Abstract
In the current study, we investigated whether the naive, poly I:C or interleukin-2 (IL-2)–induced natural killer (NK)/lymphokine-activated killer (LAK) cells use perforin and/or Fas ligand (FasL) to mediated cytotoxicity. We correlated these findings with the ability of mice to reject syngeneic Fas+ and Fas− tumor cells either spontaneously or after IL-2 treatment. The spontaneous NK-cell–mediated cytotoxicity was primarily perforin based, whereas the poly I:C and IL-2–induced NK/LAK activity was both FasL and perforin dependent. L1210 Fas+ tumor targets were more sensitive than L1210 Fas− targets to poly I:C and IL-2–induced cytotoxicity in wild-type, gld/gld, and perforin knockout mice. When L1210 Fas+ and Fas– tumor cells were injected subcutaneously (sc) or intraperitoneally into syngeneic mice, Fas− tumor cells caused mortality earlier than Fas+ tumor cells. Also, approximately 20% of the mice injected sc with L1210 Fas+ tumor cells survived the challenge(>60 days), whereas all mice injected similarly with L1210 Fas− tumor cells died. When immunotherapy using IL-2 (10,000 U, three times/d for a week, followed by once/d for an additional week) was attempted in mice injected sc with tumor cells, IL-2 treatment was very effective against mice bearing L1210 Fas+ (40% survival) but not L1210 Fas− (0% survival) tumors. These data correlated with the finding that the LAK cells from IL-2–injected mice caused increased cytotoxicity against L1210 Fas+ when compared with L1210 Fas− targets. Also, L1210 Fas+tumor-bearing mice showed increased tumor-specific cytotoxic T lymphocyte (CTL) activity when compared with those bearing L1210 Fas− tumor cells. Together our studies show for the first time that expression of Fas on tumor targets makes them more immunogenic as well as susceptible to CTL- and IL-2–induced LAK activity. The Fas+ tumor cells are also more responsive to immunotherapy with IL-2.
NATURAL KILLER (NK) cells comprise an important component of the immune system mainly involved in surveillance against cancer and viral infections.1 NK cells exhibit spontaneous cytolytic activity against certain tumor cells and virally infected target cells in a major histocompatability complex–unrestricted manner. In addition, NK cells can be activated by binding of monoclonal antibodies (MoAbs) through CD16 receptor to mediate lysis of target cells called MoAb-dependent cell-mediated cytotoxicity. The NK cells can also be activated by interleukin-2 (IL-2) and interferon-γ (IFN-γ) inducers such as polyinosonic-polycytdylic acid (poly I:C) to mediate increased lysis of NK-sensitive targets as well as kill a broader panel of target cells, including NK-resistant cells.1,2 Such IL-2–induced cytolytic property shown by NK and T cells has been designated lymphokine-activated killer (LAK) cell activity.3
Recent studies have shown that cytotoxic T lymphocytes (CTLs) and NK cells can kill target cells using two distinct lytic pathways. First, the degranulation pathway which uses perforin possibly in combination with granzymes,4,5 and secondly, the Fas-based pathway in which the interaction between Fas-Ligand (FasL) expressed on cytolytic lymphocytes and Fas on target cells triggers apoptosis and target cell death.6,7 Moreover, all cytotoxic activity measured in a 4-hour assay can be attributed to perforin and FasL.8 9
Although NK cells have been known to mediate increased lysis of target cells including those that are NK resistant when activated with IL-2 or IFN-γ inducers in vivo, it is not clear whether such lytic activity results from upregulation of perforin and/or FasL. Inasmuch as IL-2 is used to activate LAK cells in the immunotherapy of certain types of cancer,10 it is important to investigate whether such treatment augments both perforin and FasL-based pathways and therefore whether it is effective against both Fas+ and Fas− tumors.
Although the role played by FasL in the cytotoxicity mediated by CTL and NK cells is well established, the outcome of expression of Fas on the tumor cells and its ability to trigger the antitumor immunity in the host is not clear. In the current study, we used perforin-deficient and FasL-defective mice to address the role of perforin and FasL in LAK cell–mediated cytotoxicity and Fas+ and Fas− L1210 tumor cells to delineate the role played by Fas in tumor growth and induction of antitumor immunity. The data showed that IL-2 and poly I:C triggered the induction of both FasL- and perforin-based cytolytic activity. Also, the survival rate in mice injected with Fas+ L1210 tumor cells was better than that observed in mice receiving Fas− L1210 cells, due to the fact that the Fas+ tumor cells induced stronger NK/LAK and tumor-specific CTL activity when compared with Fas− tumor cells.
MATERIALS AND METHODS
Mice.
C57BL/6+/+ (+/+) and DBA/2 mice were purchased from Charles River (Boston, MA). C57BL/6 gld/gld (gld) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and perforin knockout (KO) mice11 were generously provided by Dr W.R. Clark (University of California, Los Angeles, CA). The perforin KO and gld mice were of C57BL/6 origin. The gld and perforin KO mice were bred in our facilities.12 All mice used in the current study were female and were 3 to 4 weeks old.
Cell lines.
The tumor cell lines used were as follows: YAC-1, a Moloney virus–induced lymphoma sensitive to NK cells; P815, a mastocytoma resistant to NK cells; L1210 (Fas+ and Fas−), an NK-resistant DBA/2-derived mouse lymphoma transfected with sense and antisense Fas cDNA.13 All cell lines were maintained in tissue culture medium, RPMI-1640 (GIBCO, Grand Island, NY), and supplemented by 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), 2 mmol/L glutamine, 50 mmol/L 2-mercaptoethanol, 10 mmol/L HEPES, and 40 mg/mL gentamycin, as previously described.14
In vitro growth characteristics of L1210 Fas+ and L1210 Fas− tumor cells.
L1210 Fas+ and L1210 Fas− tumor cells were cultured in tissue culture flasks at a concetration of 2 × 105 cells/mL. At 12, 24, 36, 48, 60, and 72 hours after in vitro culture, the viable cell count was performed using trypan blue dye exclusion.
Flow cytometric analysis of Fas.
The tumor cell lines were screened for the expression of Fas by incubating the cells with normal mouse serum initially to block the Fc receptors. Next, the cells were washed and incubated with anti-Fas MoAbs (Jo2; Pharmingen, San Diego, CA) at 4°C for 30 minutes. The cells were washed and stained with fluorescein isothiocyanate (FITC)-conjugated anti-hamster IgG [F(ab′)2]. The negative controls were stained with normal isotype–matched antibody and the FITC-conjugated secondary antibody. The cells were analyzed using a flow cytometer (Epics V, Model 752; Coulter Corp, Miami, FL).
LAK/NK cells.
NK cells were purified as previously described.15 Briefly, single-cell suspensions of the spleen were prepared in RPMI-1640 medium supplemented with 5% fetal calf serum16 using a homogenizer (Stomacher; Tekmar Co, Cincinnati, OH). Plastic adherence for 1 hour at 37°C was used to deplete macrophages. The cells were then passed over nylon wool columns and the nonadherent cells used as a source of spontaneous NK activity. To study the inducible NK activity, the nonadherent cells were cultured for approximately 48 hours with 1,000 U/mL of IL-2 (kindly provided by Hoffman LaRoche, Nutley, NJ). In some experiments, NK activity was induced by intraperitoneal (IP) injection of poly I:C (Sigma Chemical Company, St Louis, MO), at 15 mg/kg suspended in phosphate-buffered saline (PBS).2 In other experiments, NK cells were activated in vivo by IP injection of 10,000 U of IL-2 three times a day for 5 days.
Isolation of tumor-specific CTL.
DBA/2 mice were injected with 1 × 106 L1210 Fas+ or L1210 Fas− live tumor cells in 0.2 mL PBS subcutaneously (sc). On day 5, these mice were injected with 1,3-Bis(2-chloroethyl )-1-nitrosourea, an anticancer drug, at a concentration of 20 mg/kg body weight as described, so as to enhance the tumor-specific immune response.17,18 Five days later, the mice were killed, T cells were purified,18 and 5 × 106 cells were cultured in 24-well tissue culture plates with irradiated (2,000 R) L1210 Fas+ or L1210 Fas− tumor cells (5 × 103) in 2 mL of medium. After 5 days, the cells were obtained and viable cells were purified on Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient centrifugation. The cells were tested for cytotoxicity against 51Cr-labeled L1210 Fas+ or L1210 Fas− tumor cells. The specificity of the CTL was confirmed by testing the cytotoxicity against YAC-1 and P815 targets.
51Cr-release assay to measure cytotoxicity.
The cytotoxicity mediated by NK cells was studied using51Cr-release assay.14 Target cells (YAC-1, P815, L1210 Fas+, and L1210 Fas−) were labeled with 100 mCi 51Cr in the form of Na251CrO4, incubated at 37°C for 1 hour, washed three times, then seeded in 96-well plates (Costar, Cambridge, MA) at 5 × 103 cells/well along with varying numbers of effector cells. The plates were incubated at 37°C for 4 hours. After the incubation, the plates were harvested with the Titertech collecting system (Skatron Inc, Sterling, VA). The amount of 51Cr released by the target cells was measured using a γ-counter (TmAnalytic, Elk Grove Village, IL). Percent cytotoxicity was calculated as: (experimental release − control release)/(total release − control release) × 100. In these experiments, the control release was measured in the presence of target cells alone, which was usually less than 15%. Total release was measured by incubating target cells in the presence of 0.1% sodium dodecyl sulfate.
In blocking studies, the assays were performed in the presence of 2 μg/mL of anti-Fas MoAbs (Jo2; Pharmingen) or 100 nmol of concanamycin A (ICN Pharmaceuticals Inc, Costa Mesa, CA).19 In these experiments, the control release was measured both in the presence or absence of the inhibitors to ensure that the MoAbs or reagents alone did not alter control release.
In vivo growth characteristic of L1210 tumor cells and immunotherapy.
Groups of five DBA/2 mice were injected sc or IP with either 1 × 106 L1210 Fas+ or L1210 Fas−tumor cells suspended in 0.2 mL PBS. These mice were injected IP with 10,000 U IL-2 (kindly provided by Hoffman-LaRoche), and suspended in 0.1 mL PBS 3 times/d for 7 days, followed by once a day for an additional 7 days. The control mice received PBS in a similar fashion. The mice were observed for tumor growth and survival for 60 days, after which the experiment was terminated. These experiments were repeated with consistent results. The mean survival time (MST) was calculated for each group and compared statistically using a t-test. The MST for mice that were alive on day 60 was considered to be 60.
RESULTS
Spontaneous, poly I:C-activated, and IL-2–activated NK/LAK activity in wild-type, perforin-deficient and FasL-defective mice.
To study the role of perforin and FasL in NK/LAK-cell activity, we used wild-type, perforin-deficient, or FasL-defective (gld/gld) mice. Also, to investigate the effect of activation, the spontaneous NK-cell activity was compared with that observed after in vitro culture of cells with IL-2 or after in vivo administration of poly I:C.
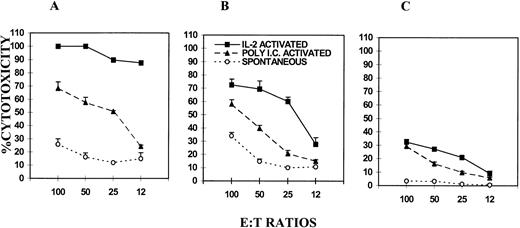
The data shown in Fig 1A, B, and C shows that IL-2–activated NK cells showed maximum cytolytic activity against NK-sensitive YAC-1 targets in all three groups of mice, followed by poly I:C-activated and spontaneous lytic activity. Furthermore, FasL-defective mice showed a similar degree of spontaneous lysis when compared with the wild-type mice. However, perforin-deficient mice showed virtually no spontaneous cytolytic activity. It should be noted that YAC-1 tumor cells were Fas+(Fig 2). These data demonstrated that the spontaneous NK activity was primarily perforin based. The fact thatgld/gld mice had similar levels of spontaneous NK activity as the wild-type mice suggested that FasL was not critical during spontaneous lysis.
Comparison of spontaneous, poly I:C-, and IL-2–induced NK/LAK-cell activity in C57BL/6 wild-type, gld/gld, and perforin KO mice. NK/LAK cells from wild-type (A), gld/gld (B), and perforin KO (C) mice were tested for cytotoxicity against NK-sensitive YAC-1 tumor targets. The same symbols were used in (A), (B), and (C) to depict different types of cytotoxicity. The cytotoxicity was studied using 51Cr-release assay and the mean percent cytotoxicity ± SEM of triplicate culture were plotted.
Comparison of spontaneous, poly I:C-, and IL-2–induced NK/LAK-cell activity in C57BL/6 wild-type, gld/gld, and perforin KO mice. NK/LAK cells from wild-type (A), gld/gld (B), and perforin KO (C) mice were tested for cytotoxicity against NK-sensitive YAC-1 tumor targets. The same symbols were used in (A), (B), and (C) to depict different types of cytotoxicity. The cytotoxicity was studied using 51Cr-release assay and the mean percent cytotoxicity ± SEM of triplicate culture were plotted.
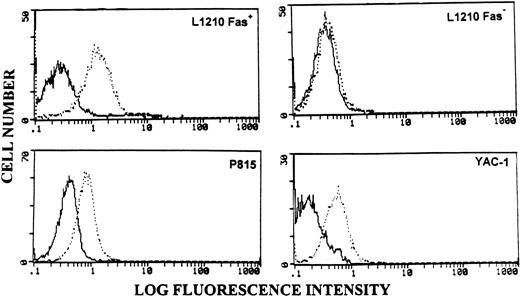
Flow cytometric analysis of tumor targets for the expression of Fas. Tumor cell lines were stained with isotype control (bold histograms) or anti-Fas MoAbs (broken histograms), followed by FITC-conjugated secondary antibodies and analyzed flow cytometrically.
Flow cytometric analysis of tumor targets for the expression of Fas. Tumor cell lines were stained with isotype control (bold histograms) or anti-Fas MoAbs (broken histograms), followed by FITC-conjugated secondary antibodies and analyzed flow cytometrically.
When poly I:C and in vitro IL-2–activated NK/LAK-cell activity were compared in the three groups of mice, it was noted that the wild-type mice showed the highest level of cytotoxicity, followed by thegld/gld mice and perforin-deficient mice. The poly I:C-induced NK activity was comparable between wild-type and gld/gld mice, whereas it was markedly reduced in perforin-deficient mice. These data suggested that in poly I:C-induced NK activity, perforin played a more important role than FasL. In contrast, the IL-2–induced NK activity was based both on FasL and perforin, inasmuch as both perforin-deficient and gld/gld mice showed significant cytotoxicity and the wild-type mice showed the highest level of cytotoxicity. However, even in IL-2–induced NK activity, perforin played a more important role because perforin KO mice had much lower level of cytotoxicity than gld/gld mice.
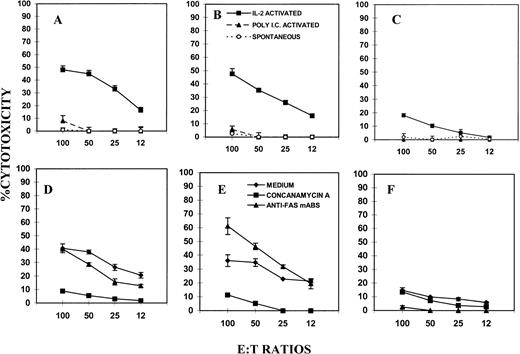
Further studies were conducted to investigate the role of perforin and FasL in cytotoxicity against NK-resistant P815 tumor cells as targets, which were also found to be Fas+ (Fig 2). The results shown in Fig 3 suggested that P815 tumor targets were resistant to spontaneous and poly I:C-induced cytotoxicity. However, in vitro IL-2–activated LAK cells were able to kill P815 cells in all three groups of mice tested. Moreover, in such IL-2–induced cytolytic activity, both perforin and FasL played a significant role, inasmuch as both perforin-deficient (Fig 3C) and FasL-defective (Fig 3B) mice showed cytolytic activity against P815. However, perforin KO mice expressed lower levels of cytotoxicity than the gld/gld mice, thereby suggesting that P815 cells were more sensitive to perforin than FasL.
Spontaneous and induced NK/LAK activity against P815 tumor targets. NK cells from wild-type (A and D), gld/gld (B and E), and perforin KO (C and F) mice were tested for cytotoxicity against NK-resistant P815 tumor targets as described in Fig 1. In (D), (E), and (F), in vitro IL-2–activated LAK cells were used as effectors and cytotoxicity was performed in the presense of medium, concanamycin A (100 nmol/L), or anti-Fas MoAbs (2 μg/mL). The symbols used in (B) are common to (A) and (C). Also, symbols shown in (E) are similar to those used in (D) and (F).
Spontaneous and induced NK/LAK activity against P815 tumor targets. NK cells from wild-type (A and D), gld/gld (B and E), and perforin KO (C and F) mice were tested for cytotoxicity against NK-resistant P815 tumor targets as described in Fig 1. In (D), (E), and (F), in vitro IL-2–activated LAK cells were used as effectors and cytotoxicity was performed in the presense of medium, concanamycin A (100 nmol/L), or anti-Fas MoAbs (2 μg/mL). The symbols used in (B) are common to (A) and (C). Also, symbols shown in (E) are similar to those used in (D) and (F).
To further address the role of FasL and perforin in the killing of P815 tumor targets by IL-2–activated LAK cells, the cytotoxicity was measured in the presence of concanamycin A, which is known to inhibit perforin-based but not FasL-based cytotoxicity,19,20 and anti-Fas MoAbs (Jo2) known to inhibit FasL-based cytotoxicity.21 The data shown in Fig 3D, E, and F indicated that anti-Fas antibody caused significant inhibition in the lysis of P815 targets by LAK cells from wild-type and perforin-deficient mice but failed to inhibit the lysis mediated by LAK cells from gld/gld mice. Moreover, concanamycin A inhibited the LAK activity from wild-type and gld/gld mice but not from perforin-deficient mice. Together, these data corroborated the observation that the lysis of P815 target cells by LAK cells was both perforin and FasL based, although perforin played a more important role than FasL.
To ensure that the IL-2–induced cytolytic activity was primarily dependent on LAK cells (T cells + NK cells) but not macrophages, the cells were analyzed for CD3, NK1.1, and Mac-3 in all three strains of mice. The data shown in Table 1 indicated that wild-type, gld/gld, and perforin-deficient mice had similar level of T cells and NK cells before and after IL-2 activation. Also, such cultures were devoid of macrophages. The data shown in Table1 also indicated that perforin-deficient and gld/gld mice had similar proportion of T and NK cells when compared with the wild-type mice. These data are consistent with recent studies from our lab in which it was noted that IL-2 treatment in vivo caused identical phenotypic changes in lymphocytes from wild-type, gld/gld, and perforin KO mice.22
Expression of NK1.1, MAC-3, and CD3 in the Spleen After IL-2 Stimulation
| Strain . | Medium . | IL-2 . | ||||
|---|---|---|---|---|---|---|
| CD3 . | NK1.1 . | MAC-3 . | CD3 . | NK1.1 . | MAC-3 . | |
| Wild-type | 76.85 ± 2.85 | 14.75 ± 2.25 | 0.2 ± 0 | 79.35 ± 6.35 | 11.75 ± 3.25 | 1.5 ± 1.2 |
| PD | 83.7 ± 10.8 | 9.05 ± 0.15 | 0.9 ± 0.5 | 85.15 ± 5.05 | 6.1 ± 2.1 | 0.65 ± 0.35 |
| gld | 83.15 ± 8.05 | 13.9 ± 4.3 | 0.75 ± 0.35 | 85.85 ± 2.35 | 15.2 ± 1.1 | 0.35 ± 0.25 |
| Strain . | Medium . | IL-2 . | ||||
|---|---|---|---|---|---|---|
| CD3 . | NK1.1 . | MAC-3 . | CD3 . | NK1.1 . | MAC-3 . | |
| Wild-type | 76.85 ± 2.85 | 14.75 ± 2.25 | 0.2 ± 0 | 79.35 ± 6.35 | 11.75 ± 3.25 | 1.5 ± 1.2 |
| PD | 83.7 ± 10.8 | 9.05 ± 0.15 | 0.9 ± 0.5 | 85.15 ± 5.05 | 6.1 ± 2.1 | 0.65 ± 0.35 |
| gld | 83.15 ± 8.05 | 13.9 ± 4.3 | 0.75 ± 0.35 | 85.85 ± 2.35 | 15.2 ± 1.1 | 0.35 ± 0.25 |
Splenic nylon wool purified T cells from wild-type, PD, andgld mice were cultured with medium or IL-2 and stained for NK1.1, MAC-3, and CD3 markers and analyzed flow cytometrically. The data are depicted as mean percent positive cells ± SEM. Data are representative of three experiments. There was no statistically significant difference between various groups.
Abbreviation: PD, perforin-deficient.
NK/LAK-cell–mediated cytotoxicity against Fas+ and Fas− tumor targets in wild-type, perforin-deficient, and FasL-defective mice.
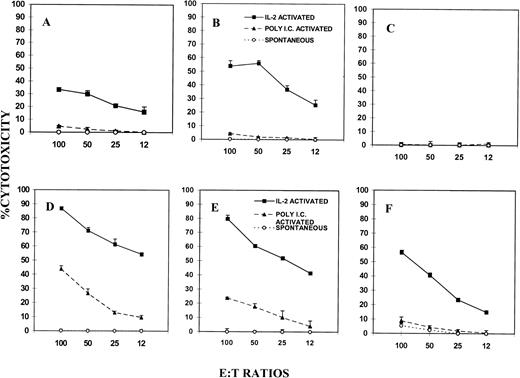
To exclude the possible variations observed in target cell susceptibility and to address the role of Fas, we used L1210 tumor cells which had been transfected with Fas-sense (Fas+) or antisense (Fas−).13 The expression of Fas on these cell lines was confirmed by flow cytometric analysis (Fig 2). As shown in Fig 4A, B, and C, Fas− L1210 tumor targets were resistant to spontaneous and poly I:C–activated killing by NK cells in all three groups of mice, similar to the NK-resistant P815 cells (Fig 2). However, after IL-2 activation, LAK cells from wild-type (Fig 4A) andgld/gld (Fig 4B) mice mediated significant lysis, whereas similar cells from perforin KO mice (Fig 4C) failed to show lytic activity. In fact, LAK cells from gld/gld mice mediated increased cytotoxicity when compared with the wild-type mice, the reason for which was not clear.
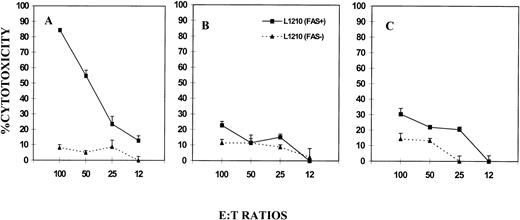
NK/LAK-cell–mediated cytotoxicity against Fas+ and Fas− tumor targets. NK/LAK cells from wild-type (A and D), gld/gld (B and E), and perforin-KO (C and F) mice were tested for cytotoxicity against L1210 Fas− (A, B, C) or L1210 Fas+ (D, E, F) tumor targets. The cytotoxicity was performed as described in Fig 1.
NK/LAK-cell–mediated cytotoxicity against Fas+ and Fas− tumor targets. NK/LAK cells from wild-type (A and D), gld/gld (B and E), and perforin-KO (C and F) mice were tested for cytotoxicity against L1210 Fas− (A, B, C) or L1210 Fas+ (D, E, F) tumor targets. The cytotoxicity was performed as described in Fig 1.
When L1210 Fas+ targets were similarly tested (Fig 4, lower panel), it was noted that they were resistant to spontaneous killing by the NK cells, similar to Fas− L1210 targets, in all three groups of mice. Interestingly, when NK cells activated with poly I:C were tested, the Fas+ targets became susceptible to cytotoxicity. Such poly I:C–induced cytotoxicity was both FasL and perforin based because both gld/gld (Fig 4E) and perforin KO (Fig 4F) mice showed significant cytotoxicity. However, such cytotoxicity was less than that observed in wild-type mice (Fig 4D). Similar results were also seen using LAK cells activated with IL-2 in vitro (Fig 4D, E, and F). Overall, when L1210 Fas+ and Fas− targets were compared (Fig 4, lowerv upper panel), Fas+ targets were found to be more susceptible to lysis after poly I:C or IL-2 activation of NK/LAK cells, thereby suggesting that activation of NK/LAK cells triggers not only the perforin-based but also the FasL-based pathway.
In vivo administration of IL-2 upregulates both perforin- and FasL-based cytotoxicity.
To further corroborate in vivo that IL-2 activation upregulates both perforin- and FasL-based cytolytic activity, wild-type,gld/gld, and perforin KO mice were administered with IL-2 for 5 days and the spleen cells were tested for lytic activity against Fas− and Fas+ L1210 tumor cells. The data shown in Fig 5 demonstrated that Fas+ targets were markedly more sensitive to cytotoxicity by in vivo IL-2–activated LAK cells from wild-type mice when compared with the Fas− L1210 tumor cells (Fig 5A). Also, the wild-type mice (Fig 5A) showed the highest level of cytotoxicity when compared with gld/gld (Fig 5B) or perforin-deficient (Fig 5C) mice, which showed moderate degree of lysis. These data indicated that in vivo IL-2 administration triggered both perforin- and FasL-based cytotoxicity and that such cytotoxicity was more effective against Fas+ tumor targets.
Cytotoxicity mediated by LAK cells activated in vivo with IL-2. Wild-type (A), gld/gld (B), or perforin KO (C) mice were injected with 10,000 U of IL-2 twice a day for 4 days and the LAK cells were tested for cytotoxicity against L1210 Fas− and L1210 Fas+ tumor targets. Cytotoxicity was studied as described in Fig 1.
Cytotoxicity mediated by LAK cells activated in vivo with IL-2. Wild-type (A), gld/gld (B), or perforin KO (C) mice were injected with 10,000 U of IL-2 twice a day for 4 days and the LAK cells were tested for cytotoxicity against L1210 Fas− and L1210 Fas+ tumor targets. Cytotoxicity was studied as described in Fig 1.
Effect of immunotherapy with IL-2 against Fas+ and Fas− L1210 tumor growth.
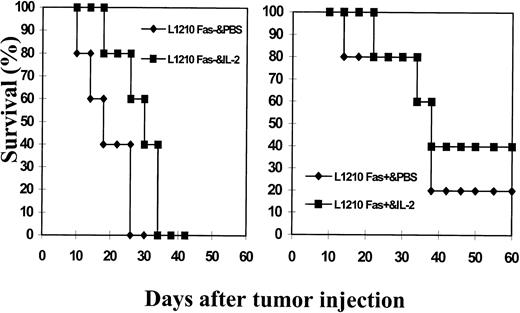
Inasmuch as the expression of Fas caused increased susceptibility of tumor cells to lysis by activated LAK cells, we next addressed whether Fas+ and Fas− L1210 tumor cells would exhibit differential ability to induce tumors in the syngeneic host and whether IL-2 immunotherapy would have varying effects on the growth of these tumor cell lines in vivo. To this effect, 1 × 106 Fas+ or Fas− L1210 tumor cells were injected sc and were administered either with PBS as a control or IL-2 (10,000 U three times/d for 1 week, followed by once/d for an additional week). The survival of the mice was monitored for approximately 60 days. As shown in Fig 6, all L1210 Fas− tumor cell + PBS-injected mice died with an MST = 18.2 ± 6.0 days. In contrast, IL-2–treated L1210 Fas−-bearing mice survived for longer periods (MST = 27.4 ± 4.6 days). This increase in MST resulting from IL-2 treatment was significant (P < .05) when compared with the MST in PBS-treated Fas− tumor-bearing mice. Interestingly, mice bearing Fas+ L1210–induced tumor survived for longer periods (MST = 37.9 ± 6.1 days), with a significant proportion (approximately 20%) surviving for more than 60 days, at which time the experiment was terminated. Thus, the MST in mice bearing Fas+ L1210 tumor was greater than those bearing Fas− tumor (P < .05). Moreover, IL-2 treatment of Fas+ tumor-bearing mice led to increased survival rate (40%) with an MST of 43.8 ± 15.5. Together, these data showed that mice bearing L1210 Fas+ tumor cells survived for longer periods than those bearing Fas−L1210 tumor cells. Furthermore, IL-2 therapy was more effective against Fas+ than Fas− tumor cells. It should be noted that IP injection of Fas+ and Fas−L1210 tumor cells into DBA/2 mice also led to distinct survival rates. The Fas− L1210 tumor cell–bearing mice all died on day 12, whereas L1210 Fas+ tumor-bearing mice died with an MST = 33.2 ± 7.6 (P < .01). However, IL-2 treatment was not effective against IP-injected tumor because IL-2–treated L1210 Fas+ tumor-bearing mice died with an MST of 31.5 ± 5.68 days, and Fas− tumor-bearing mice died with an MST of 18.4 ± 1.68 days.
Effect of immunotherapy with IL-2 against growth of L1210 Fas+ and L1210 Fas− tumor cells in syngeneic host. L1210 Fas+ and Fas− tumor cells (1 × 106) were injected sc into group of five syngeneic (DBA/2) mice. The mice were injected with PBS (control) or with IL-2 (10,000 U/mouse, 3 times a day for 1 week followed by once a day for additional 7 days). The mice were observed for tumor growth and survival. The experiment was terminated after 60 days.
Effect of immunotherapy with IL-2 against growth of L1210 Fas+ and L1210 Fas− tumor cells in syngeneic host. L1210 Fas+ and Fas− tumor cells (1 × 106) were injected sc into group of five syngeneic (DBA/2) mice. The mice were injected with PBS (control) or with IL-2 (10,000 U/mouse, 3 times a day for 1 week followed by once a day for additional 7 days). The mice were observed for tumor growth and survival. The experiment was terminated after 60 days.
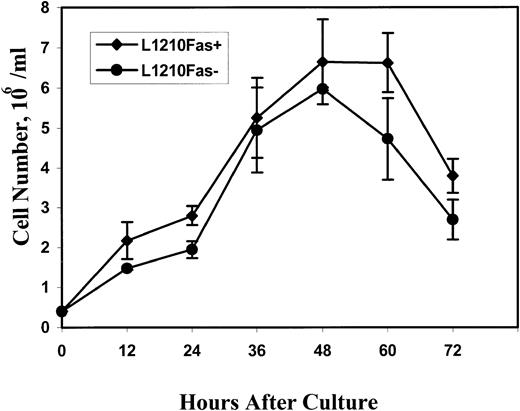
To rule out the possibility that the differences in the MST of mice injected with Fas+ and Fas− tumor cells were caused by alterations in the growth characteristics of the tumor cells, the in vitro growth pattern of the cells was investigated. The data shown in Fig 7 indicated that L1210 Fas+ and L1210 Fas− tumor cells had similar growth curves when tested for 12 to 72 hours after in vitro culture.
In vitro growth characteristics of L1210 Fas+ and Fas− tumor cells. The tumor cells were cultured in vitro as described in Materials and Methods and at various time intervals the cells were obtained and a viable count was determined. The data represent mean ± SEM of triplicate cultures.
In vitro growth characteristics of L1210 Fas+ and Fas− tumor cells. The tumor cells were cultured in vitro as described in Materials and Methods and at various time intervals the cells were obtained and a viable count was determined. The data represent mean ± SEM of triplicate cultures.
Tumor-specific CTLs also show preferential killing of Fas+ but not Fas− target cells.
Because the L1210 tumor cells could evoke CTL responses, T cells were purified from DBA/2 mice injected with Fas+ or Fas− L1210 tumor cells and cultured in vitro with respective irradiated tumor cells and tested for cytotoxicity against Fas+ and Fas− targets. The data shown in Fig 8A suggested that CTLs from Fas+ tumor bearers mediated stronger lytic activity against Fas+ as well as Fas− tumor targets, whereas CTLs from Fas− tumor-bearing mice showed weak or no significant lytic activity. These data demonstrated that Fas+ L1210 tumor cells triggered increased CTL activity when compared with the Fas− L1210 tumor cells, a finding which may account for increased survival of Fas+tumor-bearing mice when compared with the Fas−tumor-bearing mice. It should be noted that the CTL obtained from Fas+ tumor-bearing mice could kill Fas+ or Fas− targets to the same extent. This suggested that such CTL-mediated cytotoxicity was primarily perforin rather than FasL mediated. To corroborate that the cytotoxicity in the above study was mediated by CTL, we tested the lytic activity against a nonspecific H-2d tumor cell line such as P815. The data shown in Fig 8B indicated that the CTL raised against L1210 tumors failed to kill P815 target cells, thereby indicating the involvement of L1210 tumor-specific CTL.
Increased tumor-specific CTL activity in mice bearing L1210 Fas+ but not L1210 Fas− cells. Purified T cells from DBA/2 mice bearing L1210 Fas+ or L1210 Fas− tumor cells were obtained and cultured with irradiated L1210 Fas+ and Fas− tumor cells, respectively, for 5 days. Next, the harvested cells were tested for cytotoxicity against L1210 Fas+ or Fas−tumor targets (A) or against P815 (B) as described in Fig 4. In the symbols, the first column represents source of T cells, the second shows stimulator cells, and the third depicts target cells tested. For example, Fas+ / Fas+ / Fas+represents T cells from mice bearing Fas+ tumor cells stimulated with Fas+ tumor cells in vitro and tested for cytotoxicity against Fas+ targets.
Increased tumor-specific CTL activity in mice bearing L1210 Fas+ but not L1210 Fas− cells. Purified T cells from DBA/2 mice bearing L1210 Fas+ or L1210 Fas− tumor cells were obtained and cultured with irradiated L1210 Fas+ and Fas− tumor cells, respectively, for 5 days. Next, the harvested cells were tested for cytotoxicity against L1210 Fas+ or Fas−tumor targets (A) or against P815 (B) as described in Fig 4. In the symbols, the first column represents source of T cells, the second shows stimulator cells, and the third depicts target cells tested. For example, Fas+ / Fas+ / Fas+represents T cells from mice bearing Fas+ tumor cells stimulated with Fas+ tumor cells in vitro and tested for cytotoxicity against Fas+ targets.
DISCUSSION
In the current study we showed that poly I:C or IL-2 triggers NK/LAK activity by enhancing both FasL- and perforin-based cytotoxicity. Fas+ tumor targets were found to be more sensitive to poly I:C- or IL-2–induced cytotoxicity when compared with the Fas− tumor targets. Also, mice bearing Fas+ L1210 tumor cells survived for longer periods and a significant proportion of mice injected sc with the tumor rejected the tumor, when compared with the mice bearing Fas− L1210 tumor cells, which died from rapid tumor growth. Moreover, IL-2 administration in vivo was shown to trigger both FasL- and perforin-based cytolytic activity and was able to increase the MST of both Fas+ and Fas−tumor-bearing mice, particularly on sc injection. Furthermore, IL-2 therapy was more effective in sc-injected Fas+tumor-bearing mice in which approximately 40% of the mice survived for more than 60 days, when compared with Fas−tumor-bearing mice in which 0% of the mice survived.
The role of perforin in NK/LAK-cell-mediated cytotoxicity has been well established.1 In addition, recent studies have shown the existence of a perforin-independent pathway in NK/LAK-cell– or T-cell–mediated cytotoxicity based on the interactions between FasL expressed on effector cells and Fas receptor on the target cells.23-25 In the current study, we investigated the role of perforin and FasL during spontaneous and induced NK/LAK activity using a variety of NK-sensitive and NK-resistant cells, as well as Fas+ and Fas− target cells. Using NK-sensitive YAC-1 targets, we noted that the spontaneous cytotoxicity was mainly perforin dependent, inasmuch as perforin-deficient mice showed no significant levels of cytotoxicity and the gld/gldmice showed cytotoxicity comparable with or higher than the wild-type mice. However, on activation with poly I:C in vivo or IL-2 in vitro, the inducible cytotoxicity against YAC-1 and P815 targets was dependent on both perforin and FasL. Moreover, in such cytolytic activity, perforin played a more important role because the perforin KO mice showed lower levels of inducible cytolytic activity when compared with the gld/gld mice. This can be explained by the fact that YAC-1 and P815 tumor targets expressed lower levels of Fas, and moreover, not all cells within the population expressed Fas.
Recently, freshly isolated human and murine NK cells were found to express FasL and mediate Fas-based cytotoxicity.26,27However, in the current study we noted that gld/gld mice had normal levels of spontaneous NK activity against YAC-1 targets. Also, the NK cells failed to mediate spontaneous cytotoxicity against NK-resistant L1210 Fas+ transfectants despite the fact that such cells expressed high levels of Fas. The reason for the differences in previous and current studies is not clear. One possibility is that in the earlier study,26,27 the targets used were different from those used in the current study. Thus, the use of FasL by NK cells mediating spontaneous cytotoxicity may depend on the nature of target cells and not merely on the expression of Fas on target cells. Also, in the earlier study,27 the investigators used a 12-hour fluorescent dye assay to study cytotoxicity. During such long-term cytotoxicity, molecules such as tumor necrosis factor have been shown to be involved, unlike short-term assays in which the cytotoxicity is mediated exclusively by FasL and perforin.9
In the current study, comparison between Fas+ and Fas− L1210 transfectants yielded interesting results. The Fas+ L1210 transfectants were more sensitive to poly I:C- and IL-2–activated NK/LAK-cell–mediated cytotoxicity when compared with Fas− L1210 target cells. These data together suggested that Fas expression on target cells can increase the susceptibility of the tumor targets to induced NK/LAK activity. The fact that perforin-deficient mice completely failed to mediate lysis of Fas− L1210 targets and that they could mediate lysis of Fas+ L1210 cells to a lower level than thegld/gld or wild-type mice suggested that perforin may play an important role in induced NK/LAK activity. It should also be noted that despite the strong expression of Fas on the L1210 tumor cells, FasL-based cytotoxicity as observed in perforin KO mice was lower than that seen in wild-type mice. Similarly, the perforin-based lytic activity observed in gld/gld mice was also less than that seen in wild-type mice. Thus, the presence of both perforin- and FasL-based cytotoxicity was clearly an advantage to efficiently kill Fas+ tumor targets.
To further corroborate and translate the in vitro results to tumor rejection in vivo, we compared the ability of Fas+ and Fas− L1210 transfectants to grow and induce tumors in syngeneic mice and tested whether IL-2 administration would inhibit the growth of these tumor cells. In these studies, it was striking that mice injected with Fas+ tumor cells survived for longer periods than those receiving Fas− tumor cells. Furthermore, a significant proportion of mice injected sc with Fas+ tumor cells could reject the tumor, and this was further enhanced after IL-2 administration. It should be noted that the IL-2 treatment was effective against sc- but not IP-injected tumors. This may be because IP-injected tumors may metastasize faster and kill the host earlier as evident from a shorter MST, thereby preventing an effective antitumor immunity to develop and act on the tumor cells. In contrast, the sc tumors may metastasize slower as observed from longer MST, because of which IL-2 treatment may be more effective. In the current study it was also noted that Fas+ tumor-bearing mice had higher levels of tumor-specific CTL activity when compared with the Fas− tumor-bearing mice. Also, T cells from Fas− tumor-bearing mice failed to mediate cytolytic activity against Fas+ targets. These data suggested that the expression of Fas on tumor cells may make them more immunogenic. Together, the current study shows that IL-2 therapy may be more effective against Fas+ tumors. Furthermore, transfection of the Fas gene into Fas− tumor cells may offer a novel approach to trigger antitumor immunity.
Supported in part by National Institutes of Health grants (AI01392 and HL 58641).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mitzi Nagarkatti, PhD, Dept of Biomedical Sciences & Pathobiology, VA-MD College of Vet Med, Phase II, Duckpond Dr, Virginia Tech, Blacksburg, VA 24061.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal