monitor (and amplify) the expression of the characteristic Bcr-Abl message.5
The course of CML is characterized by a multistep process from a chronic phase to accelerated and blastic phases with rapidly fatal outcome. Marrow transplantation (BMT) is the only curative treatment for CML patients under the age of 45 to 50 years .6,7Autologous stem cell transplantation (ASCT) has more recently been proposed as an alternative treatment, but its efficiency is still under evaluation.8 Using recombinant interferon-α (IFN-α), it has been shown that complete hematological remission (CHR) and a cytogenetic response (minor, partial or even complete) can be achieved in some patients with newly diagnosed CML, and that some of these remitter patients have a prolonged survival.9-12 However, the contrary was found in two other studies.13 14 Thus, it would be useful to have a test to identify early those the patients who will not respond to IFN-α.
In our institution, we have now treated 116 patients with IFN-α as first line treatment and obtained a high rate of CHR as well as a high proportion of cytogenetic responses.15 We have analyzed the factors influencing survival and the response to IFN-α to identify early the CML patients who could be proposed for alternative treatments.
PATIENTS AND METHODS
Patients.
Between October 1986 and February 1997, 116 patients (males = 66; females = 50) were entered into the study. All patients (median age, 50.2 years; range, 9 to 70) had Ph+ CML in chronic phase without clinical or biological signs of acceleration or blast crisis.16 According to Sokal’s classification,17 the patients were divided into three categories: low risk (index < 0.8) (n = 57), intermediate risk (0.8 < index < 1.2) (n = 42), and high risk (index > 1.2) (n = 16) (Table 1). One patient had undergone an operation for splenectomy before diagnosis, so the Sokal index could not be calculated. These 116 patients were treated with IFN-α alone (see below).
Clinical Characteristics of Patients
| Characteristics . | Median Value . |
|---|---|
| Age (yr) | 50.2 (9-70) |
| Sex (M/F) | 66/50 |
| Enlarged spleen (0-14 cm) | 46 patients (39.6%)* |
| WBC count (×109/L) | 71.5 (14.4-500) |
| Platelet count (×109/L) | 377 (110-2,970) |
| PB blast cells (%) | 3 (0-8) |
| Peripheral basophil (%) | 1 (0-23) |
| Hemoglobin level (g/L) | 12.5 (6.7-16.7) |
| Sokal classification | n = 115* |
| Low risk | 57 (50%) |
| Intermediate risk | 42 (36%) |
| High risk | 16 (14%) |
| Characteristics . | Median Value . |
|---|---|
| Age (yr) | 50.2 (9-70) |
| Sex (M/F) | 66/50 |
| Enlarged spleen (0-14 cm) | 46 patients (39.6%)* |
| WBC count (×109/L) | 71.5 (14.4-500) |
| Platelet count (×109/L) | 377 (110-2,970) |
| PB blast cells (%) | 3 (0-8) |
| Peripheral basophil (%) | 1 (0-23) |
| Hemoglobin level (g/L) | 12.5 (6.7-16.7) |
| Sokal classification | n = 115* |
| Low risk | 57 (50%) |
| Intermediate risk | 42 (36%) |
| High risk | 16 (14%) |
One patient had a splenectomy before diagnosis.
During the same period of time, 46 other patients with previously untreated CML in chronic phase were treated differently: 13 patients were treated using straight ASCT (n = 5) or allogeneic BMT as first treatment (n = 8); 33 others were treated by hydroxyurea alone (n = 26) or combined IFN-α (n = 7), because they were more than 70 years old or they were included in an open French multicenter study comparing hydroxyurea and IFN-α.18
Treatment.
After informed consent, the patients were given recombinant IFN-α (5 million IU/m2/d, subcutaneously). All patients received IFN-α as first-line treatment. The median time between diagnosis and IFN-α was 36 days (range, 1 to 356). IFN-α doses were subsequently adjusted to maintain the white blood cell (WBC) count between 1.5 × 109/L and 5 × 109/L and the platelet count between 50 and 150 × 109/L. IFN-α was stopped when the WBC count was below 1.5 × 109/L or the platelet count below 50 × 109/L. Moreover, IFN-α was interrupted when: (1) There was no response to IFN-α with an increase in WBC count or less than 25% WBC decrease compared with initial leukocytosis over a period of 14 weeks; (2) patients failed to achieve a CHR or a cytogenetic response (complete, partial, or minor) after 6 or 12 months, respectively; or (3) an initial response was followed by a subsequent increase in WBC to more than 40 × 109/L uncontrolled by continued IFN-α therapy.
These patients were planned to undergo either allogeneic BMT (for patients under 45 years when an HLA-identical donor was available) or ASCT.19
Moreover, IFN-α was stopped when a blast crisis developed or unacceptable toxic reactions (grade 3) were observed.
For responding patients, the treatment was continued when there were no extra-hematological toxic effects (see below).
Response criteria.
Hematological and cytogenetic responses were evaluated according to the criteria reported by the Houston group.20 A CHR required the normalization of the peripheral WBC count to less than 10 × 109/L with the disappearance of immature circulating cells (blasts, promyelocytes, myelocytes, metamyelocytes), the normalization of platelet count (<450 × 109/L) and the disappearance of all signs and symptoms of the disease (in particular, palpable splenomegaly). A partial hematological remission (PHR) was defined by a decrease in WBCs of at least 50% compared with the initial leukocytosis to a value at least below 20 × 109/L. This included patients whose peripheral WBC counts had become normal but who had persistent splenomegaly or immature peripheral cells. Failure comprised nonresponders. Accelerated phase was defined according to the criteria described by Kantarjian et al16 and blast crisis was considered to be when more than 30% blast cells were present in the BM.
Cytogenetic evaluation.
BM karyotypes were performed using a routine cell synchronization technique after a 24- to 48-hour culture. Twenty-five to 30 R-or G-banded metaphases were photographed or video-printed. Neither fluorescence in situ hybridization (FISH) nor polymerase chain reaction (PCR) data were performed.
Cytogenetic response was classified according to the proportion of Ph metaphases21: (1) No response when the Ph chromosome persisted in all metaphases; (2) minor response when the Ph chromosome persisted in 35% to 95% of metaphases; (3) partial response when the Ph chromosome persisted in 1% to 34% of metaphases; (4) complete response when the Ph chromosome was not found in marrow metaphases on at least one karyotypic study.
In this report, we have considered together partial and complete cytogenetic response as “major cytogenetic response” (MCR). For cytogenetic evaluation 3 months after starting IFN-α therapy, we have defined a minimal cytogenetic response (mCR) as at least 5% Ph− cells present.
Toxic effects.
According to the World Health Organization classification, toxic effects were classified in four groups 1 to 4: mild, moderate, severe, and life-threatening. The treatment was temporarily stopped when a grade 3 or 4 toxicity appeared and definitively stopped when a grade 4 neurological toxicity occurred.
Statistical analysis.
Comparisons were made by the Log-rank method and the Chi-square test. Survival curves and cumulative incidences of hematological and cytogenetic responses were analyzed by the Kaplan and Meier method and were compared using the Log-rank test. A multivariate analysis of prognostic factors was performed according to the Cox model. We also compared results of this analysis with landmark analysis looking at survival in patients who had already survived 1 year. Cytogenetic responses were included as a time-dependent covariate (coded 0 before and 1 after the date of occurrence of the event).22 At any fixed point during the study, the effect of these events was assessed only on the basis of events that occurred before that point. Baseline variables for which P = .05 in the log-rank model were also included in this proportional hazards model using a stepwise procedure, and were tested either with threshold or continuous variables.
RESULTS
Hematological response.
For the 116 patients, the median follow-up was 42 months (range, 3 to 114). All patients received IFN-α therapy for a median duration of 24 months (range, 3 to 110).
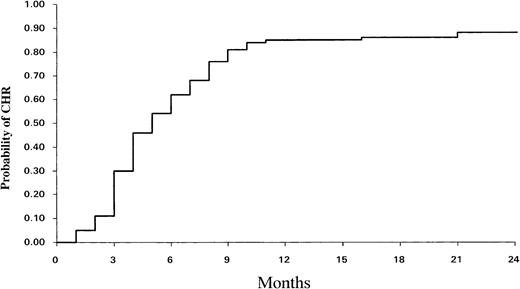
CHR was achieved in 93 patients (80%), and the cumulative incidence of CHR at 12 months was 84% ± 7% (95% confidence interval [CI]) (Fig 1). The median time to achieve CHR was 4.6 months. Among the 93 patients who achieved CHR, 41 (44%) did within 3 months of treatment (early CHR), whereas 52 other patients entered CHR later (3.5 to 9 months) (late CHR). A number of disease-related variables were tested by univariate analysis for their association with CHR. The initial individual prognostic factors (size of spleen, leukocytosis, peripheral blast [PB] cells, platelet count) and Sokal score did not influence the CHR rate.
Cumulative incidence of complete hematological response (CHR) among the 116 patients.
Cumulative incidence of complete hematological response (CHR) among the 116 patients.
For the 93 patients, the estimated chance of remaining in CHR at 3 and 5 years after achieving CHR was, respectively, 57.7% ± 11% (95% CI) and 48% ± 12% (95% CI). The Sokal risk groups (P = .028), platelet count (>380¥ 109/L) (P = .02), spleen size (P = .04), and PB blast cells (P = .018) were found to influence significantly CHR duration by univariate analysis. Using multivariate analysis only platelet count was found to influence CHR duration (P = .006 and relative risk = 2.76).
Cytogenetic response.
Of the 116 patients, 113 were available for cytogenetic evaluation. The cumulative incidence of complete cytogenetic response (CCR) at 12 and 24 months was 16% ± 7% and 38% ± 11% (95% CI), respectively (72 and 39 cases, respectively, were exposed to this probability at 12 and 24 months). Among the 93 patients who achieved CHR, 37 (32% of the whole population) subsequently achieved CCR. The estimated probability of remaining in CCR 24 and 48 months after achieving it was 81% ± 13% and 66% ± 18% (95% CI), respectively.
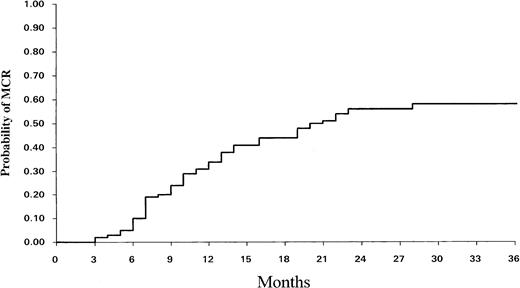
Thirteen other patients had more than 65% Ph− cells, so the total number of patients achieving MCR was 50 (43% for the whole population). Thus, the actuarial proportion of patients who achieved MCR at 6, 12, and 24 months was 10% ± 5%, 33% ± 9%, and 56% ± 11% (95% CI), respectively (Fig 2). For the 50 MCR patients, the median time to achieve the MCR was 9.6 months (range, 3 to 28). The estimated chance of remaining in MCR 24 and 48 months after achieving it was, respectively, 84% ± 11% and 70% ± 15% (95% CI).
Cumulative incidence of MCR over time among the 113 evaluable patients.
Toxic effects.
A flulike syndrome was usual and disappeared spontaneously or with paracetamol after a few weeks of treatment. In three patients, IFN-α could be continued despite clinical signs of thyroid insufficiency due to autoimmune thyroiditis.
However, 12 cases of severe intolerance were observed and the treatment had to be stopped. One patient developed hepatitis with very high levels of transaminases and gamma glutamyl transpeptidase (10×). Seven patients exhibited neuropsychiatric toxicity (1 depression within the context of manic-depressive psychosis, 1 case of undetermined transitory encephalitis, and 5 neuropsychiatric disorders). Three other patients had a cardiac dysfunction (acute pericarditis, arythmia, and congestive heart failure). Finally, we also observed a clinical symptom mimicking rhumatoid pseudo-arthritis. All these complications disappeared after a few weeks when IFN-α was stopped.
Outcome and SCT.
Forty-four patients underwent either allogeneic (n = 17) or ASCT (n = 26). Another patient was treated by autologous then allogeneic SCT. The indications for transplantation were as follows: no CHR at 3 months (4 cases), no CHR at 6 months (7 cases), no MCR at 12 months (7 cases), hematological relapse after CHR (10 cases), cytogenetic relapse after MCR (5 cases), accelerated phase (10 cases), patient request (1 case). After allogeneic (9 of 17) or autologous (8 of 27) SCT, 17 patients died: this was due to blastic transformation (4 cases); transplant-related complications, ie, veno-occlusive disease (2 cases); interstitial pneumonitis (4 cases); hemorrhage (2 cases); infection (1 case); acute graft-versus-host disease (3 cases); and multiple organ failure (1 case).
Ten other patients who did not undergo transplantation died: 9 nonresponders died before transplantation; 6 of them were in acceleration, 1 had cardiac failure, and the cause of death was undetermined for the other 2 patients. Finally, 1 patient died from hepatitis encephalopathy while he was in CCR.
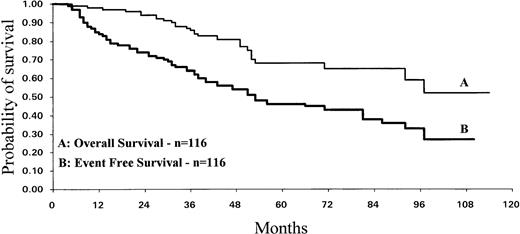
We analyzed the survival, taking into account the transplantation outcome. In both cases the median survival was not reached. When the patients who underwent transplantation were censored at the time of the transplant, the estimated 5-year survival was 85% ± 11% (95% CI) with a median follow-up of 30.9 (range, 3 to 111) months. When the patients were not censored at the time of transplant, the estimated 5-year survival was 68% ± 11% with 42 months of median follow-up (38 patients are exposed to this probability). Taking into account transplant or death as constituting events (event-free survival [EFS]), the probability of survival at 5 years was 46% ± 11% (Fig 3).
Survival of the IFN-–treated CML patients. Median survival was not reached at 110 months for the curve A but the number of observations is relatively small past 48 months. EFS of IFN-–treated CML patients was calculated using transplant and death as constituting events (curve B).
Survival of the IFN-–treated CML patients. Median survival was not reached at 110 months for the curve A but the number of observations is relatively small past 48 months. EFS of IFN-–treated CML patients was calculated using transplant and death as constituting events (curve B).
IFN-α was stopped in three CCR patients because of the toxic effects. In all three patients CCR was sustained with a follow-up of, respectively, 22, 42, and 66 months after cessation of IFN-α. Minimum residual disease was detected only by molecular analysis such as reverve transcriptase-PCR (amplification of Bcr-Abl mRNA).23
Achievement of cytogenetic response is the best predictive factor for survival.
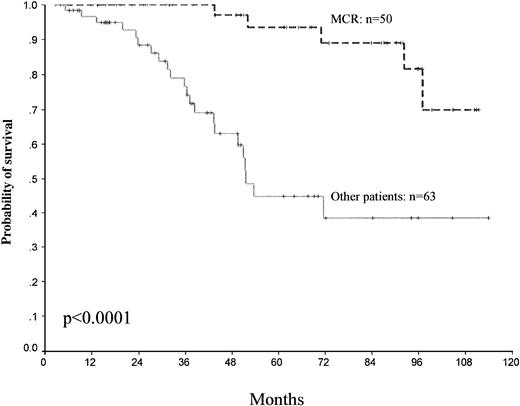
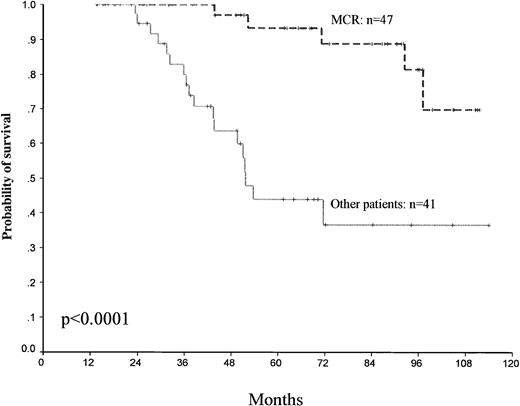
Different factors for predicting survival have been analyzed. Among the patient-related and disease-related variables studied by univariate analysis, Sokal score (P = .02) and platelet count (>700 × 109//L) (P = .16) were found to significantly influence survival. However, the best predictive factors for survival were the achievement of CHR (P < .0001), MCR (P < .0001), and CCR (P < .0001) (Table2). The 5-year survival for MCR versus other patients was, respectively, 93% ± 9% versus 44% ± 17% (95% CI) (Fig 4).
Variables Associated With MCR and CCR and Survival Studied by Univariate Analysis
| Variables . | P Value . | ||
|---|---|---|---|
| MCR . | CCR . | Survival . | |
| Sokal index | .01 | .28 | .02 |
| Sex (M/F) | .68 | .97 | .98 |
| Age | .16 | .45 | .88 |
| Enlarged Spleen | .1 | .21 | .3 |
| PB blast cells | .0067 | .01 | .016 |
| Platelets >700 (×109/L) | .63 | .98 | .016 |
| Platelets >380 (×109/L) | .45 | .54 | .95 |
| WBC > median value | .71 | .94 | .87 |
| CHR | .0015 | .0158 | .0001 |
| CHR in 3 months | <.0001 | .0001 | .006 |
| MCR | <.0001 | ||
| CCR | <.0001 | ||
| Variables . | P Value . | ||
|---|---|---|---|
| MCR . | CCR . | Survival . | |
| Sokal index | .01 | .28 | .02 |
| Sex (M/F) | .68 | .97 | .98 |
| Age | .16 | .45 | .88 |
| Enlarged Spleen | .1 | .21 | .3 |
| PB blast cells | .0067 | .01 | .016 |
| Platelets >700 (×109/L) | .63 | .98 | .016 |
| Platelets >380 (×109/L) | .45 | .54 | .95 |
| WBC > median value | .71 | .94 | .87 |
| CHR | .0015 | .0158 | .0001 |
| CHR in 3 months | <.0001 | .0001 | .006 |
| MCR | <.0001 | ||
| CCR | <.0001 | ||
Survival and cytogenetic response. Survival probability among the patients who obtained a major cytogenetic response MCR and other patients. Day 0 represents the date of starting IFN-.
Survival and cytogenetic response. Survival probability among the patients who obtained a major cytogenetic response MCR and other patients. Day 0 represents the date of starting IFN-.
Using landmark analysis, all the patients remaining alive at 12 months (88 patients) were also classified according to whether they had a MCR (47 patients) as compared with the others (41 patients), and survival was analyzed, which showed a significant advantage for MCR patients (P < .0001) (Fig 5).
Landmark analysis of survival. All patients remaining alive at 12 months (88 patients) were classified according to whether they had an MCR compared with the other patients.
Landmark analysis of survival. All patients remaining alive at 12 months (88 patients) were classified according to whether they had an MCR compared with the other patients.
For the multivariate analysis, we included either the Sokal score or the associated factors from the Sokal score (age, PB blast, spleen, platelet count) and considered cytogenetic reponse CCR or MCR as time-dependent variables. This multivariate proportional hazards regression model showed that both MCR and CCR were significantly associated with survival independently of the other factors. Indeed, according to this model, the patients who achieved MCR or CCR were at, respectively, 0.17 and 0.13 time the risk of dying of the remaining patients (P = .0001) independently of the other factors (Table3). Sokal scores alone without the threshold were entered quantitatively into this model and were excluded from the final model: Sokal score, P = .91, relative hazard (RH) = 1.0035; CCR, P =.0001, RH = 0.1273. Moreover, MCR was also confirmed to be strongly linked to survival (P < .00001, RH = 0.16) independently of the Sokal score (P = .94, RH = 0.9978).
Effect of Baseline Variables From the Sokal Score and Cytogenetic Response (MCR and CCR) Events on Survival in the 113 Patients Using a Proportional Hazards Regression Model
| . | Full Model . | Final Model . | ||
|---|---|---|---|---|
| RH . | P Value . | RH . | P Value . | |
| Age | 1.38 | .0417 | — | — |
| PB blast cells | 0.84 | .2748 | — | — |
| Spleen size | 1.19 | .0172 | — | — |
| Platelet count | 1.07 | .1152 | — | — |
| CCR | 0.15 | .0005 | 0.13 | .0001 |
| Age | 1.32 | .0761 | — | — |
| PB blast cells | 0.83 | .2496 | — | — |
| Spleen size | 1.18 | .0215 | — | — |
| Platelet count | 1.05 | .2033 | — | — |
| MCR | 0.20 | .0012 | 0.17 | .0001 |
| . | Full Model . | Final Model . | ||
|---|---|---|---|---|
| RH . | P Value . | RH . | P Value . | |
| Age | 1.38 | .0417 | — | — |
| PB blast cells | 0.84 | .2748 | — | — |
| Spleen size | 1.19 | .0172 | — | — |
| Platelet count | 1.07 | .1152 | — | — |
| CCR | 0.15 | .0005 | 0.13 | .0001 |
| Age | 1.32 | .0761 | — | — |
| PB blast cells | 0.83 | .2496 | — | — |
| Spleen size | 1.18 | .0215 | — | — |
| Platelet count | 1.05 | .2033 | — | — |
| MCR | 0.20 | .0012 | 0.17 | .0001 |
The variables were tested with different thresholds, ie, age (every 10 years), PB blast cells (every 1%), spleen size (every 1 cm), platelet (every 100 × 109/L). The relative hazard (RH) means the risk of death (multiplier) for each factor from the full and final models.
CHR at 3 months is the best predictive factor for cytogenetic response.
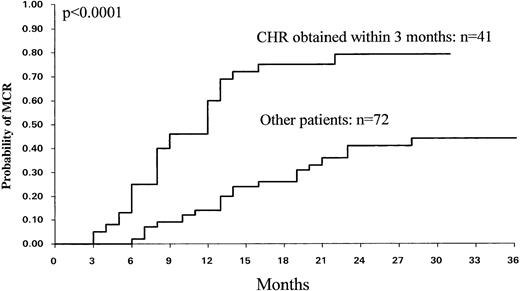
By univariate analysis a number of patient-related and disease-related variables were tested (age, sex, WBC count, platelet count, splenomegaly, PB cells, percentage Sokal score) for their association with MCR and CCR. Percent of PB cells and Sokal categories were found to influence the MCR (P = .007) and CCR (P = .01) rates. The CHR rate was also found to be significantly associated with CCR (P < .02) and MCR (P < .002). However, the CHR rate at 3 months was the most significant factor for predicting MCR (P < .0001) and CCR (P = .0001) (Fig 6). The probability of MCR for patients who achieved CHR at 3 months was 79% ± 14% (95% CI), whereas it was 44% ± 15% for the others. Consequently, CHR at 3 months was also a strong predictive factor for EFS (P = .005) and overall survival (P = .006).
Probability of MCR (n = 113) among the patients who achieved a CHR within 3 months (n = 41) and the other patients (n = 72).
Probability of MCR (n = 113) among the patients who achieved a CHR within 3 months (n = 41) and the other patients (n = 72).
Of the 113 evaluable patients, 41 achieved CHR within 3 months (group 1), 29 patients between 3 and 6 months (group 2), and 43 had not achieved CHR at 6 months (group 3). The clinical characteristics including Sokal score did not differ between the three groups (data not shown). The actuarial rate of MCR was significantly higher for group 1 than for group 2 (P = .002), and higher for group 2 than for group 3 (P = .002).
By multivariate analysis compared with the factors included in the Sokal score, achieving CHR at 3 months (P < .00001, relative risk = 4.38) and PB blast (P = .03, relative risk = 0.78) were confirmed to be the best statistically significant factors for predicting MCR.
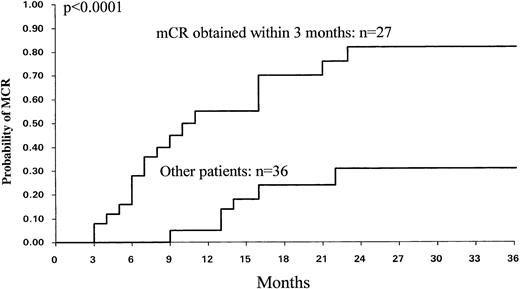
For 63 patients, a cytogenetic analysis was performed at 3 months and mCR was found in 27 cases. mCR was a significant factor for predicting MCR (P = .0001) and CCR (P = .024). For these 27 patients the probability to develop MCR was 82% ± 18% (95% CI) compared with 30% ± 20% (95% CI) for the other 36 patients (Fig 7).
Probability of MCR (n = 63) among the 27 patients who obtained an mCR at 3 months (≥5% of Ph− cells) and the other 36 patients.
Probability of MCR (n = 63) among the 27 patients who obtained an mCR at 3 months (≥5% of Ph− cells) and the other 36 patients.
DISCUSSION
In this unicentric study, we have confirmed that IFN-α can induce hematological and cytogenetic responses in a substantial proportion of untreated patients with newly diagnosed CML, and we have analyzed the factors that could predict the response to IFN-α and prolong survival. Among 116 patients with newly diagnosed Ph+ CML treated only with IFN-α, 80% achieved a CHR and 43% had an MCR. The estimated median survival for the total study group was not reached at 110 months. We confirm that achievement of an MCR is statistically associated with survival prolongation.
Our results are very close to those reported by Kantarjian et al in a unicentric study from the MD Anderson Cancer Center in Houston. In 274 patients they obtained 80% CHR and 38% MCR rates.9 These results, like ours, look better than those reported in other randomized or nonrandomized multicentric studies.10-14 Several factors may explain these differences. First, according to their Sokal classification, most of our patients belong to the low- and intermediate-risk groups, as most of the high-risk patients seen in our institution usually underwent autologous or allogeneic transplantation.19 The ratio of low- to high-risk patients, which is useful to compare the different cohorts, was higher in our study and in the Houston series compared with that of the other studies.24 Second, we used IFN-α at an initial dose of 5 mU/m2/d and tried to maintain this to obtain a large myelosuppression to maintain the WBC count around 2.5 × 109/L or 3 × 109/L rather than 5 × 109/L. This dose is higher than that used in some studies and, as reported elsewere, we think that the IFN-α dose could influence the response rate.25 Third, the mean number of patients treated in each individual center was lower in the multicentric studies than in our study or the Houston one, so the results of IFN-α treatment might have been influenced by the number of patients treated in each individual center. Indeed it has been suggested that the response to IFN-α treatment is higher in unicentric studies than in multicentric ones.26 For these reasons, we decided to pursue IFN-α in these patients, who had nevertheless been informed of the potential benefit of continuing their treatment (in the light of our previous findings).
In the Houston study, median survival was 89 months and achieving a cytogenetic response was associated with a statistically longer survival. By incorporating MCR as a time-dependent variable, it was considered as an independent prognostic factor for survival.9 In contrast, in the Cancer and Leukemia Group B study, a positive relationship between cytogenetic response and survival was not found, but the number of patients with strict MCR was small.13 In the German study, cytogenetic responders in the IFN-α arm had no significant survival advantage over cytogenetic nonresponders, and no beneficial effect of IFN-α on survival compared with hydroxyurea was found in this trial, unlike in the Italian study.10,14 These discrepancies between trials have been debated and explained.27 Other studies have reported the survival benefit of IFN treatment even among patients with no cytogenetic response.11 A meta analysis from the CML Trialists Collaborative Group based on seven randomized trials showed a survival advantage in the IFN-α group compared with the chemotherapy group.28 Recently, in a randomized trial, the French CML study group showed an advantage of the combination of IFN-α and cytarabine as compared with IFN-α alone.29 It reported that cytogenetic response was associated with a longer survival by landmark analysis in both arms of the trial.30
In our study, the achievement of cytogenetic response (MCR or CCR) was significantly associated with prolonged survival (P = .0001). Using univariate analysis, the Sokal index and platelet count were also found to influence survival, so we included the baseline variables of the Sokal score in the proportional hazards regression model with MCR and CCR as time-dependent covariates. In the final model, only MCR or CCR were included (P < .0001), showing that cytogenetic response is an independent factor for predicting survival, whatever the Sokal score. It has already been emphasized that the incidence of cytogenetic response parallels survival trials; in our study we have obtained the longest estimated median survival (>110 months) and the highest rate of MCR (43%) reported to date. However, 46 other patients were deleted in our study because they were not treated by IFN-α alone during the same period. Moreover, we have to adjust these results with the fact that follow-up of our study is relatively short compared with the estimated median survival.
We have also attempted to determine the factors that could predict the response to IFN-α and particulary MCR. Kantarjian et al30found that the percentage of PB blast cells and the degree of thrombocytosis correlated with the response to IFN therapy. The Italian Cooperative Study Group found that CHR at 8 months was also associated with a good response rate.10 In our study with univariate analysis, Sokal score and PB blast were found to influence MCR. These variables are linked because Sokal score is calculated using the PB blast percentage. Achievement of CHR within 3 months was also a statistically significant factor for predicting MCR. So multivariate analysis (including baseline variables from Sokal index) confirmed that CHR at 3 months (P < .00001) and PB blast (P = .03) were the most significant factors associated with survival. Therefore, with a physical examination and blood cell count at 3 months, it would seem to be possible to predict those patients who will respond to IFN-α. Moreover, in our study of a subset of patients, the appearance of Ph− mitosis at 3 months was also found to be statistically significant for predicting MCR and CCR.
Despite the heterogeneous results of the different trials reported to date and the insufficient follow-up in some studies, survival seems to be related to cytogenetic response, so for those patients who do not achieve an MCR, alternative treatments must be proposed. Moreover, the recent report from the Seattle group on the deleterious influence of pretransplant IFN-α on the prognosis of transplantation from an unrelated donor suggests that early prognostic factors are needed to better determine the indication of alloBMT.31 In our experience, response at 3 months (hematological and cytogenetic) is the best predictive factor.
Therefore, it is possible to identify those patients who have very high probability of achieving MCR or CCR and will survive longer (and do not need a transplant). For those patients who did not respond at 3 months a further evaluation at 6 months could be indicated.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to J. Reiffers, MD, Université Victor Segalen, Bordeaux 2, 146 rue Léo Saignat 33076 Bordeaux, France.